-
Medicinal mushrooms have gained considerable attention in recent years as potential sources for drug development due to their diverse biologically active compounds, such as polysaccharides, triterpenoids, phenolics, and sterols[1]. Historically, these mushrooms have been widely used in traditional medicine practices across various countries, such as China, Japan, and India, for strengthening immunity, delaying the aging process, combating infections, and supporting cancer therapy[2,3]. Currently, modern scientific research validates these traditional uses by demonstrating multiple pharmacological effects, including antioxidant, anticancer, anti-inflammatory, neuroprotective, and hepatoprotective properties[4−8]. In recent years, the increasing interest in sustainable and natural treatment alternatives has brought the biotechnological potential of medicinal mushrooms to the forefront. The side effects of synthetic drugs and their possible toxicity in long-term use encourage the preference for naturally sourced products[9,10]. In this context, medicinal mushrooms are considered as unique organisms that integrate traditional knowledge with modern science, placing them in a strategic position for the discovery of new bioactive molecules in the pharmaceutical sector[11,12].
The Russula genus comprises the largest and most diverse group within the Russulaceae family, and it is widely distributed throughout the world. This genus, which includes nine subgenera, recently expanded with the addition of Russula Pers. subgen. Cremeoochraceae plays important ecological roles, especially in forest ecosystems, and is quite rich in terms of species diversity[13,14]. Most Russula species exhibit ectomycorrhizal properties and establish symbiotic relationships with many tree and shrub species, especially members of the Pinaceae family and some angiosperms. These mycorrhizal associations are critical for the health and sustainability of forests. Morphologically, Russula species show great diversity in terms of cap (pileus) color and texture. However, since morphological similarities make taxonomic distinction difficult; molecular phylogenetic analyses are widely used in species identification. Studies on gene regions such as ITS, nrLSU, and RPB2 in particular contribute to the correct classification of species. The Russula genus encompasses both edible and toxic species[15−17]. While some species stand out with their high nutritional value, some species, especially Russula subnigricans Hongo, have serious toxicity and can cause fatal poisoning if accidentally consumed[15−17]. Therefore, it is crucial to identify species at both the morphological and molecular levels accurately. R. grata, an edible species, is characterized by a cap that varies in color, initially convex, flattening with age, and becoming slightly concave in the center. As the mushroom matures, the cap may develop cracks and fissures. The species features a thick stem and narrow, adnexed lamellae that are slightly attached to the stem. Its spores are broadly ellipsoidal to hemispherical or spherical, with distinctly spiny surfaces[18]. In comparison to other species within the Russula genus, such as R. cyanoxantha and R. delica, R. grata remains largely underexplored in terms of its pharmacological properties. While R. cyanoxantha is consumed across Europe for its mild taste, and R. delica has shown promising antioxidant and enzyme inhibitory activities in previous studies, R. grata has not yet been thoroughly evaluated in this context[4,19]. Notably, R. grata is ecologically significant due to its ectomycorrhizal association with Fagaceae and Pinaceae members, and it exhibits a distinct sweet odor and spore ornamentation pattern, which may reflect unique metabolite content. These aspects, combined with the lack of chemotaxonomic and pharmacological data, motivated the selection of R. grata for this study.
To date, no comprehensive biological activity profile of R. grata has been reported in the literature, particularly in relation to oxidative stress modulation, neuroprotection, and cytotoxicity. Therefore, this study aims to fill this gap by evaluating its antioxidant, anticholinesterase, and anticancer potential using standardized in vitro methods.
-
Samples of Russula grata (Fig. 1) were collected on 21 October 2024 from forested areas of Ilgaz Mountain National Park, Kastamonu province, Türkiye (41.1° N, 33.866667° E) by one of the co-authors, Prof. Dr. Ilgaz Akata, an experienced macrofungal taxonomist. The specimen was identified based on detailed macroscopic and microscopic characteristics, including pileus color and surface texture, lamellae structure and attachment, stipe morphology, and spore ornamentation, which were evaluated using standard taxonomic keys and authoritative monographs. Although molecular identification via ITS sequencing was not conducted due to technical limitations, the morphological features of the specimen were consistent with the diagnostic criteria of Russula grata Britzelm., and the specimen was deposited at the Fungarium of Ankara University under the voucher number ANK FA 194. After transportation to the laboratory, the samples were dried in an air-circulated oven at 40–45 °C. Mycochemicals were extracted from 10 g of dried mushroom sample using the Soxhlet method with 250 mL of ethanol at 50 °C for approximately 6 h. The ethanol used for Soxhlet extraction was of analytical grade with a concentration of 95%, ensuring efficient solubilization of a wide range of polar and moderately non-polar bioactive compounds. The obtained ethanolic extract was concentrated under reduced pressure using a Buchi R-100 rotary evaporator at 40 °C to remove residual solvent. The concentrated extract was then stored in the dark at + 4 °C to preserve its chemical stability prior to analysis.
Antioxidant activity tests
-
The total antioxidant status (TAS) and total oxidant status (TOS) of the ethanolic extract were quantitatively assessed using commercial kits (Catalog No: RL0017 & RL0024, respectively) obtained from Rel Assay Diagnostics (Mega Tip, Gaziantep, Turkey). All procedures were conducted following the manufacturer's instructions. Trolox was employed as the standard for the TAS determination, while hydrogen peroxide was used for calibration of the TOS measurements. As a result of the measurements, total antioxidant status values were expressed in mmol/L and TOS values in µmol/L[20,21]. The oxidative stress index (OSI) was calculated by dividing the TOS value by the TAS value after converting both measurements to the same unit[22]. TAS, TOS, and OSI assays were chosen over conventional methods such as DPPH or FRAP due to their ability to provide a more integrated and reproducible assessment of the oxidative status. These assays offer advantages in terms of automation, standardization, and physiological relevance, making them suitable for comparative antioxidant profiling of complex biological extracts.
Anticholinesterase activity test
-
The inhibitory effect of the ethanolic extract on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes was assessed based on the colorimetric method described by Ellman et al.[23]. Galantamine was used as a reference inhibitor. Test solutions were prepared by dissolving the extract at various concentrations ranging from 200 to 3.125 μg/mL. For each assay, 130 μL of 0.1 M phosphate buffer (pH 8.0), 10 μL of extract, and 20 μL of enzyme solution (AChE or BChE) were sequentially added to each well, respectively. The reaction mixtures were incubated at 25 °C, in the dark for 10 min. Afterwards, 20 μL of DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)], and 20 μL of acetylcholine iodide (AChI), or butyrylcholine iodide (BChI) as substrate were added to the wells of a microplate. Enzymatic activity was determined by measuring absorbance at 412 nm using a spectrophotometer (Thermo Multiskan Go). The inhibitory effect of the extract was expressed as IC50 values. Results were normalized against the untreated control group, which was set as 100% viability. All experiments were conducted in triplicate (n = 3), and the results are presented as mean ± standard deviation.
Antiproliferative activity test
-
The cytotoxic effect of R. grata extract on A549 human lung adenocarcinoma cells was evaluated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell viability assay. The extract was tested at concentrations of 25, 50, 100, and 200 μg/mL concentrations. When the cell cultures reached approximately 70%–80% confluency, they were detached using a 3.0 mL Trypsin-EDTA solution (Sigma-Aldrich, USA). The suspended cells were then seeded into 96-well culture plates at a density of approximately 1 × 104 cells/well. After a 24-h incubation period to allow cell attachment and adaptation, the extract solutions were added to the wells, followed by an additional 24-h incubation. At the end of the incubation, the medium was removed and replaced with 1 mg/mL MTT solution, which was incubated with the cells for 4 h. The resulting purple formazan crystals were dissolved with dimethyl sulfoxide (DMSO), and the optical density (OD) values were measured at 570 nm with an Epoch microplate reader (BioTek Instruments, USA). Cell viability was calculated as a percentage relative to untreated control cells[24].
Statistical analysis
-
All experimental procedures were conducted in triplicate (n = 3), and the results are expressed as mean ± standard deviation (SD). Descriptive statistical analyses were carried out using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA).
-
Mushroom species are recognized as a significant source of biological activity due to their rich content of phenolic compounds, flavonoids, ascorbic acid, and other antioxidant molecules[25]. The findings of this study demonstrate that the mushroom extracts tested exhibit significant in vitro antioxidant activity, indicating their potential to scavenge free radicals and mitigate oxidative stress. When compared with similar studies reported in the literature, the results further confirm that particular mushroom species possess notably high antioxidant activity. These findings underscore the potential of mushrooms as a functional food and a natural source of antioxidants[26−29]. The experimental data supporting these results on antioxidant activity are summarized in Table 1.
Table 1. Antioxidant activity of Russula grata.
Sample TAS (mmol/L) TOS (µmol/L) OSI (TOS/(TAS × 10)) Russula grata 3.718 ± 0.040 10.410 ± 0.086 0.280 ± 0.002 Note: values were studied with three repetitions, and standard deviation values are given; TAS: Total Antioxidant Status (mmol/L), TOS: Total Oxidant Status (μmol/L); OSI: Oxidative Stress Index (calculated as [TOS/(TAS × 10)]). There is no finding in the literature related to the antioxidant activity of R. grata. In the present study, ethanol extract of R. grata was used, and its antioxidant activity was determined for the first time using Rel Assay kits. The antioxidant potential of different Russula species has been reported in the literature[30−32]. In addition, TAS, TOS, and OSI values of different mushroom species have been reported. TAS values of Lactarius deliciosus (L.) Gray, Otidea onotica (Pers.) Fuckel, Hericium erinaceus (Bull.) Pers., Phellinus hartigii (Allesch. & Schnabl) Pat., Cantharellus cibarius Fr. and Candolleomyces candolleanus (Fr.) D. Wächt. & A. Melzer were reported as 7.468, 8.866, 5.426, 4.98, 5.511, and 5.547 mmol/L, respectively. TOS values were reported as 13.161, 14.724, 6.621, 9.27, 7.289, and 8.572 µmol/L, respectively. OSI values were reported as 0.176, 0.166, 0.122, 0.19, 0.132, and 0.155, respectively[33−38]. Compared to these studies, the TAS value of R. grata used in the present study was determined to be lower than that of L. deliciosus, O. onotica, H. erinaceus, P. hartigii, C. cibarius, and C. candolleanus. The TAS value is an indicator of the entirety of antioxidant compounds found in natural products[39]. It is believed that various factors, including the genetic makeup of the mushroom species, ecological conditions in their growing environments, soil and climate conditions, and developmental stages, may contribute to these differences. In addition, technical parameters such as the type of solvent in the extraction method, extraction time, and temperature may also be decisive on the antioxidant capacity obtained. In this context, the relatively lower TAS value of the R. grata species suggests that it may have a more limited content of antioxidant compounds compared to other species. However, this does not completely exclude the potential pharmacological value of the species; on the contrary, it highlights the need for more comprehensive evaluations of its various biological activities.
The TOS value is an indicator of all oxidant compounds present in natural products[39]. The TOS value of R. grata used in the present study was found to be lower than that of L. deliciosus and O. onotica, but higher than H. erinaceus, P. hartigii, C. cibarius, and C. candolleanus. The OSI value indicates the balance between oxidant and antioxidant compounds, representing the percentage of suppression of oxidant compounds by antioxidant compounds[39]. The OSI value of R. grata used in the present study was determined to be higher than L. deliciosus, O. onotica, H. erinaceus, P. hartigii, C. cibarius, and C. candolleanus. The higher TOS and OSI levels of R. grata, compared to some other species, indicate that its antioxidant system does not sufficiently counteract the oxidant compounds present in this mushroom. The OSI value is a key parameter for evaluating the balance between oxidants and antioxidants. When interpreted alongside low TAS and relatively high TOS values, the elevated OSI observed in R. grata suggests that this mushroom may exhibit a certain degree of oxidative stress potential. These findings underscore the need for further studies that focus on toxicological and biofunctional assessments, including the evaluation of oxidative biomarkers. Moreover, a high OSI value may indicate a tendency for increased reactive oxygen species (ROS) production, underscoring the importance of species–specific metabolic profiles in determining biological activity.
Anticholinesterase activity
-
In recent years, there has been a growing interest in discovering natural anticholinesterase agents, with mushrooms emerging as promising biological sources in this field[40]. In recent years, there has been a growing interest in the discovery of natural anticholinesterase agents, with mushrooms emerging as promising biological sources in this area. The inhibitory effects of mushrooms on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes suggest their potential application in managing cognitive impairments and neurodegenerative diseases[41]. Several mushroom species have been reported to exhibit strong cholinesterase inhibitory activity[42], supporting the hypothesis that they contain pharmacologically valuable bioactive compounds. In the present study, the anticholinesterase activity of R. grata was investigated, and the corresponding results are presented in Table 2.
Table 2. In vitro anticholinesterase activity of Russula grata extract.
Sample AChE (μg/mL) BChE (μg/mL) Russula grata 69.36 ± 1.46 93.58 ± 0.96 Note: values were studied with three repetitions, and standard deviation values are given; AChE: Acetylcholinesterase (μg/mL), BChE: Butyrylcholinesterase (μg/mL). To date, there is no reported evidence in the literature regarding the anticholinesterase activity of R. grata. However, acetylcholinesterase and butyrylcholinesterase inhibitory activities have been documented in other Russula species[43,44]. In this study, the inhibitory effects of R. grata on both acetylcholinesterase and butyrylcholinesterase were evaluated. The results showed that the enzyme inhibition by R. grata was lower compared to galantamine, which was used as the standard reference inhibitor. This study is significant as it provides the first evidence of the anticholinesterase effects of R. grata in the literature. The findings indicate that the inhibitory activity of R. grata against both acetylcholinesterase and butyrylcholinesterase enzymes is comparable to, or lower, than that reported for other Russula species effect. These differences may be due to the diversity of secondary metabolites between species, as well as environmental and genetic factors that affect biological activity[45]. In particular, the regulation of the cholinergic system is seen as a pharmacologically important target in the treatment of various neurodegenerative diseases, especially Alzheimer's; evaluating such naturally occurring inhibitors is valuable[46]. In this context, although R. grata has shown limited efficacy, more potent biological activities can be revealed using different extraction techniques or compound isolation strategies.
Antiproliferative activity
-
The antiproliferative effects of bioactive compounds derived from natural sources on cancer cells represent a rapidly growing area of research. In this regard, mushrooms attract attention due to their metabolites, including polysaccharides, phenolic compounds, and terpene derivatives[47]. The mushroom extracts tested in this study were found to exert suppressive effects on cell proliferation in human cancer cell lines. These findings suggest that mushrooms are valuable natural sources with potential for development as anticancer agents[48]. In this study, the effects of R. grata extract on A549 human lung cancer cells were investigated, and the results are presented in Fig. 2.
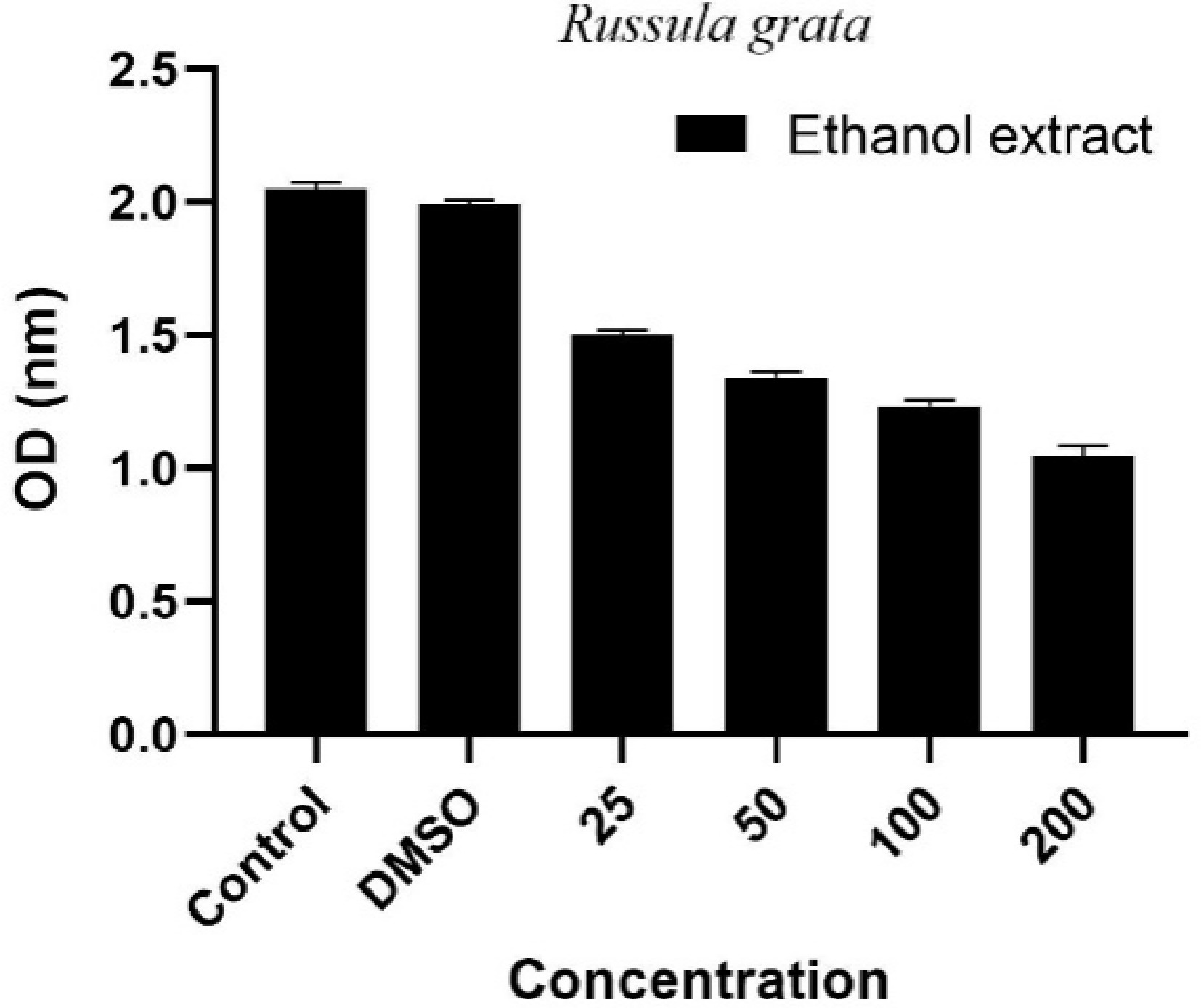
Figure 2.
Dose-dependent antiproliferative effect of Russula grata ethanol extract on A549 lung cancer cells. Control refers to cells maintained in medium without any chemical treatment. DMSO represents the vehicle control group, treated with dimethyl sulfoxide (DMSO) only. Extract-treated groups were exposed to Russula grata ethanol extract at concentrations of 25, 50, 100, and 200 μg/mL.
As a result of the study, it was demonstrated that the antiproliferative effect of R. grata ethanolic extract on A549 human lung adenocarcinoma cells increases in a dose-dependent manner. In both the control group (culture medium only) and DMSO group (solvent control), cell viability remained high, with optical density (OD) values at comparable levels, indicating minimal cytotoxicity from the solvent itself. However, in the experimental groups treated with the extract at concentrations of 25, 50, 100, and 200 μg/mL, a concentration-dependent decrease in cell viability was observed. This effect was particularly pronounced at 100 and 200 μg/mL, where optical density (OD) values showed marked reductions, indicating significant antiproliferative activity at higher extract concentrations. These results indicate that cell proliferation was significantly suppressed. The findings suggest that the phenolic compounds, terpenoids, or other secondary metabolites contained in R. grata may exert biological activity that inhibits cell division or induces cytotoxic effects in A549 cells. A review of the literature reveals that no prior studies have investigated the effect of R. grata on the A549 cell line, highlighting the originality of the present work and its novel contribution to the field. However, previous studies have reported that Russula alatoreticula K. Acharya, S. Khatua, A.K. Dutta & Paloi exhibits antiproliferative effects on the Hep3B cell line[49], and Russula delica Fr. exhibits a similar effect on the HepG2 and MCF7 cell lines[19]. These data reveal that the anticancer potential of species within the Russula genus may vary significantly. In this context, the cytotoxic effect of R. grata observed in A549 cells warrants further investigation through molecular analyses, particularly targeting apoptotic pathways, cell cycle arrest, or oxidative stress mechanisms. Such studies would provide a more comprehensive understanding of the therapeutic potential of this species.
-
In this study, antioxidant, anticholinesterase, and antiproliferative activities of the ethanolic extract of R. grata were comprehensively evaluated for the first time. The results demonstrated that while this mushroom species exhibited relatively limited antioxidant capacity, it showed a moderate cholinesterase inhibitory activity and a dose-dependent antiproliferative effect on the A549 lung cancer cell line. The scarcity on the biological activities of R. grata in the existing literature underscores the novelty of this research and highlights the potential pharmacological benefits of the species. Notably, the high OSI value and the observed antiproliferative activity suggest that certain metabolites in R. grata may be relevant to oxidative stress-related diseases and cancer biology. Future research should focus on detailed chemical profiling of the extract, investigation of the bioactivity of isolated compounds, and elucidation of their mechanisms of action at the molecular level. Additionally, assessing the therapeutic potential of this species through comparative studies across various cancer cell lines would provide a more comprehensive understanding of its biomedical relevance. However, to translate these in vitro results into clinical relevance, future research should focus on in vivo efficacy and toxicity studies, as well as isolation and structural characterization of active constituents. Mechanistic investigations, particularly regarding apoptotic pathways, oxidative stress modulation, and cholinergic system interactions, will be crucial in validating the pharmacological potential of this species.
-
The authors confirm contributions to the paper as follows: study conception and design: Baba H, Sevindik M, Gürgen A, Krupodorova T, Akata I; data collection: Baba H, Sevindik M, Gürgen A, Krupodorova T, Akata I; analysis and interpretation of results: Baba H, Sevindik M, Gürgen A, Krupodorova T, Akata I; draft manuscript preparation: Baba H, Sevindik M, Gürgen A, Krupodorova T, Akata I. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available on request from the corresponding author.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Baba H, Sevindik M, Gürgen A, Krupodorova T, Akata I. 2025. Pharmacological potential of Russula grata: antioxidant, neuroprotective, and anticancer properties. Studies in Fungi 10: e027 doi: 10.48130/sif-0025-0027
Pharmacological potential of Russula grata: antioxidant, neuroprotective, and anticancer properties
- Received: 18 May 2025
- Revised: 31 July 2025
- Accepted: 28 September 2025
- Published online: 28 November 2025
Abstract: Mushrooms have recently garnered attention as a natural source of pharmacologically active compounds due to their diverse biological properties. In the present study, the antioxidant, anticholinesterase, and antiproliferative activities of the ethanolic extract of Russula grata Britzelm. were comprehensively evaluated. Samples were collected from Kastamonu province, Türkiye, and pre-treated by drying in a ventilated oven before ethanol extraction using a Soxhlet apparatus. Antioxidant capacity was analyzed using total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI). TAS was found to be 3.718 ± 0.040 mmol/L, TOS 10.410 ± 0.086 µmol/L, and OSI 0.280 ± 0.002. These results suggest that R. grata possesses a moderate antioxidant profile. Anticholinesterase activity was evaluated using a colorimetric method; IC50 values for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) were 69.36 ±1.46 µg/mL and 93.58 ± 0.96 µg/mL, respectively. The inhibitory effect of R. grata extract was found to be lower than that of galantamine, the standard inhibitor. Additionally, antiproliferative activity was assessed against A549 lung cancer cell lines using the MTT assay, where dose-dependent cytotoxicity was observed, particularly at concentrations of 100 and 200 µg/mL. The absence of prior evaluations of R. grata in these contexts highlights the novelty of this study and suggests its pharmacological potential as a promising source for natural antioxidants, neuroprotective agents, and anticancer agents.













