-
Heterogenous distribution of crops, feed and livestock across China has halted the circulation of nutrients within the agricultural system and is responsible for massive nutrient losses[1, 2]. Generated livestock manure exceeded optimal crop requirements in 30% and 50% of over 2 300 studied counties when there was improved recycling of nitrogen (N) and phosphorus (P) in the food chain, repectively[2]. Most of these counties are located in southern and coastal areas, whereas there is a deficit of livestock manure in northern and western China. Such heterogenous distribution of crop-livestock production led to 4.0 Tg manure N and 0.9 Tg manure P[2], which are economically impossible to recycle and will end up in the surrounding environment. In addition, about 40% of feed protein consumed by domestic livestock production relied on importation, putting China’s livestock production supply at high risk in the post pandemic world[3]. Hence, China is facing the twin issues of too many manure nutrients but too little feed nutrients simultaneously. Such mismatch of feed protein demand and manure nutrient production is more severe at the regional level due to the heterogenous distribution of crops, feed and livestock within China, which may further impact sustainable livestock production.
Heterogenous distribution of crop-livestock production sites has also led to region-specific conservation activities. For example, southern China has suffered from severe water pollution, resulting from intensive watercourse and livestock production, leading to lower capacities for crop nutrient uptake[4]. Hence, the central government initiated the south-to-north pig transfer project in which southern farms were closed and northern ones established to effectively manage water pollution[5]. This will, however, increase ammonia emissions in northern China, a region already suffering from high PM2.5 levels that are in part due to ammonia emissions generated from livestock production[6, 7].
Recently, treatment and the recycling of manure have received greater attention from the public and policymakers. The central government has tightened environmental regulations on livestock manure management, aiming to promote the recycling of manure to reduce losses[8]. New regulations require manure to be treated for parasites, e. coli, flies and mosquitos prior to field application. However, this policy has overlooked the possible increase of ammonia emissions from this mandatory manure processing. Increasing ammonia emissions may have large impacts on the quality and biodiversity richness of plants in some protected and ecologically sensitive regions, contradicting the newly released ecological protection and restoration polices by the National Development and Reform Commission[9].
Current environmental regulations on livestock manure treatment overlook environmental risk as well as region-specific requirements and conditions. Treatment and circular manure systems across different regions are both necessary, but China lacks technologies and relevant system designs, despite a long history of manure application. Lessons learned abroad, where there is oftentimes less heterogeneity of crop-livestock production, may be difficult to adapt to China. For example, in the Netherlands, a country with high livestock density and a surplus of nutrients, manure recycling and processing is far-reaching and well developed. Around 25% of its annually produced manure is exported to neighboring countries after being heated at 70 °C for one hour[10]. However, such transregional transportation costs could contribute up to 10% of total production costs in livestock farms in the Netherlands[11]. In China, the lower profitability of livestock production and longer transportation distances limit the possibility of transnational or trans-provincial transportation of manure, especially when large surpluses of manure are located in South China with deficits more common in the Northeast[2]. Lessons and technology systems from the Netherlands are difficult to adapt to China, particularly given the recent controversial ban of agricultural production due to ammonia emissions in protected regions in the Netherlands[12].
HTML
-
We argue the need for designing new region-specific manure recycling and treatment strategies to combat environmental pollution, reduce reliance on imported feed and protect vulnerable ecosystems in China. The criteria for the design of such a system should seriously consider the following elements: (i) recycling nutrients in the food chain; (ii) local livestock feed self-sufficiency; and (iii) county-level human health and ecosystem vulnerability to ammonia emission.
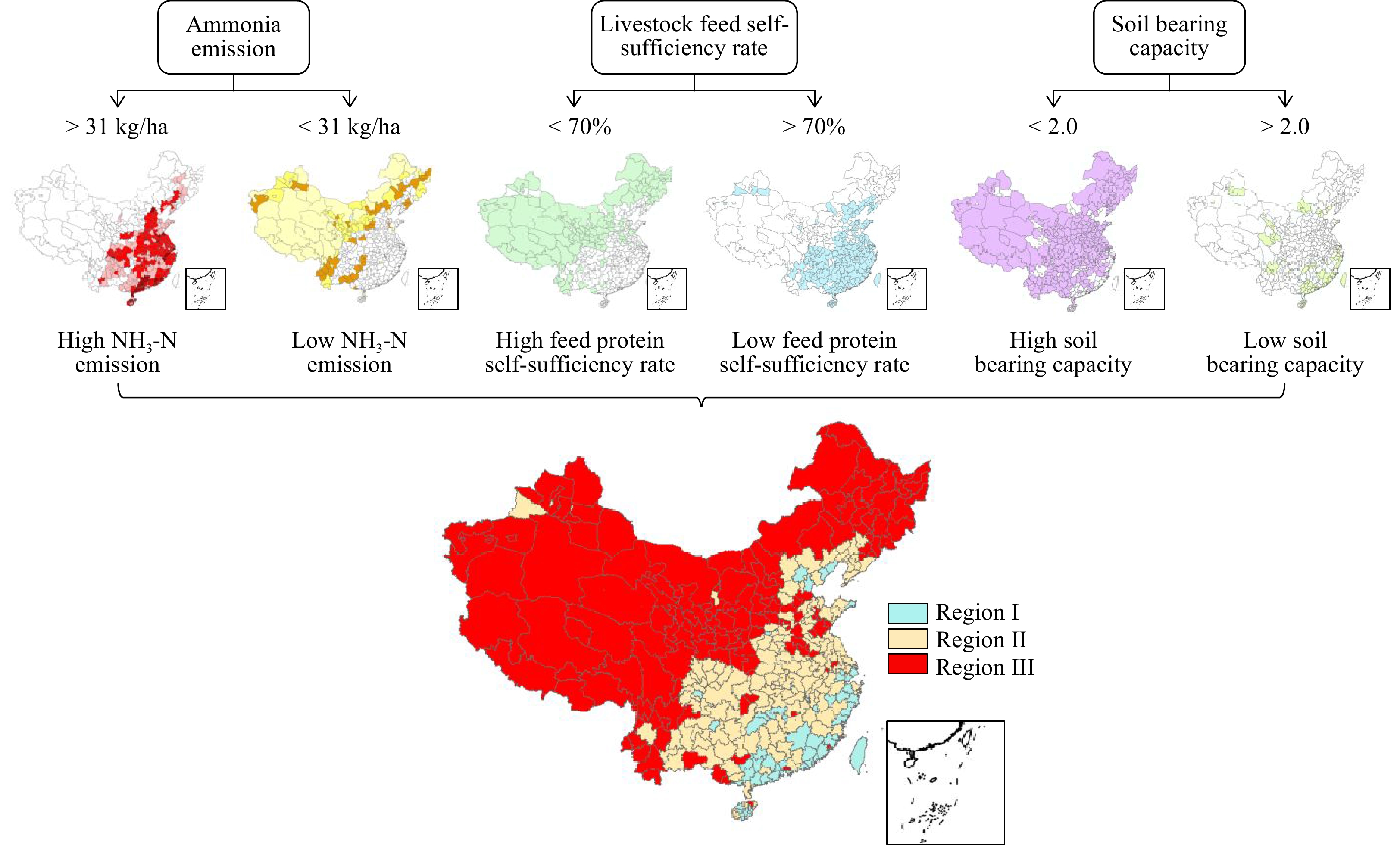
All regions are categorized into three groups according their status: regions (Region I) with an excess of manure nutrients, increased sensitivity to ammonia emissions and lack of feed that should implement new modern technologies to treat manure with low ammonia emissions, high efficiency and rapid manure dewatering, liquid manure nutrient concentration and feed protein production technologies, which will allow exporting excess manure nutrients to other regions while increasing internal recycling of manure nutrients as feed protein (Fig 1, 2); regions (Region II) with moderate soil nutrient carrying capacities featuring sensitivity to ammonia emissions that should more focus on solid-liquid manure separation and solid manure dewatering to allow exporting solid manure and in-situ application of liquid manure within region (Fig 1, 3); and regions (Region III) with a deficit of manure nutrients featuring no sensitivity to ammonia emissions and feed protein supply that should implement little or no treatment technologies to allow the full potential recycling of manure nutrients on farms (Fig 1, 4).
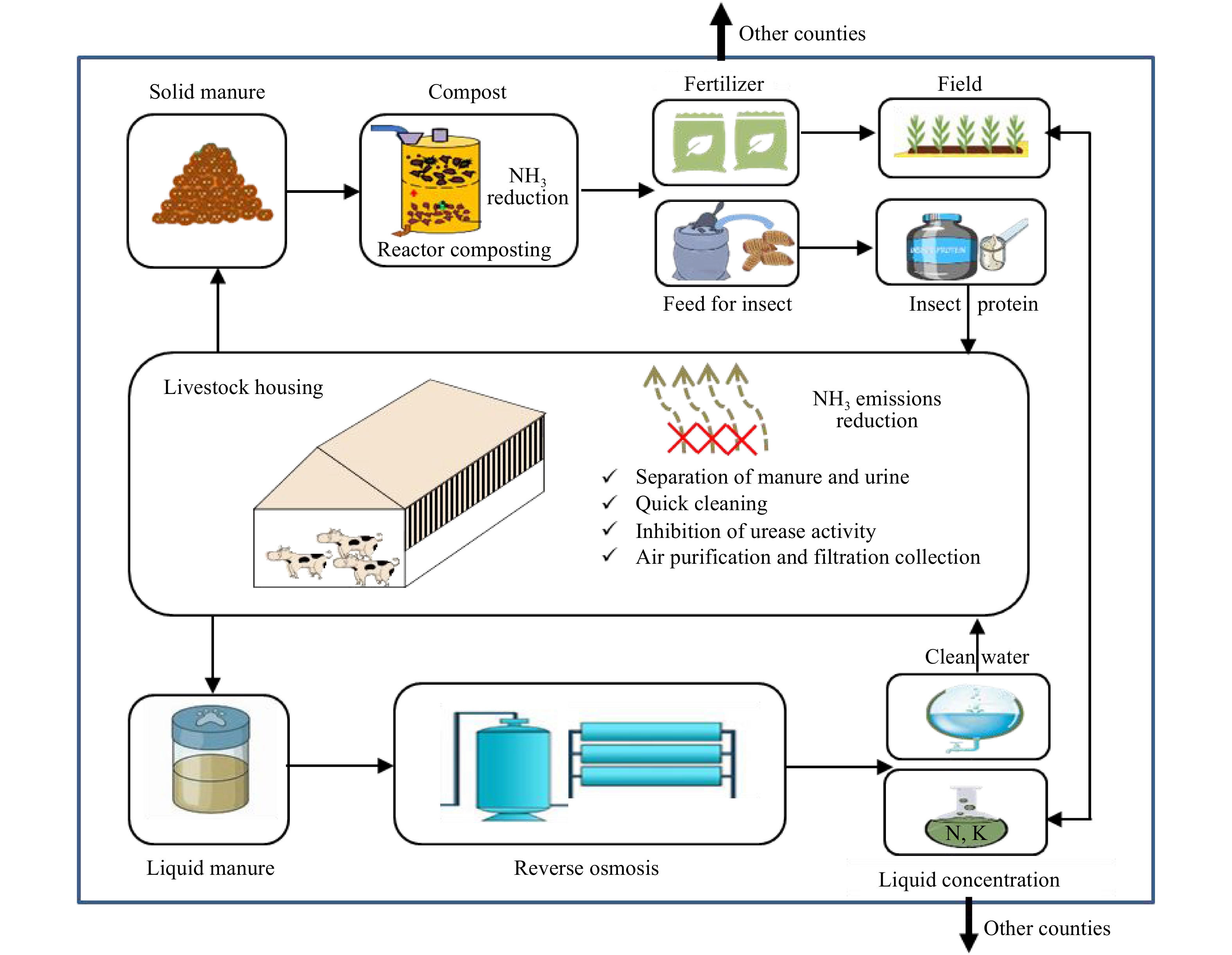
Figure 2.
Technical model diagram of region I, including: reactor composting, liquid manure concentration technologies and freed protein production.
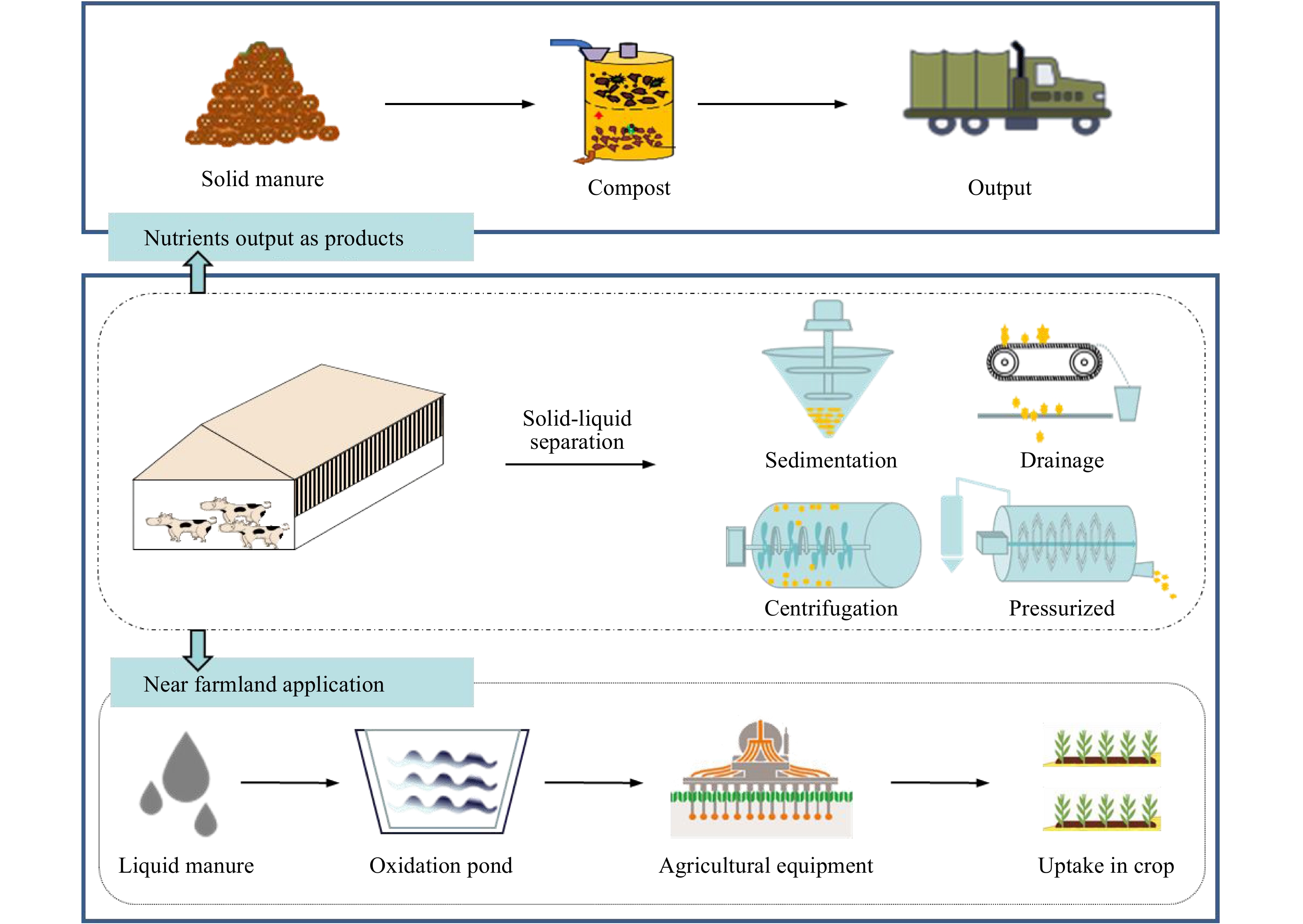
Figure 3.
Technical model diagram of region II, including: efficient solid-liquid separation and liquid returned to field.
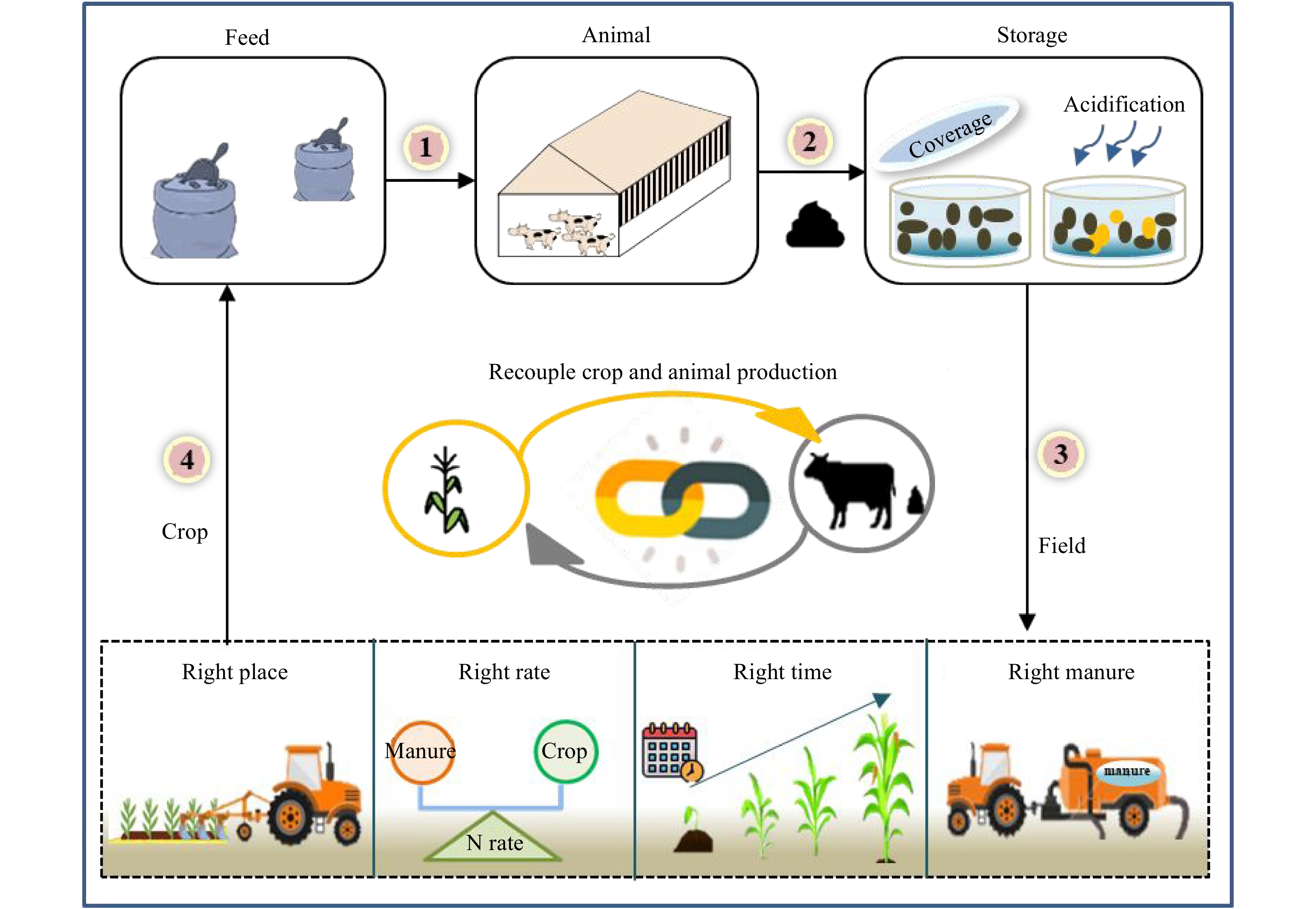
Figure 4.
Technical model diagram of region III, including: manure storage and in-situ field application.
Through using information from over 2,300 counties, including crop nutrient uptake, whole food chain nutrient management, ammonia emissions and self-sufficiency of feed protein, we have developed the first region-specific designation of manure treatment technologies in China (Fig 1). Categorization was carried out at the city level with over 360 cities included due to the strong role of city-level governments in providing subsidies to technology companies and farms. This illustrative example shows that the total area of Region I covers around 14.3 million ha cropland (12% of the manure N production), and Region II and III cover the remaining 97% and 88% of cropland and manure N production, respectively (Fig 1).
-
Region I is generally located in southeast China, mainly in Guangdong, Fujian and Hainan Provinces as well as the cities of Beijing and Tianjin (Fig 1). Technology system concepts are shown in Fig 2. Generally, livestock farms in Region I need to implement strict ammonia mitigation options in animal housing, such as frequent cleaning, air purification and filtration. Region I has a lower capacity to recycle manure, hence there is less need for storing large amounts of manure as most of it is regularly treated. Solid manure should be dewatered via advanced reactor composting technology, most of which is either directly exported outside of the region or used to produce insect feed to feed animals (Fig 2). The liquid part of manure needs to be treated via reverse osmosis technology to concentrate nutrients, allowing for long-distance transportation. Summarized, the three main proper treatments of manure in Region I are as follows: i) advanced reactor composting technology; ii) efficient liquid manure nutrient concentration technologies; and iii) feed protein production technologies.
Reactor composting. Reactor composting technology is an effective and environmentally friendly method[13]. The well-controlled temperature and aeration in closed vessels achieves the elimination of pathogens, parasites and weed seeds within 7−10 days, with more than 90% of antibiotics and their resistance genes undergoing degradation[13]. Combined with a serial exhaust gas bio-filtration system, zero emission of ammonia, GHG and odor could be achieved[14, 15]. Meanwhile, fresh manure generated in livestock farms could be feeding into reactors continuously, combined with manure cleaning systems in animal housing. Closed composting reactors play the role of both storage and treatment for manure that shortens the manure management chain. Its high efficiency and small size make it an ideal in-situ manure treatment method for intensive livestock farms. It could thus be used both in Region I and II to achieve NH3 mitigation and convert manure into high-quality organic fertilizer that benefits manure nutrient transportation and epidemic prevention.
Liquid manure concentration. Reversed osmosis (RO) is based on the ability of RO-membranes to let water pass and block salt ions. The technique is widely used for the desalination of sea water. Recently, the application of selective electrodialysis with monovalent exchange membranes on the recovery phosphate or ammonium from sewage water and livestock slurry has been investigated as a promising technique, and the technology has performed well in the Netherlands. Recovery efficiency of ammonia could reach 78% and 75% of phosphate and 87% of volatile fatty acids via using a bipolar membrane electro-dialysis system[16]. After treatment, 20% of mass was retained in the solid fraction, while 30% and 50% was retained in the concentrate and permeate material. The volume of concentrate, which contains higher concentration of ammonium-N, K and other elements, could be further reduced via use ventilating heat[17]. The effectiveness of nutrients in the concentrate was comparable to granulate chemical fertilizer (calcium-ammonium-nitrate), a common fertilizer in European countries. The Netherlands began pioneering experiments in 2009, and in total, eight large pilot plants have successfully treated slurry[17]. These could serve as good examples for Region I in China as nutrients in liquid slurry are effectively concentrated, allowing long-distance transportation to get rid of excess manure nutrients.
Feed protein production. The use of insects for animal manure management is a sustainable and low-cost technology that effectively reduces the volume and nutrient concentration of manure residue, thereby reducing potential pollution. The black soldier fly larva (BSFL), one of the most powerful recyclers, can reduce the bulk of manure residue by 56% and nutrient concentrations by 40%−55% within 14 days of manure breeding[18]. Besides effectively degrading antibiotics in the manure[19], BSFL can also greatly reduce the abundance of pathogenic bacteria[20], decrease the offensive odor[21] and inhibit the breeding of house flies[22]. Few pilot plants have initiated commercial level production. Henan Enzyme Company has developed a pilot-scale automated BSFL breeding facility to treat pig manure and produce insect proteins[23]. Through cloud technology, the device can upload data in real-time, and the controller can adjust and monitor the parameters of the breeding workshop through mobile application or computer. The facility can completely decompose 3.24 tonnes (78% moisture) of pig manure per day and produce 480 kg of insect biomass. This effectively reduces the volume and nutrient concentration of the manure residue, thereby reducing potential pollution by at least 50%−60%[18]. BSFL biomass, containing about 40% protein and 30% fat[24], can be used as a substitute for soybean and fish meal for feeding poultry and fish without causing adverse reactions[25]. Through the recycling of insects, the twin problems of excessive manure nutrients and shortage of feed in Region I can be addressed simultaneously. An alternative choice is microalgae protein production. Recently, microalgae have been considered as a promising solution for wastewater management owing to their high capacity to deplete inorganic nutrients (N and P) from a wide range of wastewater[26]. Microalgae could use wastewater effluent as a source of carbon and nitrogen to support their rapid growth and be converted to microbial protein as animal feed after proper filtration, concentration and drying.
-
Region II covers major cropland production areas in China, excluding the northeast and Region I (Fig 1). These area shows a moderate level of ammonia emission intensity, manure loading capacity and feed self-sufficiency; accordingly, attention should focus on the trans-regional recycling of manure with less emphasis on complex and high-cost treatment technologies. The core recommended technology system is solid-liquid separation in which solid manure is dewatered via closed reactor composting, transported between regions and liquid manure injected to fields adjacent livestock farms.
Efficient solid-liquid separation system. Solid-liquid separation can be divided into sedimentation, drainage, centrifugation and pressure filtration (Fig 3). Centrifugal separation is the most effective method for slurry separation, with the highest removal rate of total solids reaching up to 60%[27]. However, centrifugal equipment is used relatively less in China due to its high price, high energy consumption and demanding maintenance. Pressurized filtration, including screw press and press auger, is usually more efficient than screening technology. Improving pressure can increase the removal rate of solids and accelerate the removal of nitrogen, phosphorus, potassium and other nutrient elements. This technology is suitable for widespread application in China due to lower costs[28]. In addition, flocculent can be used before separation to improve separation efficiency.
Liquid manure injection or irrigation. Liquid manure is rich in N after separation, which can be connected to the irrigation system through the pipe network or transported to the farmland and applied with supporting agricultural machinery as liquid fertilizer. Liquid fertilizer can be diluted directly into irrigation water for field application, but the spread of nutrients on the field surface may be uneven[29]. Liquid fertilizers can be applied to fields by spraying vehicles, but it may cause the evaporation of ammonia and odors[30]. Deep soil injection could reduce ammonia emissions. Fecal injection could be combined with seeding, reducing costs and improving seeding rates, especially in dry regions and seasons. In the Netherlands, strict regulations of manure application have promoted the development and manufacturing of equipment related to liquid fertilizer. The disc-type fertilizer applicator is an injection machine with disc harrow capable of simultaneous stubble crushing, land overturning and manure injecting[31, 32]. In China, use of the liquid injection machine is relatively unexplored. However, injecting liquid fertilizer can greatly improve the utilization rate of fertilizer and reduce pollution. Li et al. (2020) showed that integrating seedings with liquid manure injection could replace 50% of mineral N fertilizer, reduce ammonia emissions by 27%−49% and increase corn grain yield by 17%−33%[33].
-
Region III, largely found across Xinjiang, Inner Mongolia and Northeast China, has an abundance of feed resources, with higher manure nutrient loading capacity and lower sensitivity to ammonia emissions. These regions usually feature larger farms, which require fewer manure treatment technologies but better manure storage facilities. This is because these regions have a single cropping system that is different from Region I and II. This indicates manure could only be applied once per year in these regions. An ideal model for Region III is shown in Fig 4. This model is characterized by manure storage and in-situ field applications. Manure needs to be stored in belowground concrete tanks for almost one year. Coverage, which reduces the timing for manure exposure in the air and resulted in reduced emissions of NH3, odour[34, 35] as well as surface acidification, further decreasing NH3 emissions with lower cost[36], are two favorable technologies to preserve N in manure during long-term storage. Applying the slurry in accordance with the principles of 4R nutrient stewardship, that is, applying the slurry at the right place, rate, time and type[37, 38], could also be used to help treat storage manure.
-
We used current data to demonstrate an approach to designing region-specific technology systems for the treatment and recycling of manure in China, and we provided an illustrative example in Fig 1. This is a preliminary analysis based on limited data and broad assumptions, and more research is needed to improve the granularity of such designs by quantifying water and airborne pollution as well as landscape and transportation costs at the regional level. Furthermore, region-specific manure management designs could become part of the recently implemented rural revitalization[39] and Blue-Sky actions[40].
The ultimate goal of manure treatment is to reduce environment impacts by recycling it as either fertilizer for crop production or feed protein for livestock production. However, in China, livestock has been largely decoupled from crop production in terms of exchange between feed and manure at both the farm household and regional scale. The share of rural households with both crop and livestock production has declined from 71% to 12% in the period between 1986 to 2017[41]. A recent nation-wide survey revealed that only 1/3 of farmers were willing to use manure as fertilizer in cereal crop production[42] due to concerns of cost, odor, antibiotics and heavy metal issues. Recycling of manure-based insect feed protein to livestock production is also a controversial issue in terms of consumer acceptance. These obstacles could be alleviated via strict feed quality control regulations and public education campaigns.
-
The criteria used in this study included soil-bearing capacity, local livestock feed self-sufficiency rate and ecosystem vulnerability. The soil-bearing rate refers to the ratio of total excretion of N by livestock and humans as well as the N withdrawal of harvested crops. In the present study, the soil-bearing capacity was estimated based on the NUFER (NUtrient flow in Food chains, Environment and Resources use) model, which calculates all nutrients for each city. The equation used to calculate soil bearing capacity was:
$ N \;soil=\frac{N\; human \;manure+N \;livestock\; manure}{{{N}}\;plant \;uptake+N\;grass} $ (1) Where N soil is soil bearing capacity, N human manure is the N content from human manure (tonnes N yr−1), N livestock manure is the N content from livestock manure (tonnes N yr−1), N plant uptake is N content taken by plants (tonnes N yr−1) and N grass is the N content taken by grass. The estimated soil bearing capacity was summarized in Fig 1. Jin et al. (2020) claimed that 2 was the threshold value for the soil-bearing capacity in China, which means areas with values higher than 2 were considered low soil-bearing capacity[2]. Across China, 19% of the total area was lower than 2 and considered as having high soil-bearing capacity. Conversely, areas higher than 2 were considered as having low soil-bearing capacity.
The feed self-sufficiency rate refers to the ratio of domestically consumed feed supplied by domestic producers. Local livestock feed self-sufficiency rate was estimated based on livestock consumption and feed production. The equation used was:
$ N\; ratio=\frac{N \;total \;cons-{N}\;imported +{N}\;exported}{{N}\;total\; cons} $ (2) Where N ratio is livestock feed self-sufficiency rate and N total cons is the total N consumption for each category of livestock (sheep, cattle, pig, poultry, horse, rabbit, mule and donkey). N imported is feed N imported from other areas. N exported is feed N exported to other areas. The distribution of livestock feed self-sufficiency rate across China is shown in Fig 2. The present study designated 0.7 as the threshold value, and values higher than 0.7 were defined as high livestock feed self-sufficiency rates. As seen in Fig 2, high livestock feed self-sufficiency rates (exceeding 0.7) were mainly located in northern and western China China.
Ammonia emissions considered here include NH3 from crop and animal production. The amount of total ammonia emissions was estimated by the NUFER model (Fig 3). The SDGs report, EU SDG index scores and ammonia data are reported for each country. Using this data, the relationship between SDG index scores and ammonia emissions data was established through multiple linear regression analysis. The statistical model (R2 = 0.91, n = 23) used was:
$ {\rm{Ammonia }}= -1.4{\rm{Score}} + 104.9 $ (3) Where Ammonia is ammonia emission per agricultural land (kg ha−1) and Score is the SDG index score. SDGs report designated a score of 60 as the threshold for European countries, and this present study assumes the European standard as the corresponding limit for China. Therefore, this statistical modeling can provide the ammonia threshold value (31 kg ha−1) for the designated 60 score. As seen in Fig 3, higher ammonia emissions (higher than 31 kg ha−1) were found in southeastern regions.
The total land area of China was divided into 3 regions, each of which in turn contains two or more ecosystem statuses (Fig 4): regions (Region I) with high ammonia emissions, low soil-bearing capacity and low livestock feed self-sufficiency rates; regions (Region II) with low ammonia emissions, low soil-bearing capacity and low livestock feed self-sufficiency rates; and regions (Region III) with low ammonia emissions and high soil-bearing capacity. This process was compiled in ArcGIS 10.6 in which areas were selected by overlaying different criteria layer (ecosystem status layers).
This work was supported by the National Key R&D Program of China (2016YFD0800106); the National Natural Science Foundation of China (31572210, 31872403, 71961137011); Key Research Program of Frontier Sciences-CAS (QYZDY-SSW-SMC014); Key Laboratory of Agricultural Water Resources-CAS (ZD201802); the Key Research Program-CAS (KFJ-STS-ZDTP-053); Hebei Dairy Cattle Innovation Team of Modern Agro-industry Technology Research System, China (HBCT2018120206); the Youth Innovation Promotion Association, CAS (2019101) and Outstanding Young Scientists Project of Natural Science Foundation of Hebei (C2019503054).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Bai Z, Wang X, Wu X, Wang W, Liu L, et al. 2021. China requires region-specific manure treatment and recycling technologies. Circular Agricultural Systems 1: 1 doi: 10.48130/CAS-2021-0001 |