-
As a common deciduous fruit tree in the north, the hormones in the leaves are changed in apple (Malus domestica) in late autumn due to the shorter daylight and lower temperature, which promotes the return of nutrients and stores them in the tree body to help it overwinter, and finally, the leaves dry up and fall[1,2]. In temperate deciduous fruit trees, temperature serves as the primary factor regulating the initiation of winter dormancy[3,4]. However, warmer winter conditions, particularly when temperatures exceed 16–18 °C, can adversely affect the accumulation of chilling requirements, potentially leading to delayed defoliation and reduced nutrient reflux. This may result in severe desiccation of fruit trees in the following year, weakened tree vigor, and increased incidence of diseases and pests[5]. Additionally, such conditions can delay dormancy entry, resulting in insufficient chilling accumulation for dormancy break, causing irregular flowering and budburst in the subsequent season[6]. Existing defoliants often lead to inconsistent defoliation among individual plants, and their efficacy tends to decline as temperatures decrease[7]. Therefore, it is of great significance to study the combination of different defoliants with Fuji concentrated defoliation in winter, to promote nutrient reflux and tree dormancy.
Dormancy during winter, and the storage of nutrients are two critical characteristics of deciduous fruit trees, which serve to protect their sensitive tissues from adverse climatic conditions. The nitrogen reserves in trees are primarily derived from root absorption and the autumn reflux of nutrients from leaves[8]. The use of physical defoliation can promote defoliation in fruit trees; however, this approach is associated with relatively high costs[9]. The application of defoliant can effectively accelerate the rate of nutrient reflux and promote defoliation. Studies have shown that artificial defoliation in autumn can effectively promote nutrient reflux from leaves, increase tree nutrient storage, and improve fruit quality and production efficiency[6]. Additionally, the application of defoliant can promote leaf senescence and abscission in maize, enabling the plants to maintain stable kernel weight even under drought stress conditions[10]. The application of defoliant compounded with thiazuron and ethephon in the process of cotton topping can effectively control the height of cotton plants, improve yield, and reduce labor costs[11]. In pepper plants, the application of the ethephon compound defoliant facilitates leaf abscission, thereby aiding harvesting during the late growth stage[12]. In cotton, the use of a defoliant mixture containing thidiazuron and ethephon prior to harvest, promotes boll opening and leaf drop, improving both harvesting efficiency and yield[13]. Castor, a perennial plant characterized by considerable height, presents challenges during harvest; the application of defoliant during the growing season induces leaf abscission, suppresses growth, and facilitates harvesting[14]. The combined application of thidiazuron and ethephon effectively promotes leaf abscission in crops, proving advantageous for mechanical harvesting[15].
Plant hormones ethylene and auxin are the main signaling molecules that regulate leaf shedding[16,17]. The application of defoliant could effectively induce the expression of ethylene biosynthesis and signal transduction genes in cotton, and the auxin level decreased with the increase of ethylene content, and regulated the expression and signal of abscisic acid (ABA) biosynthesis genes, and induced ABA accumulation[18]. ABA acts as a signaling molecule that triggers leaf senescence and abscission[19]. The use of thiurea defoliant in cotton can significantly increase the content of plant hormones such as ABA and jasmonic acid (JA) in the leaves and promote defoliation[20]. The application of defoliant promoted the increase of ABA content in old apple leaves, the increase of apical gibberellin content, and the defoliation of old leaves and the germination of apical buds[21]. Therefore, it was found that there was a significant correlation between the occurrence of defoliant action and hormonal changes in plants.
In recent years, the use of defoliant in apples is less, and urea, ethephon, and other single products are often used without compounding, so its ability to promote defoliation is relatively limited. In this study, urea, potassium (KI), and trichloroacetic (TCA) were used as the main components, and the compound formula of defoliant with the best effect on apple defoliation was selected through single-factor optimization and response surface optimization, so as to improve the yield and efficiency of tree nutrition.
-
The study was conducted in early November 2022 at the Smart Orchard of Zhongyi Fruit Industry Co., Ltd. (36.07° N, 118.14° E) in Yiyuan County, Zibo City, Shandong Province, China. The test site had a typical temperate and monsoonal climate, with four distinct seasons. The soil of the test plot was loam. In the experiment, a healthy three-year-old 'Yanfu 10/G935' with similar tree size and growth was selected as the test material, and the drugs used in the test were urea, TCA, and KI pure products, which were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). The treatment was conducted from the end of October to the beginning of November, before the leaves of the apples fell in late autumn. Each tree was sprayed with 200 mL of defoliant evenly.
Test method
-
One-factor experiments: urea (2%, 4%, 8%, w/w), TCA (0.1, 1, 2 g·L−1), KI (0.1%, 0.3%, 0.5%, w/w), 20 replicates were made for each treatment, and the defoliation rate of Yanfu 10 was determined.
$ \mathrm{Defoliation\ rate=\frac{Number\ of\ leaves\ after\ 10\ d\ of\ treatment}{Number\ of\ leaves\ before\ treatment}} $ (1) Optimization of response surface method: based on the results of single factor experiments, Design-Expert V8.0 was used for the experimental design of the response surface, and urea, KI, and TCA corresponded to the A, B, and C factors in the experiment, respectively, and the defoliation rate was the response value Y. All the defoliants used in the experiment were freshly prepared.
Verification of optimal processing
-
At the end of October 2023, 20 shoots were sprayed in each of the three treatments to measure their late autumn defoliation rate. R treatment: the optimal combination of orthocross compound was 5.00% urea, 0.30% KI, and 1.00 g·L−1 TCA. T treatment: the model predicts the optimal combination of compound was 4.75% urea, 0.34% KI, and 1.21 g·L−1 TCA. Control treatment (CK): a control treatment without defoliant spray.
Plant hormones target metabolism
-
After 10 d of defoliant spraying, five leaves were randomly selected in the front, middle, and back of each treated shoot and sent to Shanghai Meiji Biopharmaceutical Co., Ltd. (Shanghai, China) for targeted metabolism detection of plant hormones in plant leaves. The sample was weighed to 100 mg (five replicates in total), placed in a 2 mL grinding tube, 498 μL of 80% methanol, and 2 μL of SA-D4 internal standard solution (2 μg·mL−1) were added, mixed, and ground at low temperature for 3 min, ultrasonic extraction at low temperature for 1 h, 25 mg of extracted salt packet was added to the EN15662 extraction salt package and vigorously shaken on a shaker for 10 min, centrifuged at 10 °C for 10 min, and the supernatant was taken to the injection vial. LC-ESI-MS/MS (UHPLC-Qtrap) was used for qualitative and quantitative detection of targets in samples.
Statistical analysis
-
Excel 2023 software was used for data analysis and processing, and IBM SPSS Statistics 26 was used for significance and difference analysis, in which the significance level was p <0.05. GraphPad Prism 6.0 software was used for graphing. Correlation analysis: R language was used to analyze and plot the Pearson correlation coefficient.
-
The effects of urea (a), KI (b), and TCA (c) concentrations on the defoliation rate of Yanfu 10 are shown in Fig. 1a–c. As can be seen from Fig. 1, the leaves of the apple sprayed with defoliant fell significantly. With the increase of application concentration, the defoliation rate of urea, KI, and TCA increased first, and then decreased with the increase of application concentration. When urea is added at 5%, apple leaves show the maximum defoliation rate (Fig. 1a). The highest defoliation rate is at TCA concentration of 1 g·L−1 (Fig. 1b). With the increase of KI concentration, the defoliation rate of apple leaves gradually increased, and the defoliation rate was the highest when KI concentration of 0.3% was used, and then gradually decreased (Fig. 1c).
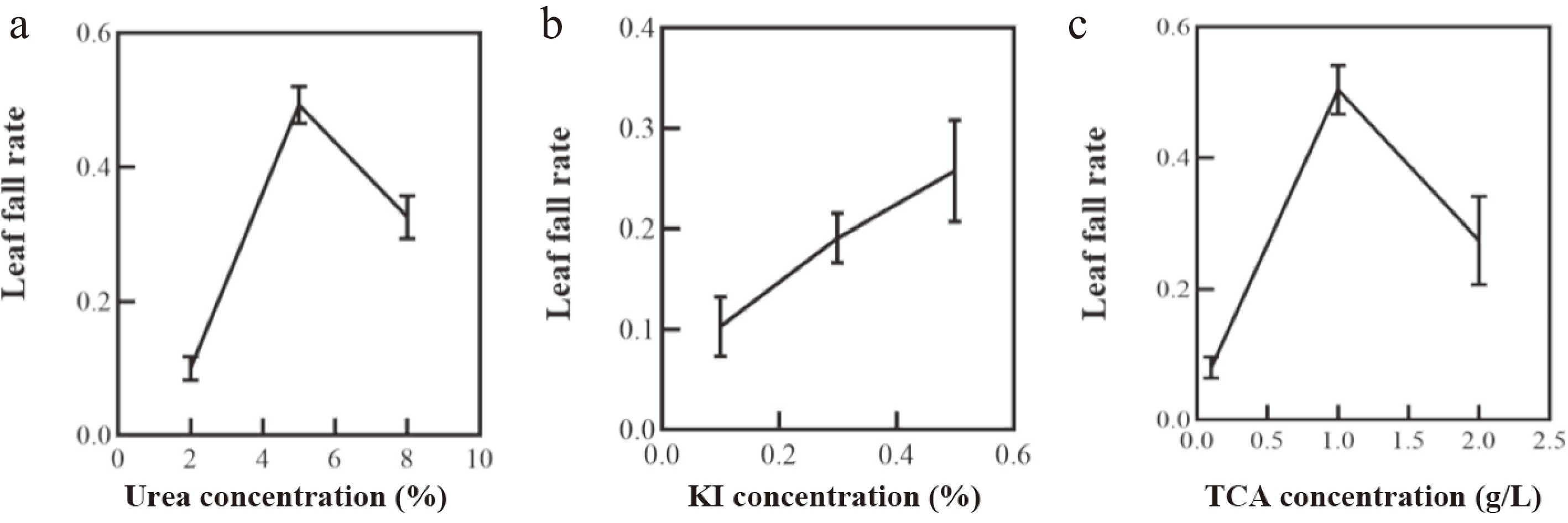
Figure 1.
The effects of (a) urea, (b) potassium iodide (KI), and (c) trichloroacetic acid (TCA) concentrations on the leaf fall rate of apples.
The response surface experiment was carried out to optimize the concentration of Yanfu 10 defoliant
Experimental design and results of response surfaces
-
The experimental design and results of the response surface of the apple defoliant compound are shown in Table 1.
Table 1. Experimental design and results of response surface.
No. A: Urea (%) B: KI (%) C: TCA (g·L−1) Leaf fall rate 1 5.00 0.40 1.50 0.57 2 2.00 0.40 1.00 0.32 3 5.00 0.30 1.00 0.49 4 2.00 0.20 1.00 0.12 5 8.00 0.40 1.00 0.19 6 5.00 0.30 1.00 0.50 7 5.00 0.30 1.00 0.60 8 8.00 0.20 1.00 0.35 9 5.00 0.30 1.00 0.52 10 5.00 0.20 0.50 0.50 11 5.00 0.20 1.50 0.48 12 5.00 0.30 1.00 0.57 13 2.00 0.30 0.50 0.30 14 8.00 0.30 0.50 0.39 15 5.00 0.40 0.50 0.49 16 2.00 0.30 1.50 0.34 17 8.00 0.30 1.50 0.32 Analysis of variance
-
From the analysis of variance results, it was found that the selected model was reliable because the selected model was significant, the lack of fit term was not significant (Table 2). Among the three factors of urea, KI, and TCA, the concentrations of urea and TCA had a significant effect on apple leaves fall off. In addition, the influence of the three factors on the late autumn leaf fall of apple is not a simple linear relationship, and there is a significant interaction between the three. Among them, the interaction between urea and KI was the most significant.
Table 2. Analysis of model variance.
Source of variation Quadratic sum Degree of freedom Mean sum of square F P Model 0.2927 9.0000 0.0325 25.1200 0.0002 Significant A 0.0042 1.0000 0.0042 3.2100 0.1162 B 0.0020 1.0000 0.0020 1.5700 0.2505 C 0.0001 1.0000 0.0001 0.0542 0.8225 AB 0.0309 1.0000 0.0309 23.8300 0.0018 AC 0.0026 1.0000 0.0026 2.0000 0.1997 BC 0.0027 1.0000 0.0027 2.0500 0.1995 Residual 0.0091 7.0000 0.0013 Lack of fit 0.0009 3.0000 0.0003 0.1551 0.9211 Not significant Pure error 0.0081 4.0000 0.0020 Cor error 0.3017 16.0000 From the analysis in Table 3, the R2 of the model = 0.97, the R2 correction = 0.93, the R2 prediction is close to the R2 correction, and the signal-to-noise ratio is 15.87 (> 4). The model showed that the fit and credibility of the regression equation were high, and the experimental error was within the controllable range, so the model could be used to optimize and predict the compound of apple defoliant. Through multiple regression fitting analysis, the multiple quadratic regression equation model of the apple defoliant compound was obtained:
Table 3. R2 comprehensive analysis.
Standard deviation
(R2)Average
(R2 correction)Coefficient
(R2 prediction)Signal-to-
noise ratio0.97 0.93 0.91 15.87 Defoliation rate (Y) = −0.97 + 0.37·A + 4.67·B − 0.34·C − 0.29·AB − 0.02·AC + 0.51·BC − 0.026·A2 − 5.93·B2 + 0.14·C2
Response surface plots and their contour plots
-
According to the regression equation, the response surface diagram and contour plot were obtained, as shown in Fig. 2, to analyze the effects of urea, KI, and TCA compounding and their interactions on the defoliation rate of apples. With the increase of urea concentration and KI concentration, the defoliation rate of apple leaves showed significant changes, which promoted the defoliation ability to first increase, and then decrease. The response surface model could determine that the optimal combination was 4.75% urea, 0.34% KI, and 1.21 g·L−1 TCA.
The defoliation promotion rate of the optimal combination was 70.91%, which was significantly better than the leaf fall effect of the unoptimized defoliant (Fig. 2).
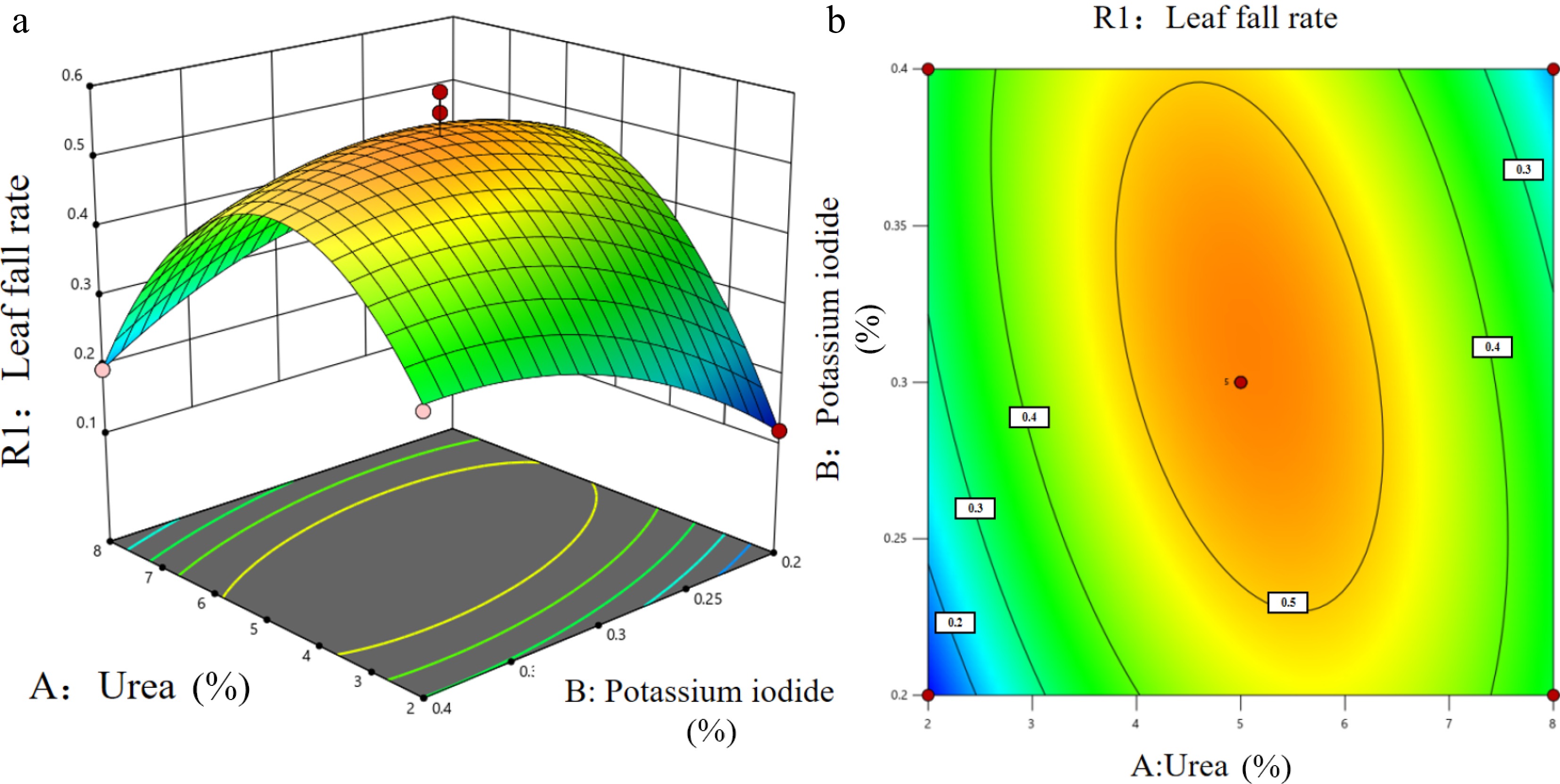
Figure 2.
Response surface diagram of interaction to (a) apple leaf fall rate, and (b) contour plot.
Changes in hormone content in plant leaves
-
Three treatments were selected to detect the hormone content in plant leaves, including the best treatment R in the orthogonal procession: 5.00% urea, 0.30% KI, and 1.00 g·L−1 TCA, the model predicts the optimal combination T: 4.75% urea, 0.34% KI, and 1.21 g·L−1 TCA, and CK (the control treatment without defoliant). OPLS-DA results revealed that the application of compound defoliant formulations R and T differentially altered the types and concentrations of hormones in plant leaves (Fig. 3b, c). Specifically, the T treatment accounted for 69.2% of the variance in Component 1 and 10.9% in Component 2, while the R treatment explained 37.1% and 38.6% in Components 1 and 2, respectively. These results indicate that the T treatment exerted a more pronounced influence on leaf hormone levels compared to the R treatment. After treatment with defoliant, the hormone content in plant leaves changed significantly, including a significant increase in the content of abscisic acid (ABA), jasmonic acid (JA), salicylic acid (SA), etc. Moreover, the changes of plant hormone content after defoliant R and T treatment tended to be consistent. The contents of ABA and SA in apple leaves were the highest among the three treatments, while the contents of JA and indole-3-acetic acid in the leaves of T treatment were significantly higher than those in the R treatment and the CK treatment, while the content of indole-3-acetic acid was significantly decreased after treatment. The contents of SA, ABA, and JA increased significantly after the application of defoliant. The contents of ABA, SA, and JA after R defoliant treatment increased by 45.65%, 82.35%, and 82.15%, respectively, compared with CK treatment. The contents of ABA and SA in the leaves of the plants treated with defoliant increased by 61.11% and 81.44%, compared with CK treatment (Fig. 4).
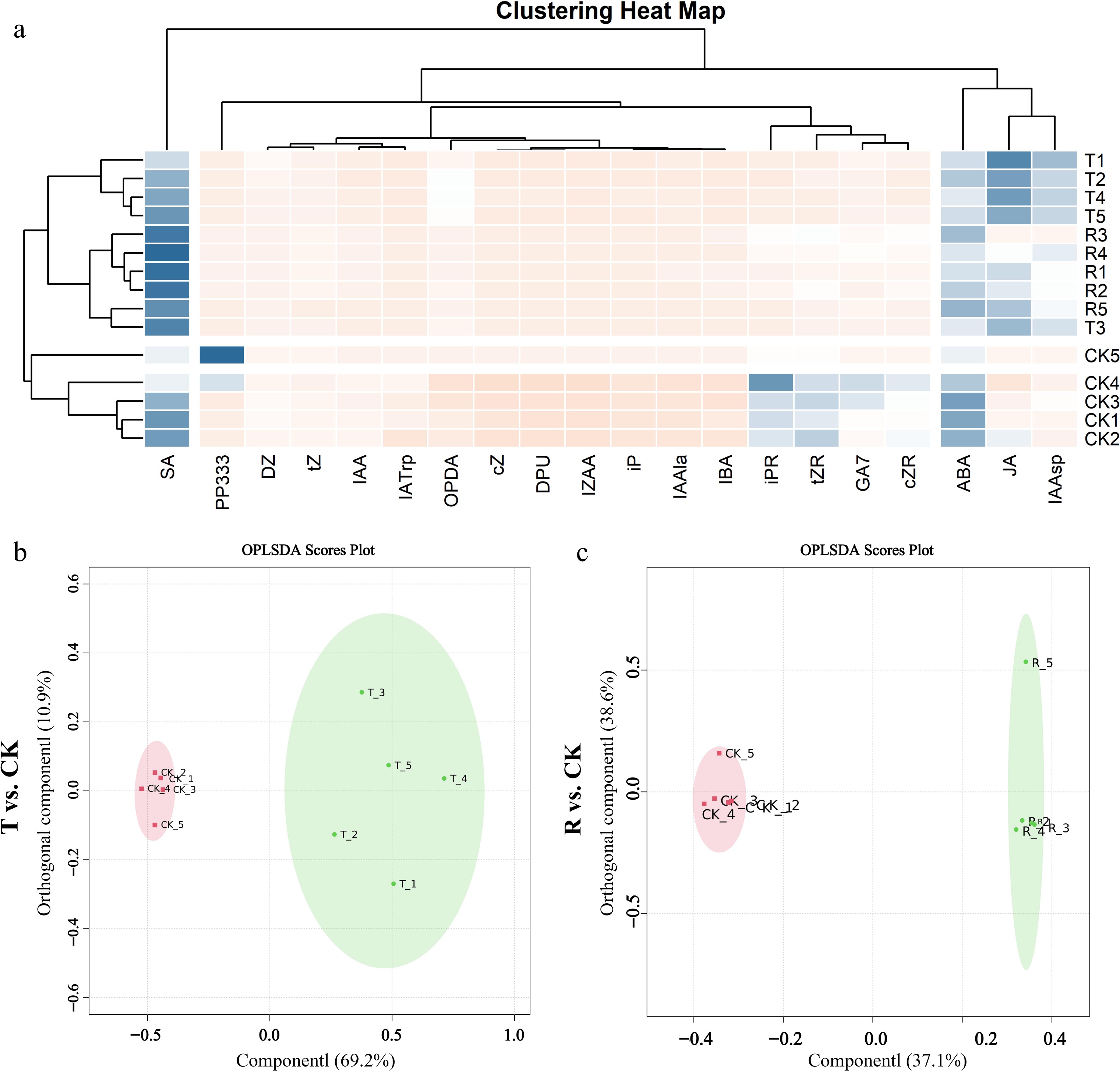
Figure 3.
Heat map of effects of defoliant on plant hormone content in leaves. (a) Differences in hormone content among different treatments. (b) T vs CK OPLS-DA analysis. (c) R vs CK OPLS-DA analysis.
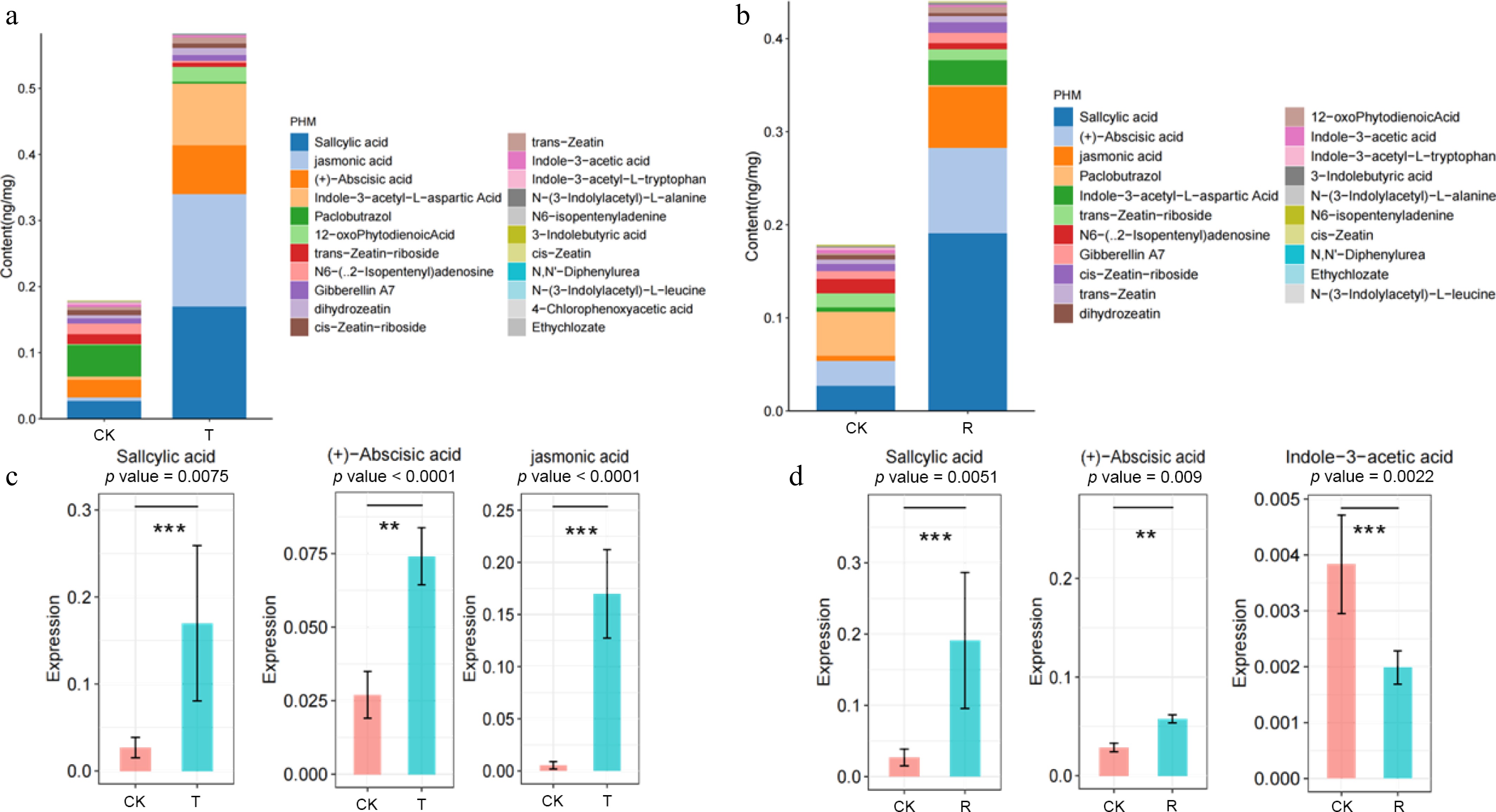
Figure 4.
Effect of defoliant on plant hormone content in leaves. (a) Effect of treatment T on phytohormones in apple leaves. (b) Effect of treatment R on phytohormones in apple leaves. (c) Content of the top three most significantly altered hormones in apple leaves under treatment T. (d) Content of the top three most significantly altered hormones in apple leaves under treatment R. The number of * indicates significant differences between treatments. ** p < 0.05, *** p < 0.001.
The hormone content in the leaves of Yanfu 10 after R defoliant treatment showed significant changes. The ABA content was significantly positively correlated with most of the hormones related to plant aging, including JA and SA (Fig. 5a). The content of SA was negatively correlated with the content of indole-3-acetic acid, and pentene adenine. ABA content was significantly positively correlated with SA, JA, and 12-oxophytodienoic acid content, but negatively correlated with most of the plant growth hormone contents. Analysis of the metabolite network for various phytohormones revealed that in R-treated leaves, the phytohormone network contained 253 positive edges, and 188 negative edges (Fig. 5b, c). Key hub metabolites identified included salicylic acid, N,N'-diphenylurea, cis-zeatin, N-(3-indolylacetyl)-L-alanine, and jasmonic acid, which exhibited significant promotive effects on the levels of other hormones within the network. In contrast, T-treated leaves showed 262 positive edges and 222 negative edges in the phytohormone network. Critical hubs under this treatment were N6-isopentenyladenine, salicylic acid, (+)-abscisic acid, N-(3-indolylacetyl)-L-leucine, and jasmonic acid (Fig. 6). Notably, the number of edges connected to these key hubs was greater in the T treated network than in the R-treated network. These results indicate that application of defoliant T significantly enhanced the content of abscission-promoting hormones such as abscisic acid and salicylic acid, and strengthened their positive correlations with other phytohormones.
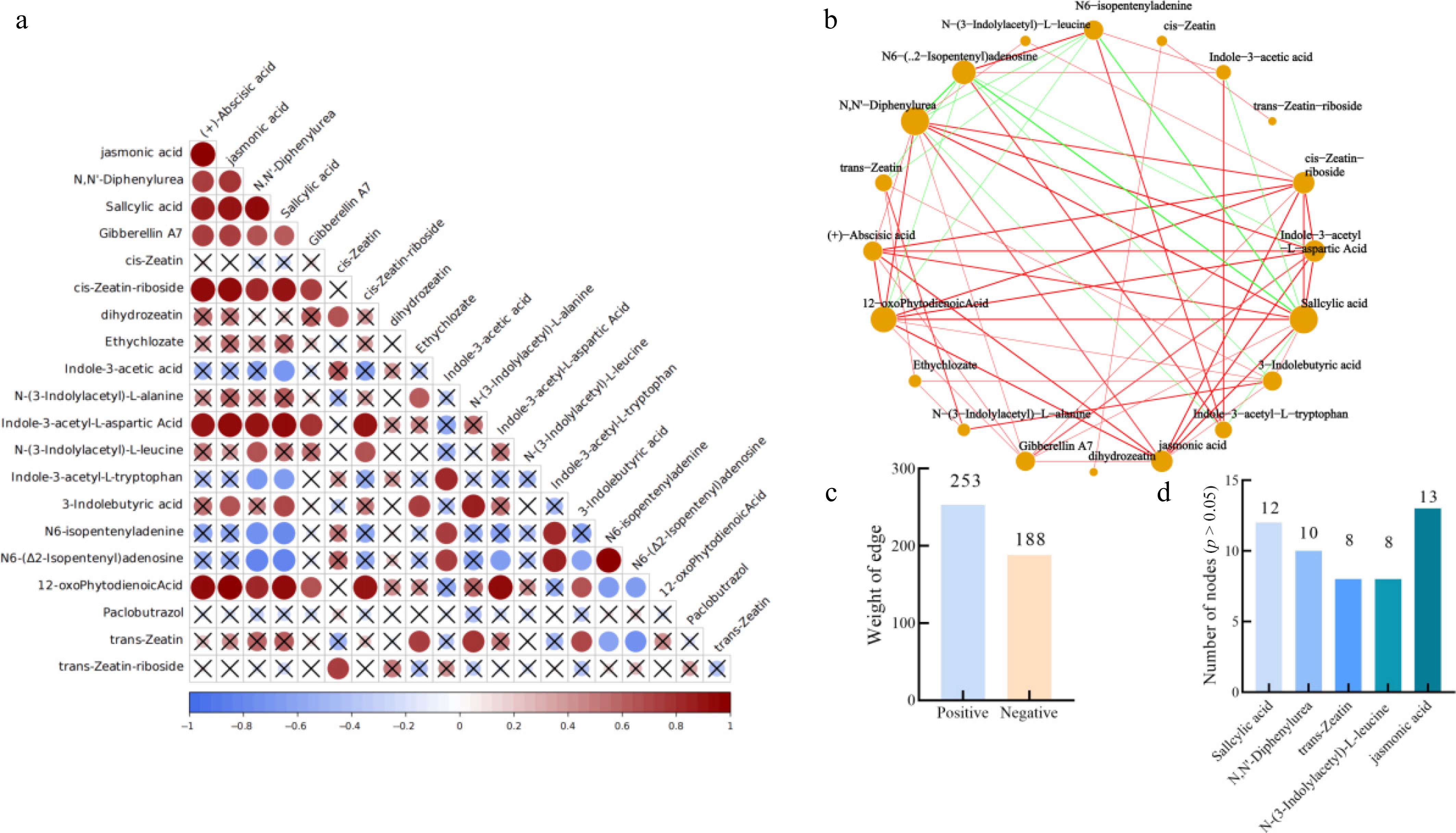
Figure 5.
Correlation between R and CK processing. (a) Correlation among phytohormones in apple leaves under treatment R. (b) Correlation network of phytohormones in apple leaves under treatment R. (c) Edge attributes of the collinearity network. (d) Key nodes with no significant difference (p > 0.05) between treatment R and CK.
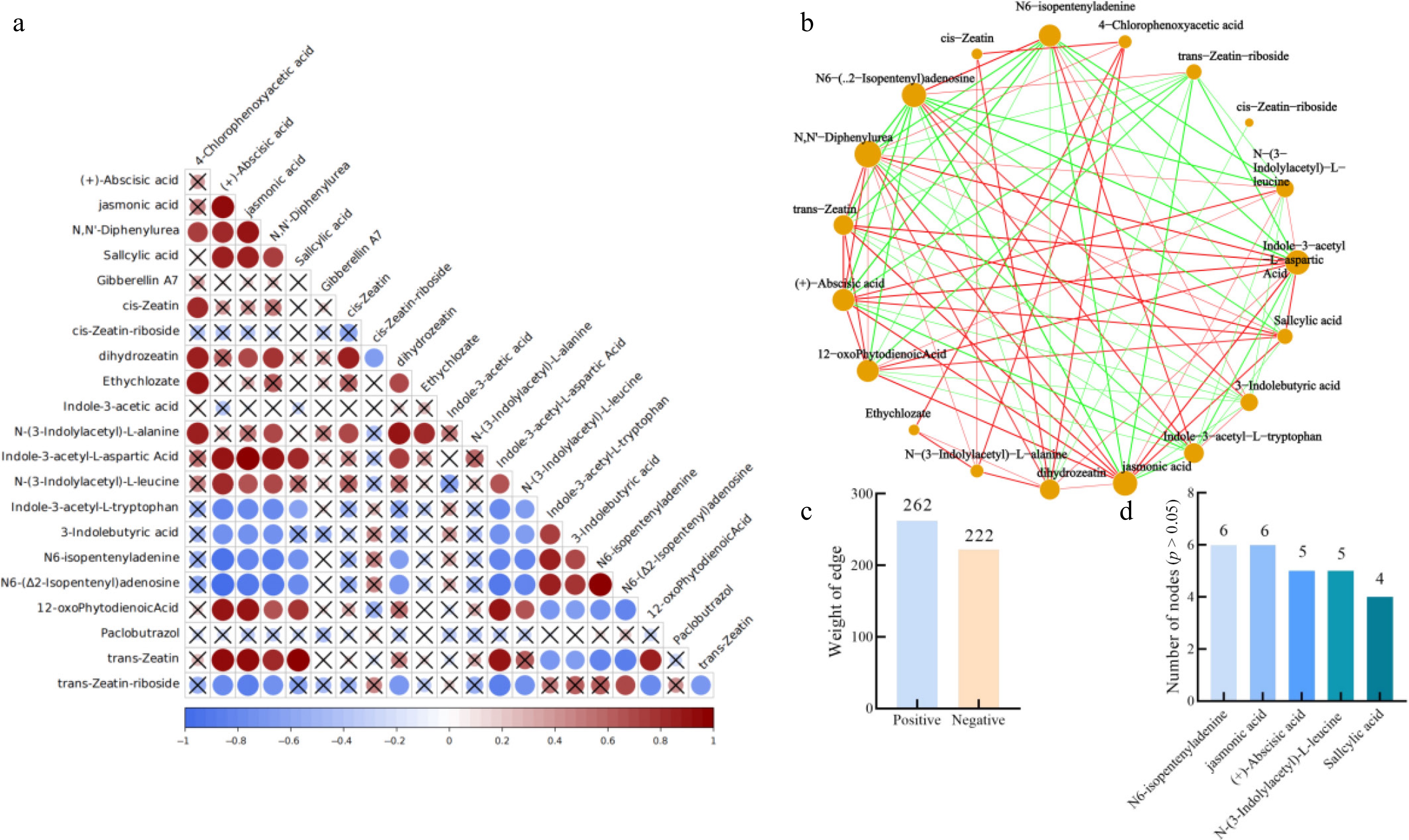
Figure 6.
Correlation between T and CK processing. (a) Correlation among phytohormones in apple leaves under treatment T. (b) Correlation network of phytohormones in apple leaves under treatment T. (c) Edge attributes of the collinearity network. (d) Key nodes with no significant difference (p > 0.05) between Treatment T and CK.
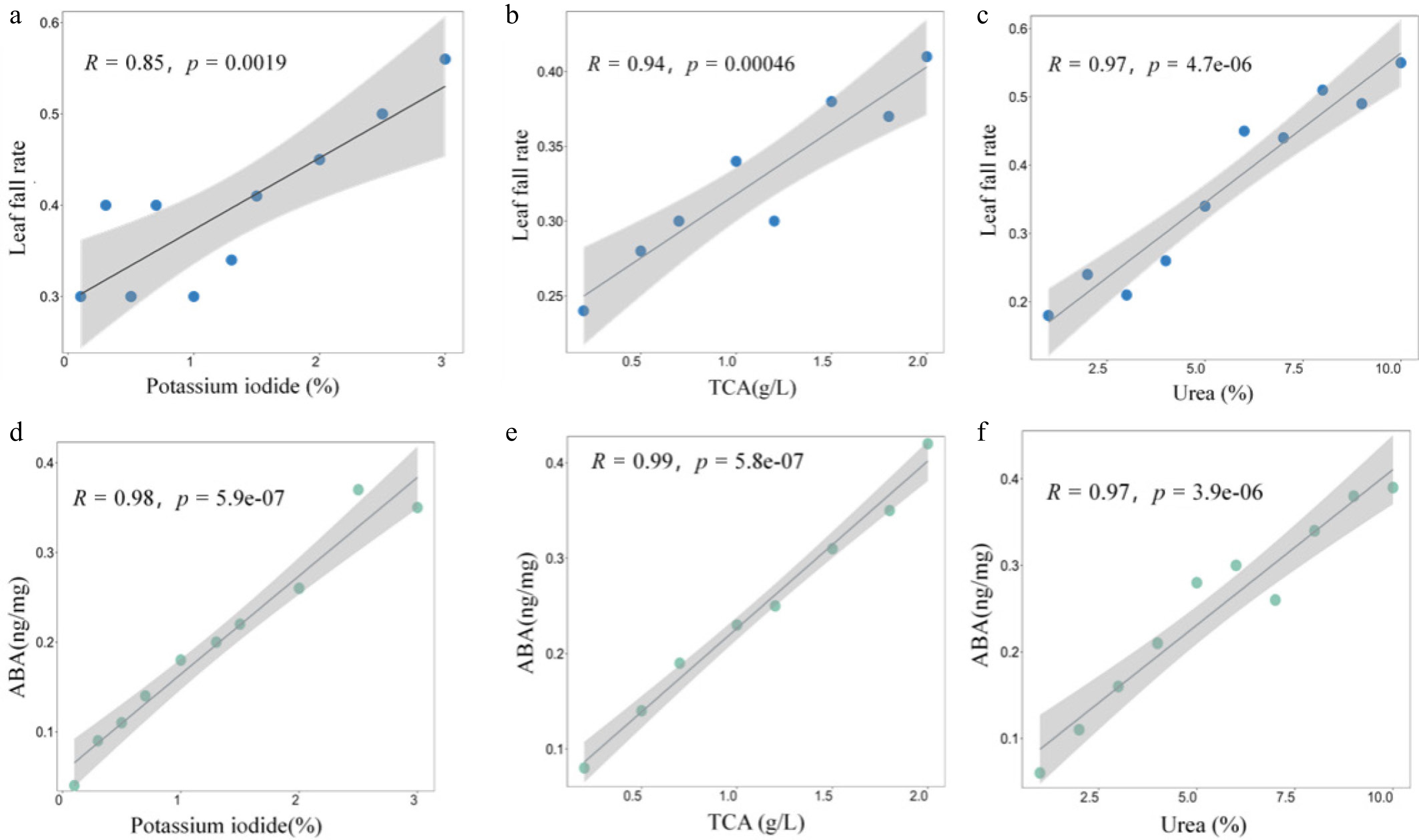
The correlation analysis between defoliant components and apple defoliation rate showed that KI, TCA, and urea were significantly positively correlated with apple defoliation (Fig. 7). Among them, the changes of TCA and urea content had a more significant effect on the change of apple leaf fall rate, and the correlation between them was calculated based on the Pearson correlation coefficient. The R values were 0.94 and 0.97, respectively, indicating that the model had a strong linear relationship, and the p values were 0.00046 and 4.7e-06, respectively, indicating that the linear relationship was extremely significant. Among them, the linear change of urea content had a more significant correlation with the defoliation rate of apples. The results of correlation analysis of the active ingredients of defoliants on ABA content directly related to plant leaf fall showed that the three defoliants had a strong positive correlation with the production of ABA in plant leaves. The R values are all greater than 0.95, indicating that the linear relationship of the model is extremely significant.
-
As a prominent temperate deciduous fruit tree, the apple is extensively cultivated worldwide. Most temperate deciduous trees shed their leaves in late autumn to store nutrients, reduce water loss, and mitigate the adverse effects of cold stress on the plants. Applying defoliants 7–10 d before natural leaf fall induces gradual leaf abscission within 5–7 d after spraying. This process facilitates nutrient reflux, improves flower bud quality, and establishes a solid foundation for the safe overwintering of apple trees. Leaf abscission is a distinct cell separation process that occurs during plant growth and development. It takes place specifically in the morphologically specialized abscission zone cells located at the base of the leaf, where the perception of signaling molecules activates the abscission process, ultimately leading to leaf detachment[6]. As a deciduous fruit tree with an extensive planting area, apple leaves in late autumn are extremely important for nutrient storage in the tree. Failure to abscise leaves in autumn results in significant nutrient consumption, which is detrimental to nutrient storage in trees, and negatively impacts flowering and fruiting in the following year[22]. Numerous studies have indicated that application of specific compounds at certain concentrations—such as urea, potassium iodide, and trichloroacetic acid—can effectively promote leaf abscission and enhance nutrient storage. The present study demonstrates that an appropriate concentration of urea significantly accelerates the defoliation process, highlighting the considerable influence of nitrogen on plant senescence and abscission. However, too high a concentration of urea will have a counterproductive effect on tree germination and growth[20]. Previous studies have shown that KI can promote the formation of leaf separation and promote leaf senescence, and the results of this study also found that the use of KI can effectively promote the shedding of apple leaves, and the higher the concentration of KI, the stronger the ability to promote defoliation. Trichloroacetic acid (TCA) promotes water loss in plant leaves and facilitates the formation of the abscission zone, effectively enhancing the abscission of apple leaves in late autumn. Studies have shown that different defoliants can significantly increase the defoliation rate of apple trees; however, the effectiveness of a single defoliant is limited. In this study, the formulations of the three defoliants were optimized by single factor and orthogonal approaches, resulting in a significant increase in the defoliation-promoting efficacy of the compounds, and the higher content of TCA and urea had a greater effect on the defoliation rate of apples, and their contents were 3% and 1 g·L−1 which had the most obvious effect on defoliation. Orthogonal experimental design serves as an effective and scientifically rational means to significantly enhance the efficacy of compound formulations[23]. By optimizing fermentation conditions and increasing the concentration of key metabolites through orthogonal experiments, the effectiveness of biological control can be substantially enhanced[24]. Therefore, orthogonal testing enables rational proportioning of defoliants, thereby maximizing their defoliation-promoting efficacy. In this study, most of the treatments in the orthogonal optimization model showed that the water content of most of the treatments in the orthogonal optimization model decreased, and the leaves yellowed and aged in advance, especially with the R treatment (5% urea, 0.3% KI, and 1 g·L−1 TCA) had the greatest increase in defoliation. This is consistent with the results of previous studies that spraying urea compound is more likely to cause plant leaves to turn yellow and promote leaf shedding, and defoliant compounds such as KI and TCA are more likely to promote the increase of ABA content in leaves, resulting in defoliation[10,13,21].
The regulation of plant growth and development involves the coordinated action of multiple hormones. As leaves senesce, pronounced alterations occur in the types and levels of phytohormones, which play vital roles in modulating plant development, and serve as key mediators of organ abscission[21,25]. The present study also found that the content of pro-aging hormones in leaves increased significantly after the application of defoliants. Perumal et al. found that the application of thiurea defoliant could effectively increase the content of plant hormones such as ABA and JA in mature cotton leaves, and promote cotton defoliation[20]. The research by Lee et al. suggests that the use of defoliants can significantly increase the ABA content in apple leaves and promote apple defoliation[21,26]. In this study, it was also found that the content of plant hormones such as ABA, JA, and SA in plant leaves increased significantly after the application of defoliants. The results indicated that the application of defoliant mainly affected the signal sensing of plant hormones in leaves, and promoted the senescence and defoliation of plant leaves. This is consistent with with the research by Xu et al. that the application of defoliant can effectively induce ethylene biosynthesis in cotton, reduce auxin content, regulate ABA biosynthesis gene expression and signaling, and induce ABA accumulation to promote cotton defoliation[27,28].
-
The defoliant formed by the combination of 4.75% urea, 0.34% potassium iodide, and 1.21 g·L−1 trichloroacetic acid had the best effect on apple defoliation in late autumn, and did not affect the leaf germination in the next year, so it was determined as the best apple defoliant formulation.
This research was funded by the Key R&D Program of Shandong Province (Action Plan for Promoting Scientific and Technological Innovation for Rural Revitalization) (2022TZXD008), the Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong Province of China (2023KJF003), and the Key R&D Program of Shandong Province (Action Plan for Promoting Scientific and Technological Innovation for Rural Revitalization) (2022TZXD0037).
-
The authors confirm their contributions to the paper as follows: conceptualization: Wang G, Gao W, Qin X; methodology: Li G, Zhang S; formal analysis: Wang G; resources: Li G, Wang G; data curation: Wang G, Lv J; writing − original draft preparation: Zhang S; writing − review and editing: Yu M; project administration and funding acquisition: Qin X, Wang G; funding acquisition: Qin X, Wang G. All authors have read and agreed to the published version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Chongqing University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang G, Lv J, Zhang S, Yu M, Li G, et al. 2025. Effects of different defoliants on defoliation and changes of hormone content in apple leaves. Plant Hormones 1: e026 doi: 10.48130/ph-0025-0026
Effects of different defoliants on defoliation and changes of hormone content in apple leaves
- Received: 24 September 2025
- Revised: 13 October 2025
- Accepted: 29 October 2025
- Published online: 11 December 2025
Abstract: The main purpose of this study is to combine and synthesize the defoliant with the best effect on apple leaves, and study the defoliant and its effect on the change of plant hormone content in leaves. Taking Yanfu 10 as the experimental material, the response surface methodology was used to optimize the concentration of urea, potassium iodide, and trichloroacetic acid to make nine single-factor treatments, and 17 orthogonal treatments, and Yanfu 10 was sprayed on the leaves. The effects of different defoliants on defoliation, and the changes of phytohormone content in its apple leaves were studied. The results showed that the defoliant rate of each treatment was significantly higher than CK treatment, and the compound defoliant treatment of 5% urea, 0.3% potassium iodide, and 1 g·L−1 trichloroacetic acid performed best after spraying for 10 d, defoliation rate is 57%. The content of hormones in leaves of orthogonal optimal treatment (R), and optimized model prediction optimal treatment (T: 4.75% urea, 0.34% potassium iodide, and 1.21 g·L−1 trichloroacetic acid, defoliation rate is 70.91%) was detected. It was found that the contents of abscisic acid, jasmonic acid, and salicylic acid in leaves increased significantly by 46.65%, 82.15%, and 82.35% compared with CK treatment. The compound defoliant of 4.75% urea, 0.34% potassium iodide, and 1.21 g·L−1 trichloroacetic acid is the best defoliant formula.
-
Key words:
- Apple /
- Defoliant /
- Response surface optimization /
- Leaf phytohormone