-
About 70% of smallholder farmers in sub-Saharan Africa (SSA) rely mainly on rainfed agriculture[1]. However, these farmers are resource-constrained but contribute significantly toward food security in developing countries hence their production methods and output are of major concern. Unfortunately, a larger proportion (> 60%) of the SSA experience persistent droughts and are characterized by inherently low fertile soils making rainfed agriculture a challenge[2]. The limited rainfall and low soil fertility are therefore twin constraints to animal and crop production in the SSA. Farmers in these semi-arid areas are encouraged to grow drought tolerant crops and early maturing crop varieties like pearl millet, barnyard millet, and sorghum[3]. Nevertheless, farmers are still opting to grow drought-prone and input-intensive but high-yielding crops e.g. maize because of their numerous benefits.
Maize is preferred by farmers due to its higher potential yield per unit area compared to small grain cereals, its dual-purpose use (grain and fodder); use as a cash crop, and raw materials for industry[4]. In many parts of the world, maize is grown in areas that receive 300−500 mm yr−1 precipitation, which is approximately the critical level for obtaining a good yield[5]. In Zimbabwe, improved maize yields are observed in areas that receive an average rainfall of > 500 mm yr−1 yield though production also depends on the variety in question[6]. To achieve high grain yield in maize, the rainfall should be effective and evenly distributed throughout the growing season. Unfortunately, climate change has resulted in uneven rainfall distribution patterns, and more rainfall is usually received at the beginning and end of the season than mid-season. In Zimbabwe, approximately 68% of summer (wet-season) maize is rain-fed and usually susceptible to the erratic behavior of rains[3]. This results in severe moisture stress at the critical maize growth stages and ultimately reduces yield. However, maize adaptation should deal not only with changes in rainfall averages but also with the increased frequency and intensity of extreme events[7]. Under normal rainfall (> 550 mm yr−1), the average maize yield in Zimbabwe is 5 t ha−1 for most varieties but very low (< 0.6 t ha−1) among smallholder farmers[5].
A large proportion (> 60%) of Zimbabwe is semi-arid and receives < 450 mm rainfall per annum which mostly occurs early in the rainy season[4]. Therefore, crops usually experience moisture stress from the pre-flowering to late grain-filling stages[8]. The moisture stress negatively affects many physiological processes e.g. photosynthesis, nutrient uptake, reproductive system, and seed set in maize[4]. Therefore, maize yield in semi-arid areas is declining due to natural intermittent water deficit stress caused by depleted soil moisture. Infield soil moisture conservation and soil fertility enhancement practices become key for improved agricultural output. Maize production can be improved through the use of in-field water harvesting techniques in combination with organic nutrient sources[9,10]. Interactive effects of rainwater harvesting methods and organic nutrient sources can be better options for addressing issues of soil moisture stress and soil fertility[11].
Coupled with effective in-field soil moisture conservation practices such as mulching and tied ridging, maize production is possible in dry areas (receiving average annual rainfall of < 650 mm) of Zimbabwe[1]. To achieve sustainable and effective soil moisture conservation in the semi-arid areas, there is a need to improve the soil status first since the soils are predominantly sandy and structureless with very low water holding capacity. Adoption of physical in-field rainfall harvesting strategies e.g. tied ridges or potholing alone will be ineffective moisture conservation strategies under such soils. In this case, addition of soil organic matter (SOM) can be ideal since it modifies soil properties e.g water holding capacity, pH, and cation exchange capacity (CEC)[12]. Higher (> 28 °C) summer temperatures in semi-arid areas exacerbate moisture depletion due to the least organic matter content[13]. Hence, organic amendments incorporation may enhance the organic matter content of the soil leading to increased moisture conservation and plant nutrient availability. The soil moisture conservation strategies should therefore aim to improve also the soil structure e.g. by increasing the soil organic matter (SOM).
There is a potential for the use of tied ridges to improve soil moisture as the method harvests rainwater stores it, and recharges water in the plant root zone[14,15]. Moisture improvement can also be augmented by the application of cattle manure which increases soil organic carbon, nitrogen content, and total porosity by reducing macropores to micropores[16]. Cattle manure reduces soil bulk density, and increases microbial population which facilitates decomposition and changes soil structure[10]. This increases the mineralization and decomposition of soil organic matter releasing nutrients and hence retaining a lot of moisture.
However, the conventional practice of applying little or no manure to the soil resulted in very low soil water storage efficiency (ratio of stored water to rainfall during the growing season)[17]. Therefore, there is a need to develop technologies that optimize the use of the limited water and soil resources to achieve sustainable crop production. Rational use of organic manure has been observed to increase water infiltration, water retention, soil water storage, grain yield, and rainfall use efficiency[16,18]. Maize production can be improved through the use of in-field water harvesting techniques in combination with organic nutrient sources[10]. Interactive effects of rainwater harvesting methods and organic nutrient sources can be better options for addressing issues of soil moisture stress and soil fertility[6,19]. Numerous infield soil moisture conservation practices such as tied ridges, potholing, fanya juu, and contour ridges have been extensively promoted among smallholder farming. The in-situ infield water harvesting is used to capture and store water as it rains. They improve soil moisture by enhancing infiltration and reducing runoff and evaporation[20].
In situ, water harvesting systems such as tied-ridging and sub-soiling improved the soil water storage in the root zone during the cropping period compared to traditional tillage by 24% and 15% respectively[14,20]. Similarly, in the semi-arid areas, tied ridges improved barley yield by 44% compared to traditional tillage[17,21]. Nevertheless, these are usually promoted in isolation ignoring the poor soil fertility aspect hence, are ineffective interventions to improve crop production in the semi-arid parts of Zimbabwe. Therefore, the present research was done to study the effects of three rates of cattle manure combined with tied ridges on dry matter accumulation and rainfall use efficiency of maize at various growth phases in drier areas of Zimbabwe. This study aimed to produce scientific evidence for providing an essential framework for farmers in semi-arid areas on how to optimize crop management practices for conserving soil water, rainfall use efficiency, and achieving high- crop yield. A realistic understanding of the soil's capacity to store water and assess available water before planting will help identify planting opportunities and potential crop yields.
-
A field experiment was done in the 2019/20−2021/22 cropping seasons (October to April) in Muzokomba, Buhera, Manicaland Province, Zimbabwe. The area is > 800 m altitude above sea level and is located in Zimbabwe’s natural farming region V which receives ≤ 450 mm of rainfall per annum. The cropping season is characterized by severe mid-season dry spells. The field was used for cereal crop production without the use of fertilizers before the experiment. The area is predominantly occupied by Lixisols[22].
Experimental design
-
The experiment had tied ridges combined with three manure application rates laid as a 2 × 4 factorial in a completely randomized block design (CRBD) with three replicates. The plots were made of tied ridges that were 2 m apart with a ridge height of 35 cm. Cross ties were put at 5 m intervals and were raised to 20 cm in height to minimize breakage from the flowing water.
An early maturity (120 days to maturity) SeedCo maize variety (SC537) was planted in the last week of October each year. The planting population was 0.8 inter-row × 0.23 in row spacing to obtain a total of 52,000 plants ha−1. Each experimental plot was 10 m × 8 m with a net area of 25 m2. Generally, in Zimbabwe maize crop requires 67 kg N ha−1 hence the quantities of fertilizer applied were calculated based on this N requirement. Inorganic fertilizer (21 kg N ha−1 was supplied through Compound D (7% N) : (14% P2O5) : (7% K2O) at 300 kg ha−1 at planting and the reminder 46 kg N ha−1 was applied through Ammonium nitrate at 100 kg N ha−1 after maize emergence). All the organic manure was applied before planting the maize. The inorganic fertilizer was applied using the blanket recommended rate (300 kg ha−1 i.e., 21 kg N ha−1) in the Muzokomba area. The organic manure application rates were also applied according to the N requirement of the maize crop. Hence, the quantities of organic manure applied were determined according to the amount of extractable NO2/NO3 (mg kg−1) in the manure (Table 1). Cattle manure was applied at 50% N (low manure), 100% N (standard manure), and 150% N (high manure) which corresponded to 7.5 t ha−1, 15 t ha−1, and 22.5 t ha−1 respectively. The cattle manure was repeatedly applied in each year of the experiment to mimic the cultural practice in the smallholder agricultural sector. Therefore, the treatment combinations for tied ridges were tied ridges + 7.5 t ha−1 low cattle manure (TLM), tied ridges + 15 t ha−1 standard cattle manure (TSM), and tied ridges + 22.5 t ha−1 high cattle manure (THM) application rates. For no-tied ridges were: No-tied ridges + 7.5 t ha−1 low cattle manure (NTLM), No-tied ridges + 15 t ha−1 standard cattle manure (NTSM), and No-tied ridges + 22.5 t ha−1 high cattle manure (NTHM) application rates. The No-tied ridges + 0% cattle manure (NT0%) and tied ridges + 0% cattle (T 0%) manure were included as positive controls.
Table 1. The initial chemical properties of the soil at the Muzokomba area, experimental field and cattle manure used in the study.
Parameter Soil Cattle manure Sand (%) 78 ± 2.3 2 ± 0.1 Silt (%) 18 ± 2.3 0.7 ± 0.2 Clay (%) 3 ± 2.3 0.01 ± 001 pH (H2O) 4.2 ± 1.2 6.98 ± 0.3 EC (dSm−1) 4.1 ± 0.03 8.12 ± 0.1 CEC (cmol(+)kg−1) 8.0 ± 0.5 314.2 ± 0.8 Total C (%) 0.7 ± 0.04 30.5 ± 0.4 Total N (%) 0.5 ± 0.03 4.16 ± 0.2 C:N ratio 0.3 ± 0.01 8.8 ± 0.7 Olsen extractable P (mg kg−1) 55.0 ± 7.3 620.4 ± 17.8 Extractable NO2/NO3 (mg kg−1) 29.2 ± 2.04 980.5 ± 8.7 Extractable NH4 (mg kg−1) 98.4 ± 0.8 386.3 ± 2.8 K (mg kg−1) 6.4 ± 0.6 3.2 ± 0.5 Ca (cmol(+) kg−1) 0.3 ± 0.05 27.1 ± 2.5 Mg (cmol(+) kg−1) 24.5 ± 1.9 10.8 ± 2.1 Na (cmol(+) kg−1) 0.45 ± 0.03 2.6 ± 0.7 Cu (cmol(+) kg−1) 110.1 ± 36.1 305.2 ± 38.6 Zn (cmol(+) kg−1) 70.2 ± 6.9 412.8 ± 0.6 Bulk density (kg cm−3) 1.52 ± 0.8 − EC, electrical conductivity; CEC, cation exchange capacity. Data are means ± standard error of the means for three replicates. Soil sampling and analysis
-
Four soil samples were taken to a depth of 0−40 cm using a soil auger in July 2019. Soil samples were mixed in a plastic bucket to produce a composite sample (1 kg) which was shade-dried for soil analysis. Cattle manure was sourced from the local farmers in the Muzokomba area and sun-dried for one week to attain uniform moisture content. Then, 500 g of manure was randomly sampled and taken for analysis while the bulky manure was stored for use. The soil and cattle manure were analyzed as explained by Parwada et al.[23]. Briefly, soil pH and electrical conductivities (ECs) were determined in a soil-water suspension (ratio of 1:5) using a TPS meter, and soil texture was analyzed by the hydrometer method as described in Okalebo et al.[24]. Total carbon (C), nitrogen (N), Olsen extractable P, and exchangeable ammonium and nitrate and nitrite in both the cattle manure were analyzed as described by Parwada & Van Tol.[12]. Bulk density (ρb) was determined using the core method.
Data collection
-
Soil water storage was measured gravimetrically (drying method, w/w) to a depth of 120 cm at 20 cm increments before sowing and at planting to emergence, emergence to tassling, tassling to silking, silking to physiological maturity, and dry-down period growth stages of maize. Three random locations in each plot were taken to measure soil water storage. Bulk density (ρb) was determined using the core method and calculated as:
$ {\rho }_{b}=\dfrac{M}{V}\, $ where,
$ {\rho }_{b} $ $ M $ $ V $ Soil water storage (0−120 cm) was calculated using the formula:
$ {S}_{w}= h \;\times\; d\;\times\; b{\text{%}} \;\times\; 10 \, $ where, Sw (mm) is the sum of soil water storages at different soil layers, h (cm) is soil layer depth; d (g cm−3) is soil bulk density in different soil layer and b% is the percentage of soil moisture in weight.
Dry matter was measured from planting to emergence, emergence to tassling, tassling to silking, silking to physiological maturity and dry-down period growth stages of maize. The maize samples collected at each respective growth stage were dried in an oven at 105 °C for 1 h and then were dried at 75 °C to constant weight. Five maize plants per plot were used (destructively sampled) for each measurement at different growth stages of maize. The dry matter accumulation (DMA) was as follows:
$ Dry\;matter\;accumulation=\dfrac{DMW\left(t\right)}{Plot\;area\;\left(ha\right)}\, $ where, DMW is dry matter weight.
Rainfall use efficiency was calculated using the following formula:
$ RUE=Y/R \, $ where, RUE represents the rainfall use efficiency for the biomass yield (kg ha−1 mm−1); Y is the dry matter accumulation of the maize and R is the rainfall.
Soil samples were collected from the surface layers (0–20 cm) of all plots during off season of maize in September each year. Four soil samples were collected for each treatment replicate, were combined into a composite sample, air-dried, and were sieved before chemical analysis. All chemical parameters were calculated based on the oven-dry (105 °C) weight of the soil. Soil organic matter (SOM) was determined using the dichromate oxidation method, total N by micro-Kjeldahl digestion, total P was determined by the wet oxidation procedure described by Rowland & Grimshaw[25], and total K by extraction with 1N ammonium acetate (NH4OAc) solution at pH 7.046.
Data analysis
-
Collected data were tested for normality and observed to follow a normal distribution and homoscedasticity, and thus, two-factor analysis of variance (ANOVA) was done to compare soil water storage, rainfall use efficiency, and growth parameters of maize under different cattle manure application rates and tied ridges. All data were analyzed using JMP version 11.0.0 statistical software. The significance of treatment effects was determined using the Duncan test at p ≤ 0.05.
-
Initial soil from the experimental field was classified as sandy loam soil with 78% sand, 18% silt, and 3% clay. The cattle manure contained some soil particles though in small quantities compared to the soil (Table 1). The soil and cattle manure had pH values of 4.2 and 6.98 respectively. The soil had a total of 0.5% nitrogen, 0.7% soil organic carbon, and 55.0 mg kg−1 phosphorous while the cattle manure had higher values of the corresponding parameters (Table 1). The cattle manure had 33.5 times more extractable NO2/NO3 (mg kg−1) than the soil (Table 1). The soil had a bulk density of 1.52 kg cm−3.
Rainfall received during the study period
-
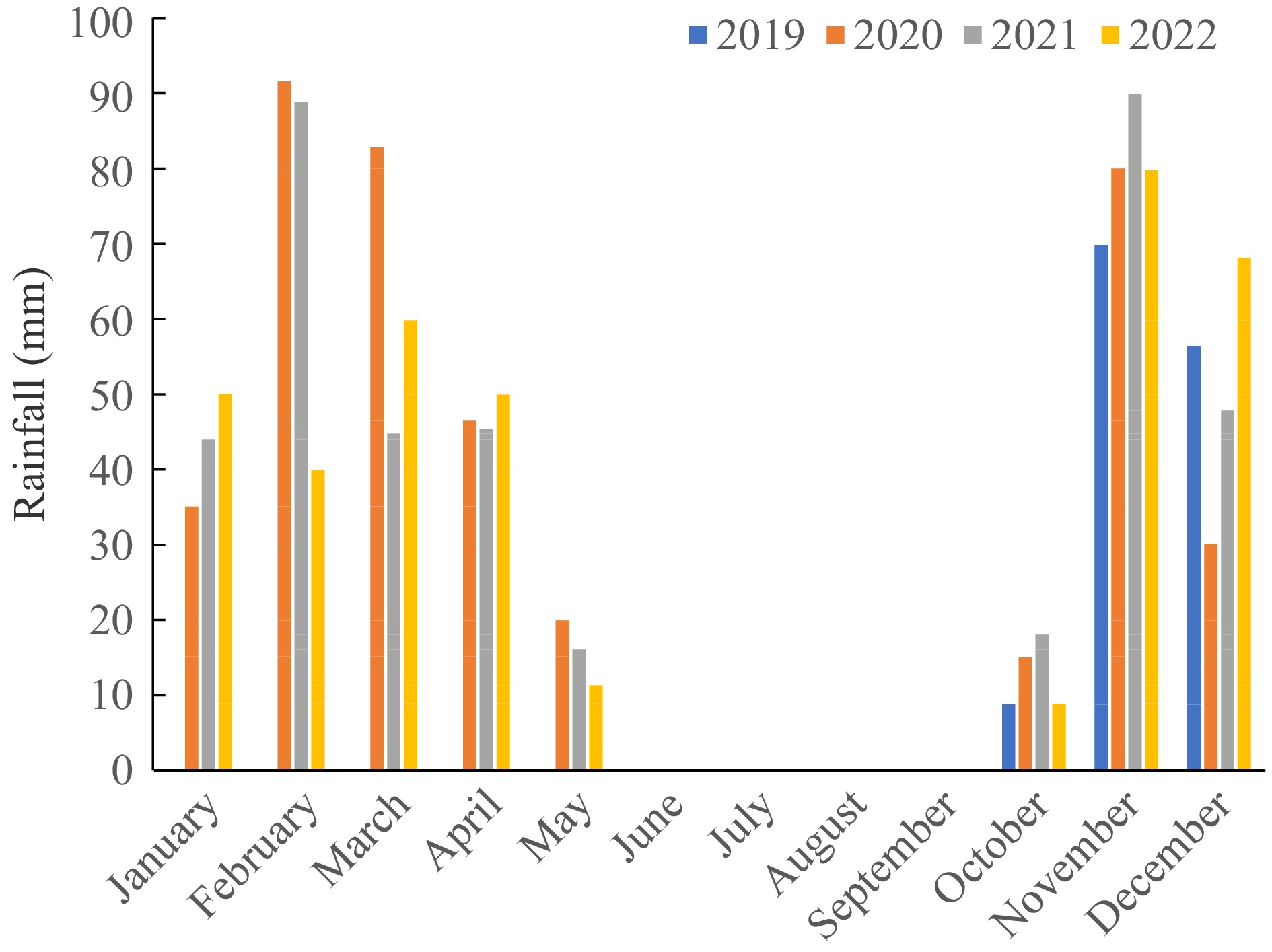
The study area received a total annual rainfall of 393.6, 350.6, and 369.9 mm in the 2019/20, 2020/21, and 2021/22 cropping seasons, respectively (Fig. 1). The rainfall was not uniformly distributed and rarely exceeded a mean of 25 mm in a pentad. A pentad was defined as having ≥ 25 mm of rain in five days and only two pentads were recorded during the 2019 to 2022 rainy seasons which translated to only 10% frequency of occurrence of 25 mm of rain in a pentad (Fig. 1). Generally, the study area received a below-normal rainfall of ≥ 400 mm per year throughout the study period.
The rainfall quantity was generally lower at the planting and maize emergence (P-E) and dry-down periods (Dry-P) (Fig. 2). Rainfall received during the emergence to tassling (E-T) was < 100 mm in all three cropping seasons.
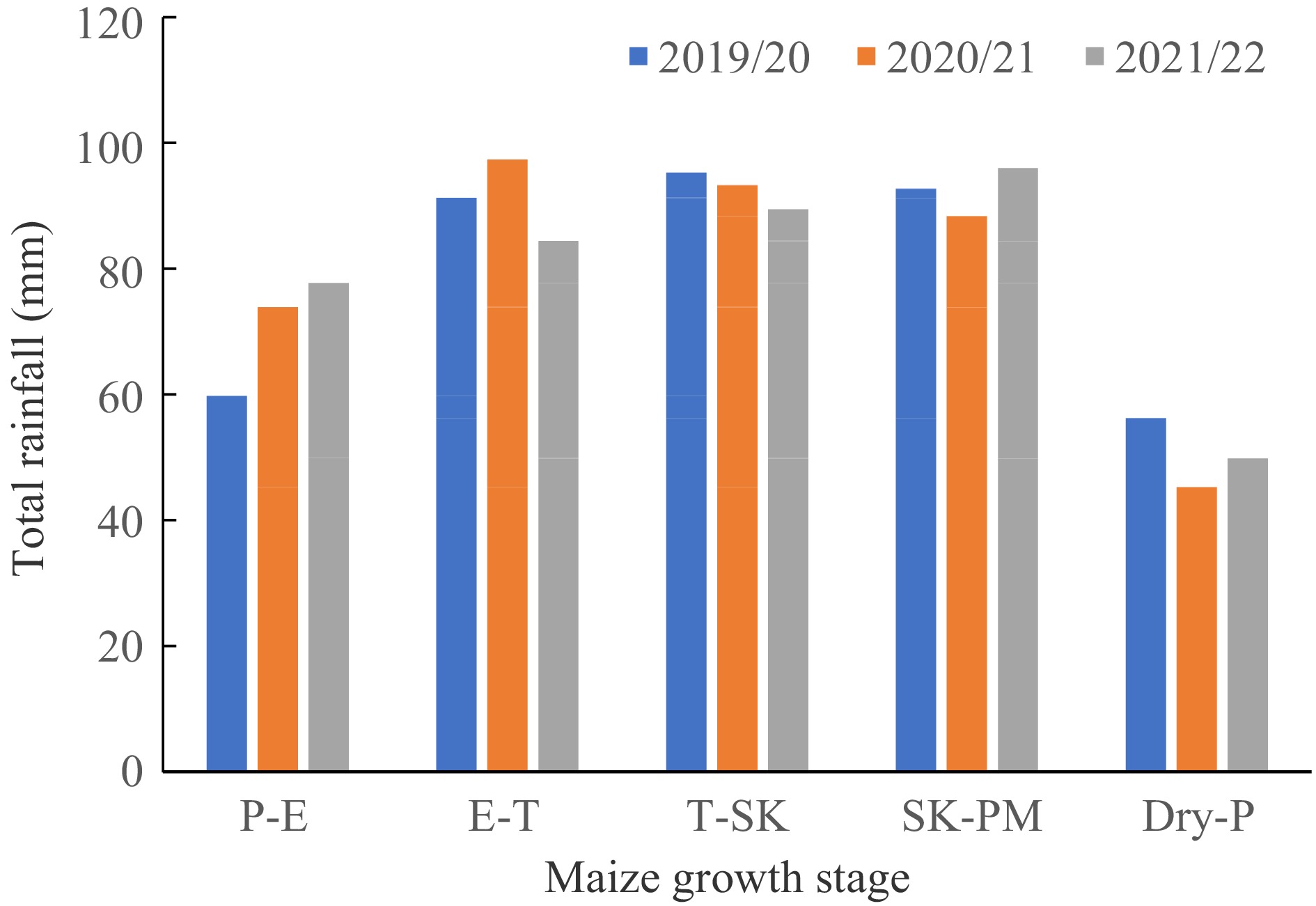
Figure 2.
Total rainfall (mm) distribution according to maize growth stages in the years of 2019/20–2021/22. P-E: Planting to Emergence; E-T: Emergence to Tassling; T-SK: Tassling to Silking; SK-PM: Silking to Physiological maturity and Dry-P: Dry-down period.
Soil water storage
-
No tied ridges + inorganic fertilizers had significantly (p < 0.05) the lowest soil water storage at all maize growth stages. The no-tied ridges + cattle manure application rates treatment combinations had significantly (p < 0.05) lower soil water storage compared to the tied ridges combined with the respective manure application rates (Table 2). The soil water storage was significantly (p < 0.05) highest at the P-E stages and thereafter showed a gradual decline with the maize growth to Dry-P in all the treatment combinations (Table 2). Tied ridges + > 7.5 t ha−1 cattle manure treatments had significantly the highest soil moisture storage. Soil water storage under NTHM and TLM application rates did not significantly differ in all the maize growth stages in the three seasons (Table 2). In the three-year study, the soil water storage was significantly (p < 0.05) increased by 6% from an average of 286.3 mm in NTHM and TLM treatments to 300 mm in tied ridges + > 7.5 t ha−1 cattle manure application rates treatments (Table 2).
Table 2. Soil water storage at 0–120 cm soil profile as influenced by manure management.
Year Treatments Soil water storage (mm) P-E E-T T-SK SK-PM Dry-P 2020 NT0% 255.2 ± 5a 240.6 ± 4a 201.3 ± 5a 176.8 ± 6a 181.4 ± 7a NTLM 269.5 ± 8b 252.2 ± 3b 236.1 ± 3b 196.2 ± 4b 205.0 ± 2b NTSM 272.1 ± 6b 258.0 ± 5b 238.3 ± 6b 198.1 ± 5b 226.1 ± 2b NTHM 284.3 ± 7c 269.3 ± 7c 249.2 ± 8c 216.4 ± 7c 231.0 ± 6c T0% 254.2 ± 5a 242.2 ± 4a 200.2 ± 5a 177.8 ± 6a 183.4 ± 7a TLM 288.6 ± 2c 270.6 ± 2c 246.4 ± 2c 220.6 ± 8c 233.2 ± 1c TSM 299.1 ± 4d 281.1 ± 4d 259.1 ± 4d 228.5 ± 1d 249.1 ± 4d THM 299.3 ± 7d 293.3 ± 7d 261.0 ± 5d 230.6 ± 8d 250.1 ± 3d 2021 NT0% 254.1 ± 5a 242.5 ± 4a 202.3 ± 4a 175.6 ± 6a 183.4 ± 6a NTLM 266.4 ± 6b 255.4 ± 6b 236.1 ± 3b 196.2 ± 4b 206.1 ± 5b NTSM 270.2 ± 4b 256.2 ± 4b 238.3 ± 6b 198.1 ± 5b 228.2 ± 7b NTHM 285.1 ± 3c 265.1 ± 3c 248.1 ± 5c 216.4 ± 7c 239.1 ± 6c T0% 256.2 ± 5a 243.5 ± 4a 201.3 ± 5a 175.7 ± 6a 180.4 ± 6a TLM 286.6 ± 2c 288.6 ± 2b 246.4 ± 2c 221.0 ± 5c 241.3 ± 1c TSM 294.1 ± 4d 290.1 ± 4b 258.3 ± 7d 226.6 ± 3d 254.2 ± 4d THM 302.5 ± 8d 302.5 ± 8c 260.3 ± 2d 233.4 ± 9d 258.0 ± 8d 2022 NT0% 254.2 ± 4a 241.5 ± 4a 201.2 ± 4a 175.6 ± 5a 180.4 ± 7a NTLM 266.5 ± 8b 249.2 ± 2b 238.4 ± 6b 198.3 ± 1b 204.6 ± 1b NTSM 270.1 ± 6b 254.0 ± 1b 237.2 ± 8b 196.0 ± 3b 226.1 ± 6b NTHM 266.3 ± 7c 264.3 ± 5c 250.1 ± 6c 217.2 ± 8c 253.0 ± 5c T0% 253.2 ± 4a 240.6 ± 4a 200.3 ± 4a 175.8 ± 5a 180.4 ± 5a TLM 285.6 ± 2c 265.2 ± 7c 247.3 ± 9c 219.8 ± 6c 255.2 ± 7c TSM 298.1 ± 4d 279.2 ± 3d 260.4 ± 6d 226.4 ± 2d 266.3 ± 4d THM 301.3 ± 7d 283.4 ± 2d 265.2 ± 3d 231.2 ± 5d 267.0 ± 5d Values in the same column and same year followed by different letters indicate significant differences (Duncan p < 0.05). Dry matter accumulation
-
There were no significant differences in dry matter accumulation (DMA) at the P-E growth stage in most of the treatment combinations except for the tied ridges + high manure application which recorded high dry matter accumulations in 2022 (Table 3). No tied ridges + 0% cattle manure application rate had significantly (p < 0.05) recorded the lowest dry matter accumulation at subsequent growth stages from the P-E (Table 3). No tied ridges + cattle manure application rates treatments combinations had significantly (p < 0.05) lower dry matter accumulation compared to the tied ridges combined with the respective manure application rates (Table 3). Dry matter accumulation was significantly (p < 0.05) increasing from the E-T stages and was highest (162.1 t ha−1) at the SK-PM in 2022 but started to decline at the Dry-P maize growth stage in all the treatment combinations (Table 3). Generally, the tied ridges + > 7.5 t ha−1 cattle manure treatments recorded significantly (p < 0.05) higher dry matter accumulation from the E-T to Dry-P maize growth stage. The DMA was significantly (p < 0.05) the same in no-tied ridges + high cattle manure and TLM application rates at all maize growth stages in the three seasons. The average DMA from 2020 to 2022 was significantly (p < 0.05) increased by 9% from 23.2 t ha−1 in NTHM to 32.7 t ha−1 in tied ridges + > 7.5 t ha−1 cattle manure application rates treatments (Table 3).
Table 3. Effect of manure management on dry matter accumulation at different growth stages and grain yield of maize.
Year Treatments Dry matter accumulation (t ha−1) Grain yield (t ha−1) P-E E-T T-SK SK-PM Dry-P 2020 NT0% 1.1 ± 0.1a 9.0 ± 1.2a 29.5 ± 2.6a 58.6 ± 2.2a 20.1 ± 2.3a 0.2 ± 0.01a NTLM 1.2 ± 0.2a 16.4 ± 2.0b 52.8 ± 3.1b 76.4 ± 3.0b 30.6 ± 3.2b 0.4 ± 0.01b NTSM 1.2 ± 0.2a 18.7 ± 3.2c 65.6 ± 3.3c 87.4 ± 3.2c 40.8 ± 3.1c 0.6 ± 0.1c NTHM 1.3 ± 0.3a 23.5 ± 3.0d 76.3 ± 3.0d 96.2 ± 3.1d 52.4 ± 3.2d 0.8 ± 0.3d T0% 1.2 ± 0.1a 8.0 ± 1.1a 28.5 ± 2.5a 56.6 ± 2.1a 20.1 ± 2.2a 0.2 ± 0.01a TLM 1.4 ± 0.3a 22.7 ± 3.0d 80.3 ± 2.3d 100.8 ± 3.1d 53.2 ± 2.1d 0.9 ± 0.3d TSM 1.4 ± 0.3a 32.3 ± 3.1e 120.4 ± 3.2e 154.6 ± 3.3e 72.6 ± 3.0e 1.2 ± 0.5e THM 1.6 ± 0.3a 33.6 ± 3.1e 125.7 ± 3.8e 160.3 ± 3.5e 75.4 ± 3.2e 2.4 ± 0.7e 2021 NT0% 1.1 ± 0.2a 8.0 ± 1.1a 28.5 ± 2.5a 59.6 ± 2.1a 21.1 ± 2.2a 0.2 ± 0.01a NTLM 1.2 ± 0.1a 16.6 ± 2.1b 50.9 ± 3.0b 77.1 ± 3.1b 31.8 ± 3.1b 0.5 ± 0.1b NTSM 1.2 ± 0.3a 17.9 ± 3.0c 66.6 ± 3.2c 86.8 ± 3.1c 42.2 ± 3.2c 0.6 ± 0.1c NTHM 1.3 ± 0.2a 24.4 ± 3.2d 75.4 ± 3.2d 97.2 ± 3.0d 45.7 ± 3.3d 0.7 ± 0.3d T0% 1.2 ± 0.1a 9.0 ± 1.3a 28.5 ± 2.6a 57.5 ± 2.2a 20.1 ± 2.3a 0.3 ± 0.01a TLM 1.3 ± 0.2a 23.3 ± 3.1d 78.1 ± 2.1d 103.2 ± 3.2d 35.5 ± 2.0d 1.2 ± 0.5e TSM 1.4 ± 0.2a 31.4 ± 3.2e 119.5 ± 3.0e 153.4 ± 3.2e 38.8 ± 3.2e 1.4 ± 0.5e THM 1.3 ± 0.2a 32.8 ± 3.0e 124.6 ± 3.5e 158.9 ± 3.3e 37.3 ± 3.1e 2.8 ± 0.7f 2022 NT0% 1.1 ± 0.1a 9.1 ± 1.2a 28.5 ± 2.6a 57.5 ± 2.2a 20.0 ± 2.1a 0.2 ± 0.01a NTLM 1.3 ± 0.3a 15.1 ± 2.2b 52.3 ± 3.2b 77.8 ± 3.1b 32.3 ± 3.1b 0.4 ± 0.1b NTSM 1.2 ± 0.2a 19.4 ± 3.1c 66.8 ± 3.2c 88.5 ± 3.5c 41.6 ± 3.0c 0.7 ± 0.1c NTHM 1.3 ± 0.1a 24.5 ± 3.1d 78.2 ± 3.1d 97.3 ± 3.0d 45.7 ± 3.1d 0.7 ± 0.3c T0% 1.1 ± 0.1a 9.1 ± 1.3a 29.4 ± 2.5a 57.5 ± 2.1a 20.1 ± 2.1a 0.2 ± 0.01a TLM 1.9 ± 0.2b 25.2 ± 3.2d 82.4 ± 2.6d 101.5 ± 3.2d 47.6 ± 2.0d 1.3 ± 0.8e TSM 2.4 ± 0.2c 31.6 ± 3.2d 121.5 ± 3.1d 155.3 ± 3.2d 48.4 ± 3.1d 2.9 ± 0.8f THM 2.6 ± 0.3c 32.7 ± 3.0e 126.0 ± 3.3e 162.1 ± 3.0e 47.3 ± 3.0e 3.2 ± 0.9g Values in the same column and same year followed by different letters indicate significant differences (Duncan p < 0.05). The DMA significantly (p <0.05) increased by 79.6% from 32.7 t ha−1 at E-T to 162.1 t ha−1 at SK-PM under the THM treatment in 2022 (Table 3). The grain yield was highest (3.2 t ha−1) in tied ridges + 22.5 t ha−1 cattle manure application rate in 2022 and lowest (0.2 t ha−1) in no tied ridges + 0% cattle manure (Table 3).
Rainfall use efficiency
-
The rainfall use efficiency (RUE) was significantly (p < 0.05) highest (1.7 kg ha−1 mm−1) under the THM at the P-E growth stage in the 2022 growing season (Table 4). Like on the dry matter accumulation generally, the no tied ridges + inorganic fertilizers had significantly (p < 0.05) the lowest RUE from the E-T to Dry-P maize growth stage compared to other treatment combinations (Table 4). The no tied ridges + cattle manure application rates treatment combinations had significantly (p < 0.05) lower RUE compared to the tied ridges combined with the respective manure application rates (Table 4). The RUE was significantly (p < 0.05) increasing from the E-T stages and was highest (92.6 kg ha−1 mm−1) at the SK-PM in 2021 under tied ridges + cattle manure treatments. The RUE started to decrease from the SK-PM to Dry-P maize growth stage in all the treatment combinations (Table 4). Generally, the tied ridges + > 7.5 t ha−1 cattle manure treatments showed significant (p < 0.05) increase in RUE from the E-T to Dry-P maize growth stage compared to other treatment combinations. The rainfall use efficiency was significantly (p < 0.05) the same in no tied ridges + high cattle manure and tied ridges + low cattle manure application rates at all the maize growth stages in the three seasons. The RUE was significantly (p < 0.05) increased by 65.8% from 45.0 kg ha−1 mm−1 in no tied ridges + high manure to 72.6 kg ha−1 mm−1 in tied ridges + 22.5 t ha−1 cattle manure application rates treatment at S-SK in the 2022 season (Table 4).
Table 4. Effect of manure management on rainfall use efficiency at different growth stages of maize.
Year Treatments Rainfall use efficiency (kg ha−1 mm−1) Grain yield (t ha−1) P-E E-T T-SK SK-PM Dry-P 2020 NT0% 0.8 ± 0.2a 5.0 ± 1.1a 14.9 ± 4.2a 31.6 ± 3.2a 17.4 ± 2.1a 0.56 ± 2.1a NTLM 0.8 ± 0.1a 9.2 ± 2.1b 28.5 ± 3.3b 42.4 ± 3.0b 28.2 ± 3.1b 1.17 ± 2.1a NTSM 0.8 ± 0.2a 10.1 ± 3.2c 36.4 ± 3.2c 48.1 ± 3.1c 22.7 ± 3.2c 1.75 ± 0.6b NTHM 0.9 ± 0.1a 12.3 ± 3.1d 41.2 ± 4.2d 53.4 ± 3.3d 48.0 ± 3.3d 2.33 ± 1.1c T0% 0.8 ± 0.2a 5.0 ± 1.1a 15.9 ± 4.2a 32.6 ± 3.2a 18.4 ± 2.1a 0.58 ± 2.1a TLM 1.2 ± 0.2a 12.8 ± 3.1d 43.9 ± 4.3d 56.0 ± 3.2d 48.9 ± 3.2d 2.63 ± 1.2c TSM 1.2 ± 0.2a 18.2 ± 3.2e 65.1 ± 4.4e 85.9 ± 3.4e 66.5 ± 3.1e 3.51 ± 1.3d THM 1.4 ± 0.2a 19.0 ± 3.2e 67.9 ± 4.2e 89.0 ± 3.6f 69.0 ± 3.0e 7.01 ± 1.5f 2021 NT0% 0.8 ± 0.2a 5.0 ± 1.1a 15.6 ± 4.1a 31.5 ± 3.2a 17.4 ± 2.1a 0.55 ± 2.1a NTLM 0.8 ± 0.1a 8.8 ± 2.2b 28.1 ± 3.1b 44.9 ± 3.2b 36.2 ± 3.0b 1.40 ± 0.8a NTSM 0.8 ± 0.2a 9.5 ± 3.1c 36.8 ± 3.0c 50.6 ± 3.2c 41.0 ± 3.0c 1.68 ± 0.9b NTHM 0.9 ± 0.2a 12.9 ± 3.2d 41.6 ± 4.1d 56.7 ± 3.1d 43.8 ± 3.0d 1.96 ± 0.9b T0% 0.8 ± 0.2a 5.0 ± 1.1a 16.2 ± 4.2a 36.2 ± 3.2a 19.3 ± 2.1a 0.59 ± 2.1a TLM 0.9 ± 0.1a 12.3 ± 3.2d 43.1 ± 4.1d 60.2 ± 3.1d 43.9 ± 3.0d 3.37 ± 1.2d TSM 1.0 ± 0.1a 16.6 ± 3.1e 66.0 ± 4.0e 89.4 ± 3.2f 42.9 ± 3.2e 3.93 ± 1.2d THM 0.9 ± 0.1a 17.4 ± 3.2e 68.8 ± 4.1e 92.6 ± 3.2f 42.4 ± 2.9e 7.86 ± 1.6f 2022 NT0% 0.8 ± 0.2a 5.0 ± 1.1a 14.9 ± 4.2a 33.5 ± 3.2a 17.3 ± 2.1a 0.56 ± 2.1a NTLM 0.9 ± 0.1a 9.2 ± 2.1b 30.1 ± 2.9b 41.8 ± 3.2b 33.3 ± 3.0b 1.14 ± 0.7a NTSM 0.9 ± 0.2a 11.8 ± 2.1c 38.5 ± 3.1c 47.5 ± 3.0c 43.0 ± 3.1c 1.99 ± 0.9b NTHM 0.8 ± 0.1a 15.0 ± 3.1e 45.0 ± 4.0d 52.2 ± 3.2d 46.7 ± 3.2d 1.99 ± 0.9b T0% 0.8 ± 0.2a 5.0 ± 1.1a 15.9 ± 4.2a 32.6 ± 3.2a 18.4 ± 2.1a 0.58 ± 2.1a TLM 1.3 ± 0.1a 15.3 ± 3.2e 44.2 ± 3.8d 54.5 ± 3.1d 47.2 ± 3.0d 3.70 ± 1.2d TSM 1.6 ± 0.2a 19.3 ± 4.2e 70.0 ± 4.2e 83.6 ± 3.5e 49.2 ± 3.0de 8.26 ± 1.2f THM 1.7 ± 0.2b 20.0 ± 4.1ef 72.6 ± 4.1e 87.0 ± 3.0e 50.0 ± 2.8e 9.12 ± 1.6g Values in the same column and same year followed by different letters indicate significant differences (Duncan p < 0.05). The RUE significantly (p <0.05) increased by 335% from 20.0 kg ha−1 mm−1 at E-T to 87.0 kg ha−1 mm−1 at SK-PM and decreased by 42.5% from the SK-PM to 50.0 kg ha−1 mm−1 at the Dry-P growth stage respectively in tied ridges + 22.5 t ha−1 cattle manure application rates treatment in 2022 (Table 4). The RUE on the grain yield was generally higher on the tied ridges + cattle manure than on no-tied ridges + cattle manure. The RUE for the overall maize grain yield was significantly (p < 0.05) was lowest (0.58 kg ha−1 mm−1) and highest (9.12 kg ha−1 mm−1) in the tied ridge control and tied ridges + 22.5 t ha−1 cattle manure application rates treatment in 2022 respectively (Table 4).
Characterisation of the soil properties under tied ridged plots after three years of manure application
-
Most of the measured soil properties changed due to the addition of the cattle manure except for the sand (%), silt (%), and clay (%) for the entire study period (Table 5). The soil pH was improved from 4.1 in the control to 6.2 in the tied ridges + high cattle manure application rates. Total N (%), extractable NO2/NO3, total C (%), K, and other measured nutrient elements increased significantly as the quantity of the manure applied increased. There was a slight increase in the measured soil parameters in the ≤ 7.5 t ha−1 cattle manure application rates treatments compared to the > 7.5 t ha−1 manure application rates treatments (Table 5).
Table 5. Soil nutrients and soil organic matter in tied-ridged plots as a function of the different manure treatments during 2020–2022.
Parameter Control Low manure Medium manure High manure 2020 2021 2022 2020 2021 2022 2020 2021 2022 Sand (%) 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 74 ± 3.1 Silt (%) 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 21 ± 2.3 Clay (%) 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 5 ± 1.3 pH (H2O) 4.1 ± 2.1 4.3 ± 2.0 4.4 ± 2.1 4.3 ± 2.1 5.1 ± 0.3 5.3 ± 2.2 5.8 ± 2.1 6.2 ± 2.2 6.6 ± 2.0 6.6 ± 2.2 EC(dSm−1) 6.2 ± 0.23 7.2 ± 0.6 7.6 ± 0.7 7.8 ± 0.6 9.1 ± 0.2 9.0 ± 0.3 9.6 ± 0.3 11.2 ± 0.6 15.1 ± 0.7 19.1 ± 0.7 CEC (cmol(+) kg−1) 4.3 ± 1.4 8.3 ± 1.4 8.2 ± 1.4 8.1 ± 1.3 19.2 ± 1.2 18.8 ± 1.3 21.1 ± 1.2 21.3 ± 1.5 23.1 ± 1.4 28.2 ± 1.5 Total C (%) 0.4 ± 0.01 0.8 ± 0.02 0.9 ± 0.01 0.8 ± 0.03 1.1 ± 0.3 1.2 ± 0.2 1.4 ± 0.3 1.2 ± 0.8 1.6 ± 0.8 2.2 ± 0.7 Total N (%) 0.2 ± 0.02 0.5 ± 0.02 0.6 ± 0.02 0.5 ± 0.02 0.6 ± 0.01 0.6 ± 0.02 0.9 ± 0.02 0.9 ± 0.03 1.6 ± 0.3 2.9 ± 0.3 C:N ratio 0.5 ± 0.01 0.6 ± 0.01 0.7 ± 0.01 0.6 ± 0.01 0.5 ± 0.7 0.5 ± 0.6 0.5 ± 0.7 0.8 ± 0.1 0.8 ± 0.1 0.8 ± 0.1 Olsen extractable P
(mg kg−1)55.0 ± 5.2 60.0 ± 5.3 59.0 ± 5.0 60.0 ± 5.3 82.4 ± 8.8 81.0 ± 9.2 87.0 ± 9.2 56.0 ± 5.2 60.0 ± 5.2 65.0 ± 5.2 Extractable NO2/NO3
(mg kg−1)25.1 ± 2.0 122.1 ± 2.0 123.1 ± 2.2 122.1 ± 2.2 250.5 ± 6.7 251.0 ± 7.0 258.3 ± 6.0 258.1 ± 7.0 265.1 ± 6.1 273.1 ± 7.1 Extractable NH4
(mg kg−1)96.2 ± 0.5 109.1 ± 0.9 108.0 ± 0.9 109.2 ± 0.8 376.2 ± 2.7 377.3 ± 2.8 381.2 ± 2.6 397.5 ± 0.6 418.2 ± 0.7 438.2 ± 0.8 K (mg kg−1) 6.4 ± 0.4 4.5 ± 0.7 4.3 ± 0.6 3.9 ± 0.6 4.8 ± 0.4 4.6 ± 0.5 5.3 ± 0.7 3.6 ± 0.3 6.2 ± 0.4 10.1 ± 0.5 Ca (cmol(+) kg−1) 0.3 ± 0.03 27 ± 4.1 26.9 ± 4.2 27.0 ± 4.1 30.3 ± 2.4 31.2 ± 2.4 39.2 ± 2.5 41.0 ± 4.4 42.2 ± 4.1 45.2 ± 3.5 Mg (cmol(+) kg−1) 20.6 ± 1.2 11.3 ± 1.4 11.3 ± 1.3 10.9 ± 1.8 10.6 ± 2.0 10.1 ± 1.6 11.5 ± 1.6 9.5 ± 1.1 8.0 ± 1.0 13.2 ± 1.4 Na (cmol(+) kg−1) 0.3 ± 0.01 2.4 ± 0.02 2.5 ± 0.01 2.5 ± 0.02 3.6 ± 0.7 3.7 ± 0.8 4.0 ± 0.9 3.9 ± 0.6 4.1 ± 0.3 5.1 ± 0.4 Cu (cmol(+) kg−1) 111 ± 9.8 201 ± 25.8 204. ± 25.8 202 ± 25.8 315 ± 38.6 312 ± 20.1 328 ± 20.2 351 ± 22.6 356 ± 22.7 360 ± 25.6 Zn (cmol(+) kg−1) 64.1 ± 5.5 330.6 ± 2.9 331.6 ± 4.1 332.3 ± 3.1 416.9 ± 0.8 415.6 ± 0.9 417.7 ± 0.8 432.6 ± 5.3 433.6 ± 5.4 438.2 ± 5.2 Bulk density (kg cm−3) 1.53 ± 0.8 1.48 ± 0.6 1.46 ± 0.6 1.42 ± 0.6 1.45 ± 0.7 1.32 ± 0.6 1.28 ± 0.8 1.32 ± 0.6 1.10 ± 0.6 0.80 ± 0.4 EC, electrical conductivity; CEC, cation exchange capacity. Data are means ± standard error of the means for three replicates. The results showed the cumulative effects of applying of high quantity (22.5 t ha−1) of cattle manure for three consecutive years. The highest values of measured soil parameters were observed in the third year (2022) in the 22.5 t ha−1 cattle manure application rate treatment (Table 5). The bulk density decreased by 47.71% from 1.52 kg cm−3 in the control to 0.80 kg cm−3 in the tied ridges + 22.5 t ha−1 cattle manure application rate in 2022.
-
No tied ridges + inorganic fertilizers and no tied ridges + cattle manure at all the application rates (7.5 t ha−1, 15 t ha−1, and 22.5 t ha−1) had significantly (p < 0.05) lower soil water storage at the five maize growth stages compared to tied ridges combined with the respective manure application rates (Table 2). Sandy soils are characterized by few and large pores[12] hence the low soil water storage recorded in no tied + inorganic fertilizer could be a result of relatively large pores of the sand soil causing rainwater to drain freely. Therefore, the observed low soil water content was observed at all the maize growth stages (Table 2). However, the combined effects of the tied ridges + cattle manure were positive on the soil water storage as the tied ridging was effective in minimizing runoff and promoting water infiltration. The cattle manure promoted the formation of soil aggregates reducing the free drainage of rain water. This counts to the general increase of soil water storage in the tied ridges + cattle manure treatments. The reduction of soil water storage with an increase in maize growth could be due to the differences in water demand and utilization at the specific growth stage. The soil water storage was highest at the planting to emergence (P-E) as there was rainfall (Table 2) with low water use and demand as the crop needed adequate moisture only for germination. The crop water demand increased as the crop reached the Emergence and Tassling (E-T) to Tassling to Silking (SK-PM) stages. These stages are characterized by high water requirements as there is rapid biomass accumulation and reproduction[8] resulting in reduced soil water content (Table 2). During the vegetative stage, the maize plants develop larger leaf surfaces increasing the demand for water and approaching maximum water use when the canopy has fully grown (40-60 d after planting (AP))[15]. The maize plant reaches peak water demand and becomes highly sensitive to moisture shortage during the flowering and early grain fill stage (60-95 d AP). The addition of cattle manure alters the soil water retention properties[26]. It was agreed that the growth and performance of crops are dependent on the soil properties, especially, soil water retention e.g. water status[11]. Under the tied ridges treatments, the soil water content was directly proportional to the quantity of cattle manure applied so that the more (> 7.5 t ha−1) the manure the more the soil water content (Table 2). These results agree with Mudatenguha et al.[5] who also showed an increase in magnetic effects of water particles as the cattle manure application rates were increased above 7.5 t ha−1 in sandy-loam soils.
Dry matter accumulation
-
The dry matter (DM) accumulation was increased from the P-E stage and peaked at the SK-PM stage before declining at the Dry-P. Generally, the grain yield was proportionally increasing with an increase in the total dry matter accumulated (P-E to Dry-P) in all the treatments (Table 3). Dry matter (DM) accumulation and its allocation to kernels are key factors that influence the final maize grain yield. Grain yield is influenced by the efficiency of many physiological processes that occur from plant germination to the maturity stage. Hybrids with short ripening periods accumulate half of all dry matter until silking and nearly the same amount until grain filling[4].
Therefore, water deficit at the flowering stage will negatively affect fertilization, and grain filling causing a low yield of maize[8]. Shumba et al.[27] noted that if soil moisture during the reproductive stage remains at the wilting point for 1−2 d or 6−8 d, the grain yield was reduced by 20% and > 50%, respectively. However, Eleduma et al.[10] observed that maize is generally tolerant to water shortage during two distinct growth phases which are at the early vegetative (until 40 d AP) and late grain fill and ripening stage (after 110 d AP). In this study, results (Table 4) showed the cumulative effects of moisture stress at subsequent maize growth stages on the final grain yield suggesting that if maize is affected by drought conditions at any growth stage from planting, the grain yield was significantly reduced.
The growing factors should be favorable for high dry matter accumulation which will be allocated to the kernels at the grain filling stage. Tied ridges + > 7.5 t ha−1 cattle manure application rates were shown to conserve soil moisture and supply plant nutrients that influenced higher DM accumulation compared to other treatments in all three seasons. The continuous (2020 to 2022 seasons) application of high quantities (22.5 t ha−1) of manure under the tied ridges has significantly modified the soil hydro-properties and the soil nutrient status resulting in the highest (3.2 t ha−1) grain yield by 2022. The observed differences in DM accumulation and grain yield under the tied ridges + 22.5 t ha−1 cattle manure application rate between the prior 2022 season and the 2022 seasons could be due to the cumulative effects of the treatment on the maize growth and yield. This could be explained by the observed increase in the grain yield with an increase in dry matter accumulation during the growth stages of the maize. These results agree with Kubiku et al.[2] who observed that the grain yield of maize was closely correlated to the seasonal dry matter accumulation. Traore et al.[16] observed double as much sorghum grain yield under residual tied-ridge treatment than in the no-tied ridged plots in drier farming areas. This confirms that the in situ water harvesting techniques are effective in soil moisture conservation in semi-arid areas.
Rainfall use efficiency (RUE)
-
The RUE factor is the quotient of annual primary production by annual rainfall, i.e. the number of kg aerial dry matter phytomass produced over 1 ha year−1 mm−1 of total rain received. All other conditions remaining equal, RUE was observed to decrease with an increase in drought conditions. Muchai et al.[18], gave some explanation on how water shortage may improve WUE and they showed that drought occurrence early in the crop growth cycle and partially closes the stomata which results in the conservation of soil water and a subsequent improved crop yield per unit of water.
In this study, no-tied ridges + cattle manure treatments recorded significantly (p < 0.05) lower soil moisture content and RUE than the tied ridges + cattle manure application rates (Tables 2, 4). This suggests that the addition of manure alone in the studied soils could not positively influence the soil water content and hence resulted in reduced dry matter accumulation. The drier conditions in the control and the no-tied ridges + cattle manure treatments resulted in low seasonal primary production by the annual rainfall received during the study period. In this study, there was an increase of RUE of maize by 4.8%, 17.2%, and 38.9% respectively for the application rate of 7.5, 15, and 22.5 t ha−1 cattle manure under tied ridges respectively in 2022 at the SK-PM stage (Table 4). The results are similar to Eleduma et al.[10] who noted an increase in water use efficiency of winter wheat by 5.1%, 13.8%, and 29.3% respectively for 60, 120, and 180 kg N ha−1 in sandy soils respectively. The results showed the cumulative effects of applying manure on the measured parameters. The soil nutrients and soil organic matter in tied-ridged plots as a function of the different manure treatments were highest in the 2022 growing season, corresponding to the season with the highest grain yield recorded (Tables 4 & 5). This indicates that continuous application of manure in the studied soils could improve the physicochemical, soil hydro-properties, and the resultant maize grain yield.
-
Lower than 22.5 t ha−1 cattle manure application rates alone did not significantly (p > 0.05) improve the soil water storage, DM accumulation, and RUE under semi-arid conditions. The soil water storage, DM accumulation, and RUE were significantly (P < 0.05) improved under the tied + > 7.5 t ha−1 cattle manure application rates. These results also proved that it is possible to obtain ≥ 3 t ha−1 of maize grain yield in the semi-arid areas of Zimbabwe if tied ridges are combined with 22.5 t ha−1 cattle manure. The combined tied ridges and cattle manure modified the soil properties which increased maize grain yield. In this study, the addition of cattle manure enhanced the soil health by building up the soil's organic carbon content. Farmers in the semi-arid areas may, therefore, apply tied ridges + > 7.5 t ha−1 cattle manure application rates for improved maize production. However, it is important to repeat the study in multi-sites to further understand the effects of cattle manure combined with tied ridges on maize production in drier farming regions of Zimbabwe.
-
The authors confirm contribution to the paper as follows: study conception and design: Parwada C, Makuvaro V, Bandason W, Chipomho J; data collection: Makore F, Parwada C, Bandason W; analysis and interpretation of results: Parwada C, Chipomho J, Makuvaro V; draft manuscript preparation: Parwada C, Chipomho J, Makuvaro V, Makore F. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
The research did not receive any specific funding, but was performed as part of employment at the Midlands State University, Zimbabwe and Marondera University of Agricultural Sciences and Technology, Zimbabwe. The authors gratefully acknowledge the Mr. Takaendesa Muzokomba for the resources to carry this study at his field.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Parwada C, Makore F, Chipomho J, Makuvaro V, Bandason W. 2024. Effects of tied ridges and different cattle manure application rates on soil moisture and rainfall use efficiency on maize growth and yield in semi-arid regions of Zimbabwe. Technology in Agronomy 4: e021 doi: 10.48130/tia-0024-0018
Effects of tied ridges and different cattle manure application rates on soil moisture and rainfall use efficiency on maize growth and yield in semi-arid regions of Zimbabwe
- Received: 11 December 2023
- Revised: 18 May 2024
- Accepted: 22 May 2024
- Published online: 02 August 2024
Abstract: A 3-year rainfed field experiment was carried out to determine the effects of combined tied ridges and cattle manure application rates on maize productivity. The experiment was laid as a 2 × 4 factorial in a completely randomized block design (CRBD) with three replicates. Treatment combinations were tied ridges + 7.5 t ha−1 low cattle manure (TLM), tied ridges + 15 t ha−1 standard cattle manure (TSM), and tied ridges + 22.5 t ha−1 high cattle manure (THM) application rates. No-tied ridges + low, medium, and high quantities of cattle manure were used as positive controls. Early maturing maize variety (SC537) was then planted at 52,000 plants ha−1 in each plot. Soil water storage, soil bulk density, rainfall, dry matter accumulation (DMA), and grain yield were measured. Rainfall use efficiency (RUE) was then calculated. Analysis of variance was carried out to determine the effects of tied ridging and cattle manure on soil moisture content, RUE, and grain yield. The addition of cattle manure in tied ridges increased the soil moisture content, RUE, DMA, and grain yield. The measured parameters were significantly (p < 0.05) increasing with an increase in the quantity of cattle manure applied. The THM had 40% higher soil moisture content, 20% more RUE, and > 50% DMA compared to TLM. Grain yields significantly (p < 0.05) increased with an increase in application rates of cattle manure with the highest (3.2 t ha−1) recorded in the 2022 season under the THM treatment. The THM had significantly (p < 0.05) higher grain yield compared to no-tied ridges combined with corresponding cattle manure application rates. Farmers can practice tied ridges and 22.5 t ha−1 cattle manure to improve RUE and maize grain yields in the semi-arid areas of Zimbabwe.
-
Key words:
- Arid conditions /
- Drought /
- Maize production /
- Organic manure /
- Soil water