-
Around 80% of plant species form symbiotic relationships with mycorrhizal fungi, which assist the plant in accessing soil nutrients beyond their roots[1]. A second, less widespread symbiosis between plant roots and N-fixing bacteria (symbiotic nitrogen fixation – SNF), allows for the further provision of N to the host plant. In return, host plants provide the symbionts with photosynthetically derived carbohydrates[2]. The improved nutrition from these symbioses enables plants to maintain higher photosynthetic rates, thus fixing more atmospheric CO2. Furthermore, if both symbioses occur in the same root system, there is an enhanced synergistic effect on plant productivity and symbiont performance[3].
This article presents the case that the co-evolution of these root symbioses contributed towards the decline in atmospheric CO2 levels at two points in the Earth's history. The potential for harnessing these symbioses to address current planetary system challenges are discussed.
-
During the Phanerozoic Eon, there were two peaks in temperature and CO2 concentrations (Fig. 1). The first peak in CO2 occurred between the Cambrian and Devonian Periods (540−420 million years ago (Ma)), coinciding with the earliest mycorrhizal records[4, 5]. The second was during the late Jurassic and early Cretaceous Periods (150−100 Ma), at approximately the same time when the first precursors of symbiotic N-fixation evolved (ca. 100 Ma) (Fig. 1)[6].
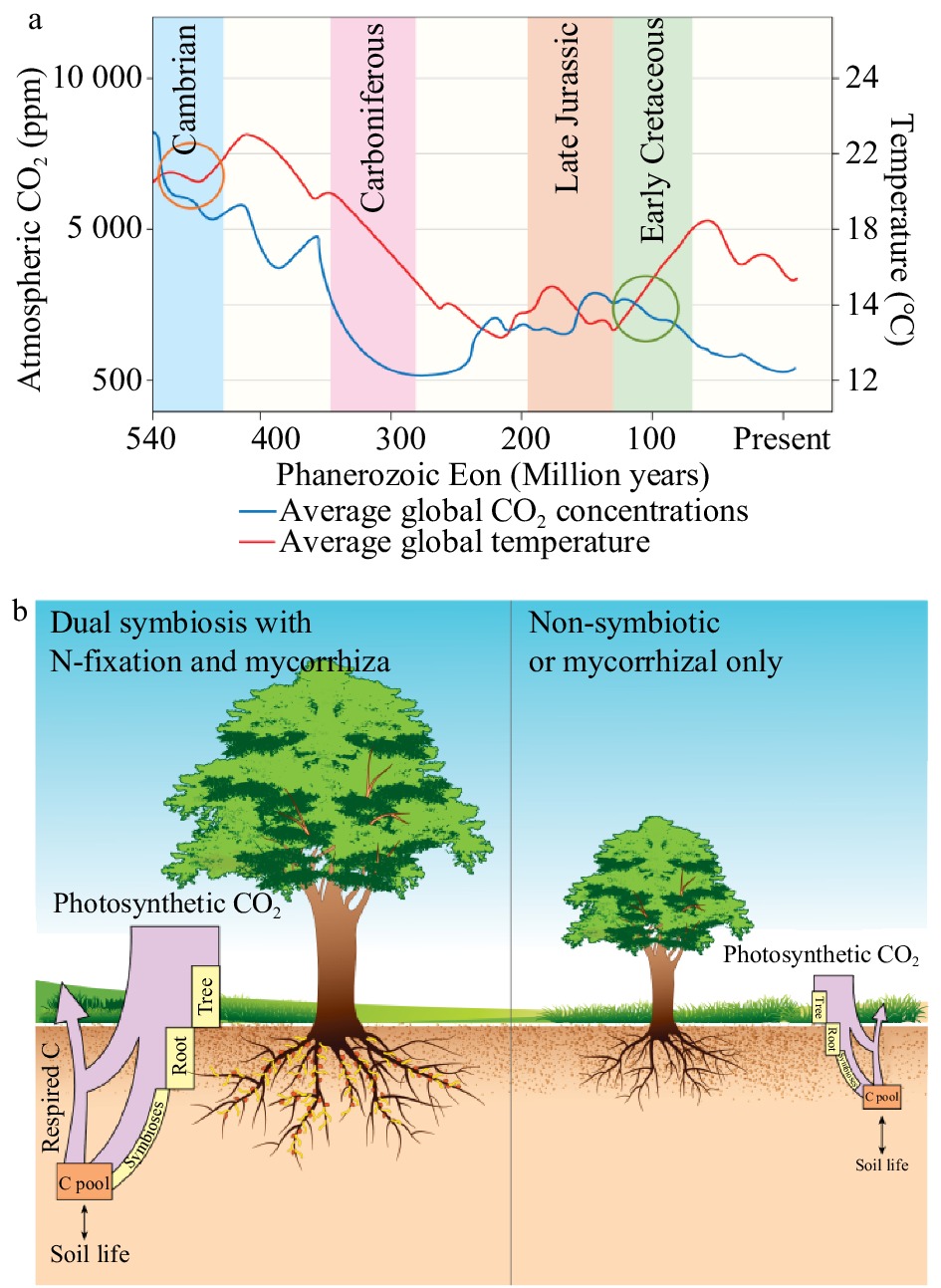
Figure 1.
Atmospheric carbon dioxide (CO2, ppm) and temperature (°C) levels during the Phanerozoic Eon (540 Ma to present) (a). Data adapted from [5], [20], [21]. The highlighted portions indicate relevant periods in the past: light blue indicates the Devonian Period, purple the Carboniferous Period, light orange the late Jurassic Period, and light green the early Cretaceous Period. The proposed periods for the development of the mycorrhizal symbioses are circled in orange and that of symbiotic N-fixation in green. The illustrative description of the C-pumps model proposed for N-fixing trees shows the relative movement of C through these systems (b). The relative proportion of C captured via photosynthesis is represented by the purple box, with as much as 50% more C fixed in dual symbiotic plants compared to non-symbiotic plants[10]. The yellow boxes represent the relative amounts of C stored in biomass, with N-fixing trees growing as much as nine times faster than their non-N-fixing counterparts[20].
These symbioses evolved during periods of elevated atmospheric CO2 concentrations, when plants could easily provide the additional C for the symbionts. During the late Devonian Period, trees and larger plants evolved and began to dominate the landscape[7]. There is evidence that mycorrhizal symbioses were critical to this process[8]. During the following Carboniferous Period (ca. 360−300 Ma), forest systems became widespread and atmospheric CO2 declined to around 300 ppm (Fig. 1a). Average temperatures also declined by about 10 °C (Fig. 1a). This climatic change led to the Carboniferous Rainforest Collapse[9], a period when many forest systems died, depositing large amounts of C belowground.
With the decline of forest systems, global CO2 levels began to rise, possibly because Agaricomycetes, the first organisms capable of degrading lignin and releasing CO2, had evolved[10]. In addition to high CO2 levels, temperatures rose to levels more than double those experienced today (Fig. 1a). The first evidence of SNF coincides with high temperatures and CO2 levels around 100 Ma. During this period, plants with N-fixing symbioses proliferated and colonized various landscapes, acting as pioneer species in soils with low N[11]. These changes were followed by another decline in global CO2 levels that continued to decline until the Quaternary Period (Fig. 1a).
-
Plants produce organic C compounds by fixing atmospheric CO2 through photosynthesis. This C is used for the plant's own growth; an additional 25% can be consumed by mycorrhiza[12] and a further 28% by N-fixing bacteria[13, 14]. To maintain these symbioses, the photosynthetic rates of dual inoculated host plants can increase by over 50%[14]. These figures only represent the C directly attributed to growth and maintenance of the symbionts. Increased C-flow through root exudates and increased plant biomass are difficult to accurately quantify but are significant additional contributions to C sequestration. Plants with both symbioses are therefore greater C sinks, moving a higher percentage of C belowground, and acquiring more C for growth (Fig. 1b). There are also beneficial downstream effects: N-fixing plants can cause improved soil nutrition and growth of surrounding plants[15].
-
The Anthropocene describes the period of recent history in which humankind's impact on the earth has been globally significant. The Anthropocene is characterised by rising temperatures, erratic weather events, biodiversity loss, chemical pollution, forest loss, and high-input agricultural systems[16]. In 'On the Possibilities of a Charming Anthropocene', Buck (2015) highlights the need for a shift in focus from the negative impacts of this period towards the possibilities for stimulating positive changes[17]. We posit the increased usage of plants with both forms of root symbioses as one such positive intervention, with particular potential in tropical regions.
The tropics is home to about 68% of legume species, which comprise the vast majority of plants capable of SNF; and more than 50% of all biological N-fixation takes place in the tropics[18, 19]. Furthermore, N is often the most limiting factor for plant growth, and therefore C sequestration, in the tropics. Batterman et al (2013) showed that in tropical forests N-fixing trees can accumulate C nine times faster than non N-fixing trees, and that younger forest systems have much higher C and N accumulation rates[20]. Thus an ideal means of landscape restoration and C sequestration would be to plant N-fixing tree species in disturbed and/or agricultural landscapes within tropical areas.
However, we could go further than simply modifying natural regeneration to favor SNF plant species. We could use new forest restoration technologies, such as superior genetic materials, pre-inoculation of saplings with symbionts, rotational forest management with sustainable timber harvest (for C storage), and superior agroecology and agroforestry systems[21].
The projections indicate that CO2 levels will continue to rise, temperature will increase, and the tropics will become drier. Evidence from past geological time periods suggest that under these conditions, we can expect the rate of C accumulation by SNF trees to increase[22]. The current direction of global climatic change provides a positive feedback for N-fixing trees, and their role in C sequestration could offer a useful buffering effect.
Improved understanding and management of N-fixing trees will enhance the contribution of agriculture and forestry to climate change mitigation, ecosystem resilience, food security and environmental sustainability. Future agricultural systems that encourage the planting of trees and diversification of agricultural landscapes will increase sustainability and productivity. We therefore propose harnessing the C pump and ecosystem enhancement capacities of N-fixing trees as a means of taking us forward into a new, positive era.
This work was generously supported by the Key Project from the Ministry of Sciences and Technology of China (No: 2017YFC0505101).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2021 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mortimer PE, Hammond J, Hyde KD, Gui H, Xu J. 2021. Root symbioses as belowground C pumps: a mitigation strategy against rising CO2 levels. Circular Agricultural Systems 1: 9 doi: 10.48130/CAS-2021-0009
Root symbioses as belowground C pumps: a mitigation strategy against rising CO2 levels
- Received: 24 April 2021
- Accepted: 25 May 2021
- Published online: 29 June 2021
Abstract: Mycorrhizal and N-fixing root symbioses evolved at two points in the past when global CO2 was highest, consistent with the high demand these symbioses place on host C. Trees hosting both mycorrhiza and N-fixing bacteria are able to fix more atmospheric CO2 and grow at faster rates than non-symbiotic plants, or plants with only mycorrhiza. We argue that on the basis of this improved C capture, N-fixing trees act as C-pumps, sequestering C and locking it in biomass, thus, if properly managed, can contribute significantly towards the mitigation of rising CO2 levels.












