-
The genus Ilex, commonly known as holly, comprises over 600 species of flowering shrubs or trees throughout temperate and subtropical regions. Many Ilex species are culturally and commercially significant for their diverse uses in the ornamental horticulture, beverage, traditional medicine, honey production, timber, and dyes[1−4]. Among these, Possumhaw (I. decidua) is a deciduous holly species native to the southeastern United States, distributing from Florida to the Midwest and even far West Texas. It thrives in alkaline soils and reaches up to 10 m in height, hanging with red, orange, or yellow berries or drupes[5,6]. Several cultivars have been developed for their distinctive features. For example, 'Warren's Red' is known for its long-lasting berries, 'Council Fire' has a bushy, round form, and 'Red Escort' is a unique male cultivar prized for its yellow fall color[7]. Yellow-fruited selections include 'Byer's golden' and 'Gold Finch', while 'Sundance' produces orange-red fruits[8]. Although Ilex species are widely used and valued, difficulties in vegetative propagation—particularly in dioecious species like I. decidua, limit their commercial potential in industry. Traditional seed propagation is often hindered by a lengthy germination period which can exceed two years due to morpho-physiological dormancy of undeveloped embryos in mature berries[9−11]. Factors such as the presence of mechanical barriers in the endocarp and inhibitors in the endosperm, testa, and endocarp significantly inhibit seed germination, requiring extended periods of stratification to break dormancy effectively[10−12]. While seed production yields roughly 1:1 male-to-female plants, additional time and effort are required to identify desirable female plants with specific traits in leaves, berries, and heights[4,13].
Vegetative propagation through cuttings offers a more efficient alternative for producing female trees in dioecious species or specifically selected genotypes. However, success in rooting Ilex species can vary greatly depending on several factors, such as the age and physiological status of the mother plants, the season of cutting collection, and the specific genotype or cultivar being propagated. For example, auxin treatment is essential for rooting in some cultivars of I. aquifolium[14−16] , while others such as I. paraguariensis may root successfully without auxin treatment[17−19]. Interestingly, the application of potassium indole-3-butyric acid (K-IBA) reduced the rooting rate of semi-hardwood cuttings of I. vomitoria 'Dare County'[20]. High irradiance has been shown to increase phenolic levels in cuttings, negatively impacting the rooting success of I. paraguariensis[21]. Other factors like the lengths of cuttings and the position of the branches also influence rooting success in the same genotype[21,22]. These inconsistencies highlight the difficulty of developing a reliable vegetative propagation protocol for I. decidua cuttings on a large scale.
Micropropagation is an efficient alternative for vegetative propagation, particularly when sexual reproduction is limited, conventional asexual propagation is challenging, mother plants are scarce, specific sex is desired in dioecious plants, or virus-free plants are required for the horticultural industry[23]. Micropropagation research on Ilex species has shown that species-specific protocols are required for both shoot proliferation and rooting. For instance, Murashige and Skoog (MS) medium supplemented with 4.5 μM zeatin (ZT) was used for the shoot proliferation of I. glabra[24], while 2.28 μM ZT was best for I. crenata[25]. However, I. aquifolium and I. dumosa prefer 6-benzylaminopurine (BA) in 1/4 strength MS medium[24−27]. Ilex khasiana responds well to MS medium supplemented with BA alone, but I. paraguariensis needs MS medium to contain both BA and α-naphthaleneacetic acid (NAA), and I. asprella needs MS medium to contain BA and adenine sulfate[28−30]. Modified woody plant medium (WPM) has been proven effective for I. guayusa[31]. If the plant growth regulators (PGRs) or medium conditions are not optimized, issues like hyperhydricity can arise during shoot proliferation due to excessive cytokinin levels and high humidity. It impairs root formation and plantlet establishment, complicating the mass production of robust and uniform plantlets for the ornamental industry. Thus, optimizing the PGRs and medium composition for I. decidua is essential to achieve high proliferation and rooting success.
Building on these documented challenges and successes in other Ilex species, this study aims to develop an efficient micropropagation protocol for female I. decidua by using epicormic shoots as explants to establish aseptic cultures, investigating various media and PGR combinations to optimize shoot proliferation and root formation. This protocol is expected to facilitate the reliable mass production of uniform I. decidua plants, ensuring their broader application for ornamental and ecological purposes.
-
A 10-year-old I. decidua 'Warren's Red' shrub located on the Texas A&M University campus in College Station, TX, USA, was selected as the mother plant (Fig. 1a). Epicormic shoots, ranging in length from 5 to 20 cm, were collected from the lower portion of the main trunk (Fig. 1b) in mid-March and used as the source of explants.

Figure 1.
(a) Ten-year-old Ilex decidua mother plant, and (b) the explants from epicormic shoots at the lower portion of trunks.
Initial aseptic culture establishment
-
After removing the leaves, shoots were cut into 1−3 cm segments. These segments were washed with running tap water for 1 h, surface disinfected by immersion in 70% ethanol for 10 s, followed by treatment with a 20% commercial bleach solution (6% sodium hypochlorite, Clorox, Oakland, CA, USA) solution for 20 min with occasional agitation. The shoot segments were then rinsed three times with sterile deionized water under aseptic conditions. After disinfection, the shoot segments were cultured on WPM media supplemented with 0.5 mg/L BA, 0.01 mg/L IBA, 30 g/L sucrose, and 6.0 g/L agar. Unless otherwise stated, all culture media, agar, PGRs, other reagents, and culture jars were purchased from PhytoTech Labs, Inc. (Lenexa, KS, USA). The pH of all media in this study was adjusted to 5.8 with 1 mol/L HCl or NaOH before autoclaving at 121 °C and 15 psi for 20 min. Two to five nodal explants were cultured in each culture jar (CultureJar™ G9, vol. 220 mL; ht. 9.5 cm; dia. 6.2 cm) containing 30 mL media (Fig. 2a). Axillary shoot formation was monitored, and the newly emerged shoots were excised and subcultured on the same media five times to generate enough shoots for subsequent proliferation experiments with different cytokinins and media.
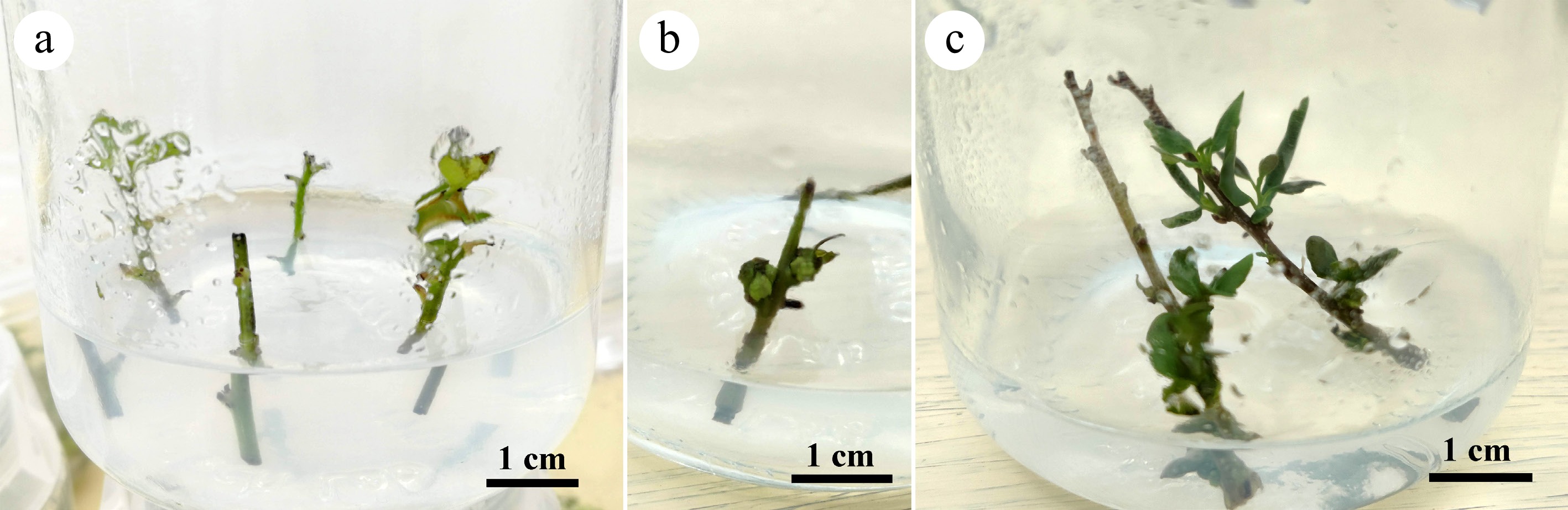
Figure 2.
The disinfected explants were inoculated on the initiation medium WPM supplemented with (a) 0.5 mg/L BA; (b) explants generated axillary buds three weeks after inoculation; (c) the buds developed into lateral shoots 1−2 cm long in the next two weeks for subculture.
Cytokinin type selection for shoot proliferation
-
To optimize shoot proliferation, WPM media supplemented with three cytokinins were investigated, including BA (0.1, 0.5, and 1.0 mg/L), 1-(2-chloropyridin-4-yl)-3-phenylurea (CPPU, 0.01, 0.1, and 0.5 mg/L), 6-(γ,γ-Dimethylallylamino)Purine (2-iP, 0.1 and 0.5 mg/L), and ZT (0.1 and 0.5 mg/L). A control group was established using WPM without PGRs (Table 1). Additionally, combinations of different concentrations of BA (0.1, 0.2, 0.5 mg/L) and CPPU (0.01, 0.02, 0.05 mg/L) were further tested to assess their effects on shoot proliferation. Each treatment consisted of 10 jars, with 8–10 explants per jar. The shoot proliferation rate and shoot growth were evaluated 30 d after inoculation.
Table 1. Effects of cytokinin type (BA, 6-benzylaminopurine; CPPU, N-(2-chloro-4-pyridyl)-N-phenylurea; 2-iP, 6-(γ,γ-Dimethylallylamino)purine; ZT, zeatin) and concentration on the in vitro adventitious shoot multiplication and shoot length of Ilex decidua.
Cytokinin Concentration (mg/L) Multiplication rate Shoot length (mm) Control 0 1.5 ± 0.13e 5.1 ± 0.5c BA 0.1 2.2 ± 0.29de 12 ± 1.5ab 0.5 6.6 ± 0.55b 10 ± 1.2b 1.0 9.1 ± 0.81ab 4.2 ± 0.3c CPPU 0.01 3.2 ± 0.37cd 16 ± 1.4a 0.1 9.7 ± 0.84ab 14 ± 1.1a 0.5 11.6 ± 1.33a 3.2 ± 0.2d 2-iP 0.1 3.5 ± 0.44cd 4.1 ± 0.5c 0.5 6.7 ± 0.57b 3.1 ± 0.4d ZT 0.1 3.7 ± 0.44c 5.2 ± 0.6c 0.5 5.6 ± 0.67bc 3.3 ± 0.5d Means in each column followed with different superscript letters are significantly different (p ≤ 0.05) according to Duncan's multiple range test. Effect of basal media on the shoot proliferation
-
To optimize the culture medium compositions for shoot proliferation, axillary or adventitious shoots established on the WPM medium with 0.2 mg/L BA and 0.02 mg/L CPPU were transferred to three basal media: Driver and Kuniyuki Walnut medium (DKW), WPM, and Long and Preece (LP). All media were supplemented with 0.2 mg/L BA and 0.02 mg/L CPPU. Each treatment had 10 vessels, with 8–10 explants per vessel. The number and growth of axillary or adventitious shoots were evaluated four weeks after subculturing.
Rooting and acclimatization
-
For in vitro rooting, shoots were transferred to rooting media after the leaves on the lower part of the shoots were removed. The rooting media consisted of 1/2 LP medium, 20 g/L sucrose, 6 g/L agar, and a pH of 5.8. IBA (0.5, 1.0, 1.5 mg/L) and NAA (0.5, 1.0, 1.5 mg/L) were tested, with 1/2 LP medium without plant growth regulators as control. Ten shoots were cultured in each jar; each treatment included a total of 10 vessels.
For ex vitro rooting, micro-shoots were cut and dipped in solutions of 0.5, 1.0, or 1.5 g/L IBA or NAA, dissolved in 50% ethanol, for 3 s. The control shoots were dipped in deionized water. Treated shoots were planted in 10 cm diameter containers filled with a substrate composed of 70% peat and 30% perlite (by volume). Three pots were placed on a misting bench in the greenhouse with intermittent misting of 2 s/min. The greenhouse was equipped with a shade net, fans and pad system, a natural gas heating system, and a climate control system. During the experiment, temperatures ranged from 20 to 35 °C, and the highest light level was 300 μmol/m/s photosynthetically active radiation. The rooting percentage was recorded four weeks after transplanting. The rooted plantlets were then moved out from the misting bench and fertilized with 3 g of controlled-release fertilizer (Osmocote plus 15-9-12, ICL Specialty Fertilizers, Summerville, SC, USA).
Culture conditions and data analysis
-
The temperature of the tissue culture room was controlled between 22−28 °C, and white LED lights provided a photosynthetic photon flux density of 80 μmol/m/s for 16 h per day. All experiments were set up in a completely randomized design, with different replications mentioned above. Each culture vessel was treated as an experimental unit for in vitro culture. One-way ANOVA was conducted to determine the difference between treatments using SPSS 19.0 for Windows (SPSS, Chicago, IL, USA). When significant differences (p < 0.05) were detected, means were separated using Duncan's multiple range test at the 0.05 significance level.
-
Uncontaminated explants (Fig. 2a) cultured on the initiation medium (WPM supplemented with 0.5 mg/L BA) generated axillary buds three weeks after inoculation (Fig. 2b). These buds developed into lateral shoots, reaching 1−2 cm in length over the following next two weeks (Fig. 2c). The newly formed shoots were then excised and subcultured on the same medium to generate sufficient materials for the multiplication experiment with different cytokinins.
Cytokinin optimization for shoot proliferation
-
The type and concentration of cytokinins in the media significantly influenced the multiplication rate, the length of the regenerated shoots, and their morphology. The explants on the control medium without any cytokinins produce only about 1.5 shoots from axillary buds (Fig. 3a), with the new shoots slowly elongated to approximately 5 mm during the four-week culture period (Table 1). The addition of BA, CPPU, or ZT to the media significantly increased the multiplication rate (Table 1). Explants on the media amended with 1 mg/L BA and 0.5 mg/L CPPU, 2-iP or ZT, proliferated more new shoots; however, most of these adventitious shoots were short and exhibited distorted or malformed hyperhydric leaves (Fig. 3d, g, i, k). Media amended with 0.5 mg/L BA or 0.1 mg/L CPPU resulted in the proliferation of 6.6 and 9.7 healthy shoots respectively, with slightly upward curved leaves (Fig. 3c, f). Most of these were axillary shoots, though a few were adventitious shoots that regenerated from callus. Explants on media with 2-iP and ZT produced short shoots with upward-curved leaves, even at 0.1 mg/L (Fig. 3h, j). Different combinations of 0.1, 0.2, and 0.5 mg/L BA and 0.01, 0.02, and 0.05 mg/L CPPU improved the quality of the proliferated shoots. Medium supplemented with 0.5 mg/L BA and 0.05 mg/L CPPU produced the highest multiplication rate, but the shoots were shortest (Table 2). In contrast, the combination of 0.2 mg/L BA and 0.02 mg/L CPPU produced the longest shoots (19 mm) with a moderate multiplication rate (4.7).

Figure 3.
Effects of cytokinin type and concentration on the in vitro shoot proliferation in Ilex decidua. (a) Control, no cytokinin; (b) 0.1 mg/L BA; (c) 0.5 mg/L BA; (d) 1.0 mg/L BA; (e) 0.01 mg/L CPPU; (f) 0.1 mg/L CPPU; (g) 0.5 mg/L CPPU; (h) 0.1 mg/L 2-iP; (i) 0.5 mg/L 2-iP; (j) 0.1 mg/L ZT; (k) 0.5 mg/L ZT. (Scale bars = 1 cm, initial subcultured shoots length is about 1 cm).
Table 2. In vitro multiplication of Ilex decidua adventitious shoots on WPM media with different combinations of BA (6-benzylaminopurine) and CPPU (N-(2-chloro-4-pyridyl)-N-phenylurea).
BA (mg/L) CPPU (mg/L) Multiplication rate Shoot length (mm) 0.1 0.01 2.8 ± 0.3d 11 ± 1.5b 0.2 0.01 6.1 ± 0.4b 13 ± 0.9b 0.5 0.01 7.6 ± 0.9ab 14 ± 1.6b 0.1 0.02 4.2 ± 0.5c 21 ± 1.8a 0.2 0.02 4.7 ± 0.3bc 19 ± 2.2a 0.5 0.02 8.2 ± 0.7a 9.1 ± 0.7bc 0.1 0.05 4.3 ± 0.4c 11 ± 1.3b 0.2 0.05 6.5 ± 0.7b 7.2 ± 0.8c 0.5 0.05 9.2 ± 0.8a 5.1 ± 0.6d Means in each column followed with different superscript letters are significantly different (p ≤ 0.05) according to Duncan's multiple range test. Effect of basal medium on the shoot proliferation and morphology
-
The basal medium had a significant impact on the multiplication rate when amended with 0.2 mg/L BA and 0.02 mg/L CPPU. WPM and LP media produced more shoots compared to DKW, without affecting the shoot length (Table 3). However, shoots regenerated on WPM exhibited yellowing leaves at the lower part, particularly when the culture period exceeded four weeks (Fig. 4a). Shoots grown on DKW medium developed large, thin leaves (Fig. 4b), while those on LP medium were healthy, uniform, and displayed medium-sized flat leaves (Fig. 4c, d).
Table 3. Effect of basal medium on in vitro shoot proliferation of Ilex decidua.
Medium Multiplication rate Average shoot length (mm) Growth observation WPM 4.7 ± 0.3a 21 ± 2.5a Small leaves, upward curved, some lower leaves yellow MS 1.7 ± 0.2b 18 ± 2.2a Leaves large and thin DKW 2.2 ± 0.2b 20 ± 1.6a Leaves large and thin LP 5.5 ± 0.4a 22 ± 1.8a Leaves mediate, healthy Means in each column followed with different superscript letters are significantly different (p ≤ 0.05) according to Duncan's multiple range test. 
Figure 4.
Effects of different basal media with 0.2 mg/L BA and 0.02 mg/L CPPU on in vitro shoot proliferation of Ilex decidua. (a) WPM medium; (b) DKW medium; (c) LP medium; (d) adventitious shoots proliferated from individual explant on LP medium. (Scale bars = 1 cm, initial subcultured shoots length is about 1 cm).
Rooting and acclimatization
-
Micro-cuttings exhibited the lowest rooting percentage when no auxin was applied, either in vitro or ex vitro (Table 4). Media supplemented with 0.5, 1.0, and 1.5 mg/L IBA or NAA produced a high rooting percentage and 9−13 roots per cutting (Fig. 5a). However, cuttings induced large callus at the base when the IBA or NAA concentration was 1.0 or 1.5 mg/L. Ex vitro rooting with a quick dip in IBA or NAA solution also produced high rooting percentages. The 1 g/L IBA treatment achieved 100% rooting, with rooting initiated just 10 d after transplanting. No significant callus formation occurred, and all roots emerged directly from the stem (Fig. 5b). Both in vitro and ex vitro rooted micro-cuttings achieved 100% survival rate after being transplanted into 10 cm plastic pots (Fig. 5c). Two months after transplanting, the plantlets had grown to about 20 cm in height (Fig. 5d).
Table 4. Rooting percentage and root number of micropropagated shoots by in vitro and ex vitro rooting with different concentrations of IBA (indole-3-butyric acid) and NAA (α-naphthalene acetic acid) in Ilex decidua.
In vitro rooting Ex vitro rooting Auxins (mg/L) Rooted cuttings (%) Number of roots Auxins (g/L, quick dip) Rooted cuttings (%) Number of roots 0 15 ± 0.8b 4.2 ± 0.3b 0 12 ± 1.1c 3.1 ± 0.2c 0.5 IBA 100a 9.3 ± 0.5a 0.5 IBA 85 ± 0.6a 7.5 ± 0.6ab 1.0 IBA 100a 11.2 ± 0.9a 1.0 IBA 100a 9.2 ± 1.2a 1.5 IBA 100a 12.5 ± 1.4a 1.5 IBA 96 ± 1.1a 8.8 ± 0.8a 0.5 NAA 90 ± 7.4a 9.6 ± 0.7a 0.5 NAA 77 ± 0.9b 6.6 ± 0.5b 1.0 NAA 96 ± 8.6a 10.7 ± 0.6a 1.0 NAA 91 ± 0.7a 8.4 ± 0.9a 1.5 NAA 100a 11.1 ± 1.5a 1.5 NAA 90 ± 0.7a 7.3 ± 0.5ab Means in each column followed with different superscript letters are significantly different (p ≤ 0.05) according to Duncan's multiple range test. 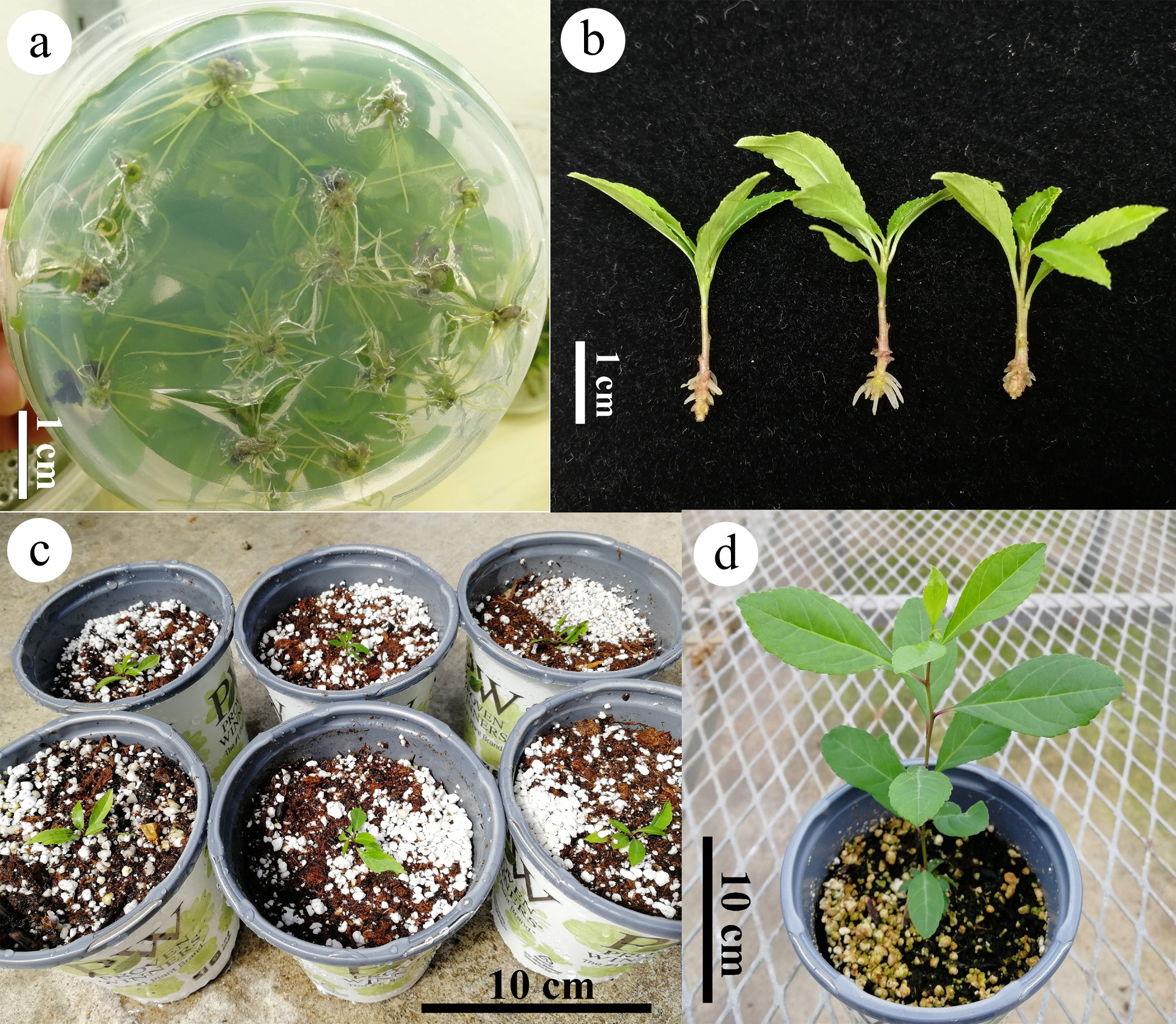
Figure 5.
Rooting and acclimatizing of Ilex decidua. (a) In vitro rooting on 1/2 LP medium with 0.5 mg/L IBA; (b) ex vitro rooting of micro-cuttings 2 weeks after quick dip in 1 g/L IBA; (c) rooted plantlets transplanted on 10 cm pots with the substrate of 1/2 peat and 1/2 perlite; (d) the plantlets grew to about 20 cm in height two months after transplanting in a greenhouse.
-
Epicormic shoots from the lower part of the main trunk were used as explant sources in this study, as they retain juvenile characteristics that enhance the successful in vitro propagation of mature woody plants[32,33]. Juvenile epicormic shoots have been utilized for micropropagation of numerous woody species, particularly those that are difficult to root, such as Eucalyptus[34,35], oaks[36], Tectona grandis[37]. In I. paraguariensis, epicormic shoots were induced from cut branches or by pruning, girdling, and coppicing for vegetative propagation[17,38,39]. The abundant epicormic shoots at the lower part of the I. decidua trunk, especially at pruning sites (Fig. 1a), make them an excellent source for propagation.
BA (6-benzylaminopurine) is the most commonly used cytokinin for Ilex micropropagation. BA at 1 mg/L has been employed successfully for I. paraguariensis[40,41], I. dumosa[27], and I. aquifolium[26], although high concentrations can inhibit shoot growth in I. paraguariensis[29]. ZT is more effective in increasing shoot number and length than BA in I. crenata[25]. However, kinetin and thidiazuron negatively affected the shoot length in I. aquifolium[26]. In this study, I. decidua produced sufficient adventitious shoots with 1.0 mg/L BA, and 0.1−0.5 mg/L CPPU, though these shoots were short and prone to hyperhydricity. ZT also induced malformed hyperhydric adventitious shoots. A combination of 0.2 mg/L BA and 0.02 mg/L CPPU provided the best balance of shoot quantity and quality.
CPPU, a synthetic cytokinin-like phenylurea, is widely used in fruit production to increase fruit size by increasing cell division[42]. Although not commonly used in micropropagation, CPPU has shown promise in stimulating shoot proliferation[43]. High shoot proliferation has been achieved in Hibiscus rosa-sinensis with 0.62 mg/L CPPU[44]. And low concentration of CPPU (0.05 mg/L) has proven effective for multiplying recalcitrant red raspberry cultivars[45]. CPPU has also been used for the somatic embryogenesis of Rosmarinus officinalis[46] and shoot organogenesis of tree peony[47]. In kiwifruit micropropagation, 0.55 mg/L CPPU yielded results comparable to 1 mg/L BA but caused hyperhydricity and short internodes at higher concentrations[48]. This finding aligns with our results for I. decidua, where CPPU concentration above 0.1 mg/L led to hyperhydricity. A combination of 0.2 mg/L BA and 0.02 mg/L CPPU resulted in a high multiplication rate without these negative effects.
The mineral compositions of basal media play a crucial role in the success of in vitro culture. WPM, 1/2MS, and modified MS are commonly used for woody plants[49]. WPM has been effective for the proliferation of I. aquifolium[26], I. crenata[25], and I. guayusa[31]. A low-salt medium (1/4 MS) was used for I. paraguariensis to reduce browning[41]. In I. decidua, slight browning was observed after several subcultures, but shoots on WPM developed smaller leaves than those on DKW. The higher nitrogen content in DKW increased leaf growth but reduced shoot number, while low-salt WPM produced smaller leaves. This is consistent with results observed in Peperomia pellucida[50], Juglans nigra[51], and Corylus avellana[52]. The LP medium, a mixed medium with half DKW and half WPM[53], successfully used in vitro proliferation of Vaccinium arboreum[54], also produced vigorous adventitious shoots with moderate leaf size in I. decidua.
Both in vitro and ex vitro rooting achieved high rooting percentages and survival rates for I. decidua and other Ilex species. IBA treatment is typically required for in vitro rooting in most Ilex species, with concentrations of 0.9 mg/L for I. guayusa[31], 1 mg/L for I. aquifolium[26], 1.5 mg/L for I. dumosa[27], and 2.0 mg/L for I. khasiana[28]. In I. decidua, a 100% rooting percentage was achieved with 0.5 mg/L or higher IBA treatments, though higher concentrations induced callus formation at the shoot base. Ex vitro rooting with a 1 g/L IBA quick dip also resulted in 100% rooting. One key advantage of ex vitro rooting is that the adventitious roots protruded from the stem directly without obvious callus formation. The roots are well connected to the vascular system and improve the survival rate during acclimatization[54]. A similar result was obtained on I. aquifolium[26]. Ex vitro rooting can also save cost and labor by skipping the in vitro rooting step, though it requires a well-controlled greenhouse facility to prevent dehydration during the rooting stage. Ultimately, there was no significant difference in the final plant growth between in vitro and ex vitro rooting for I. decidua, so deciding the optimal protocol depends on available facilities for rooting and acclimatization.
-
An efficient micropropagation method for I. decidua was successfully developed using new epicormic shoots collected in spring as explants. Explants were effectively disinfected by immersing them in 70% ethanol for 10 s, followed by 20% commercial bleach (6% sodium hypochlorite) for 20 min. Aseptic axillary buds were initiated on WPM medium supplemented with 0.5 mg/L BA. For shoot proliferation, LP medium supplemented with 0.2 mg/L BA and 0.02 mg/L CPPU provided the best balance between shoot quantity and quality. Both in vitro rooting on ½ LP medium containing 0.5 mg/L IBA and ex vitro rooting with a quick dip in 1 g/L IBA achieved 100% rooting success. When using an ex vitro rooting method, the micro-cuttings required high humidity conditions to ensure successful rooting. This optimized protocol offers a practical solution for the mass propagation of uniform female I. decidua for landscape use.
-
The authors confirm contribution to the paper as follows: study conception and design: Li Q, Gu M; data collection: Li Q, Wu B; analysis and interpretation of results: Li Q, Gu M; draft manuscript preparation: Li Q, Gu M, and Wu B. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors acknowledge the provision of plant materials, equipment and facilities by Texas A&M University.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li Q, Wu B, Gu M. 2025. Micropropagation of Possumhaw holly Ilex decidua Walter. Technology in Horticulture 5: e007 doi: 10.48130/tihort-0025-0003
Micropropagation of Possumhaw holly Ilex decidua Walter
- Received: 04 November 2024
- Revised: 16 December 2024
- Accepted: 17 December 2024
- Published online: 04 March 2025
Abstract: Possumhaw holly (Ilex decidua Walter) is an ornamental shrub or small tree known for its red, orange, or yellow fruits. However, the difficulty of vegetative propagation for specific cultivars or female specimens limits its landscape use. This study aimed to develop an efficient micropropagation method for the mass propagation of female trees. Epicormic shoots were used as explants to establish an initial aseptic culture on woody plant medium (WPM) supplemented with 0.5 mg/L BA. The best results for shoot proliferation, balancing both quantity and quality, were achieved using the Long and Preece (LP) medium with 0.2 mg/L 6-benzylaminopurine (BA), and 0.02 mg/L N-(2-chloro-4-pyridyl)-N-phenylurea (CPPU). High concentrations of BA, CPPU, or zeatin often led to hyperhydricity. Woody plant medium resulted in nutrient deficiency, evidenced by chlorotic leaves on the lower part of regenerated shoots. Conversely, shoots on Driver and Kuniyuki Walnut medium (DKW) exhibited large, thin leaves due to excess nitrogen. For rooting, both in vitro methods using half-strength LP medium with 0.5 mg/L indole-3-butyric acid (IBA) and ex vitro methods with a quick dip in 1 g/L IBA solution achieved 100% rooting success. It is crucial to maintain high humidity for micro-cuttings during the ex vitro rooting. The optimized combinations of plant growth regulators (PGRs) and culture media provide high proliferation and rooting success, facilitating the reliable mass production of uniform female propagules, and ensuring their broader application in landscaping.
-
Key words:
- Deciduous holly /
- Mass propagation /
- Tissue culture /
- In vitro /
- CPPU












