-
Medicinal plants play a pivotal role in traditional medical systems globally and they are essential for human health[1]. As traditional Chinese medicine (TCM) continues to evolve and the demand for natural remedies grows, the significance of medicinal plants has amplified. In recent years, many studies have discovered several natural active substances with anti-inflammatory, anti-cancer, and antiviral functions, with the majority of these potent ingredients being derived from medicinal plants[2−6].
The orchid family is the largest and most diverse family among flowering plants, and many orchids are rare and valuable medicinal materials[7]. In China, approximately 42 genera of orchid are used in traditional medicine. Dendrobium catenatum has effects such as antioxidation, antitumor, immunity enhancement, and blood glucose lowering, among others[8]. Cremastra appendiculata has impacts including heat-clearing, detoxification, lung moistening, cough relief, blood circulation activation, pain alleviation, and swelling reduction[9]. Bletillae rhizoma has effects of swelling reduction, bleeding cessation, and lung moistening[10]. The pharmacological activities of Gymnadenia conopsea includes antioxidant, anti-allergy properties, progenitor cell proliferation promotion, and hepatitis B virus surface antigen inhibition[11]. Pholidota chinensis has the effects of nourishing yin, promoting diuresis, eliminating blood stasis, and pain relief. It is often used for diseases such as dizziness, headache, cough, and hematemesis[12]. The main chemical compositions of the genus Bulbophylum are bibenzyls, phenylpropanoids, phenanthrenes, phenolic acids, glycosides, flavonoids as anti-inflammatory, anti-bacterial, anti-microbial, anti-oxidation, anti-cholinesterase, and other activities[13]. Gastrodia elata has analgesic, anti-epileptic, sedative, memory improvement, neuroprotective, and antioxidant activities, and is widely used in the treatment of nervous system diseases, and cardiovascular and cerebrovascular diseases[14]. Furthermore, Cypripedium henryi, Paphiopedilum malipoense, Cheirostylis chinensis, Coelogyne fimbriata, Liparis distans, and many other orchids are also precious Chinese herbal materials, which have certain effects on treating many diseases.
The Pleione genus, belonging to the orchid family, is highly valued for its medicinal properties[15]. Pleione grows on rocks or trees and possesses a pseudobulb that stores water and nutrients. The dried pseudobulb of the plant serves as the source of the TCM Pseudobulbus Cremastrae seu Pleiones (PCsP, 山慈菇)[16]. More than 1,400 years ago, in the Tang Dynasty, 'Supplements to Compendium of Materia Medica' recorded the pesticide effect of PCsP. According to the 2020 edition of the 'Pharmacopoeia of the People's Republic of China', PCsP has effects of relieving asthma, cough, inflammation, pain, and stopping bleeding[17]. Modern studies have shown that Pleione is rich in many active ingredients and exhibits pharmacological effects such as anti-tumor, anti-inflammatory, anti-oxidant effects, and reducing blood sugar levels[18]. The Pleione genus comprises approximately 33 species (including nine natural hybrids), predominantly found in China[15,19]. However, not all species are utilized for medicinal purposes. Notably, Pleione bulbocodioides (P. bulbocodioides), and Pleione yunnanensis (P. yunnanensis) have been recognized for their medicinal attributes in traditional Chinese medicine, specifically for the alleviation of asthma and anti-inflammatory effects. Furthermore, the medicinal applications of Pleione species extend beyond China to other Asian nations. In northeastern India, species such as Pleione humulis (P. humulis), Pleione praecox (P. praecox), and Pleione maculata (P. maculata) are employed for treating lacerations, wounds, colds, and liver ailments[20]. Similarly, in Nepal, both P. praecox and P. maculata serve as invigorating tonics and energy enhancers[21].
The Pleione orchid is a rich source of chemical diversity and has been extensively studied in botanical and pharmacological research[15,20]. In this review, the clinical applications of Pleione species have been critically evaluated for the first time, which has not been done in previous reviews. This is an important step to translate laboratory findings into clinical practice. The latest research progress has also been updated and concludes with the identification of research gaps and future directions, which will provide a progressive perspective. Here, the basic research on the chemical constituents, pharmacological effects, and clinical research of Pleione are reviewed, in order to provide a theoretical basis for the new drug development and clinical application of this plant. The focused review on the clinical applications of Pleione will deepen our understanding of its therapeutic potential and thus make this review a valuable addition to the field.
-
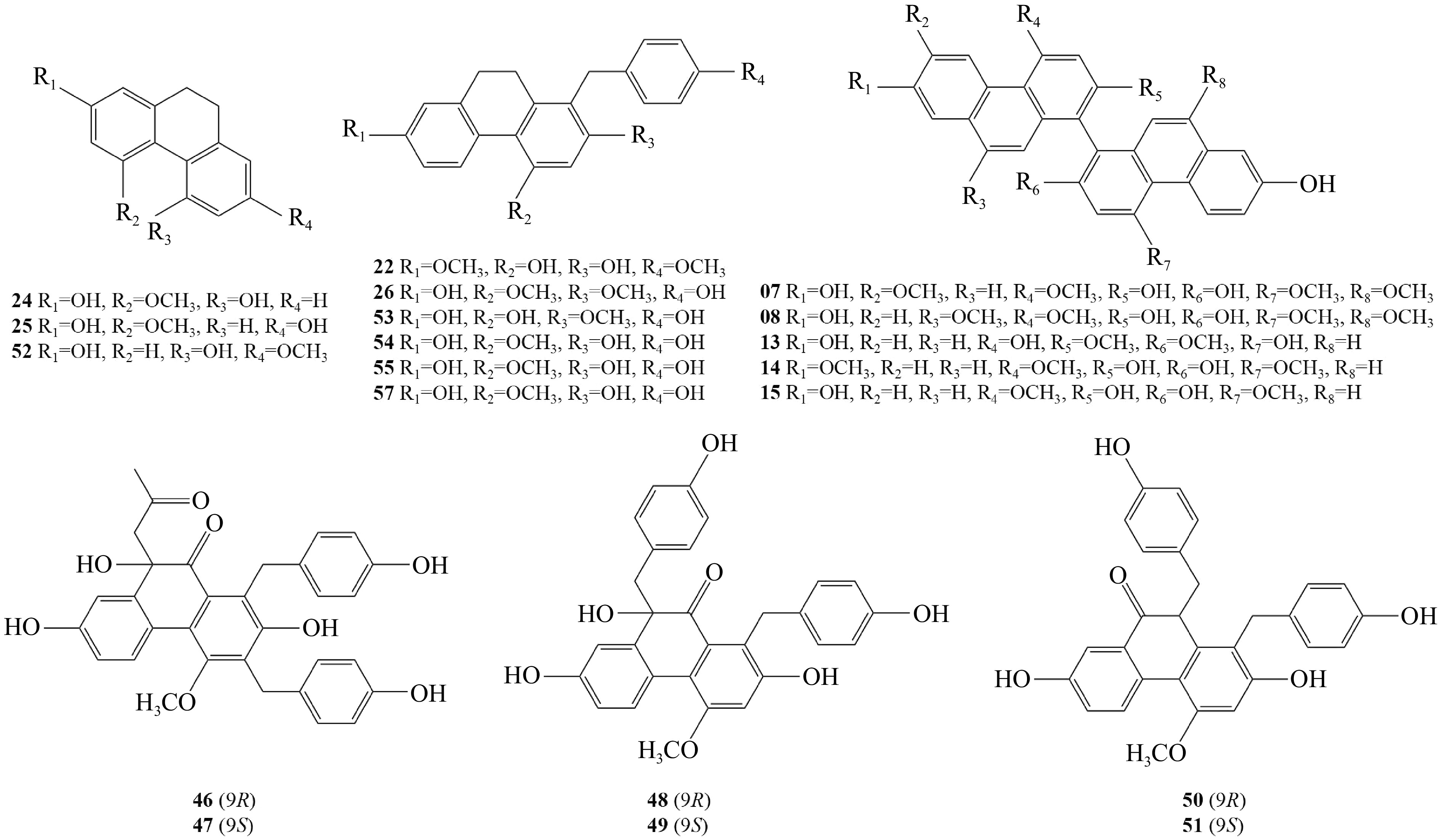
In recent years, with the increasing application of Pleione, the chemical constituents of this plant have been extensively studied by pharmacologists. So far, researchers have isolated several types of chemical components from Pleione, including phenanthrenes, bibenzyls, glucosyloxybenzyl succinate derivatives, flavones, lignans, and other compounds[22] (Fig. 1). These studies provide a reference for the basic research of Pleione and also lay a foundation for its quality control.
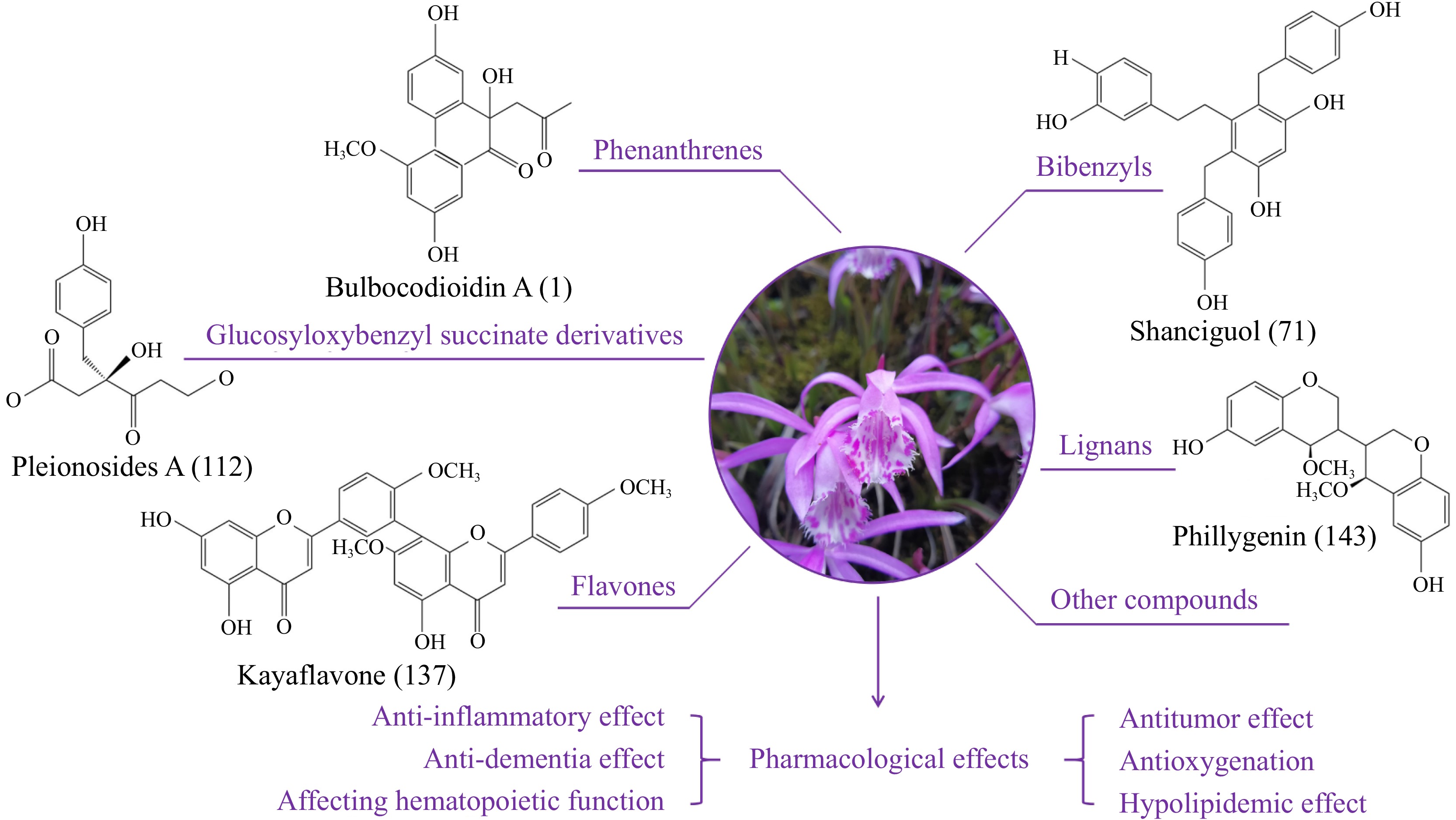
Figure 1.
Chemical constituents of Pleione. Phenanthrenes, bibenzyls, glucosyloxybenzyl succinate derivatives, flavones, lignans, and other compounds have been isolated from Pleione.
Phenanthrene compounds
-
Phenanthrene is a typical compound extracted from Pleione, and 63 phenanthrene derivatives have been isolated from this genus (Table 1). Twenty-six phenanthrenes and dihydrophenanthrenes compounds were isolated from the dried pseudobulbs of P. bulbocodioides[23,24]. Compounds 13−16, and 24 were isolated from Pleione for the first time, and compounds 1−5, 7−10, 13−14, and 20−25 exhibited potent DPPH radical scavenging activity. From the pseudobulbs of P. bulbocodioides, the following compounds have been isolated: shancilin (27)[25], bletilol A-C (28−30)[26], shanciols C-H (31−37)[27−30], two new phenanthro [2,3-b] furans (38, 39)[31,32], compounds 40−43[33], and four new pairs of enantiomers (44−51)[34] . Eleven phenanthrenes (52−61)[35,36] and two phenanthrenes (62, 63)[37] have been isolated from the pseudobulbs of P. yunnanensis and P. formosana, respectively. Compounds 24, 25, and 52 are simple dihydrophenanthrenes; compounds 26, 42, 53−55, 57, 58, 62, and 63 are benzyl-substituted dihydrophenanthrenes; compounds 16 and 43 are dihydrophenanthrene dimers; compounds 7, 8, 13−15, 56 are dimers of phenanthrene; compounds 9, 10, and 60 are phenanthrene and dihydrophenanthrene polymers; compounds 11, 12, 27, and 41 are dihydrophenanthrene and bibenzyl polymers; compound 33 is phenanthrene and phenylpropanoid polymer; compounds 44−51 are phenanthrene polymers, and other compounds are dihydrophenanthrene and phenylpropanoid polymers (Fig. 2).
Table 1. Phenanthrene compounds from Pleione.
No. Compound Ref. 1−11 Bulbocodioidins A−K [23] 12 (7'S,8'R)-7-hydroxy-7-(4'-hydroxy-3',5'-dimethoxy-phenyl)-8'-hydroxymethyl-5-methoy-9,10,7',8-tetrahydro-phenanthrene-[2,3-b]furan [23] 13 Monbarbatain A [23] 14 2,7,2'-trihydroxy-4,4',7'-trimethoxy-1,1'-biphenanthrene [23] 15 Blestriarene A [23] 16 Blestrianol A [23] 17 1-p-hydroxybenzy1-4-methoxy-9,10-dihydrophenanthrene-2,7-diol [23] 18 1-p-hydroxybenzy1-4-methoxyphenanthrene-2,7-diol [23] 19 Pleionesin E [23] 20 Shanciol H [23] 21 7-hydroxy-7'-(4'-hydroxy-3'-methoxy-phenyl)-4-methoxy-9,10,7',8'-tetrahydrophenanthrene-[2,3-b]furan-8'-yl-methyl acetate [23] 22 Pleionesin B [23] 23 Pleionesin D [23] 24 Hircinol [23] 25 Coelonin [23] 26 7-hydroxy-2,4-dimethoxy-1-(p-hydroxybenzyl)-phenanthrene [24] 27 Shancilin [25] 28 Bletilol A [26] 29 Bletilol B [26] 30 Bletilol C [26] 31 Shanciol [26] 32−37 Shanciols C−H [27−30] 38 (4'-hydroxy-3'-methoxyphenyl)-10-hydroxymethyl-11-methoxy-5,6,9,10-tetrahydrophenanthrene[2,3-b]furan-3-ol [31] 39 Hydroxy-9-(4'-hydroxy-3'-methoxyphenyl)-11-methoxy-5,6,9,10-tetrahydroohenanthrene-azaspiro[2,3-b]furan-10-yl)methylethyl [32] 40 2,7,2'-didroxy-4,4',7'-trimethoxy-1,1'-biphenanthrene [33] 41 Phoyunnanin A [33] 42 (4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol [33] 43 4,4',7,7'-tetrahydroxy-2,2'-dimethoxy-9,9',10,10'-tetrahydro-1,1'-biphenanthrene [33] 44 (9R) bulbocodioidin A [34] 45 (9S) bulbocodioidin A [34] 46 (9R) bulbocodioidin B [34] 47 (9S) bulbocodioidin B [34] 48 (9R) bulbocodioidin C [34] 49 (9S) bulbocodioidin C [34] 50 (10R) bulbocodioidin D [34] 51 (10S) bulbocodioidin D [34] 52 Lusianthridin [35] 53 4,7-dihydroxy-1-(p-hydroxybenzyl)-2-methoxy-9,10-dihydrophenanthrene [35] 54 2,7-dihydroxy-4-methoxy-1-(p-hydroxybenzyl)-9,10-dihydrophenanthrene [35] 55 2,7-dihydroxy-1-(p-Hydroxybenzyl)-4-methoxy-9,10-diphenanthrene [35] 56 Blestriarene C [35] 57 1-(p-hydroxybenzyl)-2,7-dihydroxy-4-methoxy-phenanthrene [35] 58 Shancidin [35] 59 Shancigusin G [35] 60 Blestriarene B [36] 61 Pleionesin A [36] 62 Pleioanthrenin [37] 63 (4-hydroxybenzyl)-4,7-dimethoxy-9,10-dihydrophenanthrene-2-ol [37] Bibenzyl compounds
-
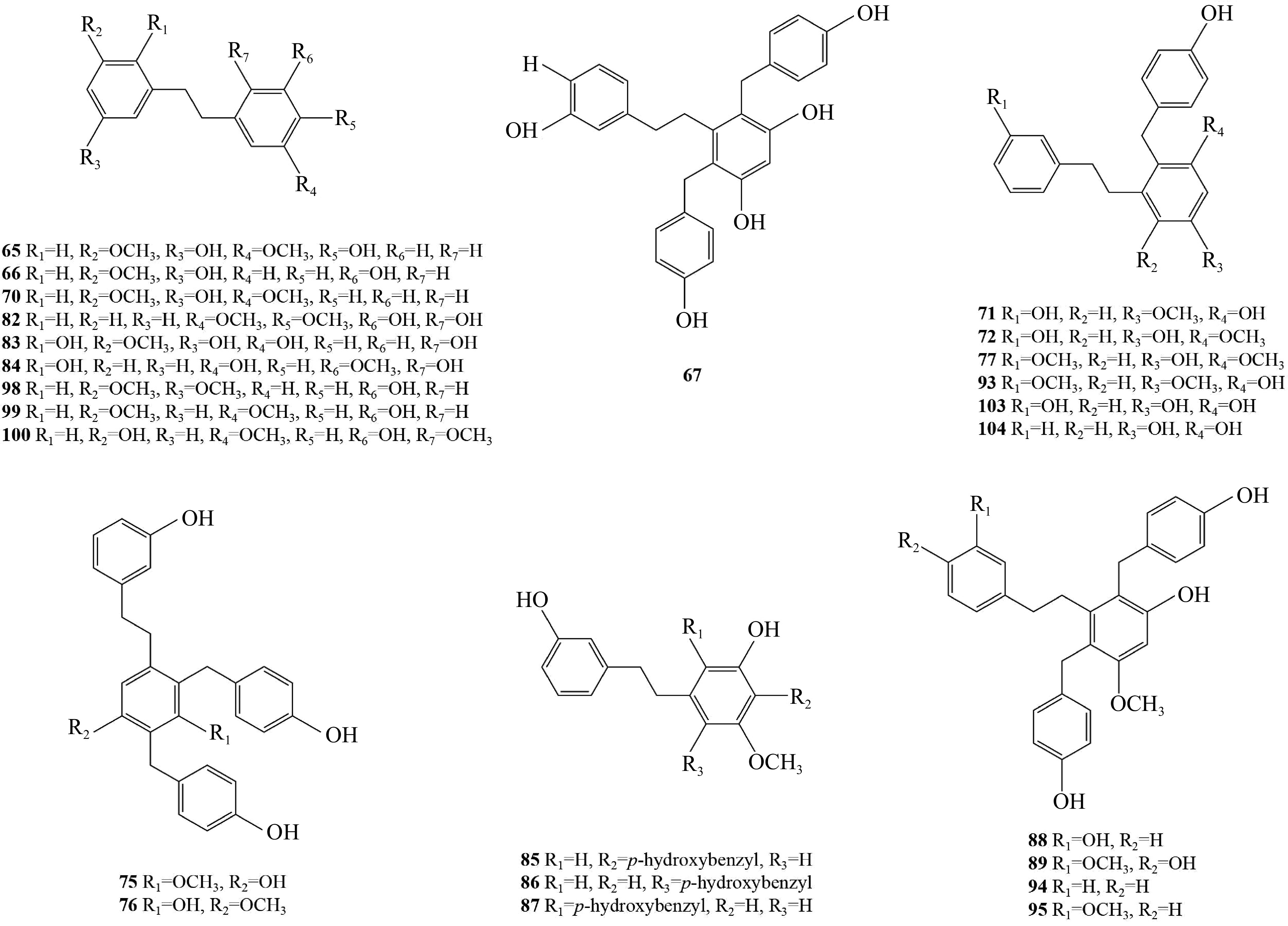
Bibenzyls are abundant in Pleione, and 43 bibenzyls have been isolated from this genus (Table 2). From the dried pseudobulbs of P. bulbocodioides, 30 bibenzyl compounds (64−93) were successfully isolated. Notably, the compound gigantol demonstrated significant DPPH radical scavenging activity[23−25,27,28,38,39,41]. Also, compounds 74−77 and 90 were isolated from this genus for the first time[28,31,39]. Additionally, two new bibenzyls (96, 97), along with two known compounds (94, 95), were isolated from the pseudobulbs of P. formosana[37]. From the pseudobulbs of P. yunnanensis, nine bibenzyl compounds (98−106) were also isolated[35,40,41]. Compounds 65, 66, 70, 82−84, and 98−100 are simple bibenzyls; compounds 64, 67, 71−74, 77, 85−89, 92−97, and 101−104 are benzyl substituted bibenzyls; compounds 75, and 76 are bibenzyl and fluorene polymers; compounds 90, 91, 105, and 106 are bibenzyl and glycoside polymers; compounds 68, and 69 are bibenzyl and phenylpropanoid polymers; compounds 78−81 are bibenzylamide polymers (Fig. 3).
Table 2. Bibenzyl compounds from Pleione.
No. Compound Ref. 64 3,3'-dihydroxy-2,6-bis(p-hydroxybenzyl)-5-methoxybibenzyl [23] 65 Gigantol [23,24] 66 Batatasin III [23,38] 67 Shanciguol [25] 68 Shanciols A [27] 69 Shanciols B [27] 70 3'-O-methylbatatasin III [38] 71 3,3'-dihydroxy-2-(p-hydroxybenzyl)-5-methoxybibenzyl [39] 72 3',5-dihydroxy-2-(p-hydroxybenzyl)-3-methoxybibenzyl [39] 73 3,3'-dihydroxy-4-(p-hydroxybenzyl)-5-methoxybibenzyl [39] 74 Bulbocodin [39] 75 Bulbocodin C [28] 76 Bulbocodin D [28] 77 Bulbocol [39] 78 Dusuanlansins A [33] 79 Dusuanlansins B [33] 80 Dusuanlansins C [33] 81 Dusuanlansins D [33] 82 Bauhinol C [33] 83 2,5,2',5'-tetrahydroxy-3-methoxybibenzyl [33] 84 2,5,2',3'-tetrahydroxy-3-methoxybibenzyl [33] 85 Arundinin [33] 86 Isoarundinin I [33] 87 Isoarundinin II [33] 88 5-O-Methylshanciguol [33] 89 Blestritin B [33] 90 2-(4''-hydroxybenzyl)-3-(3'-hydroxyphenethyl)-5-methoxy-cyclohexa-2,5-diene-1,4-dione [31] 91 6'-(3''-hydroxyphenethyl)-4'-methoxydiphenl-2,2',5'-triol [41] 92 Batatsin III-3-O-glucoside [41] 93 Gymconopin D [41] 94 Arundin [37] 95 2,6-bis-(4-hydroxybenzyl)-3',5-dimethoxy-3-hydroxybibenzyl [37] 96 Pleiobibenzynin A [37] 97 Pleiobibenzynin B [37] 98 3,5-Dimethoxy-3'-hydroxybibenzyl [35] 99 Hydroxy-3',5-dimethxoybibenzyl [35] 100 3,3'-dihydroxy-5-methoxybibenzyl [35] 101 Shancigusin A [40] 102 Shancigusin B [40] 103 Shancigusin C [40] 104 Shancigusin D [40] 105 Shancigusin E [35] 106 Shancigusin F [35] Glucosyloxybenzyl succinate derivatives
-
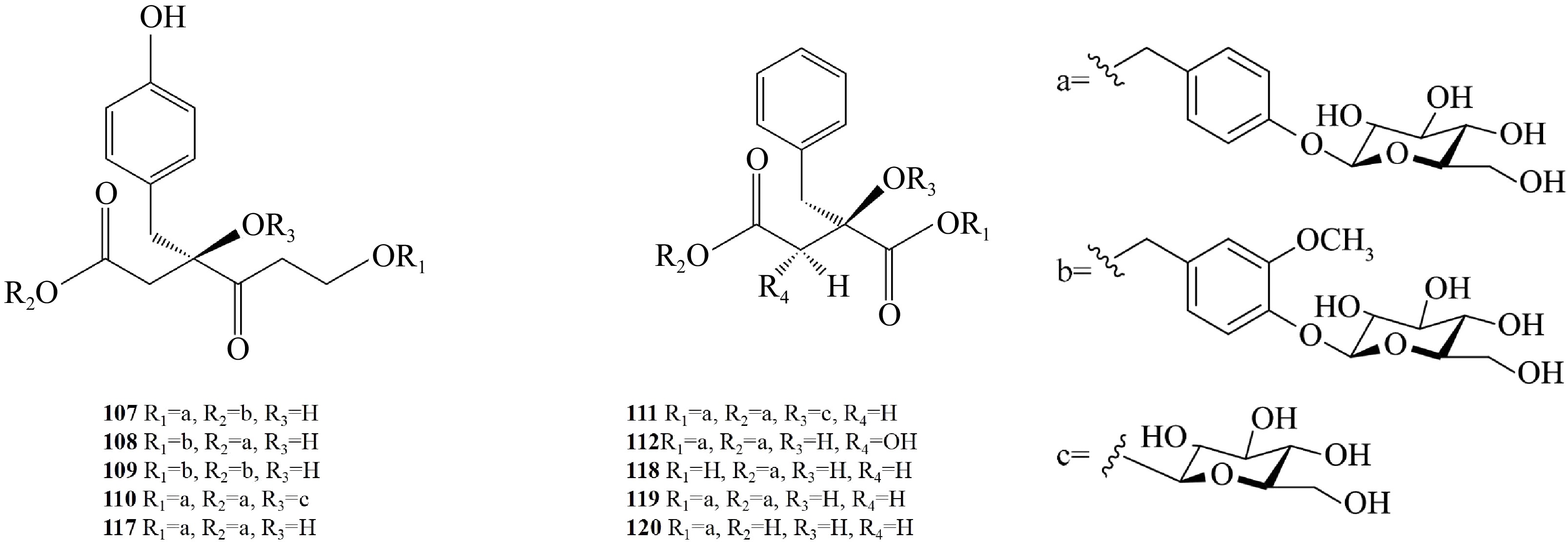
Glucosyloxybenzyl succinate derivatives are abundant in Pleione (Table 3). Pleionosides A−J (107−116) were isolated from the pseudobulbs of P. bulbocodioides and P. grandiflora[43,44]. They represent four kinds of acids, (2R)-2-p-hydroxybenzylmalic acid (107−110), (2R)-2-benzylmalic acid (111), (2R, 3S)-2-benzyl tartaric acid (112), and (2R)-2-isobutylmatic (113−116). Eight other glucosyloxybenzyl compounds (117−124) were also isolated from P. bulbocodioides[43]. Shancigusins H−I were isolated from the pseudobulbs of P. yunnanensis (125, 126)[35]. The basic structure of glucosyloxybenzyl succinate derivatives is succinic acid, which often combines with saccharides to form glycosides (Fig. 4).
Table 3. Glucosyloxybenzyl succinate derivatives from Pleione.
No. Compound Ref. 107 Pleionoside A [43,44] 108 Pleionoside B [43,44] 109 Pleionoside C [43,44] 110 Pleionoside D [43,44] 111 Pleionoside E [43,44] 112 Pleionoside F [43,44] 113 Pleionoside G [43] 114 Pleionoside H [43] 115 Pleionoside I [43] 116 Pleionoside J [43] 117 Vandateroside II [43] 118 Grammatophylloside A [43] 119 Grammatophylloside B [43] 120 Cronupapine [43] 121 Gymnoside I [43] 122 Militarine [43] 123 Dactylorhin A [43] 124 Loroglossin [43] 125 Shancigusins H [35] 126 Shancigusins I [35] Flavone compounds
-
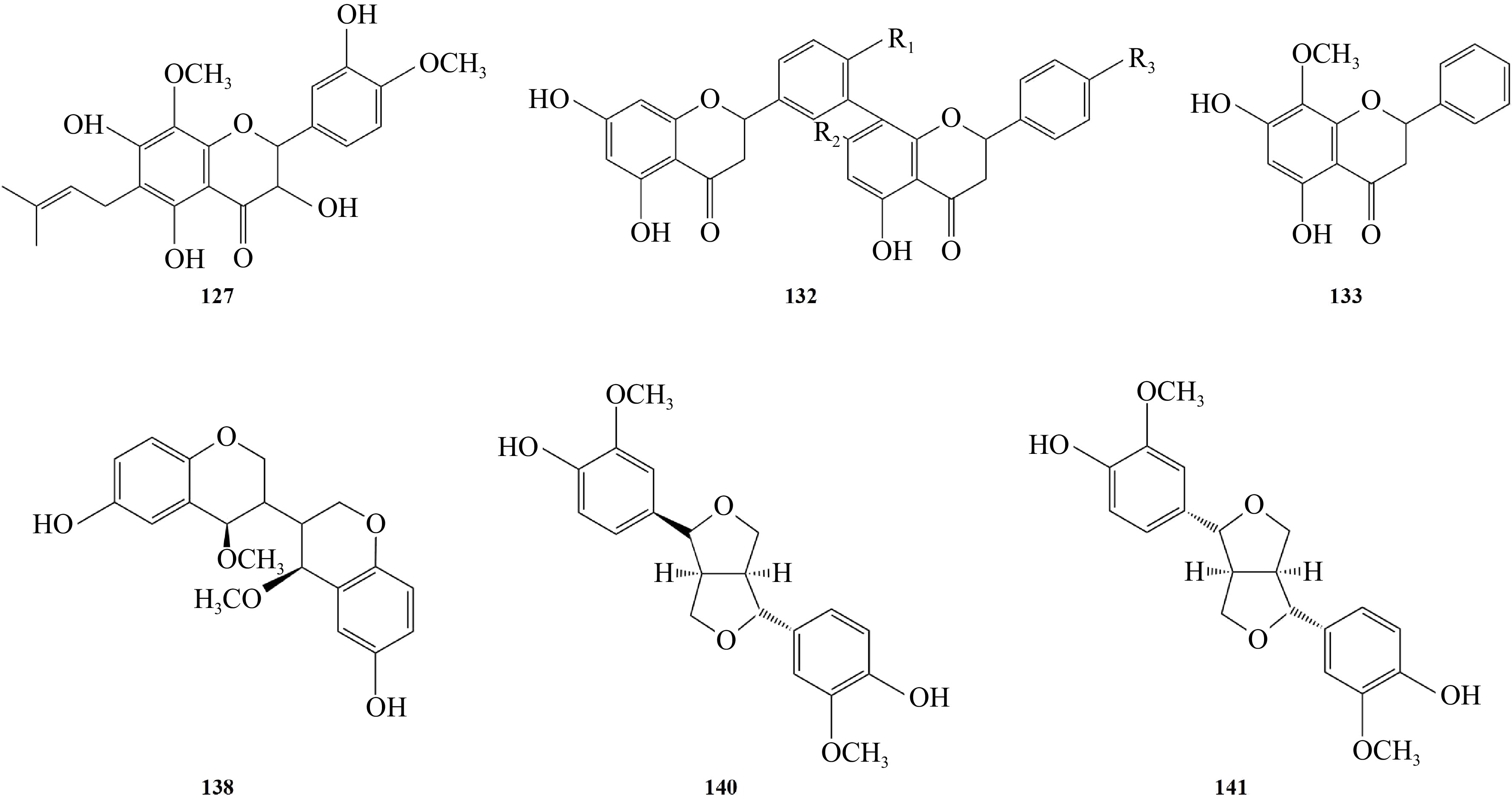
Seven flavones have been isolated from Pleione (Table 4). A new prenylated flavone (127), together with three known flavone derivatives (128−130), were isolated from the n-BuOH extract of P. bulbocodioides[45]. Amentoflavone (131), kayaflavone (132)[46], and 5,7-dihydroxy-8-methoxyflavone (133) were isolated from P. bulbocodioides[47]. Compounds 129, 130, and 133 are simple flavones; compounds 127, and 128 are prenylatedflavones; compounds 131, and 132 are bioflavonoids (Fig. 5).
Table 4. Flavone compounds from Pleione.
No. Compound Ref. 127 3,5,7,3'-tetrahydroxy-8,4'-dimethoxy-6-(3-methylbut-2-enyl)flavone [45] 128 3,5,3'-trihydroxy-8,4'-dimethoxy-7-(3-methylbut-2-enyloxy) Flavone [45] 129 Isorhamnetin-3,7-di-O-β-D-glucopyranoside [45] 130 3'-O-methylquercetin-3-O-β-D-glucopyranoside [45] 131 Amentoflavone [46] 132 Kayaflavone [46] 133 5,7-dihydroxy-8-methoxyflavone [47] Lignan compounds
-
Eight lignans have been isolated from Pleione (Table 5). Two isomerized lignan compounds (134, 135), syringaresinol mono-O-β-D-glucoside, lirioresinol, phillygenin, and (E)-p-hydroxycinnamic acid (136−139) were successively isolated from P. bulbocodioides[43,45,47−49]. Epipinoresinol (140) and syringaresinol (141) were isolated from the pseudobulbs of P. yunnanensis[35]. Compounds 134, 135, and 138 are simple lignans, and compounds 136, 137, and 139−141 are tetrahydrofuran lignans (Fig. 5).
Table 5. Lignan compounds from Pleione.
Other compounds
-
In addition to the above groups of compounds, many other compounds have been isolated from Pleione, such as aromatic, steroids, and aliphatic compounds (Table 6).
Table 6. Other compounds from Pleione.
No. Compound Ref. 142 Tetracosanol [23] 143 Gallicacid [23] 144 Tetacosanoic acid-2,3-dihydroxypropyl ester [23] 145 Chrysophanol [23] 146 Monopalmttin [23] 147 Methy(4-OH)phenylacetate [23] 148 Methyl3-(3-hydroxyphenyl)propionate [29] 149 5-hydroxymethylfurfural [29] 150 p-dihydroxy benzene [30] 151 β-sitosterol [35] 152 Daucostero [35] 153 Amber acid [35] 154 Adenosine [35] 155 (24R)-cyclomargenyl p-coumarate [37] 156 (24R)-cyclomargeno [37] 157 Pleionol [39] 158 p-hydroxybenzoic acid [41] 159 p-hydroxybenzaldehyde [41] 160 Ergosta-4,6,8(14),22-tetraen-3-one [24] 161 (7S,8R)-dehydrodiconiferyl [43] 162 Gastrodin [43] 163 Gastrodioside [45] 164 Phenl-β-D-glucopyranoside [45] 165 Hydroquinone [46] 166 Methyl4-hydroxyphenylacetate [46] 167 Physcion [46] 168 4,4'-dihydroxydiphenylmethane [47] 169 Pleionin [48] 170 3-hydroxybenzenepropanoic acid [49] 171 Cinnamic acid [7] 172 4-(ethoxymethyl)phenol [7] 173 4-(methoxymethyl)phenol [7] 174 Methyl3-(4-hydroxyphenyl)propionate [7] 175 4-oxopentanoic [7] 176 (E)-ferulic acid [42] 177 (E)-ferulic acid hexacosyl ester [42] 178 (Z)-ferulic acid hexacosyl ester [42] 179 β-daucosterol [42] 180 Pholidotin [42] 181 Triphyllol [42] 182 3-hydroxybenzoic acid [40] 183 4-(4''-hydroxybenzyl)-3-(3'-hydroxy-phenethyl) furan [40] 184 3-(3'-hydroxyphenethyl)furan-2(5H)-one [40] 185 Methyl3-(3'-hydroxyphenethyl)furan-2(5H)-one [40] -
Being rich in chemical components is an important pharmacological basis for the clinical application of Pleione[17]. With the development of science and technology, researchers have conducted in-depth studies into the pharmacological effects of Pleione in many aspects and found that this plant possesses various functions such as antitumor effect, anti-inflammatory effect, anti-dementia effect, effect on hematopoietic function, anti oxygenation, and hypolipidemic effect (Fig. 6).
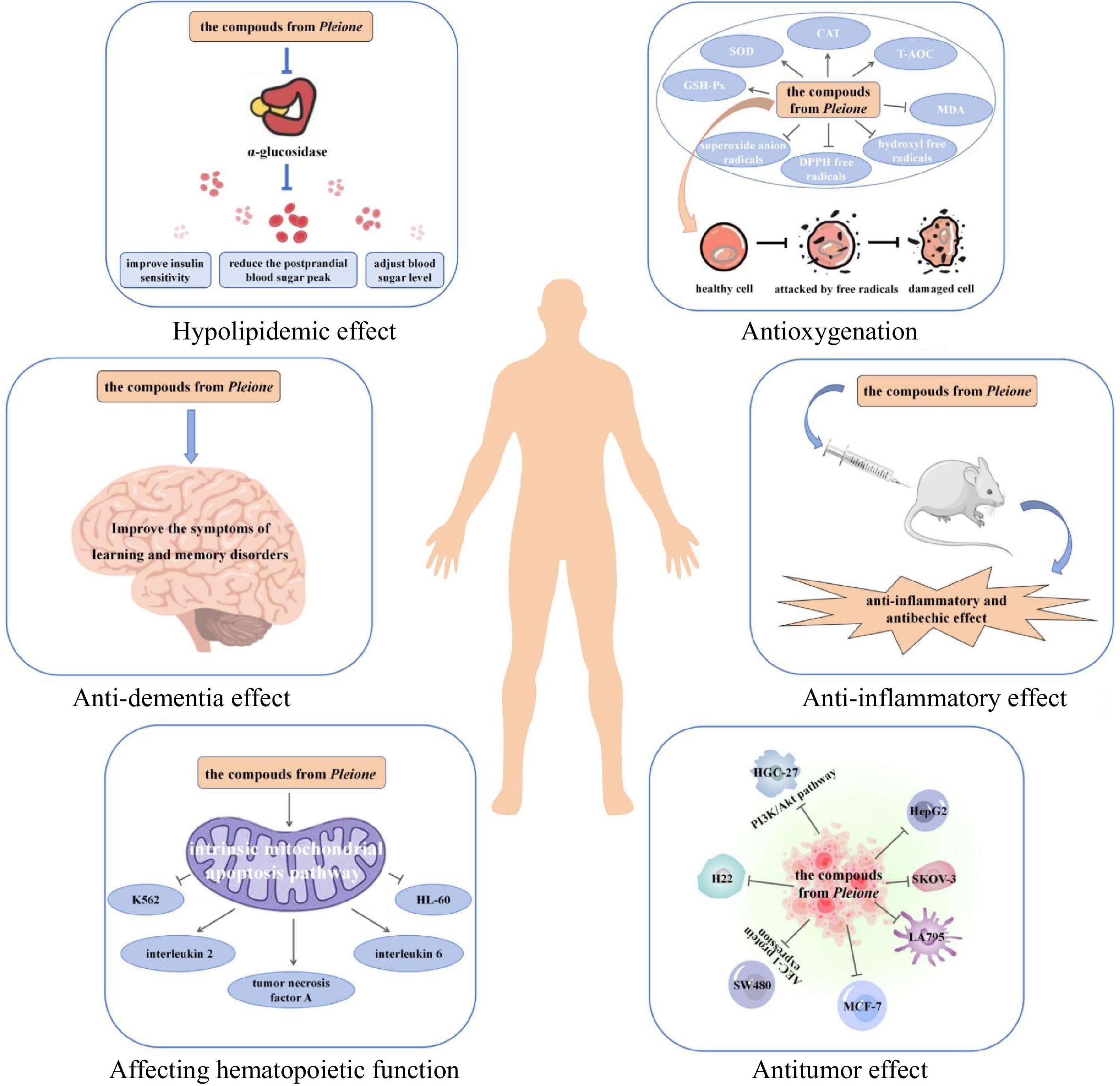
Figure 6.
Pharmacological effects of Pleione: antitumor effect, anti-inflammatory effect, anti-dementia effect, affecting hematopoietic function, antioxygenation, and hypolipidemic effect.
Antitumor effect
-
Numerous studies have shown that Pleione exhibits have an inhibitory effect on many types of tumors, such as colorectal cancer, breast cancer, liver cancer, thyroid cancer, gastric cancer, and so on (Fig. 7)[7,23,50−54]. To study the chemical constituents of P. bulbocodioides and find their antitumor bioactive compounds, 12 compounds were obtained and identified from this plant, and the antitumor activity of these constituents was studied using the MTT assay in vitro[50]. The results showed that (8R)-4,5'dihydroxy-8-hydroxymehtyl-3'-methoxydeoxybenzoin exhibited good inhibitory activity against the SKOV-3 cell line. The compounds isolated from P. bulbocodioides have some activity in inhibiting LA795 (mouse lung adenocarcinoma cells)[7]. Compounds such as phoyunnanin A, shanciol F, batatasin III, and p-dihydroxybenzene showed inhibitory effects against LA795. Hydroxy-9-(4'-hydroxy-3'-methoxyphenyl)-11-methoxy-5,6,9,10-tetrahydroohenanthrene-azaspiro[2,3-b]furan-10-yl)methylethyl and p-dihydroxybenzene showed cytotoxic activity against LA795 cells with the IC50 value of 66 and 12 μg·mL−1, respectively. Bulbocodioidins A–D were isolated from the pseudobulbs of P. bulbocodioides[51]. The cytotoxic effects of the isolated compounds were evaluated in MCF-7 cell lines, and bulbocodioidin A, and bulbocodioidin D demonstrated cytotoxic activities. Batatasin III and gigantol inhibited the growth of gastric cancer cells SGC-7901, liver cancer cells BEL-7402, leukemia cells K562, HL-60, melanoma cells M14, and lung cancer cells A569, H460[23]. Bulbocodioidin B exerted cytotoxic activities against liver cancer cells BGC-823, colon cancer cells HepG2, and breast cancer cells MCF-7 with the IC50 values of 2.3, 8.3, and 2.5 μM, respectively. Batatasin III from P. yunnanensis showed activity against the growth of LA795 cells with the IC50 value of 76.21 μM, but only moderate inhibition against BEL-7402 cells and A569 cells. Compound 1,3',5',7-tetrahydroxy-4,7'-dimethoxy-9,9',10,10'tetrahydro-2,2'-biphenanthrene from P. maculata had good inhibitory activity against three tumor cell lines, A549, MCF-7/S, and SKOV-3[52]. Wang et al. investigated the effect of polysaccharides extracted from P. bulbocodiodes on cell proliferation and epithelial-mesenchymal transition (EMT) in ovarian cancer cells and its mechanism[53]. The results demonstrated that these polysaccharides inhibited the proliferation of ovarian cancer cells by decreasing the expression levels of β-catenin and c-myc, hindered the binding of Wnt ligands to transmembrane receptors, and downregulated the expression of downstream genes, such as CyclinD1. The extracts of the dried pseudobulb of Pleione inhibited the PI3K/Akt signaling pathway affected the expression of its downstream tumor suppressor gene Bax and the anti-apoptotic genes Bcl-2 and Caspase-3, thereby inhibiting the proliferation of breast cancer cells and inducing their apoptosis[54]. These results confirm that Pleione possesses an antitumor effect.
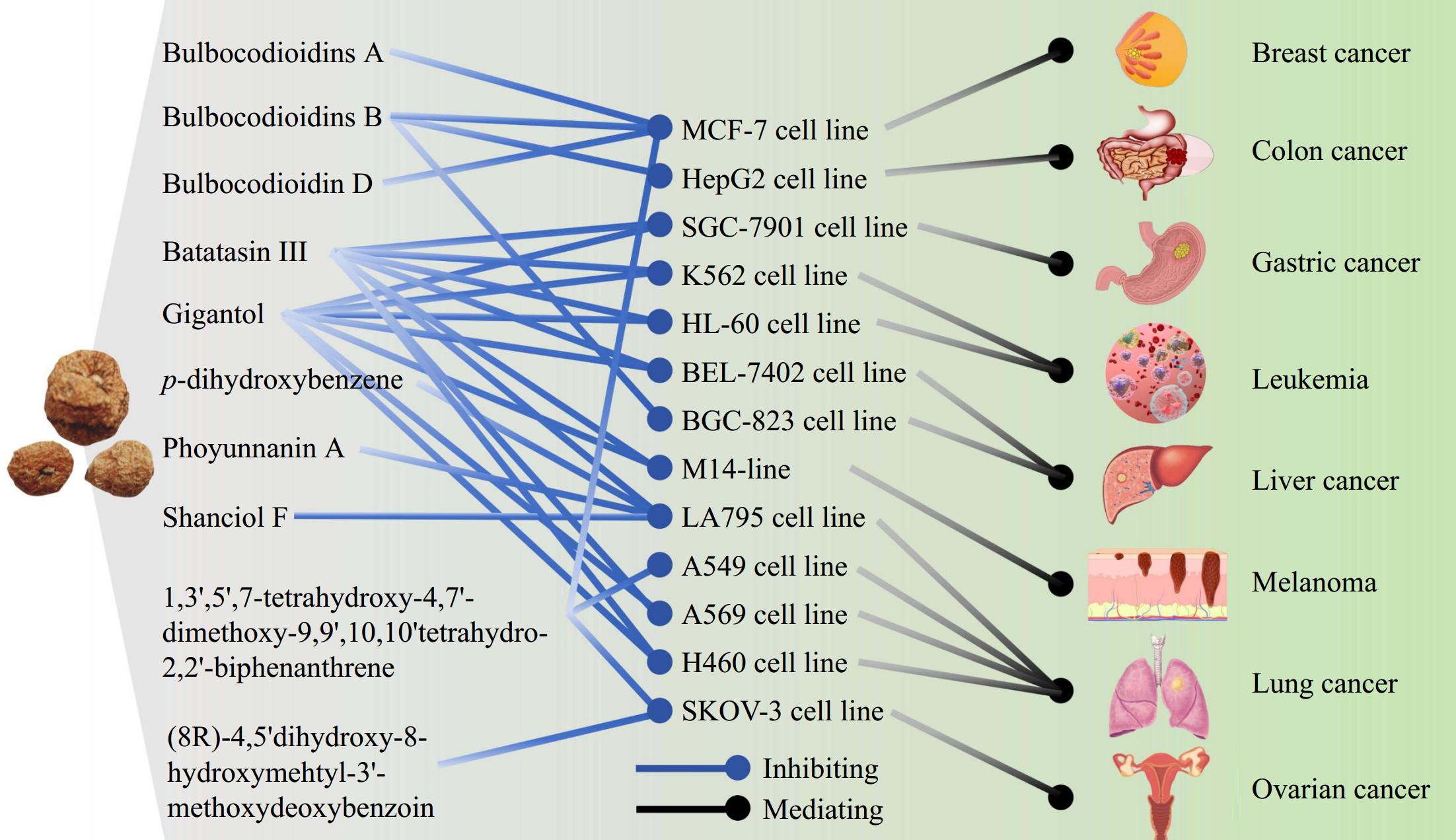
Figure 7.
Antitumor effect of Pleione. Compounds bulbocodioidins A, B, D, batatasin III, gigantol, p-dihydroxybenzene, phoyunnanin A, shanciol F, 1,3',5',7-tetrahydroxy-4,7'-dimethoxy-9,9',10,10'tetrahydro-2,2'-biphenanthrene and (8R)-4,5'dihydroxy-8-hydroxymehtyl-3'-methoxydeoxybenzoin isolated from Pleione exhibited inhibitory activity against cancer cells.
Anti-inflammatory effect
-
Researchers studied the acute toxicity, anti-inflammatory, and antibechic tests of P. yunnanensis[18]. Using maximal dosages of the suspension of p. yunnanensis extract, it was found that the dosage was more than 8.0 g·kg−1 (equivalent to 14.16 g·kg−1 of raw pharmacognosy and 106 times the common human dosage). The results indicate that p. yunnanensis has no toxic effects. Through the anti-inflammatory and antibechic tests, p. yunnanensis was proven to exhibit anti-inflammatory and antibechic effects within certain fixed dosages. Hou induced and sampled mouse peritoneal macrophages to determine the inhibitory rate of chemical components in p. yunnanensis on cell growth[55]. The results showed that the compound shanciol D had an obvious anti-inflammatory effect at the concentration of 10 μM. Coelonin, hircinol, and gigantol isolated from P. bulbocodiodes[23], possessed the potential to inhibit the LPS-induced production of nitric oxide in murine macrophage RAW 264.7 cells, with the IC50 values ranging from 9.6 to 35.7 μM. Gigantol exhibited the potent activity toward radical-scavenging and NO production inhibition, reduced inducible nitric oxide synthase mRNA expression, thus exhibited good performance in antifungal action and calmodulin inhibition. Fifteen components exhibiting moderate inhibition of NO production were isolated from P. bulbocodiodes[33,45]. 2,5,2',5'-tetrahydroxy-3-methoxybibenzyl,4,7-dihydroxy-1-(p-hydroxybenzyl)-2-methoxy-9,10-dihydrophenanthrene and 2,5,2',3'-tetrahydroxy-3-methoxybibenzyl could inhibit NO production induced by LPS in BV-2 cells with the IC50 values of 2.46 and 3.14 μM, respectively. Hydroquinone from P. bulbocodiodes had activity of anti-bacterial and anti-cytotoxicity[7]. At a concentration of 100 μg·mL−1, hydroquinone had a certain inhibitory effect on lung adenocarcinoma cells, LA795. These results show that Pleione may be a promising plant for the development of anti-inflammatory drugs.
Anti-dementia effect
-
The benzyl bisaccharide glycosides, which were isolated from P. bulbocodioides, have a significant improvement effect on the symptoms of learning and memory disorders induced by scopolamine in mice[56]. Pleionesin A and batatasin III from P. bulbocodioides exhibited neurotoxic activities on mice hippocampal neurons (SY-SH-5Y) at 10 μM[24]. Lusianthridin, shanciol H, pleionesin E, and gastrodin isolated from P. bulbocodioides and P. yunnanensis were also documented to exhibit activities against neurasthenia, neuroprotection, and epilepsy[57]. In summary, it is believed that Pleione possesses certain anti-dementia effects.
Affecting hematopoietic function
-
With the development of industry, the discovery rate of secondary aplastic anemia caused by exposure to various chemicals, drugs, or rays are increasing year by year. P. bulbocodioides can significantly reduce the toxicity of cyclophosphamide and toluene to bone marrow and can also stimulate bone marrow hematopoietic cells, causing myeloid cell lines to proliferate, which is conducive to the recovery of injured body functions. Hao et al. investigated the effects of the ethyl acetate (EtOAc) extract of P. bulbocodioides on the proliferation and apoptosis of human leukemia K562 and HL-60 cells, as well as the possible apoptosis pathway[58]. These results showed that the EtOAc extract of P. bulbocodioides inhibits cell proliferation and induces cell apoptosis in human leukemia cell lines HL-60 and K562 through the intrinsic mitochondrial apoptosis pathway. Researchers observed the pharmacodynamic effects of the 'Shancigu compound' (a compound made from the pseudo bulbs of P. yunnanensis) on mice with aplastic anemia[59]. The results showed that the 'Shancigu compound' group had an obvious function of increasing peripheral hematocytes and strengthening the hematopoietic function of bone marrow. 'Qingduyin', a compound Chinese medicine derived from Pleione can reduce the number of leukemia cells in the liver, spleen, bone marrow, and peripheral blood of L7212 mice, prolong the survival period of the model mice, regulate immune function, and improve the activity of interleukin-2, interleukin-6, tumor necrosis factor A, and their mRNA expression[60]. Li et al. observed interleukin-2 activity and lL-2 RNA expression in L7212 leukemia mice and the influence of the recipe of 'Qingduyin' on them, and they confirmed that the recipe of 'Qingduyin' can treat leukemia in the clinic as a biological response modulator[61].
Antioxygenation
-
The antioxidant activity of extracts from P. bulbocodioides has been determined by spectrophotometry[23,36]. The results showed that some compounds had scavenging effects on DPPH free radicals and exhibited good antioxidant activity in vitro. At 10 M, monbarbatain A, 2,7,2-trihydroxy-4,4,7-trimethylhydro-1,1-polyphenanthrene, shanciol H, hircinol, coelonin, and dendrobiol showed a certain free radical scavenging ability[7]. Hircinol, batatasin III, and dendrobiol possess antioxidant activity[62]. Coelonin and hircinol exhibited DPPH free radical scavenging activity[63]. Meng studied the antioxygenation effect of polysaccharide from Pleione. 80 SPF Kunming mice (20−22 g) were randomly divided into five groups (blank control group, model group, and polysaccharide with a low, middle, and high dose group)[64]. After 22 d of continuous irrigation, CAT, GSH-PX, MDA, T-AOC, and SOD in serum, liver and kidney were measured. The results showed that compared with the blank control group, polysaccharides in each dose group significantly increased in serum, liver, and kidney of CAT, GSH-Px, and SOD activity (p < 0.01) and T-AOC decreased significantly in serum, liver, and kidney MDA levels (p < 0.01). These results indicate that Pleione has a significant antioxidant effect.
Research indicates that PCsP, which originates from P. yunnanensis, has a good inhibitory effect on α-glucosidase, and inhibiting α-glucosidase can reduce the postprandial blood sugar peak, adjust blood sugar spike, and improve insulin sensitivity[65]. Further, it has been shown that the polysaccharide from Pleione has a hypolipidemic effect[64]. 70 SPF Kunming mice (20−22 g) were randomly divided into five groups (blank control group, model group, and polysaccharide with a low, middle, and high dose group). While the blank control group was fed with the full price of feed, other groups were fed high-fat feed. After 4 weeks, the levels of ALP, ALT, AST, CREA, DBil, Glu, LDH, LDL-C, TBil, TC, TG, HDL-C, UREA, and UA in the liver were measured. The results showed that the contents of TC and TG in the serum and liver of mice in the high-fat model group were significantly higher than those in the control group (p < 0.01), and the model was successful. Compared with the model group, mountain arrowhead polysaccharides with different dose groups can significantly reduce the serum TG, TC, and LDL-C levels (p < 0.01), and significantly increasee the content of HDL-C (p < 0.01). These studies indicate that Pleione plays an important role in the improvement of diabetes and lipid reduction.
-
In recent years, with the deepening of research into the biological activities of Pleione and its extracts, the compound prescription consisting of PCsP and other Chinese medicines is effective in the treatment of diseases affecting the respiratory system, digestive system, endocrine-metabolic system, and so on, and it holds a broad clinical application prospect.
Application in diseases of the respiratory system
-
In recent years, with the continuous advancement of research on TCM, it has been discovered that P. bulbocodioides, has been recognized for its therapeutic potential within the TCM framework, particularly in the treatment of respiratory conditions. Studies suggest that P. bulbocodioides is particularly effective in managing respiratory disorders, including bronchitis[66]. Additionally, contemporary research has demonstrated that P. bulbocodioides possesses anticancer properties, offering a novel perspective on its application in the treatment of respiratory system diseases. For instance, a study combined PCsP with simple targeted drug therapy in the control group to assist in the treatment of advanced non-small cell lung cancer (NSCLC)[67]. After two months of treatment, the overall effective rate and Karnofsky performance status of patients in the observation group were significantly higher than those of the control group, indicating that the adjuvant treatment with PCsP can promote improvement in patients' physical function status and has a high clinical remission rate with certain safety[67]. Furthermore, in a separate investigation involving 90 patients with advanced lung cancer, PCsP formulations demonstrated superior efficacy compared to chemotherapy[68]. The study identified qi-yin deficiency as a common syndrome in intermediate and advanced NSCLC, characterized by elements such as yin deficiency, qi deficiency, blood stasis, phlegm, and toxin, pathogenic heat, and pathogenic dampness[69]. PCsP, with the functions of promoting blood circulation, clearing heat and detoxifying, and removing blood stasis, is one of the core drug components in the treatment of NSCLC. The combination of PCsP with simple targeted drug therapy in the control group has established a solid foundation for the application of P. bulbocodioides in antitumor treatment.
Application in diseases of the digestive system
-
PCsP is widely used in diseases of the digestive system and plays a key role in the treatment of nasopharyngeal cancer, liver cancer, and colon cancer. Compound Chinese medicine containing P. bulbocodioides is notably effective in reducing fever and facilitating detoxification. This offers potential supplementary therapeutic benefits in the treatment of specific cancers, including lung cancer. For example, a compound Chinese medicine primarily composed of P. bulbocodioides has been observed to significantly decrease body temperature and enhance toxin excretion, thereby benefiting treatments for lung and liver cancer treatments[70]. The study collected prescriptions prescribed for the treatment of nasopharyngeal carcinoma outpatients in the clinic[71], aiming to investigate medication rules with TCM maintenance treatment for nasopharyngeal carcinoma. The findings indicated that the medication frequency of PCsP was very high. The research amassed authenticated initial records from specialized clinical oncology practices, focusing on colorectal cancer treatment. Upon digitization of the data into an analytical framework, a comprehensive review was conducted to discern the prevalence and correlation of frequently utilized pharmaceuticals[72]. The results highlighted that PCsP is consistently utilized as an anti-cancer detoxification agent. However, due to its inherent toxicity, the use of P. bulbocodioides should be supervised by a healthcare professional.
Application in diseases of the endocrine metabolic system
-
PCsP is widely used in the treatment of thyroid cancer, gouty arthritis, and hyperlipidemia due to its pharmacological effects such as anti-tumor, anti-gout, and hypoglycemic properties. Clinical studies have indicated that P. bulbocodioides exhibits promising antitumor effects[30,39] and may be utilized in the treatment of diseases related to the endocrine and metabolic systems. A study analyzed the underlying patterns within TCM prescriptions to elucidate the therapeutic principles governing the treatment of acute gouty arthritis[73]. In a dataset of 732 medicinal formulas, PCsP was noted to have a medication frequency exceeding 300. Additionally, the combination of 'Yi Yi Ren' (薏苡仁) with PCsP can strengthen the spleen and eliminate dampness[73]. An analysis of 254 standardized gout treatments revealed a therapeutic approach that emphasizes the regulation of the middle burner, with the acute phase being addressed by clearing heat and dampness, as well as harmonizing the middle jiao. The analysis of 254 gout treatment protocols underscored a therapeutic focus on middle jiao regulation, especially during the acute phase, where the strategy revolves around clearing heat and dampness to address the 'excess in superficiality'. This approach often involves the substantial use of PCsP to achieve the desired therapeutic effects[74]. Research has indicated that an extract from P. bulbocodioides, also known as PCsP in TCM, can inhibit the proliferation of thyroid cancer cells, such as the SW579 cell line, and promote apoptosis by regulating the expression levels of Bcl-2 protein[75]. This finding suggests that P. bulbocodioides may serve as an adjunctive therapeutic agent in the treatment of certain cancers, including thyroid cancer. In summary, research on the application of P. bulbocodioides in diseases of the endocrine and metabolic system is currently limited, necessitating further clinical studies to verify its efficacy and safety.
Application in other diseases
-
PCsP also has therapeutic effects on ovarian cancer, breast cancer, tongue cancer, cancer metastasis pain, etc. The underlying pathogenesis of breast cancer is characterized by the interplay of phlegm, stasis, stagnation, and deficiency. Consequently, a therapeutic regimen incorporating 'Trichosanthes kirilowii-PCsP-liquorice' (瓜蒌皮-山慈菇-生甘草) has been employed to prevent the recurrence and metastasis of the disease[76]. PCsP is the main component of Louci Nodule-dissipating Decoction (LCSJ). Clinical studies have found that LCSJ can improve the disease-free survival rate and overall survival time for 1, 2, and 3 years[77]. Another study found that the application of PCsP can enhance the analgesic effect in the treatment of metastatic bone pain and reduce the dose of opiates, such as Oxycontin[78]. Additionally, a variety of traditional Chinese patent medicines have been developed using PCsP as the main ingredient, such as Cigu Xiaozhi Pills (慈菇消脂丸), Shangke Wanhua Oil (伤科万花油), Zhou's Huisheng Pills (周氏回生丸), and Ziyuan Yixiao Pills (紫元益消丸)[79−82]. All of these medicines have shown promising therapeutic effects in the treatment of various diseases. It plays a significant role in improving the quality of life for survival patients.
-
As a small genus with only about 33 species, the genus Pleione is highly demanded in the medicinal market and holds great potential for development. However, the following problems persist in the development and application of Pleione (Fig. 8).
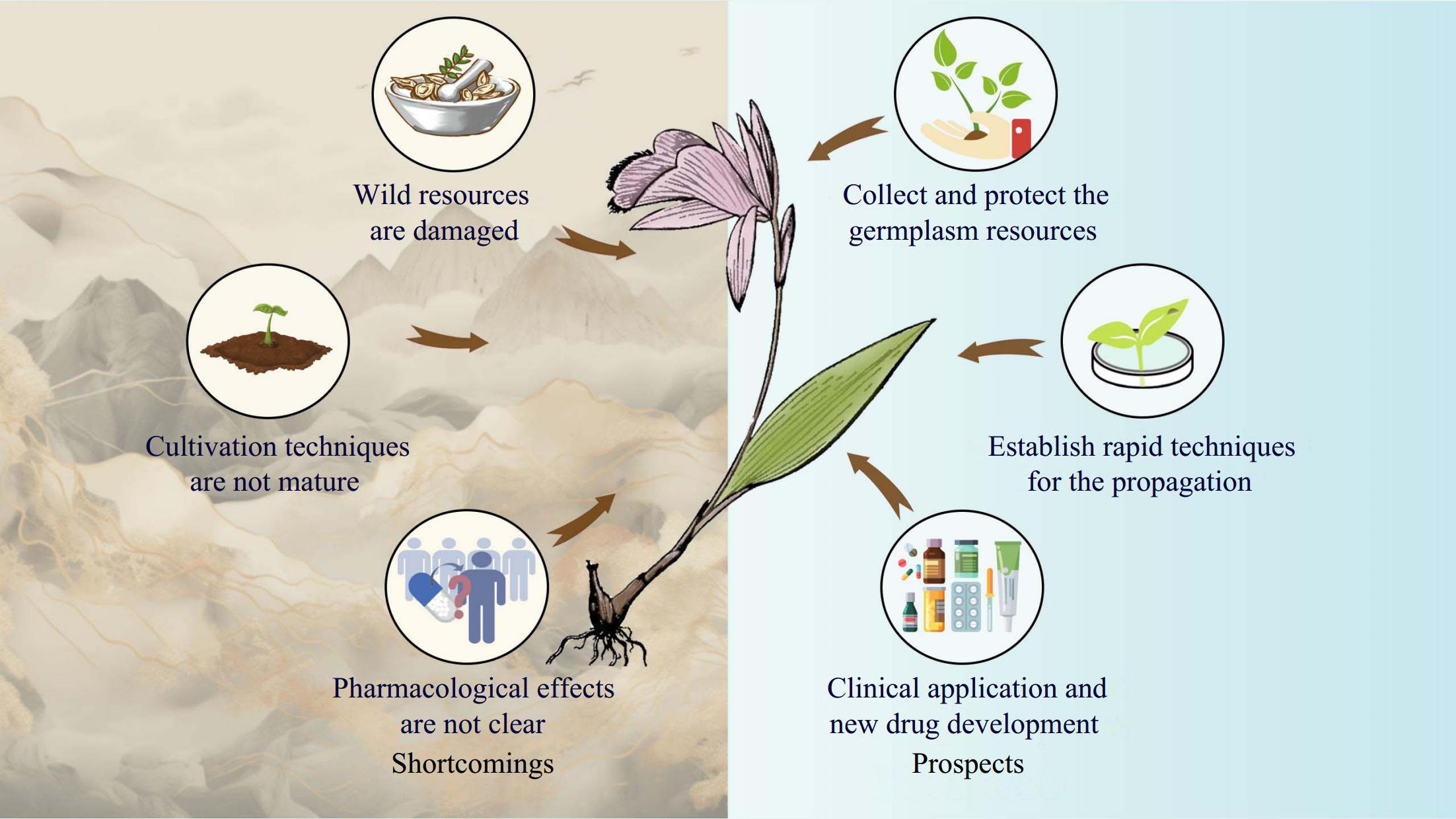
Figure 8.
Shortcomings and future development prospects of Pleione. Wild resources of Pleione are seriously damaged and need to be sustainably preserved. The tiny seeds of the Pleione make it difficult for seedlings to germinate independently in the natural state, so it is necessary to establish a rapid and modern technique for the propagation of Pleione. Pleione has a wide range of pharmacological effects and is mainly used in clinical treatments. However, studies on the pharmacological effects and the action mechanism of Pleione have not been fully understood. Therefore, further studies should focus on elucidating the pharmacological effects and the action mechanism of Pleione.
Wild resources are seriously damaged and need to be sustainably preserved
-
The Pleione genus, with its beautiful flowers, is extremely popular among gardeners. The demand for wild resources by breeders and hobbyists is increasing every year, and a large number of wild resources are harvested for private sale annually. At the same time, according to incomplete statistics, about 15,000 kg of Pleione bulbs are dug up and used in medicine, resulting in a yearly reduction of wild resources year-on-year, and their sustainability therefore worrying. Wild resources are crucial for breeding, industrialized development, and application. Consequently, there is an urgent need to conduct technical research focused on key aspects of conservation, sustainable development, and utilization of Pleione. Moreover, there is a lack of in-depth exploration of the current situation regarding Pleione resources. Considering that nearly all Pleione species are assessed as Critically Endangered (CR), Endangered (EN), and Vulnerable (VU) by IUCN criteria[83], there is an imperative need for extensive conservation measures across the genus. For instance, the collection of germplasm resources, particularly from wild populations, is a crucial aspect of conservation. Furthermore, in-situ conservation is vital in protecting and managing these species within their natural habitats, thereby complementing ex-situ conservation efforts that focus on preservation outside of their indigenous environments.
Urgent need for the establishment of efficient asexual propagation technology and cultivation technology systems.
-
The tiny seeds of the Pleione plant make it difficult to germinate seedlings independently in their natural state, so it is necessary to establish a rapid and modern technique for the propagation of Pleione. At present, there are few studies on the rapid propagation technology of Pleione, and researchers mostly use the traditional split method to cultivate P. bulbocodioides. However, this method has the risk of variety degradation due to the accumulation of viruses. Therefore, despite having mastered the basic cultivation technology of Pleione, there is an urgent need to establish an artificial pollution-free, and large-scale cultivation technology system to meet the demands of the pharmaceutical market and achieve an increase in added value.
Discovery of more active compounds and study of their pharmacological activities.
-
Pleione has a wide range of pharmacological effects and is mainly used in the clinical treatment of breast cancer, liver cancer, stomach cancer, colorectal cancer, and other tumor diseases. However, studies on the pharmacological effects and the action mechanism of Pleione have not been fully understood. Although the clinical effect of Pleione has been proven, the relevant studies largely remain at the animal or cell experiment level. Therefore, further studies should focus on elucidating the pharmacological effects and the action mechanism of Pleione and it is also of great significance to carry out large-scale, randomized controlled clinical studies, and systematic evaluation for the clinical application of Pleione and its new drug development.
This review was supported by the National Natural Science Foundation of China (Grant No. 32400317) and the Fujian Provincial Natural Science Foundation of China (Grant No. 2024J01425).
-
The authors confirm contribution to the paper as follows: study conception and design: Liu Z, Ji X; data collection: Wang S, Wu S; analysis and interpretation of results: Zeng D, Peng D; draft manuscript preparation: Ji X, Lan S, Zeng D. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ji X, Wang S, Wu S, Lan S, Peng D, et al. 2024. Review on chemical constituents, pharmacological activities, and clinical applications of Pleione orchid. Medicinal Plant Biology 3: e029 doi: 10.48130/mpb-0024-0029
Review on chemical constituents, pharmacological activities, and clinical applications of Pleione orchid
- Received: 07 October 2024
- Revised: 08 November 2024
- Accepted: 12 November 2024
- Published online: 25 December 2024
Abstract: Traditional Chinese medicine, a cornerstone of Chinese civilization, boasts a rich history spanning thousands of years. The Pleione orchid, renowned for its medicinal properties, is a primary source of Pseudobulbus Cremastrae seu Pleiones (PCsP, 山慈菇). Given its therapeutic effects, there has been a surge in research related to Pleione in recent years, underscoring the need for a comprehensive review of this medicinal plant. Here, the latest studies on the chemical constituents, pharmacological effects, and clinical applications of Pleione are summarized, and the shortcomings of current research presented. This review encompasses advancements made over the past few decades, providing a theoretical foundation for both new drug development and the clinical application of Pleione. It also aids in the effective utilization and industrialization of medicinal and edible orchids, thereby promoting their sustainable development and societal benefits.
-
Key words:
- Pleione /
- Chemical constituents /
- Pharmacological activity /
- Clinical application