HTML
-
Mycology is an imperative area that has exhibited various applications in medicine, agriculture, and environment. Natural product development has regained interest due to low production costs, structural diversity, and several uses of therapeutic compounds to treat various diseases. Medicinal plants have been used for generations to treat many diseases (Alvin et al. 2014). In current cancer therapy, there is high predominance of resistance and side effects of anticancer compounds (El-hawary et al. 2020). Endophytic fungi are interesting source of bioactive metabolites. They are able to produce similar metabolites to those produced by their host plant (Daenen et al. 2014, Ratnaweera et al. 2015, Roy 2017, Omeje et al. 2017, Deshmukh et al. 2018). Endophytes are the life forms inhabiting inside plant tissues without damage their hosts and may play critical roles in protection and development advancement (Alvin et al. 2014). Endophytes are an exceptionally common and diverse group of microorganisms that live inside tissues of the host without causing noticeable disease to the plant (Borges et al. 2009). Both fungi and bacteria are the most common microorganisms existing as endophytes, but endophytic fungi produces huge amount of secondary metabolites with medicinal properties (Staniek et al. 2008).
There are two broad groups of endophytic fungi, C-endophytes (clavicipitaceous) and NC-endophytes (non clavicipitaceous). Clavicipitaceous endophytes found in some genera of grasses and they are transmitted by vertical transfer of fungi on to offspring via infections to seed (Dias et al. 2012). Non-clavicipitaceous endophytic fungi are found in asymptomatic tissues of cryptogamic plants, conifers and most of the angiosperms (Rodriguez et al. 2009, Mishra et al. 2014). Capacity to produce useful anticancer compound by these fungi appears to depend on the host species, host genotype and environmental conditions (Saikkonen et al. 1999, Nazir & Rahman 2018).
The effect of climate on endophytic fungi population and studied the temporal changes in relative frequency of total endophytic fungi. They also stated that there is a great diversity of a population of endophytic fungi among plant kingdom. Even in same population of plant, it shows diversity according to geography and environment (Chareprasert et al. 2006, Yan et al. 2019).
The host plant protects endophytes and provides nutrients and in turn, these endophytes help plant in their growth and also produce secondary metabolites which help the plant defense against plant pathogens (Tan & Zou 2001).
The metabolic communications of endophytes with its host may support the combination of organically dynamic auxiliary metabolites. The metabolites paeonol from Chaetomium spp., citrinin from Penicillium citrinum, taxol from Taxomyces spp., piperine from Periconia spp. and many more shows bioactivity (Varma et al. 2011, Lakshmi & Selvi 2013, Alvin et al. 2014, Li et al. 2015). The host plant is being benefited by their endophytes by increasing their immunity power and by enhancing secondary metabolites production which is useful in various industries (Strobel et al. 2004, Hyde et al. 2019).
In this review, we provide a general overview on isolation, identification and cultivation strategies of endophytic fungi having anticancer potential. Herein, we discuss the significance of bioactivity and anticancer agents from endophytic fungi.
-
It has been reported that secondary metabolites of plant origin have widely used as traditional source of medically potential drugs (Strobel et al. 2004). There is a decline of interest in recent implementation in drug development. In addition, the challenges of drug resistance require very drastic drug development approaches (Cragg & Newman 2005).
In early phases of civilization, the man tried plants for their food and ethnomedicinal purpose. The current research approaches have responsible for the discovery of plant compounds having bioactivity with respect to their use in medications and treatments (Mishra & Tiwari 2011). The majority of the natural bioactive compounds anticipate disclosure with the test being the manner by which to get to this characteristic compound decent variety (Rey-Ladino et al. 2011).
The main source of bioactive natural plant products has always depended heavily upon whole plant tissues such as roots, leaves, stem, fruit, and rhizome (Dias et al. 2012). Unfortunately, use of the whole plant has serious challenges and this leads to a destruction of the whole plant over time as these plant parts are repeatedly collected without replenishment, and most of the times these valuable species become endangered and are even lost in near future. This leads to degradation of the environment such as land deterioration, ecological corruption and the typically limited yield of bioactive lead compounds (Strobel & Daisy 2003). The utilization of entire plant tissues for separation of the bioactive characteristic compound has nearly seen extraordinary wastage of research assets because of dereplication. Dereplication is the procedure of fastly identifying the known biomolecules, which results in a decrease of enthusiasm for research and is time and assets devouring (Omeje et al. 2017).
It appeared that, from last few decades, drug discovery for novel biomolecules has refocused and centered on the microorganisms that inhabit these specific host plants (Hodkinson et al. 2019). Of these living beings, endophytes were immediately perceived as veritable wellsprings of novel bioactive metabolites. Endophytes developing in different aberrant conditions have remarkable source of novel compounds possessing the natural abilities. In all these, the endophytic communities have been appeared to be astoundingly helpful in the medication revelation process. An escalated writing review uncovered that endophytic populaces of the plants in rainforests of the greater part of the mainlands have not been altogether investigated. There is a need to awaken and boost research interest in the use of these endophytic fungi for the discovery of potent bioactive molecules against the ever-increasing global disease burden (Omeje et al. 2017, Saha et al. 2019).
Also, given the quickly expanding worldwide populace and the going with interest for medications, it is fundamentally vital to recognize and create inexhaustible wellsprings of pharmaceuticals and their forerunners (Strobel & Daisy 2003, Mishra & Tiwari 2011).
-
In a period of nearly two decades, the available data reveals that more than 40% of novel bioactive compounds obtained, out of which half were derived from microorganisms (Porras-Alfaro & Bayman 2011). Moreover, over 60% of the anticancer and 70% of the antimicrobial medications right now in clinical use are herbal products or its characteristic product subsidiaries (Omeje et al. 2017). The extraordinary microbial decent diversity with energizing metabolic buildings in plant tissues has been set up over the most recent two decades and is proceeding (Porras-Alfaro & Bayman 2011). About 10 years back, the scan for novel auxiliary metabolites ought to be refocused and examine endeavours thusly fixated on the organisms that occupy one of the kind biotopes (Mishra & Tiwari 2011). Of these living beings, endophytes were perceived as assorted wellsprings of bioactive metabolites on the grounds that the majority of them involving actually a huge number of one of a kind higher plants developing in a differing strange condition. Renewability, ready availability and environmental friendliness are very interesting features of the endophytic fungi which are probable sources of biologically active natural products. It is realized that these endophytic fungi are profound pull in a plant for a critical piece of their life cycle and, they cannot create ailments in the host. This quality makes this class of microorganisms an extraordinary asset base for the disclosure and advancement of powerful anticancer particles without collecting the entire plant and its tissues (Bacon & White 2000, Souvik et al. 2012, Omeje et al. 2017).
It was recently believed that metabolic compounds are exchanged between the host plant and the endophyte by the hypothesis of flat exchange (horizontal transfer) from the host plant to its microbial symbiont (Stierle at al. 1993, Strobel 2006, Puri et al. 2006, Kusari et al. 2009b, Omeje et al. 2017). This conviction has been invalidated after the effective sequencing of the taxadiene synthase quality from the taxol creating endophyte which set up that the metabolic pathways of both the hosts and the endophytes are autonomous of one another. The ramifications of this are there are substantial open doors accessible for the control of the endophyte biosynthetic pathways to yield wide assortments of metabolites and a stage for medication disclosure process (Staniek et al. 2008, Omeje et al. 2017). As indicated by fossilized tissues of plant and related microorganisms has uncovered that endophyte-plant communications may have advanced from when higher plants initially showed up on the earth surface. De-Bary first proposed the term 'endophyte' that being of fungus or bacteria in the inner tissues of healthy plants, which are capable of living and colonizing without causing noticeable symptoms of diseases (De Bary 1866, El-hawary et al. 2020).
Since the discovery of endophytes, they have been isolated from a variety of organs from diverse plant families ranging from bryophytes, pteridophytes, gymnosperms, and angiosperms from various ecosystems. The fungi, bacteria, and actinomycetes have shown the most frequent occurrence (Arnold 2007, Li et al. 2008). It was known that the endophytic fungi were existed in the plant organs (Redecker et al. 2000). Endophytic fungi are the individual from an exceptionally differing polyphyletic group of microorganisms; they can develop asymptomatically in the plant tissues over the ground and subterranean, including stems, leaves as well as roots (Selim et al. 2012). Numerous endophytes can integrate an assorted cluster of metabolites and some of them utilized as remedial specialists against different human illnesses (Debbab et al. 2009, Aly et al. 2010, Khawar et al. 2011). Paclitaxel and podophyllotoxin are some examples of occasionally discovered endophytes derived plant secondary metabolites having therapeutic potential (Stierle et al. 1993, Puri et al. 2006).
Bioactive compound production by endophytic fungi without their host plant is important from a biochemical, ecological and molecular perspective. Direct use of medicinal plants has a common problem of dereplication. To avoid such problem, there is the necessity to exploit endophytic fungi for production of a variety of novel biologically active secondary metabolites (Omeje et al. 2017).
The endophytic fungi capacity is further limited by the presence of unknown biosynthetic pathways that remain unexpressed under in vitro conditions (Hertweck 2009). Be that as it may, distinctive strategies worried to the development and control of endophytic fungi, for example, co-culture, synthetic enlistment, epigenetic tweak, and media designing, metabolite redesigning, bioprocess innovation for scale-up, make them appropriate for the generation of known and novel bioactive compounds (El-Amrani et al. 2012).
The terrestrial ecosystems have more biodiversity in tropical and temperate rainforests (Forseth 2010). The endemic plant species have specific endophytes that may have evolved simultaneously with each other. The active species with variety of metabolites survived the most in evolutionary race due to constant chemical rearrangements in ecosystems. Tropics are the great source of novel metabolites due to high competition, limited resources and heavy selection pressure (Redell & Gordon 2000, Omeje et al. 2017).
-
Proper information is required for selection of plants for the study of endophytic fungi while searching novel bioactive compounds. Since the biochemical and metabolic pathways of both endophytic fungi and host plant are strongly correlated, it is convenient to use medicinally important plants for the bioprospecting of endophytic fungi for novel secondary metabolites having therapeutic potential.
For the isolation of endophytic fungi, there is a need to collect asymptomatic tissues from the plant such as fruit, leaves, root, secondary branches and stem in sterile plastic bags and stored at 4 ℃ temperature to ensure the integrity of the plant part (Dastogeer et al. 2020). Throughout the regular process of endophytic fungi isolation, there is a need to maintain total aseptic conditions for avoiding contaminations in the culture. The accompanying techniques are then pursued:
1. Collect an external part of host plant and wash thoroughly under running tap water for approximately 10 min to remove adhering soil particles and debris, then dry on a sterile filter paper towel.
2. Use a sterilized scalpel or hole puncher to excise pieces of the leaves and sterilize further using 95% ethanol (30 sec.), 2% sodium hypochlorite (NaOCl) (2-3 min), 70% ethanol (2 min.).
3. Wash the sterilized samples 2-3 times in sterile distilled water and allow the plant material to dry up between the folds of sterile filter papers under aseptic conditions. In practice, the isolates can be thought to be endophytic fungi when add up to surface sterilization is affirmed, i.e. no contagious development from engraving the surface sterilized plant tissues onto supplement media or refined aliquot of water from the last flushing onto supplement media.
4. Subsequently, inoculate the samples on the suitable culture media (potato dextrose agar) supplemented with an antibiotic (100-500 mg/l) to prevent the growth of unwanted bacteria (Omeje et al. 2017). Use a flame sterilized hole puncher to excise circular leaf discs from each leaf. Dice fruit pieces with a sterile scalpel to expose fresh tissue surfaces. The plating and inoculation are usually done inside laminar air flow to maintain aseptic conditions throughout the process (Sanders 2012).
5. Incubate the plates at room temperature (30-37 ℃). Subsequently, transfer the fungal mycelium growing out from leaf discs to fresh PDA/MEA plates by hyphal tip transfers and incubate further at room temperature for 1-2 weeks (Omeje et al. 2017).
6. Check the purity of isolated endophytic fungi and study their morphological and cultural characteristics. Maintain the endophytic fungal isolates in PDA/MEA slant for future studies.
-
Naturally visible and minuscule attributes assume an extremely significant job in the recognizable proof of separated endophytic fungal isolates. There are a few taxonomical order guides accessible, for example, found in these references (Ainsworth et al. 1973, Ellis 1976, Von Arx 1978, Barnet & Hunter 1998). Culture-subordinate strategies have been frequently utilized in the investigation of the decent variety of endophytic fungi as opposed to coordinate perception techniques. The investigation of endophytic fungi is a technique subordinate process. Some standard procedures like surface sanitization methods, incubation periods and sporulation limit of endophytic fungi apply their immediate effect on confinement of endophytes from the host plant. In this manner, culture seclusion strategy has a few constraints, for example, (1) It rather worksome and tedious and is improper to look at extensive quantities of samples and tests; (2) The expansive number of sterile endophytes represents an extraordinary issue, since they cannot be distinguished to any ordered classification, while different strategies have been utilized to advance sporulation of isolates so as to beat the inadequacies of some endophytes unfit to sporulate in culture (Guo et al. 1998, Taylor et al. 1999, Guo et al. 2000, 2008); (3) Some fungi might be missed because of inability to develop or some develop gradually and are effortlessly outperformed by quickly developing species in counterfeit conditions. So as to defeat the potential specialized predisposition, development autonomous methodologies, e.g., molecular techniques, to analyze endophytic fungal networks of plants are required.
Molecular methodologies have been effectively utilized in the recognition and distinguishing proof of endophytic fungi in leaves, establishes and mycorrhizal growths in roots and soil (Clapp et al. 1995, Chelius & Triplett 1999, Tedersoo et al. 2008). The obstacle in the standard culturing strategies has been overcoming by use of molecular procedures like DNA sequencing and fingerprinting techniques and so forth (Fig. 1).
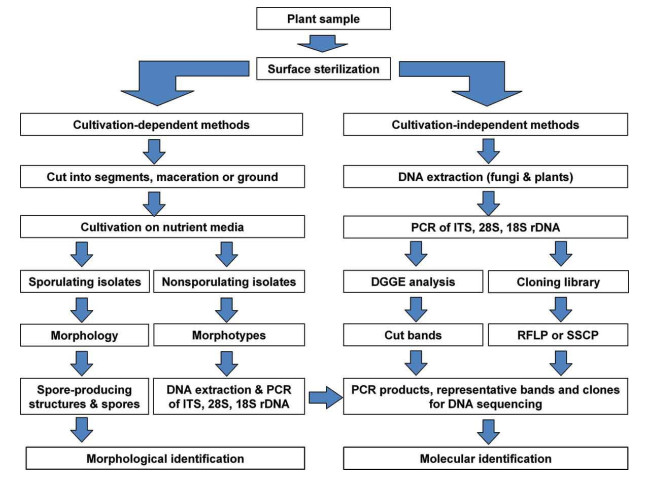
Figure 1. A schematic flowchart for isolation and identification of endophytic fungi (Sun & Guo 2012).
The newfound, high throughput sequencing (pyro-sequencing) empowers metagenomic and metagenetic investigations and gives a ground-breaking elective apparatus to molecular investigations of the fungal network in common habitats. Pyrosequencing is fast, generally modest, with a free-cloning step and high product yield, which accomplishes an around 100-overlap increment in throughput over Sanger sequencing (Margulies et al. 2005). DNA barcoding methods give a short, compelling and standardized gene region to distinguish endophytic fungi at an explicit dimension. The DNA barcode sequence region utilized ought to be a solitary locus for all gatherings of life forms in kingdom fungi.
The significantly preferred standpoint of sequence-based characterization is that it is most helpful when having plant tissues are contaminated with pathogens since specific primers are utilized for amplifying the target gene in the nearness of high DNA background of the host plant (Table 1). On another side, SSU and LSU sequences frequently don't give adequate resolution to the classification of subgeneric taxa, and genomic areas of higher diversity were scanned for. In the interim, the internal transcribed spacer (ITS) region, and to a lesser degree the IGS (inter-genic spacer) region, these two noncoding components of the nuclear rDNA have turned into the most favored sequences for concentrates on the lower ranking levels (Bachmann 1994, Schurko et al. 2003, Wattier et al. 2003).
Table 1. List of molecular techniques and their potential applications in identification of endophytic fungi
Sr. No. Technique Taxonomic level Field of application 1. Amplified fragment length polymorphism (AFLP) Species to subspecies Classification 2. Random amplification of polymorphic DNA (RAPD) Species to subspecies Classification 3. Inter simple sequence repeats (iSSRs) Species to subspecies Classification 4. Isozyme analysis Subspecies Classification 5. Single nucleotide polymorphisms (SNPs) Species to subspecies Phylogeny, classification and population studies 6. DNA barcoding Species Classification 7. Cyclooxygenase 2 (COX2) sequencing Kingdom to species Phylogeny and classification 8. Large subunit (LSU) rDNA sequencing Class to species Phylogeny 9. Internal transcribed spacer (ITS) sequencing Family to species Phylogeny and classification
-
Culture media have experienced numerous changes since its origin. Culture media are of various kinds, contingent upon the wholesome development prerequisites of the microorganisms. Microorganisms require around 10 macronutrients, for example, C, O, H, N, S, P, K, Ca, Mg and Fe (Merchant & Helmann 2012). The initial six components are utilized in the combination of Carbohydrates, Lipids, Proteins, and Nucleic acids and the staying four exist in the cell as cations and assume several of roles. Notwithstanding macronutrients, all microorganisms require a few micronutrients like Mn, Zn, Co, Mo, Ni, and Cu (Merchant & Helmann 2012). These are by and the large piece of catalysts and cofactors. Microorganisms likewise require development factors, which are natural mixes.
High sugar source, the nitrogen source is required in media for the development of fungi at pH 5-6, and a temperature from 15-37 ℃ (Basu et al. 2015). There are two general sorts of fungal culture media: natural and artificial. Natural media comprise of regular biological substrates, for example, herbaceous or woody plant parts, seeds, leaves, corn supper, wheat germ, and oats (Collins et al. 2005). Natural media are normally simple to get ready yet their obscure content is a noteworthy drawback. A few models incorporate corn meal agar, potato dextrose agar, V-8 juice agar, and dung agar. Artificial media has elements of the known contents. These sorts of media can be copied with exactness each time they are made and contain characterized amount of starches, nitrogen, and nutrient sources (Basu et al. 2015).
The mass culture of the endophytic fungi is carried out using selective/enriched media and then inoculated into a 500 ml or larger Erlenmeyer flasks containing suitable nutrients. Then will be incubated at room temperature for 20-30 days on a rotary shaker. The broth is then filtered and extracted with a suitable solvent like chloroform, ethyl acetate, petroleum ether (Song et al. 2019). The extract then dried over anhydrous sodium sulfate and then evaporated in a rotary evaporator under vacuum, to get ethyl acetate extracts. The dried extract will be purified using several solvent fractions and recovered for future biological activity. Further, the fractions are subjected to various chromatographical techniques such as LCMS and GCMS and purifications steps to isolate pure biomolecules. Then such bioactive molecules are tested and characterized using physicochemical and spectroscopic methods (Omeje et al. 2017).
So as to investigate the uncommon shrouded possibilities of the endophytic fungi as bona fide vault for anticancer compounds, tranquilize revelation approach should target 1) the choice and seclusion of samples from various of environments, 2) by controlling physiology of microorganisms to improve microbial natural metabolite biosynthetic pathways and 3) by hereditary change of strains for generation of manufactured microbial compounds. By exploiting these three methodologies, the yield of an extract can be enhanced, and thereby the chances of finding the novel bioactive compounds can be expanded (Omeje et al. 2017).
-
Cancer is a group of diseases that can harmony organ in the human body and shows uncontrolled growth of malignant cells and invasion into normal tissue. Such malignant cancer cells can travel to other body parts and produce new tumors ultimately leads to death and as such, the discovery for novel anticancer agents remains endless. This hunt has, in these advanced occasions, moved to the endophytic fungi. Novel secondary metabolites are obtained from endophytes which make them suitable therapeutic complex biochemical stage in their host plants (Tan & Zou 2001, Owen & Hundley 2004, Omeje et al. 2017).
At present endophytic fungi are considered the most promising sources of natural antitumor drugs owing to their wide distribution and diversity among all organisms (Omeje et al. 2017). Additionally, microorganisms that can survive in unusual environments synthesize bioactive compounds of potential therapeutic applications (Mushtaq et al. 2018). The greater interest has been developed from the past few decades to exploit the rich biochemical diversity possessed by the endophytes (Table 2). Since the revelation of taxol from a fungal endophyte, endeavors have relied upon the control and enhancement of the way of culture conditions and this methodology has created a few bioactive and novel drugs (Strobel et al. 2004).
Table 2. List of anticancer compounds isolated from fungal endophytes
Sr. No. Fungal Endophyte Host Plant Anticancer Compound References 1. Acremonium spp. Knema laurina Brefeldin A Chinworrungsee et al. (2008) Taxus baccata Leucinostatin Strobel & Hess (1997) 2. Alternaria spp. Polygonum senegalense Alternariol Aly et al. (2008) Alternariol 5-O-sulfate Alternariol 5-O-methyl ether Altenusin Desmethylaltenusin Clerodendrum phlomidis Squalene Kumaradevan et al. (2015) 3. Arthirnium arundinis violacea Polysiphonia Epiepoxydon Klemke et al. (2004) 4. Aspergillus clavatus Taxus mairei Brefeldin Wang et al. (2002) 5. Aspergillus fumigatus Cynodon dactylon 9-Deacetoxyfumigaclavine Ge et al. (2009) 6. Aspergillus brasiliensis Cynodon dactylon Rubrofusarin Song et al. (2004) Tabebuia argentea Lapachol Channabasava & Govindappa (2014) 7. Aspergillus Sequoia Sequoiatones Stierle et al. (1999) parasiticus sempervirens Sequoiamonascin Stierle et al. (2003) 8. Aspergillus terreus aspera Achyranthus Terrein Goutam et al. (2017) 9. Bartalinia robillardoides Aegle marmelos Paclitaxel Gangadevi & Muthumary (2009) 10. Cercospora spp. Fallopia japonica Cercosporene F Feng et al. (2014) 11. Chaetomium globosum Polysiphonia urceolata Chaetopyranin Wang et al. (2006) Imperata cylindrica Chaetoglobosin Ding et al. (2006) Ulva pertusa Cytoglobosin Cui et al. (2010) Ephedra fasciculata Globosumone Bashyal et al. (2005) Curcuma wenyujin Chaetoglobosin X Wang et al. (2012) 12. Floropilus chiversii Ephedra fasciculata Radicicol Turbyville et al. (2006) 13. Entrophospora infrequens Nothapodytes foetida Camptothecin Puri et al. (2005) 14. Eupenicillium spp. Xanthium sibiricum Eupenicillinol C, Eupenicillinol D Li & Kusari (2017) 15. Eutypella spp. Etlingera littoralis Eutypellin Isaka et al. (2009) 16. Fusarium nematophilum Camptotheca acuminata Camptothecin Su et al. (2014) 17. Fusarium oxysporum Catharanthus roseus Vincristine Yang et al. (2004) Ephedra fasciculata Beauvericin Zhan et al. (2007) Cylindropuntia echinocarpus Bikaverin 18. Fusarium solani Camptotheca acuminata Camptothecin, 9-Methoxycamptothecin Kusari et al. (2009b), Ran et al. (2017) Podophyllum hexandrum Podophyllotoxin Nadeem et al. (2012) 19. Annulohypoxylon truncatum Artemisia annua Daldinone Gu et al. (2007) 20. Lasiodiplodia theobromae Morinda citrifolia Taxol Pandi et al. (2011) 21. Neurospora crassa Camptotheca acuminata Camptothecin Rehman et al. (2008) 22. Pestalotiopsis fici Camellia sinensis Pestaloficiol Ling et al. (2009) 23. Penicillium dodgei Pinellia ternata Dehydropaxilline Gao et al. (2017) 24. Penicillium spp. Aegiceras corniculatum Leptosphaerone Penicillenone Lin et al. (2008) Annona squamosa Meleargine, Chrysogine Yunianto et al. (2014) Tabebuia argentea Lapachol Channabasava & Govindappa (2014) 25. Pestalotiopsis microspora Torreya taxifolia Torreyanic acid Lee et al. (1996) Taxus wallichiana Paclitaxel Stierle et al. (1993) Artocarpus heterophyllus Hydroxypestalotin Riga et al. (2019) 26. Pestalotia photiniae Roystonea regia Photinides Ding et al. (2009) 27. Pestalotiopsis spp. Rhizophora mucronata Pestalotiopsone Xu et al. (2009) 28. Pestalotiopsis terminaliae Terminalia arjuna Paclitaxel Gangadevi & Muthumary (2008) 29. Phialocephala fortinii Podophyllum peltatum Podophyllotoxin Eyberger et al. (2006) 30. Phomopsis longicolla Dicerandra frutescens Dicerandrol Wagenaar & Clardy (2001) 31. Phomopsis spp. Musa acuminata Oblongolide Taridaporn et al. (2010) Tectona grandis Phomoxanthone Isaka et al. (2001) 32. Phyllosticta spinarum Platycladus orientalis Tauranin Wijeratne et al. (2008) 33. Rhinocladiella spp. Tripterygium wilfordii Cytochalasin Lee (1995) 34. Rosellinia sancta-cruciana Albizia Jammosporin A lebbeck Sharma et al. (2018) 35. Stemphylium globuliferum Mentha pulegium Alterporriol Debbab et al. (2009) 36. Teratosphaeria spp. Pinus clausa Teratosphaerone A, Monosporascone Padumadasa et al. (2018) 37. Canariomyces subthermophilus Hypericum perforatum Hypericin, Emodin Kusari et al. (2009a) 38. Trametes hirsuta Podophyllum hexandrum Podophyllotoxin Puri et al. (2006) The in vitro anticancer activity of compound terrein (4, 5 dihydroxy 3 (1 propenyl) 2 cyclopenten 1 one) was evaluated against human lung cancer cell line (A 549) with the IC50 value of 121.9 ± 4.821 μgml-1 (Goutam et al. 2017). The in vitro cytotoxicity assay of chaetoglobosin X was effective against MFC (gastric cancer cells in mice) and H-22 (hepatic cancer cells in mice) cell lines. It displayed the strongest cytotoxicity against H 22 cells with IC50 value 3.125 μgml-1 and exhibited moderate cytotoxicity against MFC cells with IC50 value of 6.25 μgml-1 (Wang et al. 2012).
Camptothecin from endophytic fungi Fusarium solani was assayed in vitro for cytotoxicity against vero cell and human prostate cell line PC3. It displayed remarkable inhibitory effects at inhibiting cell growth and inducing apoptosis on vero and PC3 cell lines in a dose dependent manner (Ran et al. 2017).
In this review, we limit our dialog to a couple of selected imperative bioactive metabolites produced by fungal endophytes from some restorative plants. In the last, some decade's different analysts have detailed work on endophytic fungi as a potential wellspring of novel bioactive hotspots for anticancer, antiparasitic, antitubercular, cancer prevention agent, insecticidal agents and immunoregulatory agents (Omeje et al. 2017).
In the present review, we are displaying a report on endophytic fungi as a potential wellspring of extraordinary anticancer compounds. Following the disclosure of the first anticancer compound, taxol from the endophytic fungus Taxomyces andreanae, a few different scientists have been considered as authentic vaults of anticancer compounds. The strong anticancer compound paclitaxel (Taxol) has a place with the diterpenoid class of plant-inferred bioactive agents (Stierle et al. 1993, Omeje et al. 2017).
Taxol was isolated and purified for the first time, from the bark of yew plant Taxus brevifolia from South America, before, being isolated from the endophytic fungus Taxomyces andreanae. The medication was afterword endorsed by the Food and Drug Administration (FDA), USA, for the treatment of chose malignancies (Cremasco et al. 2009, Omeje et al. 2017). The diverse sources of novel anticancer agents from endophytic fungi are enlisted in Table 2.
The extensive variety of chemical compounds have been disengaged from an assortment of endophytic fungi has a place with different hosts and synthetic groups, for example, aldehydes, alkaloid, chromones, cyclohexanones, depsidones, depsipeptides, ergochromes, esters, lactones, lignans, peptides, polyketides, quinones, diterpenes, sesquiterpenes, xanthones and so forth.
Today the greater part of the bioactive agents enrolled in Table 2 are at various dimensions of phase Ⅰ, phase Ⅱ clinical trials (Perfect 2017) and there are apparent expectations that the vast majority of them will be affirmed for helpful use sooner rather than later. Besides, the bioactive compounds obtained from the variety of endophytes isolated from host plants will be proven as big armor against the disease of cancer.
-
The present review demonstrates the potential of endophytic fungi as a source for anticancer drug development. It is assumed that nature continues to provide bioactive compounds as bio-resources for the further development of novel and improved drugs. Plant endophytic fungi offer an exciting new resource and research continues to reveal that most of the significant drugs originally thought to be produced by plants are most likely are interaction products with endophytes residing in the tissues of the host plant. With the help of modern biotechnology, biochemical techniques, bioprocess engineering, and fermentation technology, we can better understand and explore such significant fungal bioresources and make it more beneficial for human welfare. The fungal source of a desired anticancer agent is of distinguished value; as fungal fermentation provides a virtually inexhaustible source of metabolites of interest.
-
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
- Copyright: © 2021 by the author(s). This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| SD Palwe, MY Borde, HB Sonawane. 2021. Endophytic fungi: a source of potential anticancer compounds. Studies in Fungi 6(1):188−203 doi: 10.5943/sif/6/1/12 |












