-
Five billion tons of plastic polymers have been discarded as waste[1]. Their persistence and negative ecological effects are of substantial concern[2], and recovery and reuse strategies are being actively explored. Most of them are thermoplastics (polyethylene, polypropylene, polystyrene, etc.) amenable to some forms of recycling[3] when available in clean and uncontaminated condition. While polyurethanes (PU) comprise only about 7% of current production[1], such thermosetting plastics offer limited recycling options, and their combustion for bioenergy produces toxic gases[3]. Furthermore, PU generally resists biodegradation[4,5] and can persist in environments long term[6]. Fungal and bacterial incubations in industrial settings produce valuable chemical derivatives from plastic polymers[3,7], but energetic and infrastructural costs are absent from the ‘natural’ biodegradation of these materials
Several studies have published rates of consumption and degradation of various plastic polymers by various insects (see Table 1 below). Insect-based recycling has been partially reviewed[8]. They summarized rates of consumption for polyethelyene (PE) and polystyrene (PS) by Tenebrionid beetle larvae. They also identified studies in which the incorporation of carbon from polymers into insect biomass has been demonstrated. They also highlight studies in which microparticles or decomposition products have been shown in insect frass. Another study[9] included Pyralid moth larvae as well as Tenebrionid beetle larvae, but it did not broadly summarize results. Use of these insect groups to degrade plastics was considered to be uneconomical[10]. However, they used atypically low degradation rates in their analyses. They suggested that the economics could be improved if glycerol or other small hydrocarbons could be derived from processing. Most importantly for our analysis, while they did cite studies showing insect protein to be useful for animal feed and human food, this aspect was excluded from their analysis.
Table 1. Rates of plastic polymer degradation from published sources and from this study. PS = polystyrene, PE = polyethylene, PVC = poly vinyl chloride, PU = Polyester polyurethane, tire crumb = vulcanized natural latex rubber, v-SBR = vulcanized butadiene-styrene elastomer, WC = honey bee wax comb, micro = microparticulate. Food additives and mixtures are indicated; B = bran. * weight from other studies. ** weight is our estimate. One study included ultraviolet light-pre-treated polymer, and its result is highlighted in red. Where rates in these units were reported in original, they are listed directly here. Reference numbering is contiguous with text.
Ref. # Polymer type Insect type Individual live
weight (mg)Insect total live
weight (mg)Test duration
(d)Consumpt. (mg) /
live weight (g) / day[20] PS foam Land snails Achatina fulica 26 080 26 080 28 0.024 [10] PE Yellow mealworm larvae (Tenebrio molitor) 85 8 500 38 0.06 [10] PE Yellow mealworm larvae (Tenebrio molitor) 85 8 500 38 0.27 This study PU foam Gryllus bimaculatus cricket adults 650 32 500 18 0.28 [21] tire crumb Yellow mealworm larvae (Tenebrio molitor) 70* 17 500 21 0.57 [22] PS plate Yellow mealworm larvae (Tenebrio molitor) 30 3 000 21 0.63 [23] PE foam Yellow mealworm larvae (Tenebrio molitor) 75 7 500 40 0.64 [10] PE Wax moth larvae (Galleria mellonella) 100* 1 000 4 0.85 [24] PS (PE PS mix) Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 0.88 [21] v-SBR Yellow mealworm larvae (Tenebrio molitor) 70* 17 500 21 1.00 [25] PS foam Superworm larvae (Zophobas atratus) From text ---> 1.04 [23] PE foam Yellow mealworm larvae (Tenebrio molitor) 75 22 500 60 1.09 [26] PS foam Yellow mealworm larvae (Tenebrio molitor) 70* 35 000 30 1.14 [27] PS foam + B Wax moth larvae (Galleria mellonella) 100* 12 000 21 1.19 [22] PS powder Yellow mealworm larvae (Tenebrio molitor) 30 3 000 21 1.27 [23] PS foam Yellow mealworm larvae (Tenebrio molitor) 75 22 500 60 1.33 [25] PS foam Yellow mealworm larvae (Tenebrio molitor) From text ---> 1.40 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 1.55 [29] PS foam Superworm larvae (Zophobas atratus) 400 96 000 28 1.45 [30] PE micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 250 28 1.72 [25] PS foam + B Superworm larvae (Zophobas atratus) From text ---> 1.79 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 1.83 [27] PE + B Wax moth larvae (Galleria mellonella) 100* 12 450 21 1.87 [24] PS foam Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 1.88 [28] PS Yellow mealworm larvae (Tenebrio molitor) 79 7 900 32 1.90 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 1.96 [24] PE (PE PS mix) Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 2.00 [22] PS sheet Yellow mealworm larvae (Tenebrio molitor) 30 3 000 21 2.00 [25] PS foam + sugar Yellow mealworm larvae (Tenebrio molitor) From text ---> 2.03 [28] PS Yellow mealworm larvae (Tenebrio molitor) 79 7 900 32 2.03 [31] PLA plates Yellow mealworm larvae (Tenebrio molitor) 72 1 440 21 2.08 [31] PVC pieces Yellow mealworm larvae (Tenebrio molitor) 64 1 280 21 2.12 [25] PS foam + B Yellow mealworm larvae (Tenebrio molitor) From text ---> 2.14 [28] PS Yellow mealworm larvae (Tenebrio molitor) 79 7 900 32 2.15 [28] PS Yellow mealworm larvae (Tenebrio molitor) 79 7 900 32 2.15 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 2.16 [30] PE micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 600 28 2.23 [27] PS foam + wax Wax moth larvae (Galleria mellonella) 100* 13 200 21 2.27 [32] PS foam + cofeed Superworm larvae (Zophobas atratus) 129 12 900 87 2.35 [22] PS foam Yellow mealworm larvae (Tenebrio molitor) 30 3 000 21 2.38 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 2.44 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 2.56 [30] PS micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 600 28 2.60 [30] PVC micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 250 28 2.79 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 2.8 [24] PE Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 2.88 [33] PS foam Superworm larvae (Zophobas atratus) 469 18 760 20 2.90 [28] PS Yellow mealworm larvae (Tenebrio molitor) 79 7 900 32 3.04 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 3.04 [25] PS foam + sugar Superworm larvae (Zophobas atratus) From text ---> 3.19 [30] PE micro Yellow mealworm larvae (Tenebrio molitor) 70* 29 050 28 3.20 [24] PS + half B Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 3.38 [31] PS plates Yellow mealworm larvae (Tenebrio molitor) 70 1 400 21 3.64 [34] PS foam Yellow mealworm larvae (Tenebrio molitor) 63 8 000 31 3.86 [30] PS micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 600 28 3.86 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 3.92 [24] PE + half B Yellow mealworm larvae (Tenebrio molitor) 80 8 000 32 4.00 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 4.12 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 4.15 [30] PVC micro Yellow mealworm larvae (Tenebrio molitor) 70* 33 425 28 4.27 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 4.28 [30] PVC micro Yellow mealworm larvae (Tenebrio molitor) 70* 28 350 28 4.28 [32] PE Superworm larvae (Zophobas atratus) 137 13 700 33 4.28 [32] PE + cofeed Superworm larvae (Zophobas atratus) 129 12 900 87 4.43 [32] PS foam Superworm larvae (Zophobas atratus) 137 13 700 33 4.49 [33] PS foam Yellow mealworm larvae (Tenebrio molitor) 46 2 300 20 4.80 [27] PE + wax Wax moth larvae (Galleria mellonella) 100* 12 450 21 4.82 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 4.85 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 4.90 [30] PS micro Yellow mealworm larvae (Tenebrio molitor) 70* 29 400 28 4.92 [34] PS foam Dark mealworm larve (Tenebrio obscurus) 63 8 000 31 5.14 [32] PE + cofeed Superworm larvae (Zophobas atratus) 137 13 700 33 5.25 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 32 5.31 [10] PE Wax moth larvae (Galleria mellonella) 100* 1 000 3.71 5.37 [32] PS foam + cofeed Superworm larvae (Zophobas atratus) 137 13 700 33 5.61 [10] PE Wax moth larvae (Galleria mellonella) 100* 1 000 9 5.67 [28] PS Yellow mealworm larvae (Tenebrio molitor) From table ---> 35 5.92 [27] PS foam Wax moth larvae (Galleria mellonella) 100* 6 600 21 6.35 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 24 7.17 [28] PS + B Yellow mealworm larvae (Tenebrio molitor) From table ---> 35 7.64 [27] PE Wax moth larvae (Galleria mellonella) 100* 10 800 21 8.60 [10] PE Wax moth larvae (Galleria mellonella) 100* 1 000 0.71 9.18 [35] PS foam Darkling beetle larvae (Plesiophthalmus davidis) 130** 14 18.8 [36] PE Wax moth larvae (Achroia grisella) 52 5 200 8 27.0 [36] PE + WC Wax moth larvae (Achroia grisella) 56 5 600 8 39.1 [37] PE Rice moth larvae (Corcyra cephalonica) 12** 300 20 41.7 [36] WC Wax moth larvae (Achroia grisella) 56 5 600 8 50.2 [38] PE Wax moth larvae (Galleria mellonella) 32 3 200 0.5 57.5 [39] PE Wax moth larvae (Galleria mellonella) 100* 1 500 7 81.4 [39] PE UV trt Wax moth larvae (Galleria mellonella) 100* 15 000 7 240 [39] WC Wax moth larvae (Galleria mellonella) 100* 1 500 7 394 Other reviews consider microorganisms extracted from insect guts that can be cultured independently to degrade plastic polymers[11−15]. This is an incomplete list because reviewing external cultures and use of insect-derived microbes are not our main focuses here. However, we nonetheless return to the topic later because most attention has been paid to bacterial enzymes and very little to fungal enzymes.
For insects fed plastic polymers exclusively or with co feeds, valorization only occurs if insects generate downstream value either as feed or frass. Indeed, most insect species examined for plastic degradation were selected because they are already used as animal feed. For this reason, they are readily obtainable in large numbers and with uniform characteristics, and suitable rearing conditions are known. However, the obvious conclusion has not yet been drawn: that feed insects can have their diets supplemented with plastic wastes, and their already-recognized value can be enhanced by including this novel form of nutrition.
Here, we more completely summarize published insect consumption results for PE and PS, and include effects of mixing supplemental feed with the polymers. We add additional information on consumption of polyvinyl chloride (PVC). We present new results for insect consumption of Polyester polyurethane (PU) which has not been previously shown susceptible to insect degradation, and this is the first demonstration of identified gut fungi being responsible. In addition, we summarize results for other polymers (rubber tire crumbs, butadiene-styrene elastomer (SBR), and wax combs from honeybees. These together present the broadest examination to-date of insect degradation of hydrocarbon polymers, both natural and man-made.
We further suggest that the insects examined thus far for polymer degradation represent a very narrow selection of potential candidates. Notably absent are xylophagous insects with wood-degrading gut fungi. Wood is the most abundant hydrocarbon polymer and most extensively degraded by any insect groups by a wide margin. Those insects are the most prominent examples of fungal-assisted polymeric degradation. Other prominent food-source insects (e.g., black soldier fly, Hermetia illucens) have yet to be examined for potential as degraders of plastic polymers[16].
We stress that all insects studied so far have been used as they were received for experimentation. No attempts have been made to improve their degradation ability by modifying their gut microbiota, either with bacteria or fungi. This will be an important area for future research into valorization of plastic wastes.
At the same time, there is concern over potential negative environmental effects of microplastics. The extent to which microplastics become incorporated into insect biomass or frass is in urgent need of elucidation.
All man-made plastic polymers contain plasticizers and other additives[17]. These chemicals have not been examined in the context of insect biodegradation and may impact their suitability as feed or frass.
Insects are widely recognized as components of human and animal food chains[18]. Whether their value can be further increased as degraders of plastic wastes depends on matters raised in this review.
-
Plastic foam, made of polyester polyurethane (PUF), was purchased from Jinfeng Plastic Insulation Materials Co., Ltd, Shenzhen, China.
A population of Gryllus bimaculatus De Geer,
1773 was purchased from the Sanmao Cricket Insectary in Wuxi City, Jiangsu Province, China. Identification of the crickets was based on morphological characteristics and taxonomic classification was made with reference to Integrated Taxonomic Information System[19]. The crickets were maintained under controlled conditions of temperature 24 ± 2 ℃, relative humidity 75 ± 2%, and 8:14 hours light to dark ratio for 18 days.Two duplicate experiments, with three replicates in each, were performed in parallel. Six incubators were prepared with 50 crickets in each incubator (each cricket was 45 days old). Each experiment varied only in the material fed to the crickets during the 18-days incubation period. As a control, the crickets received 4 g/50 crickets as a diet comprising wheat bran, wheat germ, and yeast powder, with a ratio of 10:3:1, respectively. Additional supplementation of 0.2% of the total amount of vitamin powder was added into the control diet, and water was kept in all petri dishes. In the experimental treatment, the crickets received 4 grams of 100% PUF/50 crickets as their sole source of carbon, and water (about 2 ml/day in the 90 mm Petri dishes) was also provided. The PUF was sterilized by exposing it to UV for five minutes prior to incubation. The number of crickets that had survived after 3, 6, 9, 12, 15 and 18 days was recorded.
Percentage mortality, fecula weight, and biomass change of crickets
-
Weight of produced fecula was recorded every three days for a period of 18 days. Changes in cricket biomass were determined by measuring the weight of five randomly selected crickets at the beginning of the experiment and after every three days for a period of 18 days, and the mean change in cricket biomass was determined for each time period. Furthermore, the percentage mortality (% M) of the crickets was determined every three days.
Isolation of cricket gut fungus
-
Using crickets from the PUF treatment, 20 healthy adults were selected and placed in a refrigerator until they become motionless. Their surfaces were washed and sterilized with 75% ethanol. Cut were made in the ventral side of the abdomen to open the exterior layer, and pinned to a cork surface. The anterior and posterior ends of the alimentary canal were removed using scissors and forceps. The entire alimentary canal was divided into foregut, midgut, hindgut and rectum. Microbiota were isolated from the alimentary canal by spreading each segment on nutrient agar and potato dextrose agar (PDA) plates containing tetracycline antibiotic. Prior to placement on the PDA plates, each segment was washed in saline solution. The resultant cultures were sub-cultured on fresh plates and incubated at 30 ℃ for one month. The process was repeated, until a single fungal strain was obtained on each plate.
Insect degradation of plastic polymers
-
Data sources come from those referenced an earlier review[8], their citations, and from additional searching with Microsoft Academic. Studies not reporting consumption rates but with information on insect survival, growth and fecundity are not included in Table 1 but rather as ancillary data. For consistency, all studies in Table 1 are converted to polymer consumption rates per grams of live insects per day. Where conversion to gram live weight could not be performed from information presented in individual studies, sources of information needed (milligram weight per individual) are noted. Duration of studies is also reported, as studies vary in duration and consumption rates tend to decline over time. Differences resulting from supplementing plastic with other feeds are described in text.
Fig. 1 Summarizes results for PE, PS, PVC and PU in terms of mean values and standard errors for each insect species and feeding condition. Outlier values are mentioned individually in text.
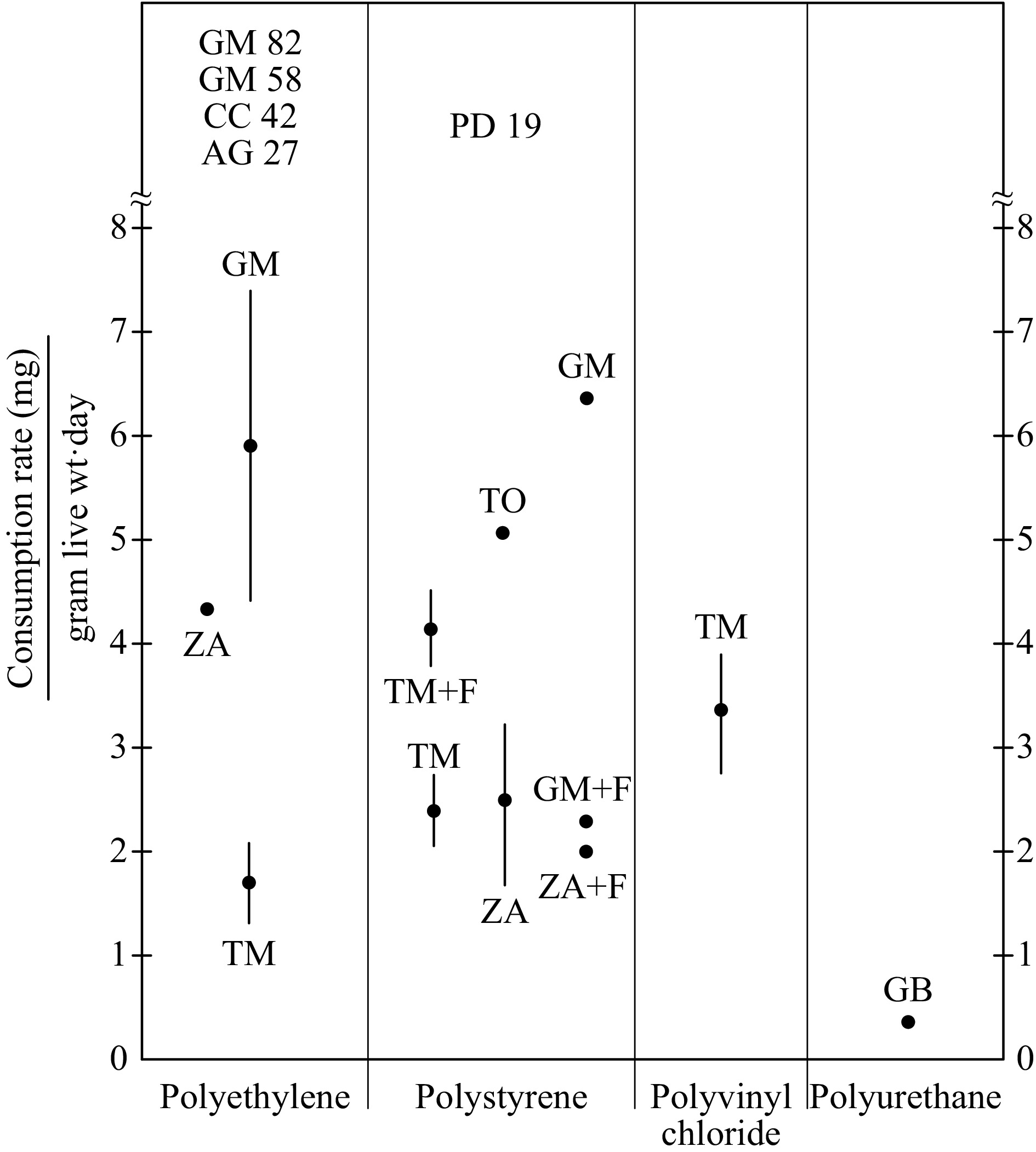
Figure 1.
Means and standard error for insects and plastic polymers with multiple results reported. Individual values are shown by insect abbreviations. Values above 8 mg/gram live weight day show measured values. AG = Wax moth larvae (Achroia grisella), CC = Rice moth larvae (Corcyra cephalonica), GB = Cricket adults (Gryllus bimaculatus), GM = Wax moth larvae (Galleria mellonella), PD = Darkling beetle larvae (Plesiophthalmus davidis), TM = Yellow mealworm larvae (Tenebrio molitor), TO = Dark mealworm larve (Tenebrio obscures), ZA = Superworm larvae (Zophobas atratus).
Only one xylophagous insect species, Ambrosia beetle (Cnestus mutilatus) adult has been shown to consume PE and is included, although consumption rates were not reported. One non-insect species, the land snail Achatina fulica, has been shown to consume PS foam and is included. These snails are raised commercially for feeding ducks.
-
Highest rates are from Galleria mellonella moth larvae (mean 5.9 milligrams per gram live weight per day, standard error 1.5, N = 5). A single study of Zophobas atratus beetle larvae reported 4.3 mg/g/d. Tenebrio molitor beetle larvae mean rate was 1.7 mg/g/d (standard error 0.4, N = 7). Much higher rates were reported by single studies for Achroia grisella moth larvae and Corcyra cephalonica moth larvae; 27 and 42 mg/g/d, respectively. High rates of 82 and 58 mg/g/d were reported in single studies of Galleria mellonella moth larvae, but they are considered outliers only in a statistical sense. Galleria mellonella moth larvae and others showing such high rates may be made routinely capable under improved conditions.
One particularly low reported Tenebrio molitor beetle larvae rate of 0.06 mg/g/d is notable[10] because their conclusion that plastic consumption by insects is impractical at large scale was based in part on that result.
The highest reported PE degradation by Galleria mellonella moth larvae was 240 mg/g/d following ultraviolent (sunlight) pre-treatment[39]. This is higher than any other report for man-made plastic polymers. It contrasts with some reports that PE is chemically resistant to photodegradation[9] and suggests that photodegradation may be a useful adjunct for polymer degradation by insects.
Insect Consumption of PS
-
This polymer was more slowly degraded than PE overall. Zophobas atratus beetle larvae degrade PS at a mean rate of 2.5 mg/g/d (standard error 0.8, N = 4). Mean rate by Tenebrio molitor beetle larvae was 2.4 mg/g/d (standard error 0.3, N = 19). With supplemental feeding, mean rate by Tenebrio molitor beetle larvae increased to 4.1 mg/g/d (standard error 0.4, N = 15). In contrast, supplemental feeding decreased Galleria mellonella moth larvae mean rates from 6.4 to 2.3 mg/g/d. Single studies of PS degradation by Plesiophthalmus davidis beetle larvae and Tenebrio obscurus beetle larvae yielded degradation rates of 19 and 5.1 mg/g/d, respectively.
Only one non-insect has been demonstrated to degrade plastic polymers. It is the land snail Achatina fulica. It degrades PS at a rate of 0.024 mg/g/d.
Insect Consumption of PVC and PU
-
Larvae of Tenebrio molitor beetles degrade PVC at mean rate of 3.4 mg/g/d (standard error 0.5, N = 4). This seems lower than PE but broadly similar to PS. More insects and feeding conditions should be examined for this polymer.
Our report here is the first for insect degradation of PU. Gryllus bimaculatus adult crickets consume PU at mean rates of 0.28 mg/g/d. It also shows that these crickets survive on PU as their sole carbon source. The fungus Aspergillus flavus was extracted from the gut of Galleria mellonella wax moth larvae and demonstrated to degrade PE in external cultures[40]. These are the only two reports of an insect gut fungus being identified as degrading plastic polymers. Possible reasons for that are considered in Discussion. Gryllus bimaculatus adult crickets consume PU at rates lower than other insects on PE, PS and PVC (Fig. 1). This may relate to difficultly of degradation of PU. It also could indicate that ideal conditions in terms of insects, gut microbes, and feeding practices are yet to be developed for PU.
Other insect consumption of polymers not reporting rates.
-
An early study synthesized PS, polymethyl acrylate and, phenol formaldehyde from 14C- labeled compounds[41]. They detected no labeled CO2 production from the isopod Oniscus asellus, a millipede Diploiulus sp.; a snail Oxychilus draparnaldi, the slugs Limax maximus and Deroceras reticulatum, nor earthworms Eisenia foetida, Eudrilus eugeniae, Pheretima spp. This technique is sensitive, but only where carbon is completely oxidized to CO2, not merely depolymerized. PE consumption was observed by Plodia interpunctella Indian mealmoth larvae, but rates were not quantified[42]. PE consumption was also observed by Cnestus mutilatus ambrosia beetle adults but not quantified[43]. Several other studies of unquantified polymer consumption have been published but are not mentioned here.
Galleria mellonella wax moth larvae survived and gained weight on PE and honey bee wax comb with supplemental nutrition, but consumption was not quantified[44]. They further asserted that polymeric degradation was independent of the microbial gut community. This is important for future work on Galleria mellonella. Tenebrio molitor beetle larvae were reported to survive and become fecund on a pure PS diet[45]. Circular insect culture based on plastic wastes will not require complete life cycles, but this information may nonetheless be useful.
Insect consumption of other polymers
-
There is no value in degrading the natural polymer wax comb of honeybees. However, its presence for millions of years along with consuming insects and their gut microbiota may provide general limits on hydrocarbon degradability rates. Achroia grisella moth larvae were reported to consume 50 mg/g/d[36], and Galleria mellonella wax moth larvae were reported to consume 394 mg/g/d[39].
Rubber tire crumb consumption rates by Tenebrio molitor beetle larvae were reported as 0.57 mg/g/d and butadiene-styrene elastomer as 1.0 mg/g/d[21]. It is possible few man-made polymers have insect (with non-optimized gut microbiota) degradation rates of such low levels.
New experimental results for crickets and polyurethane
Cricket consumption of, survival on and fecula production with polyurethane foam as food
-
Over 18 days, 50 crickets consumed 0.163 g of PU foam, about 4.1% of the material supplied (Fig. 2). Control crickets consumed 2.53 g of their total feed (4 g) or 63%.

Figure 2.
a, b. Gryllus bimaculatus feeding on polyurethane foam. The holes, valleys and hollows formed in the PUF blocks showed that the cricket used PUF as their diet.
At day 3, 6 and 9 of the experiment, the percentage fecula weight of the experimental crickets (fed PUF) was lower than control-diet crickets. However, at day 12, 15 and 18, the percent fecula weight of the control was lower than PUF fed ones (Fig. 3a). Significant differences were observed between experimental and control cricket percent fecula weights through time intervals days (p <
0.0001 ). No significant difference was found between body weights of the experimental crickets with controls (Fig. 3b) (p = 0.135). Mortality was higher with a pure PU foam diet (Fig. 3c).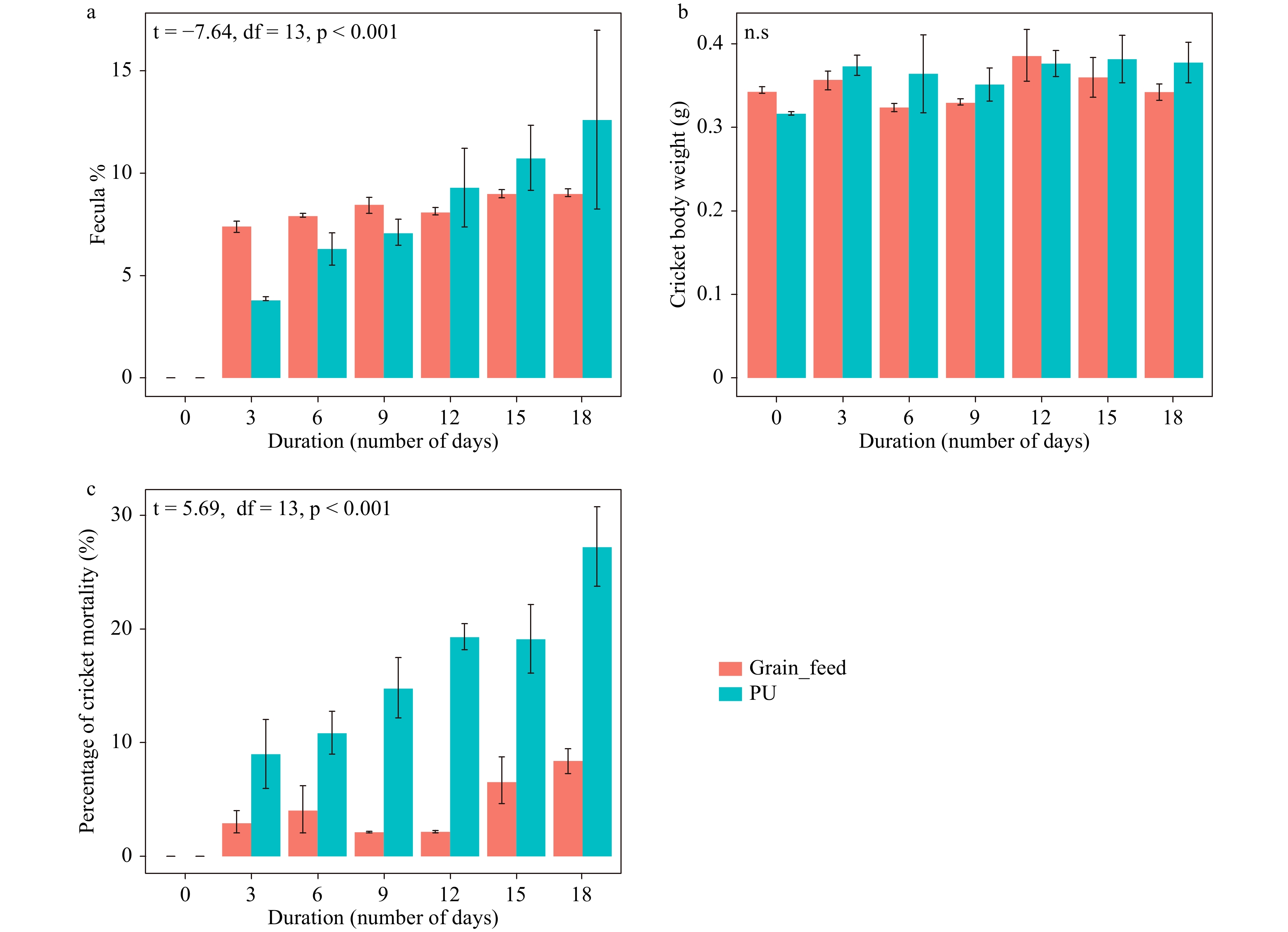
Figure 3.
Cricket performance through time with PUF or grains as feed. (a) Percentage fecula weight of crickets. (b) Mean weight of crickets. (c) Percentage mortality for both treated and untreated crickets.
Isolation and identification of fungi from cricket guts
-
Nine different microorganisms, comprising five bacterial, three fungal and one yeast species, were isolated from the gut of the crickets fed with PUF as a sole source of carbon. Screenings for PU-degrading activity by these microbes were subsequently performed by analyzing their colonization on the PU film, weight loss in PU films after incubation, and the SEM analysis of PU surfaces exposed to each microbe. The results (data not shown) identified only one fungal species that was capable of colonizing and biodegrading the PU films. The fungus was identified to be Aspergillus flavus G10 based on morphological characteristics, and phylogenetic analysis (Fig. 4a−h, Fig. S1). Further details are presented in supplemental results.
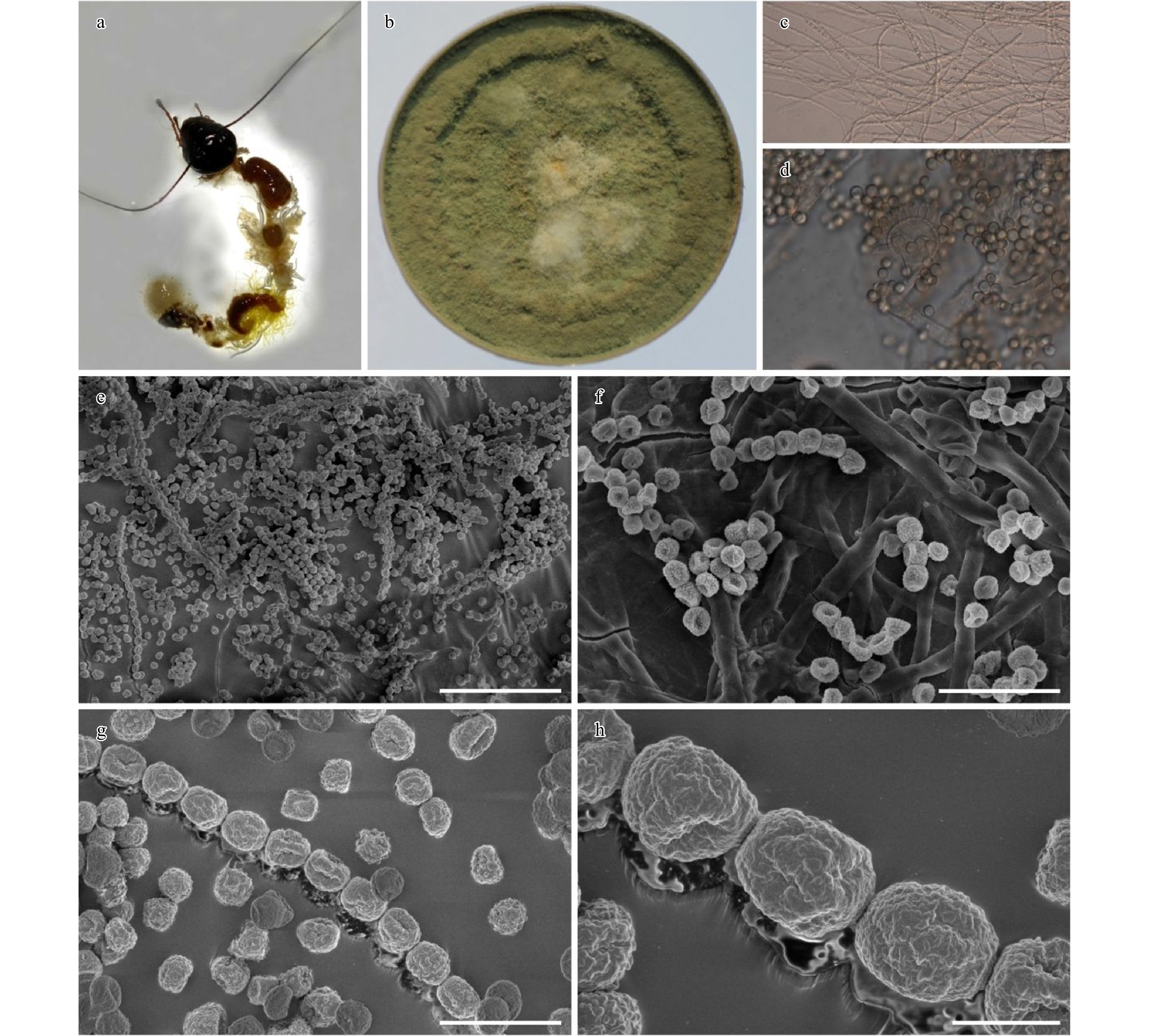
Figure 4.
Aspergillus flavus G10 (HMAS 101889). (a) Alimentary canal of the dissected cricket (Gryllus bimaculatus). (b) Culture on MEA (20 days). (c) Hyphae. (d) Conidiogenesis (light microscope). (e–h) Conidiogenesis and conidia (SEM). Scale bars e = 50 µm, f = 20 µm, g = 10 µm, h = 3 µm.
Molecular identification and phylogenetic classification of A. flavus G10 are presented in supplemental results. Evidence for polyurethane biodegradation by Aspergillus flavus G10 is presented in supplemental results.
-
Insects are already a growing component of nutritional supply for humans and their animals[18]. They can feed on and thus valorize agricultural wastes[46]. For insects to also valorize plastic wastes, they must be shown to:
- Consume (aided by gut microbiota) plastic polymers rapidly
- Convert plastic-polymer carbon (and nitrogen where present) into biomass or useful frass
- Avoid transferring microparticulate plastics to biomass or frass, if those are shown harmful
- Avoid transferring plasticizers or other additives to biomass or frass, if those are shown harmful
Insects’ best claim on this role is that they and their gut microbial symbionts have been converting hydrocarbon polymers to insect biomass for hundreds of millions of years[47−49]. No man-made polymers present novel chemistries that insects and their gut microbes have not already long been exposed to.
The research reviewed here has examined an incomplete list of man-made polymers. It addresses an incomplete list of insects already valorized for food and feed. Importantly, it has examined insects and their gut microbiota only ‘as received’ from feed-insect-rearing facilities or as collected in the field. As reared insects are optimally fed, there is no certainty that their gut microbiota are suited to the matter at hand. No study has looked for insects in waste dumps where plastic-degrading microbes have famously been isolated[50].
Previous studies not reviewed here have identified many bacteria and fungi competent in degrading the full range of plastic polymers. No study has attempted to inoculate those into insect species, including those used for food or feed. It must be said that while almost all studies cited here find insect-gut bacteria to be important, those same studies use bacterial DNA primers to prove their results. Fungal primers might tell a different story, but these are parts of a larger picture that must be seen holistically. It remains remarkable that, despite the broad range of decomposition by fungal enzymes, only this study and one other[40] identified effective fungi from insect guts.
Some research cited here shows higher plastic degradation rates with co-feeding, but others show lower rates. In no case has optimal plastic conversion to insect biomass been demonstrated. Emphatically, this has also not been demonstrated with insect gut microbiomes intentionally optimized for plastic degradation. Plastic waste in the environment are mixed in identity, and they co-exist with other organic wastes. If insects or any other approach can valorize mixed wastes, they must do so based on real-world substrates.
Toward this goal, all the chemically competent xylophagous insects with gut fungi[51] remain to be examined.
High rates reported here for microbially non-optimized feed insects suggest a bright future for valorizing plastic wastes by insect consumption. Much fundamental research, however, remains to be done.
This work was supported by Key Research Program of the Ministry of Sciences and Technology (Grant No. 2017YFC0505101) of China, Chinese Academy of Sciences, President’s International Fellowship Initiative (CAS-PIFI), Grant No. 2019PC0011, 2017PC0035 and Key Research Program of Frontier Sciences, CAS, Grant No. QYZDY-SSW-SMC014. We thank the National Science Foundation of China (NSFC) for funding this work under the project codes Y4ZK111B01, 41761144055, 3181101433, 41771063, 31650410651, 41761144055 and 31550110215. We are thankful to Zhijia Gu, Key Laboratories for Plant Diversity and Biogeography of East China, Kunming Institute of Botany, Chinese Academy of Sciences for scanning electron microscopy. G.G.O. Dossa thanks China Postdoctoral Foundation Grant No. 2017M613021, and the young international staff Chinese Academy of Sciences (CAS) president international fellowship initiative (PIFI) grants # 2019FYB0001 and 2017PC0035. Heng Gui would thank the CPSF-CAS Joint Foundation for Excellent Postdoctoral Fellows (Grant No. 2017LH029), the China Postdoctoral Science Foundation (Grant No. 2018M633435) and the 2018 Yunnan Province Postdoctoral Science Research Foundation. Heng Gui would also like to thank the support from the Human Resources and Social Security Department of Yunnan Province, German Academic Exchange Service (DAAD) under the program: Research Stays for University Academics and Scientists, 2018 (Ref. No. 91691203) and the China Scholarship Council under the State Scholarship Fund (Ref. No. 201804910259).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Sehroon Khan, Yang Dong, Sadia Nadir
- Fig. S1 Phylogram generated from maximum likelihood analysis based on combined partial LSU, RPB2, ITS, BTUB and CAM sequences data. Related sequences were obtained from Frisvad et al. (36). 40 strains are included in the combined sequence analyses, which comprise 3271 characters with gaps. Aspergillus muricatus (NRRL 35674) is used as the outgroup taxon. The best scoring RAxML tree with a final likelihood value of -16016.105263 is presented. The matrix had 1036 distinct alignment patterns, with 20.83% of undetermined characters or gaps. Estimated base frequencies were as follows; A = 0.232666, C = 0.252689, G = 0.268734, T = 0.245911; substitution rates AC = 1.198516, AG = 3.642609, AT = 1.139202, CG = 0.716346, CT = 6.351285, GT = 1.000000; proportion of invariable sites I = 0.430234; gamma distribution shape parameter α = 0.954354. Bootstrap support values for ML equal to or greater than 60% is given above the nodes. Newly generated sequence is in blue color.
- Supplemental results Molecular identification and phylogenetic classification of A. flavus G10 .
- Copyright: © 2021 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Khan S, Dong Y, Nadir S, Schaefer DA, Mortimer PE, et al. 2021. Valorizing plastic waste by insect consumption. Circular Agricultural Systems 1: 7 doi: 10.48130/CAS-2021-0007
Valorizing plastic waste by insect consumption
- Received: 08 January 2021
- Accepted: 09 February 2021
- Published online: 13 April 2021
Abstract: Insects first began evolving hundreds of millions of years ago, and aided by gut microbes, they have been consuming hydrocarbon polymers ever since. Few man-made plastic polymers are chemically novel, so it is reasonable that insect/microbe systems can be found or developed to degrade them rapidly. However, remediation of global plastic waste problems should involve more than just conversion into CO2. Some industry-scale microbial enzymatic degradation of plastic polymers may yield valuable monomers, but the plastic waste starting material must be of uniform chemistry and clean. This adds cost to the process. Many insect species can be utilized for animal feed as well as human food. Some of these insects have the capability to degrade plastic polymers. However, valorizing plastic wastes by producing edible insects or useful frass has largely been overlooked. Here we assemble the current knowledge of plastic degradation rates by insects. In addition, we also show the first instance of insect degradation of polyurethane and the first identification and isolation of insect gut fungi as directly aiding insect degradation.
-
Key words:
- plastic degradation /
- crickets /
- Aspergillus













