-
Plants contain a variety of biologically active compounds (pigments, polyphenols, flavonoids, glucosinolates, etc.)[1]. These substances are used in medicine, pharmaceuticals, and the food industry[2,3]. In turn, plants are an important source of biologically active substances for the human body and animals, which cannot synthesize them on their own[4,5]. Each plant has an individual composition of antioxidants, depending on external environmental conditions (light, temperature, humidity, soil composition, etc)[6]. Under the influence of various unfavorable factors of different nature (low and high temperatures, drought, salinity, heavy metals, pathogens, high light intensity, etc.), free radical oxidation processes develop in plants[7]. Due to the presence of effective antioxidants in the plant cell that protect it from oxygen radicals and peroxidation processes, plants can resist oxidative damage[8]. The antioxidant system of plants ensure the functioning of mechanisms to resist oxidative stress and consist of high molecular weight compounds (enzymes superoxide dismutase, catalase, peroxidases, glutathione reductase) and low molecular weight compounds (ascorbic acid, phenolic compounds, flavonoids, etc.)[9−11].
Leafy vegetables are important sources of minerals, both macronutrients and micronutrients, proteins, dietary fiber, carbohydrates, and vitamins for human nutrition[12,13]. Most importantly, they are rich in natural antioxidants such as phenols, xanthophylls, violaxanthins, ascorbic acid, carotenoids, betacyans, betaxanthins, chlorophyll a, chlorophyll b, and β-carotene, which have high radical scavenging properties[14,15]. Phenolic compounds, secondary metabolites found in foods, have long been known for their biochemical and pharmacological importance. Phenolic compounds are believed to play a vital role in the human body's defense responses, including anti-inflammatory, anti-aging, anti-proliferative, and antioxidant mechanisms[16]. These include coumarins, phenolic acids such as hydroxybenzoic acid and hydroxycinnamic acid, flavonoids such as flavonols, flavones, flavanols, flavanonic acids, isoflavones, anthocyanins, chalcones and non-flavonoids such as tannins, lignans, and stilbenes[17,18]. Flavonoids are also the most common group of phenolic compounds that are widely found in vegetables and fruits. Epidemiological and clinical data confirm their importance for health promotion and disease prevention[19].
Eruca sativa Mill. and Diplotaxis tenuifolia (L.) DC. are popular Brassicaceae crops cultivated throughout the world[20,21]. Crops are rich in phytochemicals such as carotenoids, flavonoids, glucosinolates, ascorbic acid, and others[22−24]. The VIR collection includes 71 Eruca accessions and 24 Diplotaxis accessions of five species: D. tenuifolia (L.) DC. – 20 acc., D. erucoides (L.) DC. – 2 acc., D. tenuisiliqua Delile – 1 acc., D. muralis (L.) DC. – 1 acc., from Russia and European countries.
The purpose of the present study is a comparative study of the antioxidant activity and accumulation of low-molecular compounds of Eruca and Diplotaxis in the greenhouse and open field.
-
The research work was carried out in the Vavilov Federal State Scientific Research Institute of the Russian Academy of Sciences (VIR) at the Pushkin and Pavlovsk Laboratories of VIR (Pushkin, Leningrad Region, Russia) in 2023 in an open field and a greenhouse. The research material was 20 Eruca accessions and five Diplotaxis accessions from the VIR collection.
Sowing in the greenhouse was carried out on March 3, in the open field - on July 3, and harvesting of plants was carried out on days 30−35. The sowing pattern is 10 cm × 5 cm, repeated three times, 20 plants each. The light period in March was 10−13 h, and 13−16 h in April. The average temperature was 22 ± 2/20 ± 2 °C (day/night). In the greenhouse peat nutrient substrate 'Agrobalt-C' (Pindstrup, Russia) was used as a soil, with a pH value ranging from 6.0 to 6.5. The soil contained nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), and calcium (Ca) at concentrations of 120, 80, 140, 30, and 170 mg/m3, respectively. Irrigation was carried out with water. Soil humidity was 60%−70%, air humidity was 60%−65%. The light period in July was 18.4−16.0 h, and 16.0−14.0 h in August. The average temperature in July in the afternoon was 16–26 °C, at night − 11.0−20.0 °C, in August in the afternoon 17.0−33.0 °C and at night 12.0−25.0 °C. Air humidity was 70%−75%, soil humidity 80%−85%. The soil of the open field was soddy-podzolic, with a pH value of 6.0–6.5, and the contents of nitrogen (N), phosphorus (P), and potassium (K) are 100, 72, and 120 mg/m3, respectively.
Biochemical analysis
Ascorbic acid
-
This method is based on the extraction of vitamin C with a solution of hydrochloric acid, followed by visual titration with a solution of sodium 2,6-dichlorophenolindophenolate (DCPIP, Tillman's reagent) until a light pink color is established[25]. A solution of hydrochloric acid with a mass fraction of 1% was used as an extraction solution. The solution for titration of 60 mg of sodium 2,6-dichlorophenolindophenolate was dissolved in pre-boiled water (150 mL) for 30 min, cooled and the solution was made up to 200 mL. The shelf life of the solution was not more than 10 d. The titre of the 2,6-dichlorophenolindophenolate sodium solution was established using a standard ascorbic acid solution with concentrations of 1.0 and 0.1 g/L on the test day.
Extraction
-
A 10 g sample weight was weighed with an accuracy of ± 0.01 g and poured with a 1% hydrochloric acid solution, and allowed to infuse for 120 min. Then homogenized for not more than 2 min with a small amount of extraction solution and transferred into a 100 mL volumetric flask, washing the homogenizer with a small amount of extraction solution, then stirred and filtered.
Ten mL of the prepared extract was pipetted and placed in a conical flask. Then the extract was titrated with a solution of sodium 2,6-dichlorophenolindophenolate. Titration was repeated three times; the arithmetic mean was calculated. Then the amount of ascorbic acid in each sample was calculated according to Eqn (1). The liquid extracts of samples were titrated by calibrated titrant. Then, the amount of AA (mg) could be calculated in 100 g of the natural plant sample utilizing Eqn (1):
$\rm Amount\;of\;AA\;(mg/100\;g)=\dfrac{100\,\times\, a\,\times\, T\,\times \,V}{V1\,\times\, m} $ (1) where: a, volume of sodium 2,6-dichlorophenolindophenolate (mL); T, the titer of sodium 2,6-dichlorophenolindophenolate by ascorbic acid; V, the total volume of extract (mL); V1, the volume of the extract taken for titration (mL); m, the mass of the analyzed material (g).
Pigment composition
-
Chlorophylls and carotenoids of experimental plants were extracted with 100% acetone[25]. Fresh leaf (1 g) was homogenized in 10 mL of 100% acetone, then the vegetable mass was ground and filtered. The amount of acetone that was rubbed was considered. Absorption of filtrate was measured on an Ultrospec II spectrophotometer at different wavelengths (nm): 645, 662 – for chlorophylls a and b, 440 – for carotenoids, 454 – for carotenes (total carotenes determined by paper chromatography), 454 – for β-carotene. The paper chromatography: 2 mL of filtrate was applied to chromatographic paper and separated in petroleum ether. The upper strip was separated, re-dissolved in 5 mL of 100% acetone, and measured on a spectrophotometer and calculated using a calibration curve.
$\rm Chl\; a\;({\rm mg/100\;g})=9.784\,\times\, {\rm D}_{662}-0.99\,\times\, {\rm D}_{645} $ (2) $\rm Chl\; b\;({\rm mg/100\;g})=21.426\,\times \,{\rm D}_{645}-4.65\,\times\, {\rm D}_{662} $ (3) $\rm Total\; Carotenoids\;({\rm mg/100\;g})=4.695\,\times\, {\rm D}_{440}-0.268\,\times\, (Chl\;a + Chl\;b) $ (4) where: Dx, absorbance at x nm.
The amount of pigment was calculated using the following formula:
$\rm X =\dfrac {C \,\times \,V \,\times \,V2 \,\times \,100}{m\,\times \,V1} $ (5) where: С, concentration of pigment (mg/1,000 g); V, the total volume of extract (mL); V1, the volume of the extract taken for titration (mL); V2, volume of diluted extract (mL); m, the mass of the analyzed material (g).
Extraction of anthocyanins
-
Anthocyanins were extracted by 1% hydrochloric acid. The solvent to the mass of the analyzed material ratio was 10:1 (v/w). The extract was then filtered and centrifuged at 4,500 rpm at 4°C for 10 min. Then measured by spectrophotometry (Novaspec II, LKB, Biochrom, Cambridge, England) at a wavelength of 510 nm, in terms of cyanidin-3,5-diglycoside, with a correction for the content of the green pigment at 657 nm[26]. The difference between absorbencies (A) was determined using the following equation:
$ \rm A = D_{510} - 1/3 \,\times\, D_{657} $ (6) where: Dx, absorbance at x nm.
The amount of anthocyanins in mg/100 g was calculated by:
$\rm Amount\;of\;Anthocyanins\;(mg/100\;g)=\dfrac{A\,\times \,V\,\times \,100}{E\,\times \,m} $ (7) where: V, the total volume of extract (mL); E, the specific absorption index of cyanidin-3.5 diglycoside at a wavelength of 510 nm in a 1% aqueous hydrochloric acid solution equal to 453; m, the mass of the analyzed material (g).
Total phenolics (TP)
Extraction of phenolics and flavonoids
-
The cut sample (10 g) was mixed with 50 mL of 80% aqueous ethanol. The sample was then homogenized for 5 min and infused for 24 h in the dark, then transferred into large centrifuge tubes and centrifuged in a Centrifuge 5804 R (Eppendorf, Hamburg, Germany) for 20 min.
The total contents of the phenolic compounds in the 80% EtOH extracts and their fractions were determined by the modified Folin-Ciocalteau assay as gallic acid equivalents (GAE)[27]. The determination of phenolic compounds is based on the reduction of Mo6+ to Mo5+, as a result of which the analyzed solution becomes blue and can be measured optically. 0.5 mL of extract samples were mixed with 2.5 mL Folin-Ciocalteau's reagent (1:2 diluted with water) and after 3 min, 2 mL of 7% Na2CO3 was added to the mixture. As a control, reagent without adding extract was used. After incubation of the samples at room temperature in the dark for 120 min, their absorbances were measured at 765 nm (Novaspec II, LKB, Biochrom, Cambridge, England). For the calibration curve, 10 mg of gallic acid was dissolved in 10 mL of 80% EtOH as a stock solution. Experiments were reported three times for every dilution and a calibration curve was created. Total phenolics were expressed as mg gallic acid equivalent (mg GAE/g).
$ \rm TP\;(\mathrm{m}\mathrm{g}\;\mathrm{G}\mathrm{A}\mathrm{E}/100\;\mathrm{g})=\dfrac{a\,\times\, V}{m\,\times\, 1\;000}\,\times\, 100 $ (8) where: a, concentration of phenolic compounds according to the calibration curve (mg GAE/L); V, the total volume of extract (mL); m, the mass of the analyzed material (g).
Total flavonoids
-
The flavonoid content of the EtOH extracts was determined using a modified colorimetric assay and rutin used as a standard. The method is based on the formation of a flavonoid-aluminum complex[28]. Extracts or standard solutions (0.5 mL) were mixed with 5% AlCl3 solution (2 mL), 15% acetic acid (0.1 mL) and finally 70% EtOH was added to make a volume of 25 mL in a 25 mL volumetric flask. After incubation of the samples at room temperature for 30 min, the absorbances of the samples were read spectrophotometrically (Novaspec II, LKB, Biochrom, Cambridge, England) at 410 nm against a blank and the total flavonoid content was expressed as mg per 100 g in terms of rutin (according to the calibration curve) and all tests were carried out in duplicate:
$\rm X=\frac{D\,\times\, 100\,\times \,C1\,\times\, 100\,\times\, 100}{D1\,\times \,V\,\times\, m\,\times\, (100-W)} $ (9) where: D, optical density; D1, optical density of rutin; C1, the content of rutin in the dry matter sample (1,455 mg, of rutin 97%); m, the mass of the analyzed material (g); W, sample humidity (%); V, volume of extract (mL).
Antioxidant activity
-
Quantitative assessment of the antioxidant activity of ethanolic extracts of fresh plant biomass were carried out spectrophotometrically using the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and antioxidant level. The reduction in absorbance, visible as a change of color from purple to yellow, was measured. DPPH (23.8 mg) was dissolved in 96% ethanol (100 mL) to obtain a concentration of 0.6 mM. Experimentally, 3.9 mL of the DPPH solution was added to 100 μL of various extract concentrations (0, 1, 2, 3, 4, and 5 mg/mL), keeping the mixture in the dark for 30 min. Subsequently, the absorbance was read by a UV-Vis spectrophotometer (Novaspec II, LKB, Biochrom, Cambridge, England) at 515 nm at room temperature. Ascorbic acid was used as positive control and all tests were carried out in duplicate. The results obtained were expressed as either the level or % inhibition value (the rate of oxidation) of the DPPH radical[29,30].
The antioxidant level was expressed as mcg ascorbic acid equivalent (μg AAE/g) and was calculated using the following formula:
$\rm AOA\;({\text{μ}}\mathrm{g}\;AAE/g)=\dfrac{C\,\times \,V}{m} $ (10) where: C, μM from the calibration curve; V, the total volume of the extract (mL); m, weight of the attachment (g).
The scavenging activity was calculated using the following formula:
$\begin{split}&\rm Free\;radical\;scavenging\;activity\;({\text{%}})=\\&\dfrac{\rm\left(Absorbance\;of\;control-Absorbance\;of\;sample\right)} {\rm Absorbance\;of\;control}\,\times \,100 \end{split}$ (11) All data are presented on raw material.
Statistical analysis
-
Data analysis was performed using STATISTICA v software. 12.0 (StatSoft Inc., USA). Descriptive statistics (mean, standard deviation, range of variability, and coefficient of variation) were calculated for all biochemical parameters. Data testing for normality of distribution was performed using the Shapiro-Wilk test and quantile-quantile plot (QQ Plot). Mean data values were compared using analysis of variance (ANOVA), and Pearson's correlation coefficient was used for correlation analysis. The Tukey HSD (honestly significant difference) post hoc test was used to identify the differences between the means for each characteristic. The principal component method was used to reveal the main factors that reveal the variability of accessions based on a set of characteristics.
-
This article discusses Eruca and Diplotaxis accessions being introduced into the Russian market. Table 1 and Supplementary Table S1 present the results of a biochemical study of Eruca and Diplotaxis accessions grown in the greenhouse and the open field. Plants were collected at the technical ripeness stage. During this period, they are characterized by the largest mass of leaves and a high concentration of biologically active substances.
Table 1. Biologically active substances Eruca and Diplotaxis in the open field and the greenhouse in North-West Russia (N* = 3).
Traits Place Eruca (20 acc.) Diplotaxis (5 acc.) Mean ± SD Range CV** (%) Mean ± SD Range CV (%) Ascorbic acid, mg/100 g Greenhouse 17.1 ± 6.8 4.2−35.2 39.9 37.0 ± 3.6 30.8−39.6 9.8 Open field 16.5 ± 3.1 10.3−22.7 18.9 18.3 ± 3.9 14.4−22.7 21.7 Total chl, mg/100 g Greenhouse 135.6 ± 37.6 34.0−179.9 27.7 137.8 ± 21.5 103.7−163.0 15.6 Open field 108.3 ± 24.5 71.8−165.6 22.6 73.8 ± 4.1 67.1−78.2 5.6 Chl.a, mg/100 g Greenhouse 98.8 ± 25.5 26.6−123.7 25.8 107.5 ± 16.4 81.5−126.4 15.3 Open field 84.9 ± 16.7 57.0−114.0 19.7 59.2 ± 3.0 54.7−63.1 5.1 Chl.b, mg/100 g Greenhouse 36.8 ± 12.6 7.4−56.2 34.3 30.4 ± 5.1 22.2−36.1 16.8 Open field 23.4 ± 8.6 14.8−51.6 37.0 14.6 ± 1.3 12.3−15.5 8.8 Carotenoids, mg/100 g Greenhouse 41.0 ± 17.3 7.0−61.3 42.2 46.0 ± 8.4 33.6−57.2 18.4 Open field 15.9 ± 4.7 9.4−29.1 29.6 10.9 ± 2.6 7.0−13.4 23.9 Carotenes, mg/100 g Greenhouse 6.8 ± 0.8 5.0−8.7 11.7 8.5 ± 1.6 6.2−10.5 18.8 Open field 8.1 ± 2.0 3.9−10.9 24.9 5.1 ± 2.4 3.4−9.4 47.5 β-carotene, mg/100 g Greenhouse 5.9 ± 1.7 1.4−7.8 28.0 6.0 ± 0.9 4.5−6.9 15.4 Open field 4.8 ± 0.9 3.4−7.2 19.9 3.3 ± 0.2 3.0−3.6 6.1 Anthocyanins, mg/100 g Greenhouse 2.4 ± 0.8 0.6−3.6 32.4 1.8 ± 0.3 1.6−2.3 14.8 Open field 8.3 ± 6.6 3.8−29.5 79.2 4.2 ± 1.9 2.8−7.5 45.3 Total phenolic compounds, mg GAE/100 g Greenhouse 58.7 ± 9.6 43.3−76.9 16.4 73.3 ± 10.0 63.2−88.4 13.7 Open field 274.9 ± 76.1 120.0−402.6 27.7 242.2 ± 37.1 200.0−287.0 15.3 Total flavonoids, mg/100 g Greenhouse 51.1 ± 33.5 5.9−120.9 65.6 20.5 ± 10.2 8.0−33.6 49.9 Open field 185.7 ± 48.3 103.9−308.8 26.0 71.1 ± 18.1 53.2−95.8 25.5 * N, replicates number; **CV, coefficient of variation. Ascorbic acid plays many roles in organisms and its content in plants varies greatly depending on the variety, growing conditions, maturity at harvest, and post-harvest handling and storage[31].
In the present study, the range of variability of ascorbic acid varied from 4.2 to 39.6 mg/100 g. The highest accumulation of ascorbic acid was observed in Diplotaxis grown in the greenhouse, while in Eruca, depending on the growing conditions, this indicator did not change significantly. The Eruca accessions 'Aromat' (k-36, Russia) and 'Barokko' (k-14, Russia) – 35.2 and 26.4 mg/100 g and Diplotaxis – 'S olivkovym listom' (k-1, Russia) and 'Local' (i: 630841, Armenia) – 30.8 and 39.6 mg/100 g were characterized by a high content of ascorbic acid in the greenhouse. In the open field, high content was found in Eruca accessions 'Pikantnaya' (k-12, Russia) and 'San Remo' (k-25, Russia) – 22.7 and 20.6 mg/100 g. The Diplotaxis accession 'Roket' (k-2, Russia) was characterized by a high content of ascorbic acid in both growing conditions – 39.6 and 22.7 mg/100 g. These cultivars are important for human dietary nutrition and should be better stored when selling them in the market.
The absorption and transformation of solar energy during photosynthesis is carried out by photosynthetic plant pigments, in particular chlorophylls and carotenoids, which are among the secondary metabolites and are present in our daily diet, especially due to the growing consumer trend towards healthy eating.
The pigment complexes of Eruca and Diplotaxis are of high interest. In the studied accessions, there is three times more chlorophyll than carotenoids. The total chlorophyll content in the leaves varied significantly between accessions – 34.0–179.9 mg/100 g, averaging 135.6 (Eruca) and 137.8 (Diplotaxis) mg/100 g in the greenhouse; and in the open field – 108.3 and 73.8 mg/100 g, respectively. The chlorophyll a content was more than 73%. The accumulation of chlorophyll in the greenhouse in the Eruca and Diplotaxis accessions, was almost the same, while Diplotaxis plants in the open field had a significantly lower total amount of chlorophylls a and b. Eruca accessions with high content of chlorophylls a and b in both growing conditions were identified – 'Dikovina' (k-30, Russia) and 'No name' (k-31, China).
Of the 40 carotenoids that come from food, the main ones are carotenes – β- and α-carotenes, lycopene, and xanthophylls – lutein, zeaxanthin, β-cryptoxanthin. Carotenoids make an important role in protecting plants from photo-oxidative processes, and they are effective antioxidants that absorb singlet molecular oxygen and peroxyl radicals[32].
In the studied Eruca and Diplotaxis accessions, the total carotenoids are low (from 7.0 to 61.3 mg/100 g) and its maximum value was observed in accessions in the greenhouse. No significant differences were found between crops. Carotenoids included carotenes in the amount of 3.4−10.9 mg/100 g (represented mainly by β-carotene: 1.4−7.8 mg/100 g). Considering the total carotenes, it should be noted that their maximum value was for Eruca in the open field, and for Diplotaxis in the greenhouse. For the β-carotene, the general tendency for pigment accumulation remained the same – the amount was higher in the greenhouse. It should be noted that for Diplotaxis these values were significant. Eruca accessions 'No name' (k-31, China) and 'Orekhovaya' (k-28, Russia) were identified with a high content of carotene in the open field: 10.9 and 10.2 mg/100 g; β-carotene in the greenhouse: 'Barokko' (k-14, Russia), 'Sitsiliya' (k-29, Russia) and 'Rucola' (k-11, Germany). Under both growing conditions, Eruca accessions 'Sorrento' (k-26, Russia) and 'Local' (i: 640875, Russia) were characterized by a high content of carotenes. Eruca accessions 'Poker' (k-9, Russia) and 'Ampir' (k-15, Russia) were characterized by a high content of carotenes and β-carotene in both growing conditions. The Diplotaxis accession 'Local' (i: 630841, Armenia) stood out for its content of carotenes and β-carotene in the greenhouse.
The next group of pigments are anthocyanins, heterocyclic glycosides, widely represented in higher plants as polyphenolic pigments, giving flowers, fruits and leaves a variety of colors: from reddish-blue to violet-black. There are more than 600 forms of anthocyanins found in nature, most of which are derived from six aglycones: cyanidin, delphinidin, pelargonidin, peonidin, malvidin, or petunidin. In plants, anthocyanins serve as optical filters that protect the photosynthetic apparatus from high-energy photons, and also act as inhibitors of reactive oxygen and nitrogen species.
In the present study, the anthocyanin content of Eruca and Diplotaxis was low, with only a few Eruca accessions having a slight purple tint to the leaves and stems. The anthocyanins variability in the accessions varied from 0.6 to 29.5 mg/100 g. Anthocyanin content was two times higher in Eruca accessions in the open field. With an increased amount of anthocyanins in the open field, Eruca accessions from Russia 'Viktoriya' (k-27): 21.1 mg/100 g, 'Poker' (k-9): 13.9 mg/100 g, 'Orekhovaya' (k-28): 12.4 mg/100 g, 'Evrika' (k-37): 10.4 mg/100 g were selected.
Polyphenols include several classes of weakly acidic chemicals associated with or built on a phenolic ring[33]. Reactive oxygen species readily oxidize them to quinones, a property that helps explain their ability to scavenge free radicals. Polyphenols perform diverse functions in plants and are a major class of secondary plant metabolites. The antioxidant potential of phenolic compounds is now a scientifically established reality.
The results of the present study indicate that phenolic compounds vary greatly depending on both cultivars and growing conditions. Eruca and Diplotaxis accessions had their maximum phenolic content in the open field – from 120.0 to 402.6 and from 200.0 to 287.0 mg GAE/100 g, respectively. An excess of more than three times was observed in the accumulation of phenolic compounds in accessions in the open field over those in the greenhouse.
The most multiple and widespread group of phenolic compounds in plants are flavonoids. They are a class of plant polyphenols that have a broad spectrum of activity due to their antioxidant, antibacterial, and fungicidal properties. They perform protective functions, protecting plants from various adverse environmental influences.
The range of variability in the amount of flavonoids in the studied crops varied from 5.9 to 308.8 mg/100 g. Comparing crops with each other, the amount of flavonoids was two times higher and averaged 51.1 (Eruca) and 20.5 mg/100 g (Diplotaxis) in the greenhouse; in the open field – 185.7 and 71.1 mg/100 g, respectively. The highest amount of total flavonoids was observed in accessions in the open field of both crops. In accessions in the greenhouse, the total flavonoids were three times lower.
Green leafy crops for humans serve as an important source of antioxidants that can neutralize reactive oxygen species and the products of their interaction with organic molecules and nitrogen oxides.
As a result of the present study, the values of antioxidant activity (AOA) of Eruca and Diplotaxis accessions were determined in the open field and the greenhouse in the Leningrad region. Antioxidant activity against DPPH free radicals was as follows: Diplotaxis (open field) > Eruca (open field) > Diplotaxis (greenhouse) > Eruca (greenhouse).
It was revealed that in the open field the speed of oxidation of the DPPH radical was higher in accessions of both crops: 61.2% in Eruca accessions and 63.0% in Diplotaxis accessions; in the greenhouse – 9.7% and 39.7%, respectively (Fig. 1a). When determining the level of antioxidant activity (in terms of ascorbic acid), it was revealed that Diplotaxis accessions had slightly higher AOA in the greenhouse – 53.4 ± 7.2 μg AAE/g, compared to the open field – 47.7 ± 5.9 μg AAE/g (Fig. 1b). In the greenhouse, Diplotaxis accessions were characterized by higher AOA values and the rate of binding of free radicals – 39.7% (CV = 13.4%) and 53.4 μg AAE/g (CV = 13.5%), compared to Eruca accessions – 9.7% (CV = 32.7%) and 25.1 μg AAE/g (CV = 39.4%) (Fig. 1a & b). The high rate of radical oxidation in the open field may be associated with an increased content of phenolic compounds, including flavonoids and anthocyanins.
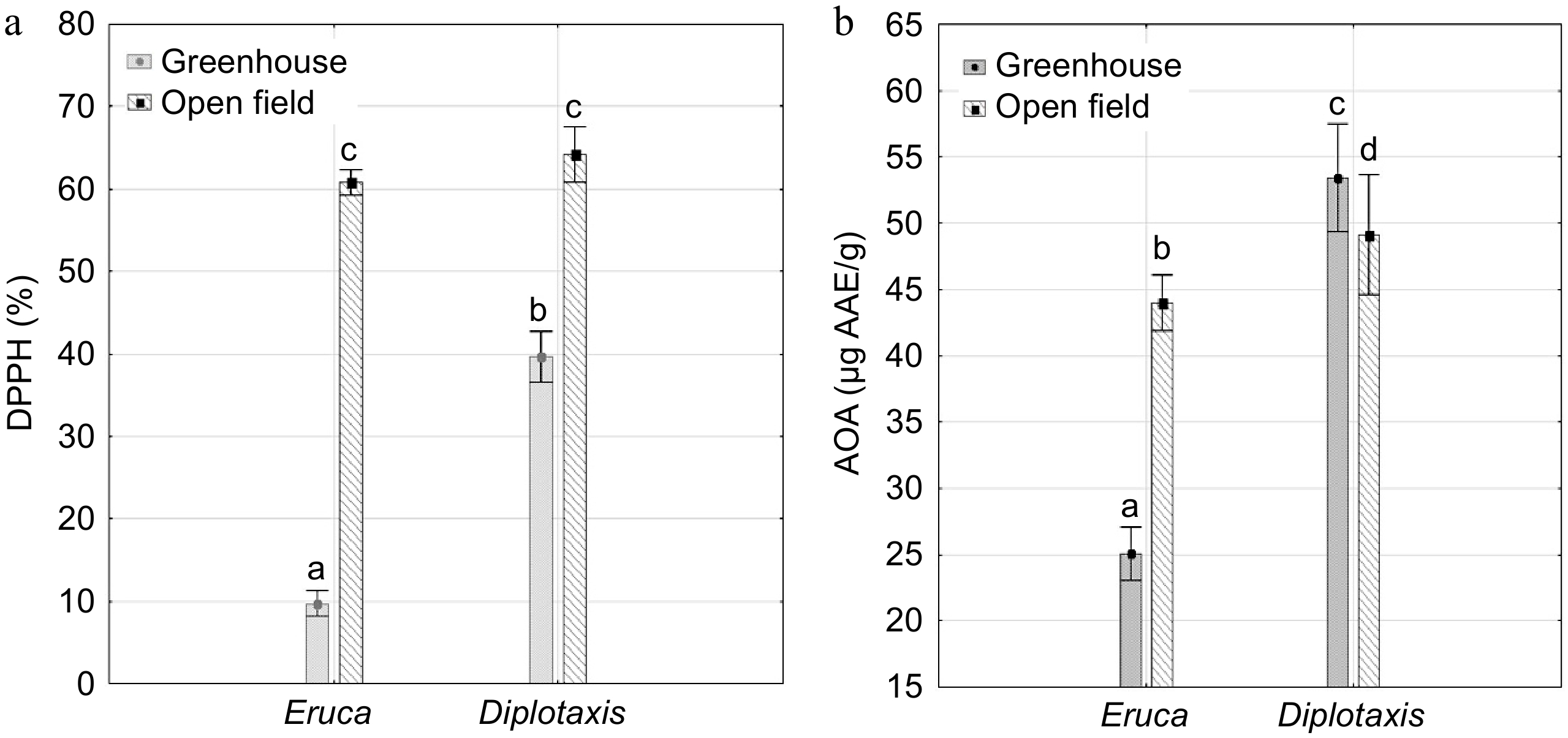
Figure 1.
Levels of antioxidant activity in Eruca and Diplotaxis accessions in the open field and the greenhouse. (a) Rate of oxidation of the DPPH radical (%), (b) level of antioxidant activity (μg AAE/g). Mean ± SE. a−d Values with different superscript letters were significantly different p < 0.05 (Tukey's HSD test).
As a result of the analysis of variance, it was revealed that the studied factors (genotype and place of cultivation) contribute to varying degrees to the formation of the level of antioxidant activity of Eruca and Diplotaxis. The level of antioxidant activity and the rate of radical oxidation in Eruca accessions strongly depend on the place of cultivation (50.4% and 93.4%). In Diplotaxis, the level of antioxidant activity depends mainly on the genotype (88.2%), and the rate of radical oxidation depends on the place of cultivation (85.7%).
As a result of correlation analysis, several positive and negative relationships were identified between the parameters of antioxidant activity and the accumulation of ascorbic acid, pigments, and phenolic compounds under different growing conditions (Table 2).
Table 2. Correlation analysis of pigments and phenolic compounds with antioxidant activity of Eruca and Diplotaxis in the open field and the greenhouse.
Traits Place Eruca Diplotaxis AAE DPPH AAE DPPH Ascorbic acid Greenhouse 0.55* 0.60* −0.74* −0.65* open field −0.22 −0.17 −0.70 −0.77 Total chlorophylls Greenhouse 0.19 0.09 0.67 0.70 Open field 0.42 0.09 0.27 0.22 Chl.a Greenhouse 0.23 0.15 0.65 0.70 Open field 0.37 0.03 0.41 0.35 Chl.b Greenhouse 0.09 −0.05 0.50 0.46 Open field 0.49* 0.20 −0.02 −0.09 Carotenoids Greenhouse −0.14 −0.43 0.62 0.67 Open field −0.21 −0.47* −0.08 −0.03 Carotenes Greenhouse 0.10 0.09 0.18 0.20 Open field −0.13 0.17 0.60 0.59 β-carotene Greenhouse 0.15 0.05 0.50 0.47 Open field 0.42 0.11 0.44 0.42 Anthocyanins Greenhouse −0.35 −0.46* 0.56 0.60 Open field 0.01 0.21 0.60 0.51 Total polyphenolic compounds Greenhouse 0,02 0.23 0.88* 0.90* Open field −0,53* −0.13 0.80 0.83 Total flavonoids Greenhouse 0.13 −0.07 0.90* 0.85* Open field 0.70* 0.57* 0.53 0.58 * significant at p ≤ 0.05 (Pearson's correlation coefficient). Thus, in the greenhouse, Eruca accessions showed positive correlations with the parameters of antioxidant activity (both DPPH and AAE) with the content of ascorbic acid (r = 0.60 and 0.55) and a negative correlation between DPPH and accumulation of anthocyanins (r = −0.46). In the open field, positive correlations were revealed with the parameters of antioxidant activity (both DPPH and AAE) with the total flavonoids (r = 0.57 and 0.70); AAE with chlorophyll b (r = 0.49). Negative relationships were found with DPPH and the accumulation of carotenoids (r = −0.47), AAE and, with the total polyphenolic compounds (r = −0.53).
Diplotaxis accessions showed correlations only in the greenhouse. Thus, positive relationships were determined between DPPH and AAE and the total phenolic compounds (r = 0.90 and 0.88) and flavonoids (r = 0.85 and 0.90) and negative relationships with the content of ascorbic acid (r = −0.65 and −0.74).
In breeding research, the features of the coupled inheritance of biochemical composition traits with the level of antioxidant activity is of interest. As a result of the analysis of 12 biochemical characteristics by the principal component method, it was found that their variability is determined by three factors (Table 3).
Table 3. Dispersion of the principal components of the biochemical characteristics of Eruca and Diplotaxis.
Component Eigenvalue Total (%) Cumulative Cumulative (%) 1 6.00 49.97 6.00 49.97 2 2.54 21.16 8.54 71.13 3 1.46 12.19 10.00 83.32 Together, they account for 83.32% of the total variance. In this case, the first component determines it by 49.97%, which characterizes the content of pigments − chlorophylls, total carotenoids, and β-carotene (Fig. 2a). The second main component determines the accumulation of anthocyanins, carotenes, total polyphenols, total flavonoids, and DPPH. The third component characterizes the accumulation of ascorbic acid and AAE. Thus, principal component analysis indicates an independent relationship between anthocyanins and carotenes from other pigments; ascorbic acid from other elements of the chemical composition.
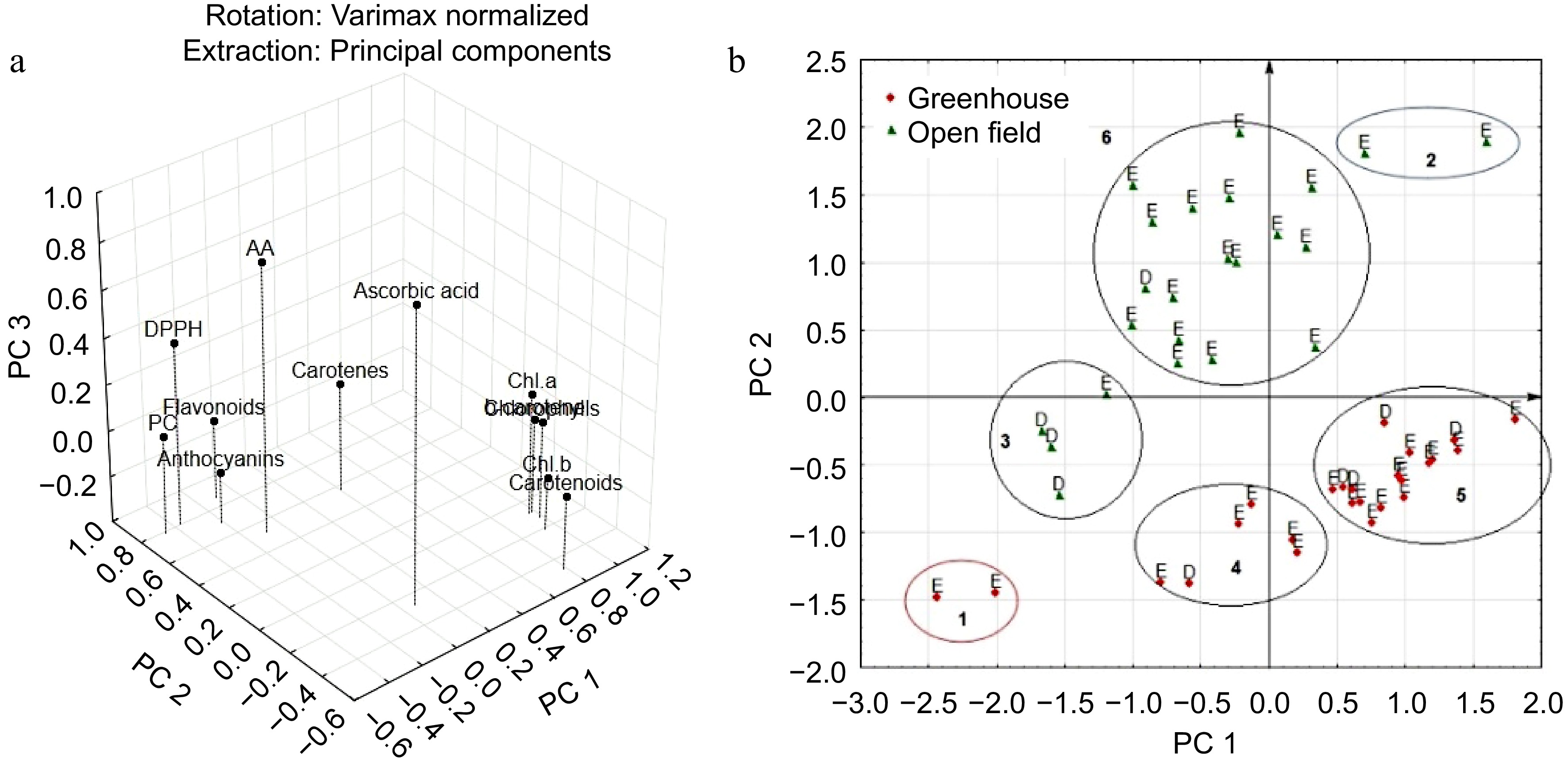
Figure 2.
Distribution of (a) features, and (b) accessions of Eruca (E) and Diplotaxis (D) in the space of the first two components. Places of cultivation are indicated by color.
Because the first two components account for most of the trait variability, the arrangement of accessions in their space were examined (Fig. 2b).
In the space of two factors, the accessions were clearly divided according to places of cultivation. In this case, two groups of accessions are distinguished. The first group is represented by Eruca accessions 'San Remo' (k-25) and 'Sorrento' (k-26) from Russia, which are characterized by a stable low content of ascorbic acid (on average 13.2 mg/100 g), all pigments, total phenolic compounds, and low values of antioxidant activity, and the average content of total flavonoids in the greenhouse (Table 4). The second group includes two Eruca accessions 'Ampir' (k-15, Russia) and 'No name' (k-31, China), which were characterized by a high content of chlorophylls (on average 154.9 mg/100 g), carotenes, β-carotene, flavonoids and high values antioxidant activity in the open field.
Table 4. Characteristics of principal component groups of Eruca and Diplotaxis.
Traits Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Ascorbic acid* 13.8 ± 0.8a 12.9 ± 3.6a 17.8 ± 3.7b 25.7 ± 11.0c 20.6 ± 10.3c 17.0 ± 2.8b 13.3−14.3 10.3−15.5 14.4−22.7 15.4−39.6 4.2−39.6 12.4−22.7 Total chl* 39.1 ± 7.3a 154.9 ± 15.2d 76.0 ± 8.3b 116.8 ± 15.1c 152.2 ± 12.1d 100.4 ± 18.1c 34.0−44.3 144.1−165.6 67.1−87.1 97.6−131.4 131.8−179.9 71.8−135.2 Chl.a* 30.5 ± 5.5a 113.0 ± 1.4c 61.0 ± 6.2b 90.0 ± 11.0b 111.3 ± 7.0c 80.1 ± 14.0b 26.6−34.4 112.0−114.0 54.7−69.6 75.1−98.5 97.4−126.9 57.0−106.7 Chl.b* 8.6 ± 1.8a 41.8 ± 13.8c 15.0 ± 2.2a 26.8 ± 4.5b 40.9 ± 7.2c 20.4 ± 4.3b 7.4−9.9 32.0−51.6 12.3−17.5 22.2−32.9 30.1−56.2 14.8−29.3 Carotenoids* 12.5 ± 3.1a 17.4 ± 5.4b 9.5 ± 2.0a 23.9 ± 16.0b 50.4 ± 4.8c 15.7 ± 4.5b 10.3−14.7 13.6−21.1 7.0−11.8 7.0−41.9 42.7−61.3 11.2−29.1 Carotenes* 6.2 ± 1.8a 9.1 ± 2.5c 4.0 ± 0.4a 6.3 ± 0.7a 7.5 ± 1.1b 8.4 ± 1.7b 5.0−7.5 7.4−10.9 3.4−4.5 5.3−7.0 6.3−10.5 5.2−10.2 β-carotene* 1.6 ± 0.3a 6.7 ± 0.6c 3.4 ± 0.3b 5.0 ± 0.7c 6.7 ± 0.5c 4.6 ± 0.8b 1.4−1.9 6.3−7.2 3.0−3.9 4.2−5.8 5.9−7.8 3.4−5.9 Anthocyanins* 2.6 ± 1.4a 6.4 ± 1.1b 3.8 ± 0.8a 1.8 ± 1.1a 2.3 ± 0.5a 9.0 ± 7.0c 1.6−3.6 5.6−7.1 2.8−4.8 0.6−3.6 1.6−3.2 3.8−29.5 Total PC, mg GAE/100 g 50.0 ± 6.9a 177.2 ± 51.9b 250.6 ± 30.3c 62.6 ± 7.9a 62.6 ± 11.9a 296.1 ± 59.6c 45.1−54.9 140.5−214.0 210.6−278.6 48.8−68.6 43.3−88.4 142.8−402.6 Total flavonoids* 50.5 ± 39.4b 273.4 ± 50.0d 88.7 ± 41.3b 24.4 ± 14.6a 50.1 ± 33.9b 171.7 ± 43.1c 15.6−85.4 238.0−308.8 60.9−148.3 5.9−38.7 8.0−120.9 95.8−251.4 DPPH, % 6.5 ± 0.9a 73.6 ± 6.2c 61.3 ± 5.9c 18.2 ± 11.2b 16.0 ± 14.4b 60.0 ± 7.9c 5.9−7.2 69.2−78.0 54.3−68.1 7.2−33.0 5.0−46.5 43.0−71.3 AOA, μg AAE/g 18.6 ± 2.1a 61.8 ± 5.3c 46.8 ± 4.5c 32.3 ± 11.8b 31.7 ± 16.1b 42.4 ± 7.2c 17.1−20.1 58.0−65.5 41.4−52.1 17.1−44.4 10.9−62.6 30.3−55.5 * mg/100 g. All data are presented as Mean ± SD, Xmin−Xmax. a−d Values with different superscript letters were significantly different p < 0.05 (Tukey's HSD test). The third group is represented by three Diplotaxis accessions (k-2, Russia; vr.11, Russia; i: 630841, Armenia) and Eruca accession from the UK (k-6), which were characterized by medium content of ascorbic acid, chlorophylls, β-carotene, anthocyanins, and total flavonoids, low content of carotenoids, carotene, and high content of phenolic compounds in the open field. The accessions were also characterized by a high level of antioxidant activity. The fourth group included four Eruca accessions from Russia (k-34, k-36, k-37) and UK (k-5) and Diplotaxis accession 'Roket' (k-2, Russia). The accessions were characterized by high content of ascorbic acid, chlorophylls, β-carotene, medium content of carotenoids, and low accumulation of carotenes, anthocyanins, total phenolic compounds, and flavonoids under greenhouse conditions. The level of antioxidant activity was medium. The fifth group included four Diplotaxis accessions and 14 Eruca accessions grown in the greenhouse. The accessions were characterized by a high content of ascorbic acid, chlorophylls, carotenoids, β-carotene, and medium content of total flavonoids and carotenes. Antioxidant activity parameters had medium values. The sixth group included 16 Eruca accessions and Diplotaxis accessions from Russia ('Positive', k-3). Accessions of this group were characterized by a high accumulation of chlorophylls, anthocyanins, total phenolic compounds, and flavonoids in the open field. The accessions were also characterized by a high level of antioxidant activity.
It was revealed that, based on a set of characteristics, the optimal biochemical composition in both growing conditions is characterized by Eruca accession from China (k-31) and Diplotaxis accession 'Roket' (k-2, Russia). In addition, these accessions in two growing conditions were characterized by a medium-sized rosette of leaves (height 12−17 cm, diameter 9−15 cm), plant weight from 12 to 18 g, and a vegetation period of 25−27 d. These phenological, morphological, and biochemical characteristics make these accessions promising materials for implementation in the production process.
-
The content of bioactive compounds and the nutritional quality of food can be influenced by several factors such as genetics, degree of ripeness, agronomic and environmental conditions during growth. Growing conditions include a large number of variable agronomic and environmental factors: climate, soil type, hours of sunlight, irrigation, nutrient supply, growing location, cultivation methods, time of harvest, and others[34]. These variables can affect both crop productivity as well as the nutritional profile of plants.
This article presents Eruca and Diplotaxis accessions being introduced into the Russian market. To determine their antioxidant potential, the following studies were performed: ascorbic acid content, pigments, phenols, flavonoids, and antioxidant activity.
L-Ascorbic acid (AA) is an antioxidant, a cofactor for redox enzymes, and a precursor for the biosynthesis of several important metabolites[35]. Humans are completely dependent on AA from food since they cannot synthesize it due to the absence of gulonolactone oxidase. Research into the physiological functions of L-ascorbic acid has classically focused on this compound's role as an antioxidant. A large amount of data has been accumulated on the positive effect of AA on yield performance and stress resistance of plants. The generally accepted mechanism by which AA can control physiological processes is considered to be its effect on the level of H2O2 and other reactive oxygen species (ROS) involved in cellular redox metabolism and pathophysiological oxidative processes[35]. Ascorbic acid also plays a role in regulating leaf aging. At high concentrations, leaf death occurs later than in leaves with low concentrations[36].
Ascorbic acid, along with flavonoids, carotenoids, and xanthophylls such as lutein, has antioxidant properties[37]. For this reason, nutritionists recommend diets that include Eruca and Diplotaxis leaves as a means of preventing cardiovascular diseases and cancer[38,39].
Guijarro-Real et al.[40,41] studied the effect of different cultivation environments on the nutritional quality of Diplotaxis accessions. It was found that the content of AA increased when grown in the field compared to the greenhouse. In the present study, on the contrary, accessions in the greenhouse were characterized by a higher content of AA. This pattern was typical for both crops. Salvatore et al.[38] found that the Diplotaxis plant accumulates on average 13.9 mg of AA/100 g; Martínez-Sánchez et al.[42] revealed the content of AA 73 mg/100 g (Diplotaxis) and 52 mg/100 g (Eruca). Kurbakov and Molchanova[43] determined the content of AA under the Moscow region to be 84.7 mg% (Eruca) and 83.8 mg% (Diplotaxis). The data from the present study were also comparable to previously reported results of AA content for Diplotaxis by Spadafora et al.[44] (22 mg/100 g FW) and Durazzo et al. (21−81 mg/100 g FW)[39].
During the adaptation of plants to the action of abiotic factors, many metabolic processes are involved, including changes in photochemical activity, regulated by the concentration of pigments in photosynthetic membranes[45]. Chlorophyll reacts to all changes in metabolism and, under unfavorable conditions, both its total content and the ratio of individual forms (a/b) change[46]. The structure and configuration of chlorophylls influence their antioxidant activity, mainly for chlorophylls a and b. Pérez-Gálvez et al.[47] showed that chlorophyll a is three times more effective at quenching radicals than chlorophyll b, which is consistent with previous results obtained using singlet oxygen lipid peroxidation assays. However, some researchers have found that chlorophyll b exhibits higher antioxidant activity, suggesting an unknown role of its C7 aldehyde group in antioxidant capacity[48−50]. Along with the ability to reduce the level of oxidation in the main biomolecules, chlorophyll derivatives are effective enhancers of the activity of the main enzymes involved in the antioxidant defense mechanism at the cellular level. In addition to scavenging free radicals, chlorophylls perform the function of trapping mutagens based on their flat structure, which reduces the availability of harmful compounds in the cell, i.e. performs metabolic activation of detoxification pathways[47]. A decrease in light levels in a greenhouse is an unfavorable factor for photosynthetic activity[51].
The understanding of carotenoids as antioxidant compounds has evolved in recent years, from simply radical scavenging molecules to biomarkers associated with reduced incidence of various degenerative diseases, including lung, gastrointestinal, pancreatic, breast, and prostate cancers; cardiovascular diseases; and age-related macular degeneration[47].
Wagstaff[52] used two controlled plant stresses (light and temperature) to examine the impact these had on growth habit and leaf pigment. Some cultivars in this study increased carotenoid levels in response to high light stress, and there was variation in the levels of chlorophyll at harvest and in response to postharvest storage. In general, chlorophyll level was higher in accessions grown under high light and decreased during shelf life as the leaves senesced. In contrast to chlorophyll levels, leaf carotenoid levels were generally not photosensitive, although they did decrease during postharvest storage. Galmes et al.[53] reported that the amount of chlorophyll decreases in high natural light.
In the present study, low levels of natural light in the greenhouse could be an unfavorable factor for photosynthetic activity. Therefore, the increase in the content of chlorophylls and carotenoids in the greenhouse in the studied accessions can be considered as an adaptation of plants to unfavorable conditions.
Phenols are aromatic compounds containing one or more hydroxyl groups directly attached to a benzene ring. Depending on the number of hydroxyl groups, phenols are classified into diatomic, triatomic, and polyatomic. To date, thousands of polyphenolic compounds have been isolated from plants. Plant polyphenols may have beneficial and/or harmful effects on mammals. Once plants die, phenolic compounds can persist for several weeks and influence decomposition. They also influence the shelf life of harvested products[33].
Disciglio et al.[54] reports the total polyphenol content of Diplotaxis was 1,477−1,699 mg GAE/kg FW. The total flavonoid content was 0.65 mg/g FW (Eruca) and 0.29 mg/g FW (Diplotaxis); total polyphenols – 208.1 and 100.1 mg GAE/100 g FW, respectively[55]. Pasini et al.[56] reported an average total flavonoid content of 12.35 g/kg DW, the Eruca accessions exhibited ample variability of total flavonoid content, with an average 23.53 g/kg DW and range from 9.99 to 31.39 g/kg DW. The more abundant flavonoid group was represented by kaempferol derivatives, in agreement with Martínez Sánchez et al.[57], ranging from 8.47 to 26.0 g/kg DW.
The results of the present study indicate that phenolic compounds vary greatly depending on both cultivar and growing conditions. The content of flavonoids in accessions in the greenhouse was three times lower. This could be due to the influence of the edaphic factor. Both positive and negative effects of fertilizers on the accumulation of flavonoids have been identified. It was also found that nitrogen deficiency in the soil stimulates the accumulation of phenolic compounds, including flavonoids[58]. An increase in the level of flavonoids in the aboveground phytomass of plants may also indicate the influence of the light factor (protection from ultraviolet radiation). In the present study, DPPH free radical levels were high in the open field, possibly due to day length (decreasing from 18 to 14 h) and fluctuations in day and night temperatures during the growing season. Low values of DPPH free radicals in the greenhouse could be due to the effect of stress factors being insignificant.
Concentrations and structural classes of phytochemicals are affected by various stresses such as light, nutrient supply, growing conditions, and especially UV light. Therefore, some of the differences between the data presented in this paper and previous reports could be due to differences in light quality and intensity and growing conditions.
-
Thus, the study found that Eruca and Diplotaxis accessions grown under different growing conditions differed in their antioxidant levels. Thus, accessions grown in a greenhouse were richest in chlorophylls, carotenoids, and beta-carotene, while accessions grown in the open field accumulated more phenolic compounds, including flavonoids and anthocyanins. Distribution of the studied crops according to their antioxidant activity: Diplotaxis (greenhouse) > Diplotaxis (open field) > Eruca (open field) > Eruca (greenhouse). Eruca accessions from China (k-31) and Diplotaxis accession 'Roket' (k-2, Russia) with optimal biochemical composition were identified.
The biologically active substances Eruca and Diplotaxis that have been considered in this study allow us to classify these plants as functional food products that contain physiologically active, valuable, and safe ingredients with known physicochemical characteristics, for which beneficial properties for preserving and improving health have been identified and scientifically substantiated.
The study was carried out within the framework of State Demand No. 0481-2022-0003 World resources of vegetable and cucurbit crops of the VIR collection: effective ways to reveal the ecological and genetic patterns of diversity formation and the use of breeding potential.
-
The authors confirm contribution to the paper as follows: study conception and design: Kurina AB, Solovyeva AE; data collection: Ermolenko KA; analysis and interpretation of results: Kurina AB, Solovyeva AE; draft manuscript preparation: Kurina AB, Solovyeva AE. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Biologically active substances Eruca and Diplotaxis in the open field and the greenhouse, mg/100 g (N = 3).
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Kurina AB, Ermolenko KA, Solovyeva AE. 2025. Comparative study of antioxidant activity of VIR Eruca sativa Mill. and Diplotaxis tenuifolia (L.) DC. collections. Technology in Horticulture 5: e006 doi: 10.48130/tihort-0024-0032
Comparative study of antioxidant activity of VIR Eruca sativa Mill. and Diplotaxis tenuifolia (L.) DC. collections
- Received: 09 July 2024
- Revised: 20 November 2024
- Accepted: 21 November 2024
- Published online: 25 February 2025
Abstract: Eruca and Diplotaxis are popular Brassicaceae crops rich in phytochemicals such as carotenoids, flavonoids, glucosinolates, ascorbic acid, and others. This study aimed to compare the antioxidant status of Eruca and Diplotaxis under different growing conditions (greenhouse and open field). For this purpose, the content of ascorbic acid, pigments, phenols, flavonoids, and antioxidant activity were studied. The highest accumulation of ascorbic acid was observed in Diplotaxis in a greenhouse, while in Eruca this indicator did not change significantly. Total phenolic and flavonoid contents were higher in the open field. Antioxidant activity against DPPH free radicals was as follows: Diplotaxis (open field) > Eruca (open field) > Diplotaxis (greenhouse) > Eruca (greenhouse). It was revealed that in the open field, the speed of oxidation of the DPPH radical was higher in accessions of both crops: 61.2% in Eruca accessions and 63.0% in Diplotaxis accessions; in the greenhouse – 9.7% and 39.7%, respectively. The level of antioxidant activity and the rate of radical oxidation in Eruca accessions strongly depend on the place of cultivation (50.4% and 93.4%). In Diplotaxis, the level of antioxidant activity depends mainly on the genotype, and the rate of radical oxidation depends on the place of cultivation. A number of positive and negative relationships were identified between the parameters of antioxidant activity and the accumulation of ascorbic acid, pigments, and phenolic compounds under different growing conditions. Eruca accessions from China (k-31) and Diplotaxis accession 'Roket' (k-2, Russia) have been identified with optimal biochemical composition.
-
Key words:
- Eruca /
- Diplotaxis /
- Antioxidant activity /
- Growing conditions /
- Low-molecular compounds













