-
As one of the most important palm species in the world, the coconut (Cocos nucifera L.) belongs to the Cocoideae subfamily within the Arecaceae (palm) family and stands as the sole species in the Cocos genus[1]. Its origin is believed to be traced back to Polynesia or the Indian Archipelago[2]. However, in the present day, coconut palms thrive in over 90 countries worldwide[3]. Referred to as the 'tree of life', the coconut palm is highly versatile[4], with almost every part of the tree serving a purpose. It not only meets essential daily needs but also caters to various luxuries in life. Coconut products are diverse, including coconut oil, coconut milk, coconut water, coconut sugar, coconut mats, coconut fiber, coconut shell activated carbon, building materials, furniture, and coconut carving handicrafts[5].
The variant characterized by a jelly-like endosperm in its fruit is referred to as 'makapuno' (or macapuno), a term originating from Filipino, meaning 'tends to fullness'[6]. This particular variety was initially identified in the Philippines, as documented by Gonzalez in 1914, who described the fruit as having a cavity that is either completely or partially filled with a white, gelatinous endosperm[7]. It was observed that not every fruit from a makapuno-bearing palm displayed these characteristics[8]. Intriguingly, similar varieties with different local names are found in various countries, such as Dừa Sáp (Vietnam), Dikiri Pol (Sri Lanka), Kopyor (Indonesia), Maphrao Kathi (Thailand), Dahi Nariyel (Myanmar), Thairu Thengai (India), Dong Kathy (Cambodia), and Niu Garuk (Papua New Guinea)[9]. Makapuno coconut cannot germinate in natural conditions. Makapuno embryos exhibit morphological normalcy, but its inability to germinate stems from biochemical and physical characteristics of the exceptional endosperm, which fails to support germination[10]. The non-germination of the makapuno embryo is attributed to the haustorium's failure to develop, resulting in the loss of connection between the viable embryo and the abnormal endosperm[11]. Makapuno is the most economically valuable coconut variety worldwide and deeply loved by tropical people, sometimes makapuno serves as a gift for its costliness in producing countries[12].
-
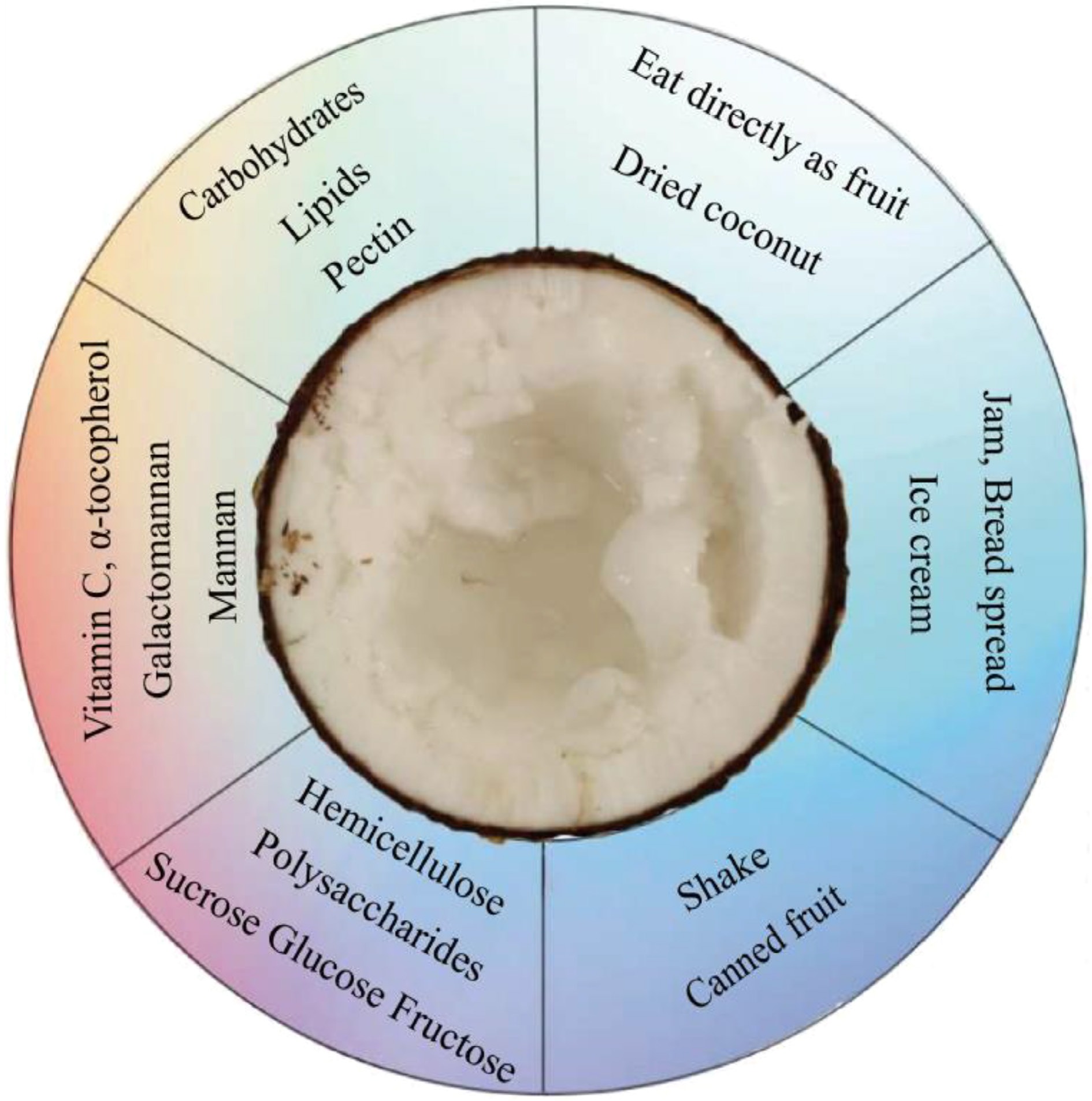
Elite coconuts like makapuno and aromatic varieties are not just prized for their scarcity. The delicious makapuno endosperm (as shown in Fig. 1), with its melt-in-your-mouth texture and rich, nutty flavor, makes them highly sought-after for fresh consumption and a range of attractive processed products[13]. When Sri Lanka is used as an example, it becomes evident that this elite variety commands a higher price due to its distinctive flavor. Sri Lankan dikiri coconut is gaining a reputation as a high-potential coconut type in the coconut sector because of its high composition of pectin which physico-chemical properties are comparable with commercial fruit-grade pectin. The dietary fiber components available in dikiri coconut are pectin and hemicellulose while normal coconut contains cellulose and lignin. Lignin is comparatively very low in dikiri coconuts. The high amount of methoxyl pectin indicates the potential in food products like shakes, jam, bread spread and ice cream without adding extra pectin. Dikiri kernel-based ice cream provides a substitute for dairy ice cream which is preferred by consumers with lactose intolerance[14]. The ice cream prepared from dikiri kernel is rich in fat and pectin with quality attributes like appearance, taste, texture, mouth feel and overall acceptability, not significantly different from those of dairy ice cream[15]. Dikiri kernel has a lower composition of fat and a higher composition of carbohydrates, than the ordinary coconut which is a favorable characteristic in food processing, as risk of rancidity in storage is reduced[16].
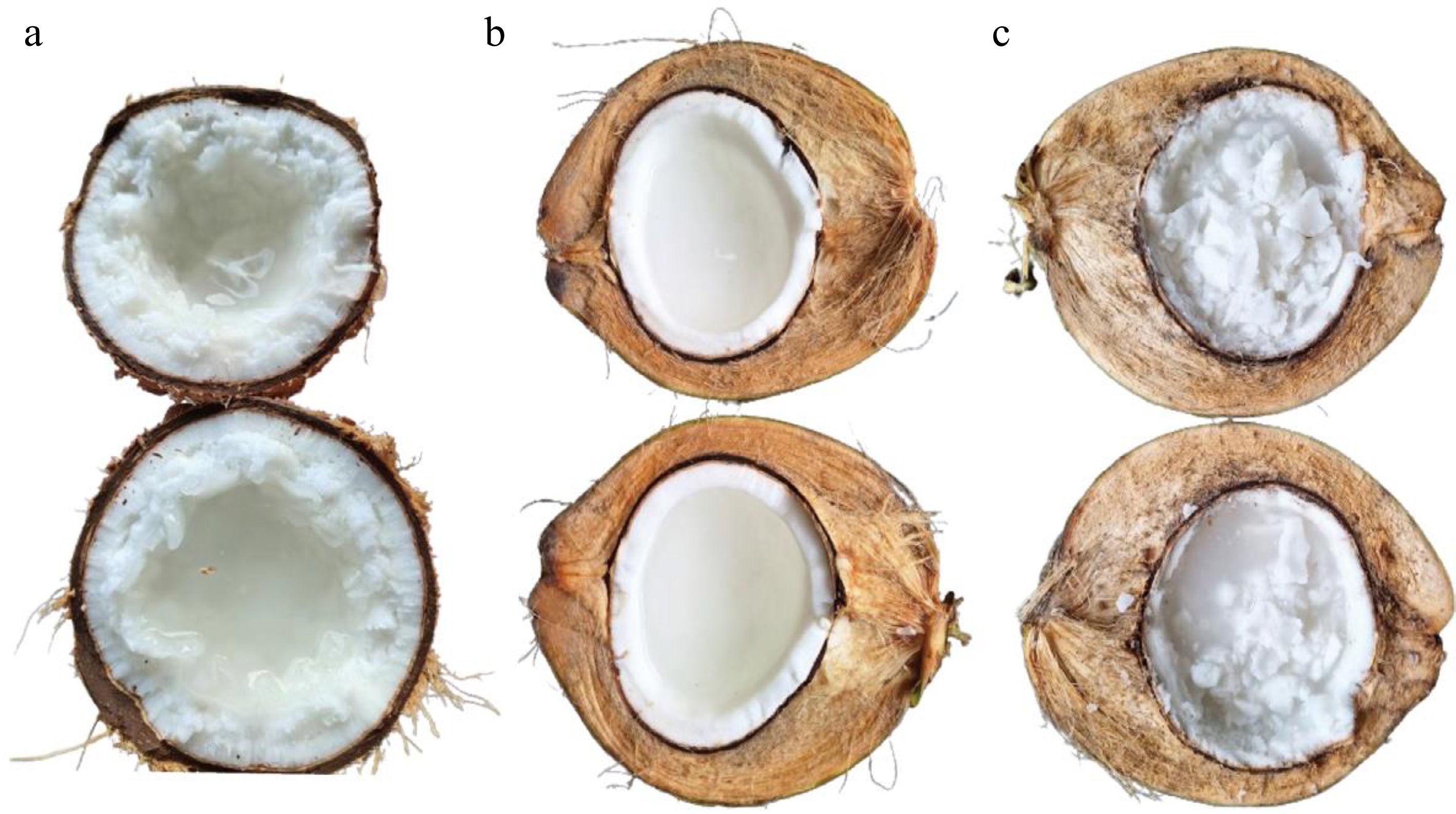
Figure 1.
The comparison between normal coconut and makapuno coconut. (a) Fruit of Thai makapuno coconut with jelly-like liquid endosperm, (b) fruit from normal tall coconut variety, (c) special makapuno variety from Indonesia called kopyor.
Nutritional values of makapuno coconut
-
The nutritional composition analysis of makapuno was initiated early and conducted comprehensively, revealing its rich nutritional profile (as illustrated in Fig. 2). Some nutritional components present in makapuno are even absent in regular coconuts. Makapuno meat primarily consists of carbohydrates, followed by lipids, which is in contrast to the normal mature meat that serves as a source of oil[17]. They also noted that the main cell wall material of normal mature coconut meat is hemicellulose, while that of makapuno is pectin[18]. Previous studies indicated that the polysaccharides in coconut meat are primarily cellulose, with high amounts of galactomannan and mannan[19]. The vitamin content of makapuno meat is also similar to that of normal mature meat but exhibits significantly higher levels of vitamin C and α-tocopherol[17]. The mineral composition of makapuno meat is comparable to that of young or normal mature meat, making it a good source of dietary minerals, with potassium being the chief mineral[20]. The sweet taste of normal coconut is largely attributed to sucrose, and makapuno meat has higher contents of sucrose, glucose, and fructose compared to normal mature meat[20,21].
Makapuno type coconut is deficient in the function of α-D-galactosidase, an enzyme that converts galactomannan into mannan during nut maturation. The absence of α-D-galactosidase activity results in the accumulation of galactomannan, whereas in the normal coconut, it converts galactomannan to mannan[22]. Accumulated galactomannan makes the solid endosperm soft, fluffy, and gelatinous and the liquid endosperm highly viscous in makapuno type coconut[23]. Galactomannan functions as a dietary fiber since the molecules are not digested by digestive secretions in the small intestine of humans[24]. Galactomannan and citrus pectin are considered 'super fibers' known for altering gut microbiota composition and improving glucose and lipid metabolism. The dietary fiber of makapuno meat is comparable to that of oat bran, chickpea, or other legumes. Makapuno contains high dietary fiber in its meat which was found to be hemicellulose, whereas in young meat, it is cellulose[20]. Therefore, due to the higher dietary fiber content makapuno type coconut has the potential to be used as a functional food as well as in prebiotics to support gut health[25]. It is well known that the intake of dietary fiber is associated with health benefits. Furthermore, soluble dietary fiber, like galactomannan is fermented by gut microbiota and it has important health benefits due to its hypo-cholesterolemic properties. Due to these nutritional and health properties, dietary fiber is widely used as a functional ingredient in the food industry[26]. Makapuno meat contained about 3-fold lower fat, than what has been reported for mature coconut. This is reflected in the calories of makapuno meat, which was approximately 3-fold lower than that of mature coconut[25]. According to Gunathilake, dikiri kernel has a significantly lower fat content (34.45% dry basis) compared to that of the normal coconut kernel (66.11% dry basis)[14]. Therefore, makapuno meat has high potential in foods such as ice cream, pastries, cakes, candies, and beverages like smoothies and shakes with authentic coconut taste. Makapuno galactomannan can be exploited as a plant-based biopolymer and a hydrocolloid material, having potential as a new commercial source of galactomannan[23]. Gunathilake also reported that dikiri contains a considerably high amount of pectin (22.36% ± 1.2%) compared to other commercial pectin sources[14].
-
Kopyor is an Indonesian type of coconut with a soft and friable endosperm, and it is said to be distinct from makapuno coconut. Two main areas have been known as central production areaa, i.e. Pati District of Central Java Province which mostly produces about 3 to 5 thousand kopyor nuts per week and South Lampung, Sumatera which produces less than a thousand nuts per week[27]. With the high demand for kopyor nuts in Indonesia and the limited number of nut production per year, the price of kopyor nuts increases rapidly every year. In 2014, the price of a kopyor nut was about 20 to 30 thousand rupiahs (USD
${\$} $ ${\$} $ Embryo culture has been applied to coconut seedlings production for several reasons such as germplasm collection and exchange[29,30], germplasm storage[31−38], physiological study on coconut including biotic and abiotic stresses[39]. In Indonesia, embryo culture has been applied widely on producing true-to-type of certain coconut cultivars such as makapuno in the Philippines[13,40,41] or kopyor in Indonesia[42−46]. In general, kopyor seedling production using the embryo culture approach consists of two steps, in vitro and ex vitro steps (Fig. 3). In vitro step mostly needs about 6 to 9 months for culture initiation, embryo germination, and seedling development. After the coconut seedlings produce more than two open leaves then culture can be moved to the ex vitro step which consists of a rooting and acclimatization process, followed by transplanting in nursery. This ex vitro step needs another 9 months before the plants reach about 1 m height and are ready for field planting.
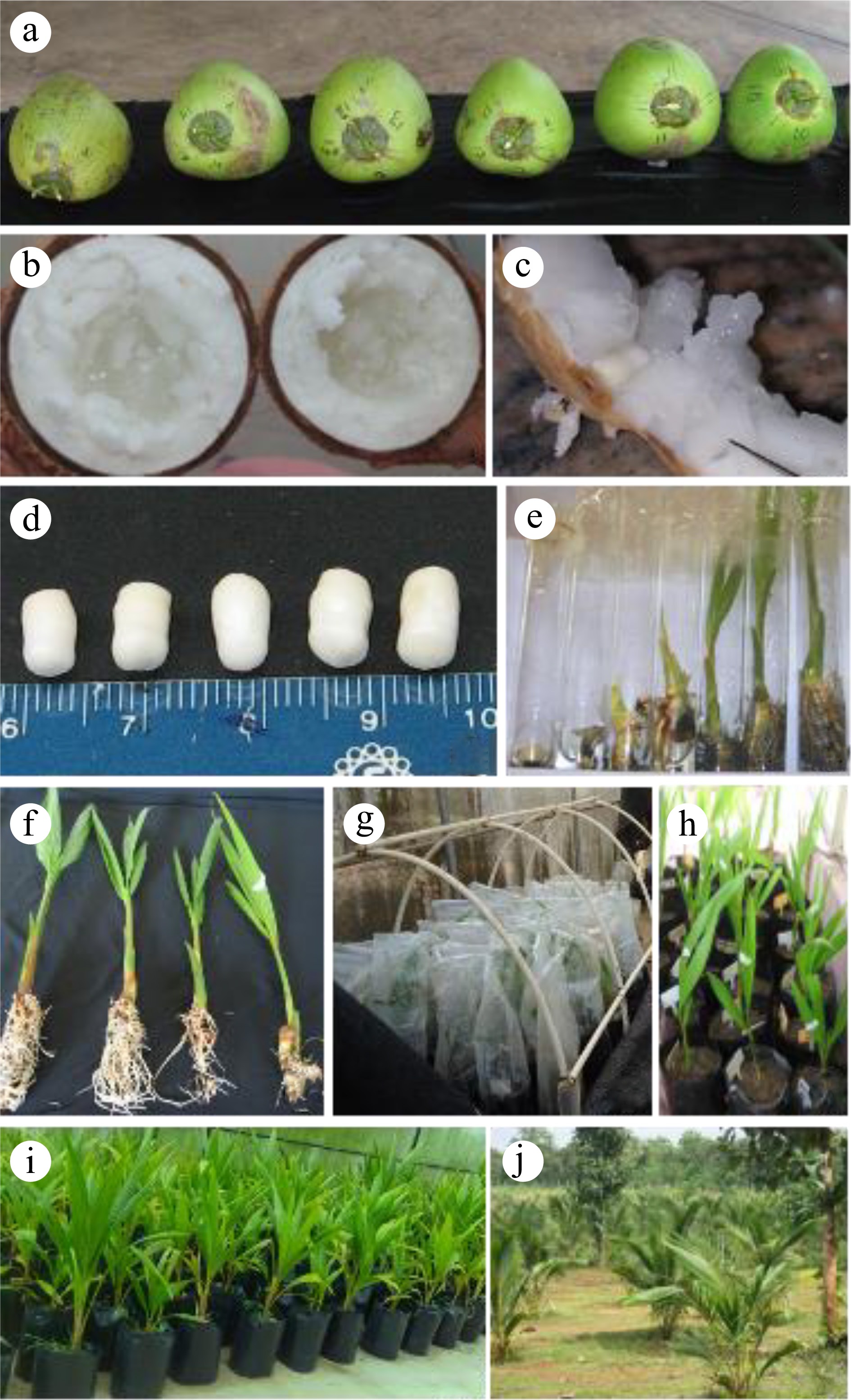
Figure 3.
Makapuno coconut propagation methods (Sri Lanka). (a) Mature dikiri nuts at 11−12 months postpollination, (b) dikiri kernel, (c) extraction of dikiri embryo, (d) dikiri embryos taken from kernel, (e) dikiri plantlets from embryo culture at different stages, (f) plantlets at growth stages 7−8-month in vitro period, (g) initial acclimatization in polybags, (h) potted plants, (i) fully acclimatized plants ready for selling, (j) field establishment of dikiri plants in Coconut Research Institute, Sri Lanka.
The most common in vitro culture medium used for kopyor seedling production is hybrid embryo culture (HEC) medium in which Y3 macro- and micro-nutrients are combined with UPLB vitamins and high sucrose concentration (60 g/L), without addition of plant growth regulators[47]. However, minor changes have been reported such as the HEC medium with added coconut water[42,45], indole butyric acid (IBA), and kinetin during seedling development[44], or added with IBA during rooting and acclimatization process[43]. In several Indonesian tissue culture laboratories, in vitro rooting steps have been reported[42,45]. However, a more efficient protocol has been reported by combining rooting and acclimatization through ex vitro rooting[43]. To date, there are several laboratories in Indonesia commercially producing true-to-type kopyor seedlings using embryo culture with the potential to produce more than 30,000 seedlings per year.
Dikiri in Sri Lanka
-
A makapuno-type coconut called dikiri with a gelatinous kernel is originally found in some villages of southern Sri Lanka. In dikiri coconut, the solid endosperm is a soft translucent gelatinous pulp and amorphous which fills almost the entire central cavity of the coconut, and the liquid endosperm is viscous and colorless. The thickness of meat differs among the nuts, from filled nuts with no or very little liquid endosperm to thick and soft but not amorphous kernels, with a considerable amount of thick liquid endosperm. Only a few tall-type palms were identified initially with a potential of bearing about 2%−5% of dikiri nuts from the harvest. Dwarf varieties bearing similar nuts were not reported in Sri Lanka. Dikiri nuts were collected from these palms and the extracted embryos were in vitro raised, acclimatized, and field planted in two experimental plots in research centers for conservation. Since 2006, a limited number of embryo-culture dikiri plants have been distributed among interested growers for domestic use. In Sri Lanka, dikiri kernel is consumed as fresh with sugar, treacle, or jaggary. Since the nuts are scarce, these nuts are sold at higher prices (approximately USD
${\$} $ To propagate true-to-type plants dikiri is propagated through embryo culture technology (Fig. 3). The embryo of the dikiri nuts is excised from the kernel and cultured aseptically in solid (with 0.3% gelrite) Y3 nutrient medium[48] supplemented with activated charcoal (0.1%) and sucrose (6%) for germination. The germination percentage is above 70%. The germinated plants with roots are transferred to Y3 liquid medium. Sometimes rooting hormones (naphthaleneacetic acid, 200 μM) are supplemented to acquire well developed root system. In vitro raised plantlets with 2−3 leaves and a good root system are transferred to the potting media (sand : coir pith : compost : paddy husk charcoal, 1:1:1:1) after 6−8 months of culture initiation. After the hardening process, the plants are ready for field planting. The Coconut Research Institute of Sri Lanka collects the dikiri nuts from the fields established at research centers and raises the plants in vitro at the Tissue Culture Laboratory. An acclimatized poly-bagged plant is sold at about USD
${\$} $ Maphrao Kathi in Thailand
-
In Thailand, the makapuno coconut, also known as 'maphrao kathi', stands out with its unique soft and fluffy meat, earning it favor among consumers and a premium price in the market due to its rarity. The makapuno market in Thailand features two primary varieties: the landrace makapuno and the aromatic makapuno[25]. The landrace type is characterized by its coconut oil-like aroma, large fruit size, and tall plant height, with a harvesting cycle of approximately six years. Conversely, the aromatic makapuno emits a delightful pandan scent, matures faster at around three years, and can be harvested at 7−8 months for consumption as young aromatic coconuts or, if delayed, as aromatic makapuno at 12 months.
Makapuno cultivation is widespread across various provinces of Thailand, particularly in central regions like Samut Songkhram and Prachuap Khiri Khan, as well as southern regions such as Chumphon, Surat Thani, and Nakhon Si Thammarat. In Thai cuisine, makapuno is a prized ingredient in traditional desserts, thanks to its creamy texture and flavor. Moreover, its frozen flesh is popularly used in ice creams, enriching the consumer experience. Due to its rarity and unique characteristics, makapuno commands a significantly higher price compared to regular coconuts, sometimes reaching up to 50 times more expensive[49]. This has led to a growing consumer interest and increased demand, with local farmers selling makapuno at prices ranging from USD
${\$} $ ${\$} $ For farmers, cultivating makapuno yields substantial profits, with estimated returns per hectare surpassing those from aromatic coconuts and normal coconuts; an estimated USD
${\$} $ ${\$} $ ${\$} $ 
Figure 4.
Makapuno coconuts and the products derived from them. (a) Freshly cracked makapuno coconut, (b) packed makapuno flesh from Thailand, (c) makapuno sweetmeat from Vietnam, (d) jelly made from makapuno liquid endosperm, (e) homemade makapuno ice cream, (f) kopyor cake from Indonesia, (g) frozen kopyor endosperm from Indonesia.
Dừa sáp in Vietnam
-
In the dynamic landscape of the Vietnamese agriculture, an unusual coconut variety with a distinct type of endosperm, resembling the renowned makapuno variety in the Philippines, was discovered in Tra Vinh province in the early 1900s and garnered significant attention for scientific research and agronomic cultivation. Albeit its morphological and physiological similarities to other native tall coconuts, this exceptional coconut, thriving specific soil and climate conditions in the region exhibits unique traits with exceptionally thick, spongy, butter-like endosperm which occupies the majority of space within the nut while remaining only a small amount of glutinous coconut water with a slight fragrance. Due to its distinctive properties, this novel variety is commonly referred to as 'Dừa Sáp' (wax coconut), 'Dừa Kem' (cream coconut), or the 'Vietnamese Makapuno'[51,52].
For years, Vietnamese makapuno coconut has become a famous specialty of Tra Vinh province with significantly beneficial values. Its unique, butter-like, and highly nutritious endosperm is widely preferred for direct consumption, preparation of tasteful desserts and beverages, as well as the production of numerous processed products such as makapuno jams, sweetmeats, candies, nutrition bars, packed yogurts, and ice cream. These products have been strategically positioned in nationwide supermarkets, e-commerce platforms, and have even been exported to global markets[53,54]. Furthermore, makapuno coconut serves as the key ingredient for the production of low-calorie cream powder and purified galactomannan which contribute significant values in the cosmetic and pharmaceutical industries[55]. Additionally, recent studies have also discovered the potential of biodiesel production from makapuno coconuts, suggesting a promising approach for renewable energy development in the Mekong Delta[56].
Under high demand of fruit production, makapuno coconut is highly promoted for cultivation in Vietnam. Due to gemination failure in natural conditions, the key approach for seedling production of this elite variety is through embryo culture (Fig. 4). Since the early 2000's, the Institute for Oil and Oil Plants (Vietnam Ministry of Industry and Trade) and Tra Vinh University have proceeded as the leading institutes for researching and supplying embryo-cultured makapuno coconuts for planting purposes[57,58]. To date, seedling production via this technology has been widely expanded to industrial scale by various agricultural companies that are capable of producing 10,000–20,000 seedlings annually for each facility with an average price of USD
${\$} $ Furthermore another approach for coconut cloning, via somatic embryogenesis, has recently been developed in Vietnam[60−62]. This advanced technique enables the propagation of makapuno seedlings with a superior multiplication rate compared to embryo culture. As this innovative technology is transitioning to commercialization, the mass production of elite makapuno seedlings could be notably facilitated with outstanding efficiency and significantly lower prices in the near future, providing a large potential supply of high-quality planting materials for the cultivation of Vietnamese makapuno coconuts.
Driven by substantial consumption potential, the price of makapuno coconuts in Vietnam have experienced a steady increase, reaching up to USD
${\$} $ ${\$} $ ${\$} $ ${\$} $ -
Interestingly, makapuno coconuts cannot germinate in nature due to their special structure (as illustrated in Fig. 3). To propagate makapuno, scientists devised coconut embryo culture technology. The main steps of coconut embryo culture are illustrated in Fig. 4. Serving as the most crucial, if not the sole, method for cultivating makapuno, coconut embryo culture technology has undergone significant development over the past few decades[13]. The recalcitrance of coconut to in vitro manipulations is well-known. To overcome that, various plant growth regulators (PGRs) could influence the initial in vitro response of coconut embryos in Y3 media[65]. Various embryo culture systems for coconut propagation have been documented. The journey towards successfully isolating and culturing coconut embryos began with Cutter & Wilson in 1954[66], and it took another decade for Guzman & Rosario to achieve successful plant regeneration from makapuno embryos[67]. Subsequent modifications were made by Philippines Coconut Authority (PCA), Coconut Research Institute of Sri Lanka (CRISL), the University of Queensland (UQ) and other industry organizations such as the Food and Agriculture Organization (FAO) of the United Nations, the International Plant Genetic Resources Institute (IPGRI), the Australian Centre for International Agricultural Research (ACIAR), and Bioversity International[68]. Towards the conclusion of the previous century, embryo culture technology had evolved into a method facilitating the transfer of germplasm among various research laboratories, though its adoption within the industry was not commonplace. Leveraging this well-established technology allows for the collection, preservation, and sterile transportation of valuable and distinctive germplasm, which can subsequently be cultured in any laboratory worldwide[69].
Products from elite coconut are becoming more popular and valuable, which could help coconut farmers and the industry[13]. Special types of coconuts that taste good and smell nice are in high demand and can be sold for much more than regular coconuts. A technique called embryo culture is used to grow these special coconuts, and scientists are working on improving this process[68]. Scientists successfully cultured makapuno embryos in the 1960s using a special nutrient mixture[70]. Their efforts bore fruit two years later when De Guzman and Del Rosario utilized a basal medium developed[67], using medium compositions described by White[71] and Nitsch[72]. These studies demonstrated favorable shoot development in the medium, albeit with limited root growth[73]. Subsequent advancements have been achieved since the 1960s, including enhancements in surface sterilization techniques[74], consideration of embryo age during culture[75], and the implementation of additional methods to foster root growth and seedling adaptation[76].
Cutter & Wilson were the pioneers in documenting the process of coconut embryo culture[66]. The embryo culture technique initially developed for other coconut varieties has proven successful for makapuno[77]. Reports of success extend to similar varieties like 'Dahi Nariyel' in the Andaman Islands (India) and Kopyor in Indonesia[78]. In the latter case, excised embryos were first cultured on White's basal medium to encourage germination[79]. An embryo culture procedure typically involves four main stages: (i) initiating embryo germination; (ii) inducing shoot formation; (iii) promoting root development; and (iv) facilitating acclimatization[13]. The commonly followed procedure involves extracting the embryo from a small portion of solid endosperm, often referred to as a cylindrical plug or core, using a cork borer[80]. Research comparing different embryo ages has determined that the 11-month-old makapuno embryo is most suitable for in vitro growth[75]. Surface sterilization typically consists of two stages. Initially, the cylindrical plug undergoes surface sterilization in the field using a concentrated commercial bleach solution (approximately 5% sodium hypochlorite), followed by triple rinsing in sterile water. After transportation to the laboratory and placement in a laminar flow cabinet, the embryos are isolated and subjected to surface sterilization using a milder bleach solution (ca. 0.5% sodium hypochlorite) and 70% ethanol, followed by at least three rinses with sterile water[13]. Although various basal media formulations, such as those by White[71], Nitsch[72], and Murashige & Skoog[81], have been experimented with, the Y3 medium by Eeuwens[48] has emerged as the most advantageous and is now widely utilized for makapuno embryo culture[74]. This medium offers a higher concentration of micro-elements essential for tissue growth, including cobalt, copper, and notably iodine. Optimal germination and the attainment of a desirable shoot-to-root ratio have been linked to a high sucrose level (6%)[82]. To prevent tissue browning, activated charcoal (0.10%−0.25% w/v) is commonly incorporated into the medium[83,84]. Furthermore, studies have indicated that the addition of gibberellic acid to the liquid medium can enhance germination rate[82]. Autoclaved coconut water and high concentrations of indole acetic acid (IAA) and other IAA-like plant growth regulators[85], such as naphthalene acetic acid (NAA) and indole-3-butyric acid (IBA), have been found to significantly enhance root formation[86,87].
Recently, a novel method has emerged involving the longitudinal division of the kopyor embryo, followed by culturing both halves in a medium supplemented with plant growth regulators. This approach has shown success in generating two kopyor plantlets in most cases[43]. However, its effectiveness hinges greatly on precise embryo cutting, making it unsuitable for large-scale plantlet production. Additionally, an innovative embryo transplantation technique has been proposed, wherein a zygotic embryo from a non-germinable fruit is aseptically transferred to a viable fruit[88]. This alternative to embryo culture allows for the nurturing of non-viable fruits without the need for artificial support, resulting in a considerably more cost-effective outcome[13]. Automation technology has been employed in coconut embryo culture techniques and has yielded excellent results, enhancing the efficiency of embryo culture and seedling propagation and acclimatization[89].
-
China's economy has been growing at a rapid pace in recent years. This has led to an increase in disposable income for many Chinese consumers[90]. As a result, they are now more willing to spend money on premium products. China's fruit production accounts for 29.16% of the world's total, but the average per capita consumption of fruit in China is less than 50 kg, which is still significantly lower than the average of 80 kg or more in developed countries[91]. In recent years, with the change of consumption habits of urban residents, especially those in large and medium-sized cities, are increasingly consuming imported fruits[91]. More and more high-quality imported fruits are pouring into the Chinese market. Blueberries, strawberries, and raspberries, representing small berries, clementines representing citrus fruits, and apples representing temperate fruits, have become the representatives of high-end fruits in China[92]. China's rapid economic growth and the growing demand for green organic fruits have led to a sustained increase in the scale of China's sustainable agriculture[93]. China has invested heavily in the development and utilization of fruit germplasm resources. The protection and utilization technology of tropical economic crop germplasm resources has also reached the world-leading level, among which tropical fruits lychee, longan, and loquat are all ranked first in the world[94].
Coconut products, especially high-value items like coconut oil, have become true luxury goods in China, enjoying popularity among the middle class[95]. Coconut products are a popular choice for Chinese consumers, as an entirely new concept to Chinese enthusiasts, and have entered the coconut market in China (as shown in Fig. 4). Coconut is a healthy and nutritious food source, and they are also considered to be a medicine for certain infections and diseases[96]. China is now the world's largest importer of coconut products[97], and the growth of China's coconut industry is having a positive impact on the economy of many coconut-producing countries. This trend is expected to continue to grow in the coming years[98,99]. Thus, coconut producers are seeing increased sales and profits. As per data provided by China customs, the period from January to July 2022 saw a notable rise in coconut imports to China, totaling 566,000 tonnes. This marked a significant increase of 35.3% when compared to the corresponding period in 2021. Indonesia, Thailand, Vietnam, Philippines, and Malaysia, being the key origins of China's coconut imports in 2022. Indonesia contributes over 40% of China's annual coconut imports (ca. 17.16 million metric tons)[100]. The major contributors to China's coconut supply were primarily Thailand, Indonesia, and Vietnam, constituting 48.6%, 32.5%, and 18.4%, respectively[101]. Indonesia contributes to almost 30% of the global coconut production and serves as a significant provider of raw materials for Chinese coconut processing industry[102]. Vietnamese coconut growers and industries are enthusiastic about scaling up production to meet the rising domestic and international demand. The director of Mekong Fruit Import-Export Ltd. Co. in Ben Tre highlighted that China stands as the world's largest coconut importer, importing approximately thousands of containers of fresh coconuts annually from Vietnam[103]. According to the 2020 China Coconut Oil Import Source Countries Statistics from the Philippine Embassy in Beijing, the Philippines is the second-largest provider of coconuts to China, holding a 27% share of the overall market[104].
The development of the coconut industry is encouraged at both the national and provincial levels in China. As an important oil crop, the central government of China places great emphasis on the development of the coconut industry[105]. The Hainan provincial government is encouraging farmers to plant more coconuts and develop coconut forest tourism. This will help to increase farmers' income by selling coconuts and generating tourism revenue. The government is providing financial incentives to farmers who plant coconuts, and it is also working to promote coconut forest tourism. This includes developing infrastructure for the coconut industry, such as roads, trails, and restaurants, and promoting the region's coconut culture[106]. Given the favorable climate, soil conditions, and diverse resources in Hainan, coconut cultivation is well-suited to the region. Despite the imbalance in coconut processing and planting sectors, the Chinese coconut industry is currently presented with significant opportunities arising from the establishment of the Hainan free trade zone and the implementation of the national rural revitalization strategy[107]. As home to 99% of China's coconut farms, Hainan Island of China boasts a 2000-year legacy of cultivating this versatile palm. It also takes center stage in China's burgeoning coconut industry; an annual production value has reached CNY 20 billion in 2022[108]. With an annual coconut yield of 56.3 billion across 12 million hectares, the potential for coconut-based products is vast, drawing stakeholders together to harness this tropical treasure's economic and health benefits[109]. Despite producing 99% of China's coconuts, Hainan Island's booming coconut processing industry relies heavily on imported raw materials (CNY 20 billion coconut annually)[110]. Recognizing this, the Chinese Academy of Tropical Agricultural Sciences (CATAS) held the 2023 China (Hainan) International Coconut Industry Forum and mobilized local stakeholders to promote integrated development and reduce reliance on imports[111].
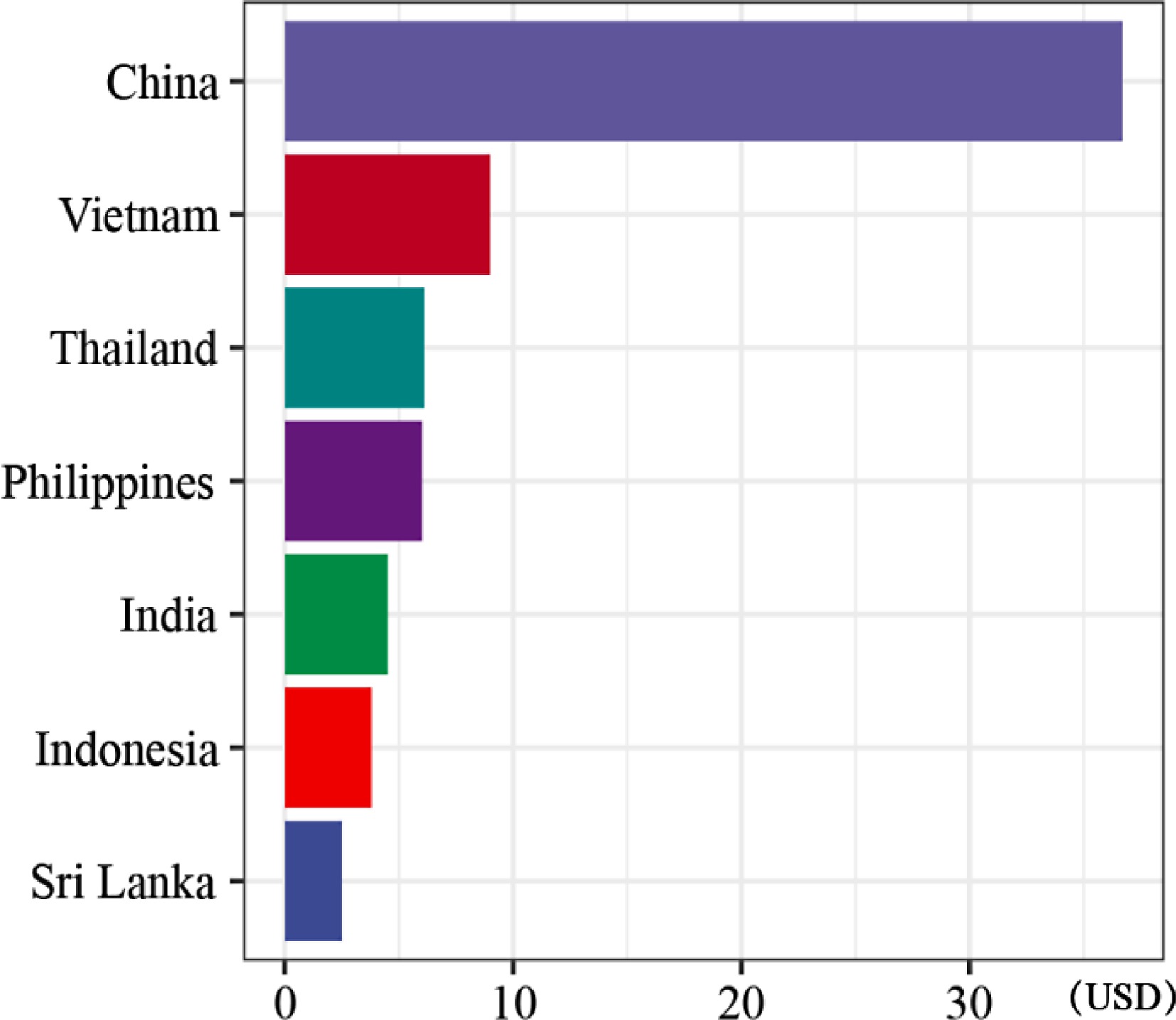
The explosive growth of the coconut market in China became one of the hot topics of news in 2023. The skyrocketing prices of coconut water catalyzed the booming Chinese coconut market[85]. Previously relegated to a processing byproduct, coconut water surged through China's summer beverage market, achieving explosive popularity and transitioning from obscurity to a coveted blockbuster[112]. After coconut water became a hit in the European and American markets, some coconut brands tried to enter the Chinese market, but all failed[113]. Consumers, increasingly attentive to ingredient provenance and natural attributes embraced coconut water's inherent goodness: its refreshing taste, natural appeal, and rich vitamins and potassium content, solidifying its image as a health beverage leader[114]. A remarkable 92.5% (37 out of 40) of China's top new beverage brands incorporated coconut-based drinks into their portfolios, which led to a staggering 66% year-on-year increase in the number of coconut-themed beverage stores in 2022[115]. Beyond coconut water, the coconut's versatility thrives with coconut milk, dense coconut cream, powdered forms, functional blends, and coconut-infused fruit and vegetable juices, solidifying its dominance as a top ingredient in 40 Chinese freshly made beverage brands, according to the Report on Beverage Industry of China 2022[115]. Because macapuno is not legally imported from China, only a small amount of macapuno is currently circulating in the Chinese market, which leads to a very high price in China, as shown in Fig. 5. This rapid proliferation underscores the immense potential of coconut within China's dynamic beverage market and suggests a sustained consumer appetite for its health and taste attributes[116].
Challenges and resolute solutions of the makapuno industry of China
-
The main challenges facing the development of the makapuno industry in China are twofold: technological obstacle (including embryo culture and cultivation techniques) and sourcing of germplasm resources. It has been demonstrated that embryo culture is the cornerstone coconut tissue culture technique due to its many applications (propagation, conservation, and genetic transformation). In China, this technology has already been successfully applied by the Chinese Academy of Tropical Agricultural Sciences (CATAS)[117] and Hainan University[89]. Although the technique has been widely documented, embryo culture is being underused for germplasm movement and conservation. In addition, it has been found that most protocols have room for improvement. Key enhancements which are needed include ensuring consistently low contamination rates and high survival rates for a wide range of genotypes, in several laboratories. Protocols for in vitro steps during coconut seedling production using embryo culture technique have been established successfully with a high success rate for the conversion of embryos to plantlets. Losses are still particularly high during acclimatization steps and when plants are transferred from tissue culture facilities into field conditions. The ex-vitro steps especially during the transfer of in vitro plantlets into the nursery remain a major bottleneck for most laboratories[76,118,119].
The rooting step is the most crucial among these processes, bearing significant importance for the survival of embryo-cultured seedlings[87]. Several approaches have been applied to improve in vitro rooting process such as increasing sucrose content in the medium[120], exposure for plant growth regulators especially IBA[121], adding polyethylene glycol into culture medium[122] or by removing haustorium during culture process[123]. However, the success rate during in vitro rooting remains low. Moreover, several protocols have also been developed during the acclimatization process, i.e. increasing humidity around the seedlings by using a plastic bag cover or humidity tent[41], using a wooden box chamber[122] or increasing the photosynthetic level by pumping carbon dioxide into the box chamber in a photoautotrophic system[76]. However, the success rate after acclimatization during those approaches remains low. Ex vitro rooting, a more efficient protocol by smart combining of in vitro rooting and acclimatization, then showed a better result on the conversion of in vitro plantlets into the nursery[43]. The last method is quite simple, seedlings with two or three opened leaves (about 6–8 months old) were taken from an in vitro system, washed gently with tap water then dipped into 2% fungicide for 20 min, then planted into small plastic pots containing a combined cocopeat and rice charcoal medium. The pots were then put into a glass box (70 cm × 40 cm × 40 cm), flooded with 12 L macro- and micro-nutrient liquid medium added with 1 μM IBA then closed the lid tightly for about a month. To maintain the air exchange and relative humidity inside the box, the medium was aerated using an air pump. A month later the lid was opened gradually every week to decrease the relative humidity until fully opened in the following months. The seedlings were kept in the glass box for 3 months before transferring the seedlings into bigger pots and growing in the nursery for another 6 months. This method was efficient in producing kopyor seedlings to the field (90% success rate), with less contamination risk and lower labor cost[43]. This method has been successfully applied in several coconut in vitro laboratories in Indonesia, but its application in other countries may not result in the same success rate of ex vitro transplanted seedlings. Several climate factors such as humidity and light intensity and also the vigor of plantlets produced from in vitro steps have to be considered aside from this ex-vitro rooting protocol.
Coconut biotechnology, such as embryo culture, is anticipated to offer significant assistance in the identification and development of novel coconut genotypes like makapuno[124]. Further refinement is necessary to fully utilize the potential of embryo culture, innovative approaches like marker-assisted breeding should be explored. The recent increase in genetic information for marker gene identification enables more efficient molecular screening and identification of candidate cultivars compared to the past[125]. Hybridization of recognized coconut varieties is currently underway to enhance fruit production and harvest index. In addition to increasing the production of improved planting materials, meticulous quality control measures for products are warranted. The limitation of market reach due to the shelf life of these fruit types remains a challenge, impeding the full realization of their market potential. Enhancements in pertinent food processing techniques will further facilitate the access of these uniquely captivating coconut varieties to markets beyond tropical regions[126].
Reports on makapuno from China are scarce, thus China is considered to be severely lacking in makapuno germplasm resources. Thus, major coconut producers near China may be strong competitors in the makapuno industry, for instance, Thailand, the Philippines, Vietnam, and Indonesia[127]. The coconut market in China also faces competition from imported products from other countries, which may exert pressure on domestic producers. Finally, with increasing attention to sustainable development and environmental protection, the coconut industry in China may need to address challenges related to land use, water resource management, and ecological conservation. In addition, climate conditions that coconuts typically thrive in tropical or subtropical regions, and certain parts of China may not have sufficiently suitable climates, limiting the range for coconut cultivation and distribution. Hainan island is the southmost province in China, and it accounts for 99% of coconut cropping area in China[128]. However, only 42.5% land area of this island are tropical and subtropical land. The population of the province is predominantly rural, at around 68.5% of the total population. The annual average temperature is recorded as 23−25 °C, with mean annual rainfall of above 1,600 mm[129]. Coconuts require specific types of soil and land conditions for healthy growth, and some areas in China may lack these conditions. Furthermore, yield and quality control must be considered. Coconut cultivation in China may be affected by factors such as farming techniques, management practices, and quality control measures, potentially impacting market competitiveness and product quality.
As with other plantation crops, it is recommended to use good agriculture practices (GAP) to enhance the yield and quality of makapuno. GAP on makapuno (kopyor) farming mostly include site selection and management, sourcing and selecting of plant materials, farm establishment, farm maintenance, harvesting and post-harvest handling, and waste management. Makapuno farming should be in areas suitable for food production and market. For optimum growth, it is also preferable that makapuno farming is better within an altitude of not more than 600 m above sea level. The types of makapuno varieties to be grown should also be selected based on the market requirements, adaptability to local conditions, and farmer preferences. Several makapuno varieties (e.g. kopyor) have been known as water-sensitive varieties while other varieties have been known as fertilizer-sensitive varieties. Makapuno market has its own requirements. Indeed, the selection of the correct variety with superior quality become key factors for the success of makapuno farming. As makapuno has been known as a recessive trait, the use of true-to-type makapuno seedlings coming from embryo culture or other vegetative tissue culture approaches as planting materials becomes the main key for the success of makapuno farming. Mother palms used for embryo culture should be selected from the homogenous bearing kopyor areas with average of at least 10 nuts per bunch for the tall varieties and 15 nuts per bunch for the dwarf varieties.
Farm establishment is another important factor for optimum growth for makapuno, including land preparation, proper field layout and planting of either square or triangular systems with recommended distance between palms being about 8 to 10 m. Farm maintenance including soil conservation such as minimum tillage, contour planting, cover cropping, fertilizer management, and water management are important growth factors. The use of fertilizer materials at different stages of growth such as nitrogen, phosphorus, and potassium are highly recommended for optimum growth and production of nuts. In some areas of Indonesia, Oryctes rhinoceros and red weevil (Rhincoporus) remain the main insect pest for makapuno farming. Integrated pest management including various strategies to control insect pests, mites, pathogens, and rodents are also important in maintaining makapuno farming. Makapuno fruits should be harvested at a certain time, mostly 10−11 months after fertilization. During this time, the solid endosperm is at the optimum stage, the taste is also fresh. The younger fruits will give a better taste, but less solid endosperm can be harvested, meanwhile the older fruits will give non-consumable endosperm. Similar results will also be found for longer fruit storage, more than 3 weeks storage. Furthermore, time of harvesting and postharvest handling are also important to enhance the quality of makapuno fruits. As the expertise of the tissue culture practitioner influences the success of embryo culture, the key to further coconut embryo culture development and widespread adoption is likely to involve the adequate training and perpetual support of genebank staff in the use of the technique[68]. These valuable experiences from coconut-producing countries will have a positive impact on the localization of makapuno in China.
Furthermore, as sequencing technology has advanced, various omics techniques have emerged, enabling scientists to delve deeply into the genetic foundations and regulatory mechanisms behind unique plant phenotypes. In the case of makpunuo, which displays distinct characteristics from regular coconuts, employing a range of omics approaches such as genomics, metabolomics, transcriptomics, variant omics, epigenomics, etc., can reveal the metabolite composition and levels in makpunuo's special coconut meat and water. This analysis can also pinpoint the genes responsible for these compounds and identify crucial mutation sites. Subsequently, the development of molecular markers linked to beneficial allelic variations will facilitate the cultivation of more flavorful and favorable makpunuo coconuts, supported by data and theoretical insights.
-
Makapuno coconut stands out as one of the most economically valuable coconut varieties globally. Currently, this special type of coconut has great popularity worldwide. Amidst China's rapid economic development, there exists a growing demand for high-end coconut varieties like makapuno. Despite its significance, the makapuno industry in China has yet to gain substantial traction. Leveraging biotechnological methods such as embryo culture presents a promising avenue to establish a thriving makapuno coconut industry in China. By harnessing the potential of biotechnology (embryo culture, cryopreservation, and micropropagation), we can overcome existing challenges and capitalize on the opportunities presented by the burgeoning market for elite coconut varieties. With concerted efforts and strategic investments, China can position itself as a significant player in the makapuno coconut industry, contributing to both its economic growth and agricultural diversification.
-
The authors confirm contribution to the paper as follows: study designing and writing: Mu Z, Yang Z, Xu H (contributed equally); draft manuscript preparation: Khongmaluan M, Tran B, Vidhanaarachchi V, Sisunandar S; figure and table modification: Xu H, Yang Z, Xia W, Yang S; manuscript review and editing: Xia W, Luo J, Mu Z. All authors reviewed and approved the final version of the manuscript.
-
All data used in this review paper were derived from the Hainan University institutional repository, the Kasetsart University institutional repository, the Coconut Research Institute of Sri Lanka institutional repository, the University of Muhammadiyah Purwokerto institutional repository and the Vietnam National University of Ho Chi Minh City institutional repository. All data were freely available and accessible without restrictions.
This research was supported by Hainan Seed Industry Laboratory and sponsored by the Hainan Province Science, Hainan Yazhou Bay Seed Lab. (JBGS + B21HJ0903), Technology and Innovation Project for Talent (KJRC2023L09 and KJRC2023D16), Postdoctoral Research Funding Program of Hainan Province (No. B23B10015), Science and Technology Innovation Special Project of Sanya City (2022KJCX53), PhD Scientific Research and Innovation Foundation of Sanya Yazhou Bay Science and Technology City (HSPHDSRF-2023-12-002) and '111' Project (No. D20024).
-
The authors declare that they have no conflict of interest. Jie Luo and Siwaret Arikit are the Editorial Board members of Tropical Plants who were blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of these Editorial Board members and the research groups.
-
Received 6 May 2024; Accepted 1 July 2024; Published online 4 September 2024
-
# Authors contributed equally: Zhihua Mu, Zhuang Yang, Hang Xu
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mu Z, Yang Z, Xu H, Khongmaluan M, Arikit S, et al. 2024. Prospects and challenges of elite coconut varieties in China: a case study of makapuno. Tropical Plants 3: e029 doi: 10.48130/tp-0024-0028
Prospects and challenges of elite coconut varieties in China: a case study of makapuno
- Received: 06 May 2024
- Revised: 24 June 2024
- Accepted: 01 July 2024
- Published online: 04 September 2024
Abstract: Makapuno, one of the rarest and economically valuable coconut, boasting superior qualities like soft, jelly-like endosperm, creamy texture, and special flavor, presents significant opportunities for the Chinese market. Through a comprehensive analysis of available evidence in coconut-producing countries, this study evaluates the potential for makapuno cultivation in China, considering factors such as the application of embryo culture techniques, economic acceptance, market demand, and agricultural and climate suitability. Furthermore, this paper identifies the key challenges hindering the widespread adoption of biotechnologies involved in makapuno cultivation, including embryo culture, acclimatization of in vitro seedlings, limited germplasm resources, inadequate technical expertise, and regulatory constraints. By leveraging makapuno as a case study, this paper provides insights into the broader issues surrounding the development of elite coconut varieties in China. It offers recommendations for researchers, policymakers, and industry stakeholders to overcome these challenges and unlock the full potential of makapuno cultivation in the country.