-
Plastic is a material used almost ubiquitously in our lives. Consequently, millions of tons of plastic are produced every year. As of 2019, global plastic production has reached 359 million metric tons[1]. Given wide use and production, plastic debris, especially micro (< 5 mm) and nano (< 100 nm) sized particles have become a menace for terrestrial and aquatic ecosystems[2,3]. Plastics are produced on land and then transported to terrestrial and aquatic ecosystems. It is suggested that terrestrial ecosystems receive ~4–23% more microplastics than aquatic ecosystems[4]. Another survey[5] reported that microplastic abundance in soil remained one order of magnitude higher than in sediments. Consequently, microplastic pollution in terrestrial ecosystems may present a rather larger problem, thus demanding more attention[5]. However, research has only just started to embrace this new focus on terrestrial ecosystems after ignoring them for at least a decade; almost all past research has focused on aquatic ecosystems[6]. Therefore, our understanding about microplastic-associated toxicity in terrestrial ecosystems is limited.
In terrestrial ecosystems, soil grapples with an onslaught of pollutants, including microplastics. The proportion of microplastics in soil environments continues to increase. In particular, agricultural soils have become permanent sinks of microplastics[5]. Microplastic pollution stems there from different sources. Direct sources include soil amendments and irrigation water that supplies primary microplastics, i.e., those manufactured in micro sized[7]. However, indirect sources include mulching and littering, supplying secondary microplastics, i.e., those fragmented from large plastic materials[5,8]. Some sources like sewage sludge supply both primary and secondary microplastics[9,10]. Nonetheless, all these sources supply highly variable types and rates and shapes of microplastics that are difficult to record. These particles exist in different degrees of the aging phenomenon under environmental conditions[5] which may continuously change their size and shape and ultimately offer unique challenges.
The repercussions of soil microplastic contamination are likely to be far-reaching. Plants are important living components of soil and serve as basic food for humans/animals. Recent research shows that microplastics presence in soil can induce phytotoxicity in some of crop plants[11,12]. Additionally, microplastic interactions with soil environmental factors are likely to exacerbate phytotoxicity[13,14]. Plastic fragments contain certain chemical additives (Table 1) that are released slowly into environments[15]. These additives are also of environment concern, especially with regard to crop performance[16]. Pervasive use of plastics represents a mounting threat for food web exchanges[17]. Microplastics are capable of ending up in plants[18]. It is, however, unclear that all plants can be affected by plastic particles and have the ability to uptake these pollutants.
Table 1. Examples of additives released from different polymers.
Additives Polymers Reference Irgafos 168 (tris (2,4-ditert-butylphenyl) phosphite) Polyethylene and
Polypropylene[19] Diisoheptylphthalate, Benzyl butyl phthalate, Di butyl phthalate, dipentyl phthalate, di-(2-ethylhexyl) adipate, di-octyladipate, diethyl phthalates, diisobutylphthalate, Tris (2 chloroethyl) phosphate, dicyclohexyl phthalate, butyl benzyl phthalate, diheptyl adipate, and heptyl adipate Polyvinylchloride [20,21] Irganox 1010 (tetrakismethylene-(3,5-di-t-butyl-4- hydroxyhydrocinnamate) methane)Linear low-density polyethylene and
High-density polyethylene[20] Soil underpins land-based lives and ecosystem functions that ultimately benefit humans[22]. Microplastic pollution now poses serious problems for key soil ecosystem functions[14]. However, research has hitherto largely focused on soil physical functions[11,22,23] rather than on biochemical processes. Hence, consequences for nutrient-cycling are largely unclear[6]. Despite potential toxicity of plastic fragments for microbes[11,22], we know little about shifts in microbial abundance and diversity. Moreover, effects on soil organic-matter turnover are plausible but remain widely unknown. It is therefore of great importance to conduct studies that can clarify existing uncertainties about soil ecological risks posed by microplastics.
In this review, we intend to evaluate emerging microplastic ecotoxicological concerns in agroecosystems. Specifically, we develop a conceptual model (based on available studies) that describes various effects crops can experience. From the nature of effects, i.e., positive or negative, we categorize crops into sensitive and tolerant groups. This will inform the choice of crops in microplastic polluted lands. We even discuss bioaccumulation of microplastics. Because of strong link between plant performance and soil functions, we present conceptual mechanisms of ecosystem functions disturbances. Such a review, particularly about crops, is scarce and thus important to support further research on microplastic ecology.
-
Although research on microplastic toxicity in crops is rapidly expanding, a mechanistic understanding in this area is still in its infancy. Accordingly, we develop a conceptual model based on previous studies (Fig. 1) that describes various effects of microplastics on crops such as positive, toxic, lethal and no effect. It is meaningful in the model to think in terms of scales of size at which different types/shapes of microplastics affect crops. Moreover, based on observed crops responses in previous studies, we categorize them into tolerant and sensitive groups (Table 2). We will next separately discuss each effect type according to our model and crop category.
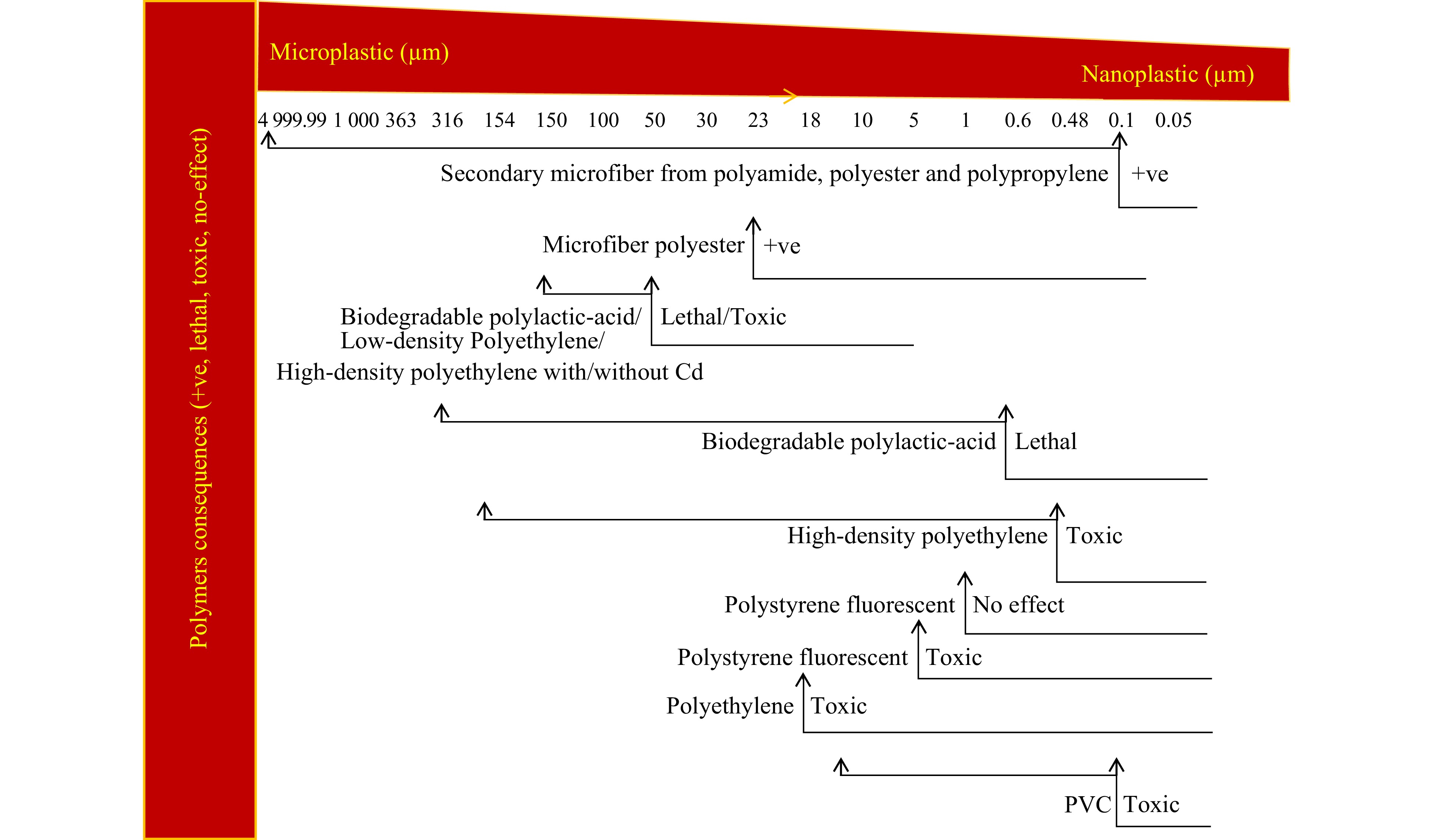
Figure 1.
Conceptual model of various effects of microplastic on crops. Positive (+ve) effect related to microfibers in onion and carrot[14,24], lethal effect linked to biodegradeable plastic in wheat, ryegrass and maize[8,25,26], toxic effect related to low-density polyethylene in wheat[8], high-density polyethylene in ryegrass[25], polystyrene fluorescent in broad bean[27], PVC, polyethylene in lettuce[12,16,26], and no effect associated to polystyrene fluorescent in wheat[28].
Table 2. Hypothesized classification of different crops based on their observed responses to polymers (Fig. 1).
Crops Polymers Observed effect directions
(+, –) for crop growthExpected category Tolerant Sensitive Onion Polystyrene, polyethylene terephthalate, polyamide, microfiber polyester + √ Carrot Microfibers: polyester, polyamide, polypropylene + * * Wheat Low-density polyethylene, biodegradable polylactic-acid – √ Maize Polyethylene, biodegradable polylactic-acid – √ Ryegrass Biodegradable polylactic-acid – √ Broad bean Polystyrene fluorescent – √ Lettuce Polyethylene – √ * unclear. Positive effects of microplastics on crops are plausible. Our model shows that microfibers in wide ranging sizes can pose positive effects on crops (Fig. 1). Importantly, none of the fiber features are likely to harm the crops as shown in the model. In carrots, microfibers from polyester, polyamide, polypropylene ranging between 0.1 µm and < 5 mm appeared to promote plant biomass[14]. Polyester fiber of 30 µm size has shown to positively affect spring onion (Allium fistulosum), especially root length, biomass and arbuscular mycorrhizal fungi (AMF) associations[11]. Even in drought conditions pollutant-enhanced onion (Allium cepa) showed aboveground biomass and root AMF colonization[24]. Besides microfibers, polystyrene pellet ranged from 547−555 µm also appeared to promote these traits in spring onion[11]. Additionally, polyethylene-terephthalate pellets ranged from 222−258 µm and polyamide bead ranged from 15−20 µm increased total plant biomass. From such effects, we imply that onion is quite tolerant (Table 2) and can be a good growing choice in microplastic-polluted soil. However, due to lack of evidence we are unclear about carrot potential against microplastics other than microfibers.
Toxic effects of microplastics are more common as shown in model (Fig. 1). At different sizes, many types of microplastic alone and in combination with other pollutants can be toxic for crops. Low-density polyethylene residues between 100−154 µm appeared toxic for vegetative and reproductive growth of wheat[8]. High-density polyethylene range = 0.48 −316 µm induced phytotoxicity in ryegrass[25]. Soil exposure to polystyrene fluorescent microspheres at 0.1 and 5 µm sizes decreased broad bean (Vicia faba) root biomass and catalase activity[27]. Higher concentration of PVC ranged from 0.1−18 µm reduced the abilities of light energy absorption, dissipation, capture and electron transfer in lettuce[12]. In lettuce, polyethylene beads at 23 µm induced cell membrane damage in both roots and leaves but more seriously in roots[16]. Exposure to polyethylene and di-n-butyl phthalate in combination appeared to be more toxic across a wide range of plant growth (e.g., weight of roots and leaves) and physiological (e.g., photosynthesis rate, stomatal conductance, transpiration rate, chlorophyll content and activity of Rubisco) processes. Microplastic × heavy metal interactions are possible. In maize, high-density polyethylene (100−154 µm) and Cd interactions appeared to affect root biomass[26]. Additionally, microplastic appeared to increase Cd contamination in wheat[29]. These negative directions of effects on wheat, broad bean and lettuce demonstrate the sensitivity of these crops (Table 2). The interaction between microplastics with other contaminants demonstrates serious threats for crops. Farmers should thus avoid selecting the land altogether polluted with microplastic and other chemical pollutants.
The model shows that microplastic effects on crops can be lethal (Fig. 1). Surprisingly, various sizes of biodegradable plastic are likely to be lethal. In wheat, residue of biodegradable plastics mulch (
1000 −50 μm) appeared more harmful than nondegradable plastics to reduce leaf area, plant height and biomass as well as to delay tillering and lower seed setting[8]. In perennial ryegrass (Lolium perenne), biodegradable polylactic-acid (0.6−363 μm) over high-density polyethylene and clothing microfiber also appeared to more drastically reduce seed germination and shoot height[25]. Moreover, higher concentration (upto 10%) of biodegradable polylactic-acid (100−154 μm) reduced maize biomass and chlorophyll content[26]. These negative effects demonstrate sensitivity of crops from the Poaceae family (Table 2). It is now clear that the use of biodegradable plastic is not an effective solution for rising plastic pollution in the environment. Their presence in agricultural soils could cause irreversible damage to agroecosystems. Consequently, its advocacy on a large scale as an environmentally friendly product should be carefully reconsidered.Because polymers are of a diverse nature, it is likely that microplastics cannot harm the crop (Fig. 1). Recently, microplastic polystyrene fluorescent at 1 μm size showed no effect on wheat growth up to 1−2 leaf stage; however their strong association with roots might affect plant growth at the lateral stage[28].
-
Research (Fig. 2) has only recently started to embrace the direct toxicity of micro/nanoplastics, having initially focused on other materials. Accordingly, our understanding remains limited.
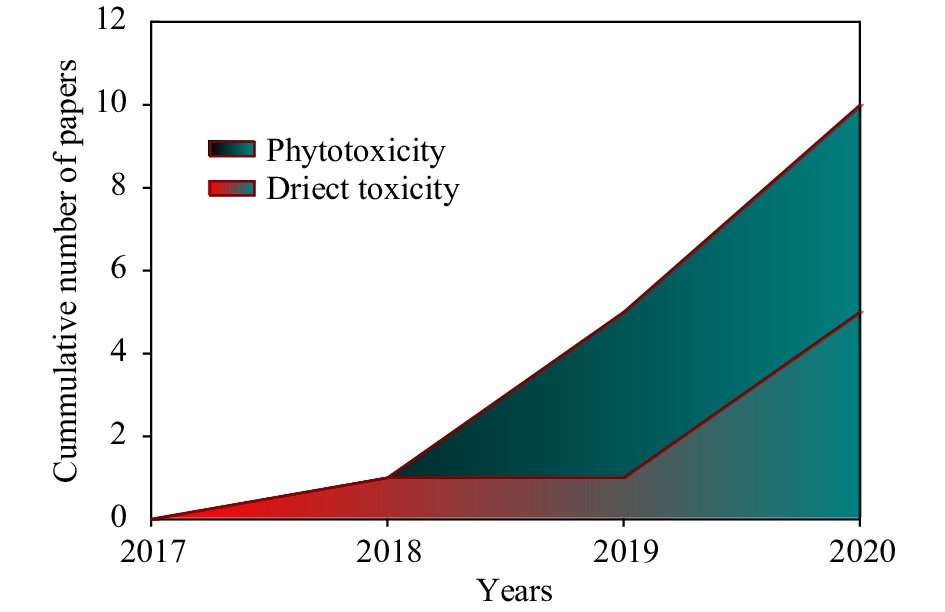
Figure 2.
Cumulative publications numbers on microplastic consequences and direct toxicity for food crops.
Nanoplastics are capable of entering into plants and generating stress. A recent study found that nanoplastics polystyrene fluorescent (100 nm) is able to accumulate in broad bean root and subsequently block cell wall pores, obstructing the transportation of nutrients[27]. Rubber ash nanoparticles also appeared to accumulate in cucumber root cells[30]. Thus, we can assume that soil nanoplastic pollution is a great threat for crop performance and their safety for human/animal consumption.
Recent research has disproved the widely held notion that microplastics cannot enter the plant body[31]. Submicrometre (0.2 μm) and even micrometer sized (2 μm) plastics can in fact enter plant[3]. Such sized particles of polystyrene and polymethylmethacrylate appeared to penetrate cracks at lateral root emergence sites in wheat and lettuce grown with wastewater. Due to crack features, it is assumed that plastics even larger than one micrometer can enter roots. Subsequent to root entry, the transport from root to shoot of these particles is supported by transpirational pull. Consequently, at higher rates of transpiration, the preferential uptake of microplastics is expected. Major concerns are related to cropping systems that frequently utilize soil amendments. Soil amendment of industrial compost has been shown to intensify microplastic uptake in cabbage[32]. Several sizes (0.2−1.2 mm, 0.05−1.5 mm, and 0.4−1.5 mm) of polyethylene have been observed in plant tissue samples. We can therefore speculate that waste amendments are likely not sustainable. Unchecked accumulation of plastic particles in soils and plants occur with amendments that are likely to reduce soil productivity and increase food toxicity, respectively.
Concerns about microplastics contaminating food continue to surge. Microplastic-contaminated fruits and vegetables are currently freely available in market. Recently, many fruits and vegetables in local markets the city of Catania tested plastic-positive[18]. In particular, apples and carrots appeared to be highly contaminated and lettuce least contaminated among the sampled fruits and vegetables. Carrots had the smallest microplastics (1.51 μm), while lettuce contained the largest (2.52 μm). Thus, the time has come that we must establish new benchmarks for food safety. This must include development of a precise food testing system.
-
Soil helps to deliver a wide range of ecosystem services. Given the longer residence, microplastics can pose various biogeochemical, biodiversity and ecotoxicological threats to soil ecosystems (Fig. 3).
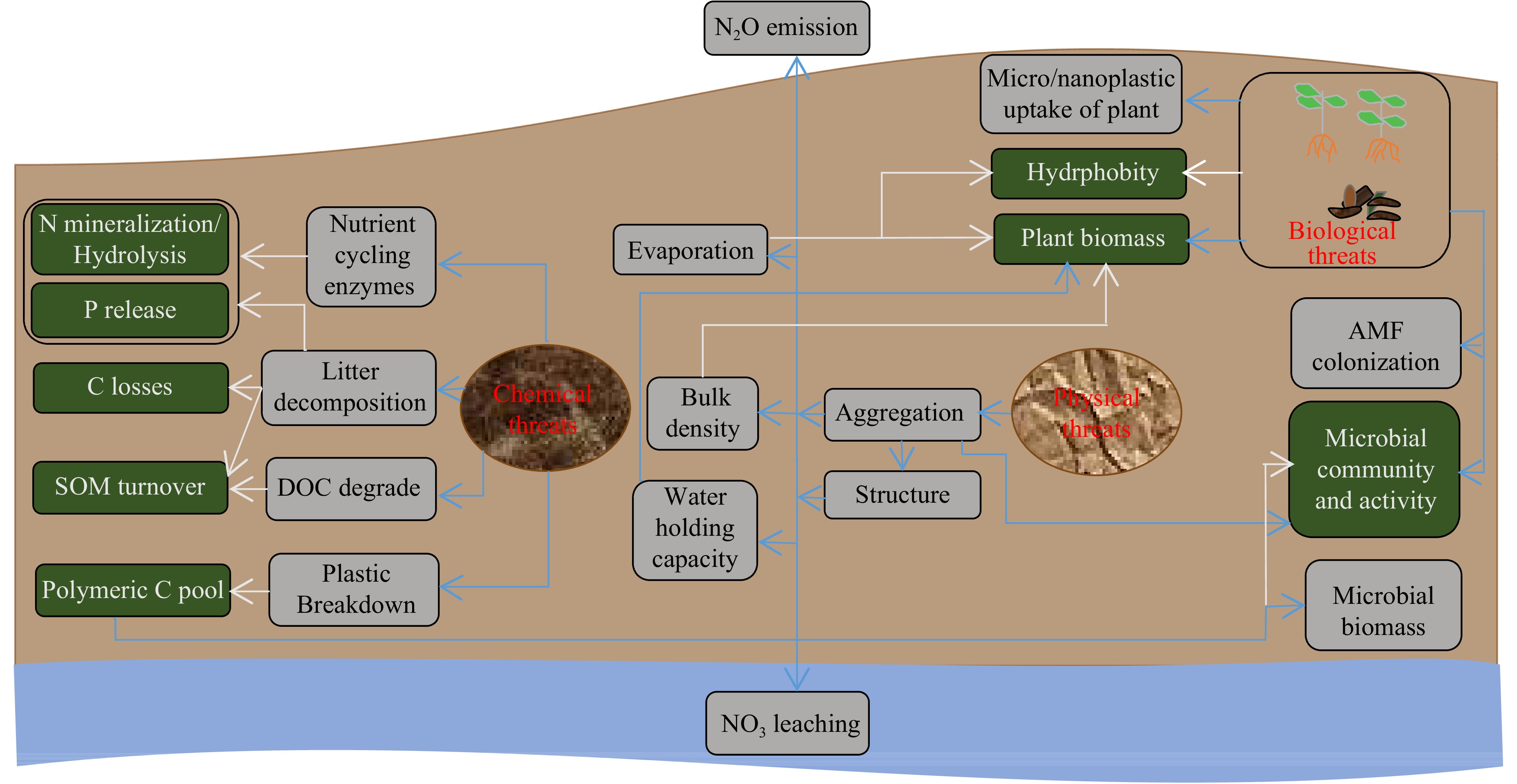
Figure 3.
Diagram disclosing various mechanisms through which microplastic can affect ecosystem functions and plant performance. Blue arrows show observed effects in previous studies while white arrows show conceptual mechanisms.
Nitrogen (N), phosphorous (P) and carbon (C) cycling
-
In soil, element cycles are often affected by external nutrient additions. Because of their inert nature and minimum nutrient contents, microplastic incorporation into soils is less likely to affect nutrient cycling[6]. Instead, through several other plausible mechanisms nutrient-cycling can be affected (Fig. 3).
Microplastics tend to alter the activity of many N transformation enzymes in soil. In particular, polystyrene and polyethylene appeared to suppress the activity of leucine aminopeptidase and N-acetyl-b-glucosaminidase[33,34]. We thus assume that these pollutants can reduce N mineralization (Fig. 3). On the contrary, polyethylene and polyhydroxyalkanoates may also promote N hydrolysis depending on soil type (Fig. 3) as they appeared to increase urease and leucine aminopeptidase activities, respectively[35−37]. Polystyrene and polytetrafluorethylene reduced the N availability mainly driven by reducing urease and proteases activity[38]. Alternatively, polypropylene can increase soil N concentrations, as it has been shown to augment PO and FDAse activity[39]. Many soil functions are inherently sensitive to soil structure[40]. As a result of aggregates formation, microplastics primarily alter soil structure[6,11]. This develops a steady link between microplastic pollution and N transformations and transport (Fig. 3). Previously, microfibers seemed to decrease NO3 leaching (70%) mainly caused by increased soil aggregation[13]. This pollutant also appeared to increase aeration and subsequently decreased N2O production[41]. Furthermore, microplastic incorporation can affect soil microbial communities and thus N processes (Fig. 3). Soil exposure to polyethylene for 30 days has been shown to increase the production of N2O, mainly owing to a reduction in Gemmatimonadacea[42].
Given the lack of evidence, effects of microplastics on P cycling are less certain. A first study showed that polypropylene (< 180 µm) presence in soil affects the cycling of dissolved P[39]. However, we did not gain mechanistic insights related to P cycling from this study. Any change to enzyme activities in soil is more likely to affect P cycling (Fig. 3). Importantly, microfiber polyester in soils has led to decreasing and increasing the phosphatase activity in well-hydrated and drought condition, respectively[13]. However, PO4−3 leaching seemed unaffected by this pollutant. Polystyrene and polytetrafluorethylene reduced P availability was mainly caused by reducing phosphatase activity[38]. This implies that microplastics can interact with soil hydraulic conditions to form P release. Arbuscular mycorrhizal fungi (AMF) scavenge nutrients for plants. In a study, microplastic polyester and polypropylene were found to be increased, while polyethylene terephthalate reduced AMF colonization around onion roots[11]. Thus, alteration of root AMF association demonstrates another possible mechanism of change in P cycling via microplastic pollution[6].
Unlike stability notion, microplastics are able to breakdown. In a recent research, polystyrene showed to breakdown within few days in agar media[28]. Additionally, polyhydroxyalkanoates, polyethylene and poly-butylene adipate-co-terephthalate provided C (after breakdown) to microorganisms to increase their biomass[37,43]. Thus, microplastics should consider a corresponding C-pool in soil that may affect global C cycle (Fig. 3). Moreover, microplastics can affect the decomposition of organic materials. Microfibers appeared to increase and decrease litter decomposition under well-watered conditions and drought conditions, respectively[13]. Accordingly, we speculate that microplastics can stimulate C losses depending on soil hydrological condition (Fig. 3).
Soil structure and water dynamics
-
While microplastics are largely known as physical contaminant, these materials alter soil aggregation to varying degrees depending upon climatic conditions (Fig. 3). Microfiber polyester and polyacrylic have shown to decrease stable water stable aggregates[23,44]. Greater decrease was observed by polyester fiber with increasing temperature[44]. Contrarily, this pollutant increased soil aggregation under drought[13]. Beyond this, microplastics polyester, polyamide, polypropylene, polyethylene, polyethylenterephthalat, polyurethane, polystyrene and polycarbonate in the shapes of fiber, foam, film and fragments appeared to decrease soil aggregation[13]. Consequently, soil aggregation and thereafter soil structure modification is inevitable in microplastic pollution. This could exacerbate climate change effects on soil-plant system.
Microplastic contamination may change water cycle in soil, exacerbating moisture limited condition[45]. Soil exposure to polyester fibers and polyamide increased evaporation and water holding capacity more over high-density polyethylene, polyethylene terephthalate and polystyrene[11]. Soil structural changes may explain these increases. Polyethylene microfilm also appeared to increase evaporation by creating channels in soil[45]. Smaller (2 mm) over larger (5 and 10 mm) films promoted more evaporation. Consequently, microplastics can lead to reduced plant biomass resulting from frequent water losses (Fig. 3). Additionally, microplastic incorporation could lead to the inaccessibility of water and soil nutrients for plants due to the impermeability of these pollutant materials[46]. Thus, microplastic pollution could exacerbate climate change conditions[47]. Soil cohesiveness is important to overcome external forces and maintain pore spacing for the circulation of air and water. Given the soil physical changes (such as bulk density and aggregation), microplastic could potentially affect rates of erosion and infiltration[40]. Hence, we conclude that microplastic induces hydrophobity for crop growth and production (Fig. 3).
Microbial communities and activities
-
Soil teems with microbiomes that perform many ecosystem functions. Microplastics are likely to alter their community structure (Fig. 3). In recent study, polyhydroxyalkanoates appeared to increase abundance of bacterial Acidobacteria and Verrucomicrobia phyla[37]. Polystyrene and polytetrafluorethylene reduced Proteobacteria and increased Chloroflexi and Acidobacteria abundance[38]. Polyethylene and polylactic acid have shown to affect the diversity and community structure of AMF genera[26]. Increasing concentration of both microplastics increased Glomeraceae and Ambispora abundance. The polyethylene increased more Glomeraceae over polylactic. Another study tested the effects of polypropylene, low density polyethylene, polystyrene and polyamide on the structure of bacteria, fungi and protozoa via phospholipid-derived fatty acids, PLFA[48]. Bacterial PLFA over fungi and protozoa was highly variable across these microplastics. Thus, it is much clear that microplastics, depending on types and concentration, are highly toxic to microbial structure.
Microplastics pollution can also shift microbial activities (Fig. 3). During 5-weeks of incubation, polyester, polyacrylic fibers, polyamide beads and polyethylene fragments led to change the microbial activity[23]. Predominantly, polyacrylic and polyester lowered the actives over control. Alternatively, polypropylene appeared to increase the microbial activity[39]. de Souza Machado et al.[11] compared the effects of microplastic in bulk and rhizosphere soils. In the rhizosphere, polyamide beads, high-density polyethylene and polyethylene terephthalate fragments appeared to decrease microbial activity. Both in rhizosphere and bulk soil, these microplastics, especially polyester, increased rates of AMF colonization. Additionally, microfibers also increased AMF colonization around onion roots[24]. Hence, we speculate that microbial activities are under great threat because of rising soil microplastics pollution.
Soil organic matter (SOM) turnover
-
Microplastics can participate in the formation of SOM via the decomposition of large C materials (Fig. 3). Polyester and polypropylene appeared to decompose surface litterbags[49]. Moreover, microfibers have also led to an increase in litter decomposition under well-watered conditions[13]. The dissolved organic carbon (DOC) pool represents an important part of SOM. Exposure of high-level of polypropylene appeared to degrade DOC by increasing activity of phenol-oxidase[39]. However, polyethylene appeared to affect relative functional groups of C rather than DOC[42]. Polystyrene and polytetrafluorethylene reduced rihizospehre SOM[38]. Thus, alteration of SOM turnover is inevitable under microplastic pollution.
-
Research on microplastic ecotoxicology in agroecosystems is a recent advancement. To fully understand microplastic ecotoxicology, we must holistically characterize their effects on crops. Our model produces the scaffolding around which different effects can be characterized. This model is flexible and applicable to other ecosystems. Additionally, we extend the model to develop a system (Table 2) that groups different crops. For example, wheat, lettuce and broad bean are very sensitive while onion is tolerant to soil microplastic pollution. However, classifying all crops is a big challenge because of limited data. As such, more work is needed to generate data on the phytotoxicity of crops to feed our system. Considering the rising direct toxicity in crops, scientists must develop systems that set benchmarks for food safety. At the ecosystem level, microplastic impacts are widely unknown; however, there are some possible mechanisms that likely devastate ecosystem functions. As a result of changes in enzyme actives and soil physical properties, microplastics are likely to affect N and P cycling. Microplastic accumulation in soil is likely to act as corresponding pool of C. It would follow that microbes could utilize this C pool as a substrate. With an increase in concentration, these pollutants can affect soil microbial communities and activities. In addition, impacts on SOM turnover are also possible. Based on these potential changes, ecosystem functioning should be a research priority.
This work was generously supported by the Key Project from the Ministry of Sciences and Technology of China (No. 2017YFC0505100). Authors are also thankful to Yunnan Human Resource and Social Security Department for providing funds. In addition, Dr. Shahid Iqbal and Dr. Sehroon Khan acknowledge funds from the Chinese Academy of Sciences for the President’s International Fellowship Initiative (Grant nos. 2021PB00094 and 2019PC0011) for his postdoctoral research.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2021 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Iqbal S, Xu J, Khan S, Arif MS, Yasmeen T, et al. 2021. Deciphering microplastic ecotoxicology: impacts on crops and soil ecosystem functions. Circular Agricultural Systems 1: 8 doi: 10.48130/CAS-2021-0008
Deciphering microplastic ecotoxicology: impacts on crops and soil ecosystem functions
- Received: 16 April 2021
- Accepted: 22 May 2021
- Published online: 25 June 2021
Abstract: Review on microplastic toxicity in agroecosystems is scarce. Thus, we develop a conceptual model (based on literature to date) that describes various microplastic effects using a size-scale. We also classify crops depending on their observed responses, and discuss several conceptual mechanisms of soil functions. The model shows that microplastic effects on crops can be positive, toxic, lethal and no-effect. Predominantly, microfibers in a wide range of sizes can positively affect crops. However, toxic effects of microplastics with/without other pollutants are more common at different sizes. Surprisingly, biodegradable plastic effects are lethal, calling into question their environmental friendliness. No-effect on crops is also possible but less observed. Unlike other crops (e.g., wheat, maize and bean), only onion seems resistant to microplastics. Crop uptake of micro/nanoplastic demands a clear benchmark to ensure food-safety. Furthermore, mixed effects are observed on soil functions. Alternation in soil enzymes and litter decomposition can affect nutrients and organic matter biogeochemistry. Hydrophobicity can be induced by increasing evaporation. Shifts in microbial community structure and activities are inevitable.
-
Key words:
- agroecosystems /
- crops /
- crop uptake /
- toxicity /
- microplastic /
- soil functions












