-
Tibetan pig is a precious genetic resource in China, mainly living in the semi-agricultural and semi-pastoral area of the Qinghai-Tibet plateau at an altitude of 2,500−4,300 m[1]. As an excellent local breed in China, Tibetan pig is characterized by strong environmental adaptability, high fertility and rich intermuscular fat, which has attracted extensive attention in the domestic pig breeding industry. Shen et al.[2] compared three kinds of pigs living at different altitudes: Tibetan Pig, Liangshan pig and Duroc pig. It was found that Tibetan pig and Liangshan pig of high altitude breed had higher pH, redder meat color and lower shear force than Duroc pig. The difference was attributed to the lower proportion of glycolytic fiber in plateau pigs, which reduced the post-mortem glycolysis process, so they had better post-mortem meat quality. Different pig breeds have different interspecific characteristics due to their different genetic background, growing geographical environment and feeding management. Previous studies have found that there are specific breed characteristics in lipid metabolism between Korean native pigs and Western Landrace pigs[3]. The difference in fat content between the two pig breeds may be related to the difference in liver fat metabolism function. Fat deposition, as an important target economic trait in pig genetic improvement, directly affects pig growth efficiency and pork quality[4, 5].
Liver is an important lipid metabolism organ for animal body weight, and its fat production affects fat deposition in muscle and carcass[6]. The process of fat deposition is also influenced by genetic selection, nutrient level and environmental conditions[7]. The study on lipid metabolism of Tibetan pigs can provide reasonable nutritional regulation and has certain significance for practical production.
In this study, we compared fatty acid composition of longissimus dorsi and liver lipid metabolism-related enzyme gene expression between DLY pigs and Tibetan pigs from three different regions, aiming to provide theoretical guidance for production and processing of Tibetan pigs and to provide theoretical basis for comparing the interspecific characteristics of lipid metabolism among different pig breeds.
-
All animal procedures and care were performed in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China).
Nyingchi Tibetan pigs (n = 10), Gannan Tibetan pigs (n = 9) and Aba Tibetan pigs (n = 10) at the normal development age of one year old were selected under the natural grazing condition at about 3,000 m altitude (Nyingchi Tibetan pig: provided by Bayi District, Nyingchi City, Tibet Autonomous Region, with an altitude of 3,100 m; Gannan Tibetan pig: provided by Hezuo City, Gannan Prefecture, Gansu Province, with an altitude of 2,950 m; Aba Tibetan pig: provided by Xiaojin County, Aba Prefecture, Sichuan Province, with an altitude of 3,100 m). Ten-month-old Duroc × Landrace × Yorkshire castrate boar were purchased from a commercial pig farm (Jiangsu Food Group Co., Ltd., Huaian, China, with an altitude of 80 m).
All pigs, before slaughter, were kept in lairage with full access to water but in fasting for 12 h. The blood was collected via anterior vena cava, and the samples were centrifuged at 2,000 g for 20 min at 4 °C to obtain serum. After slaughter, about 8 g of tissue were collected from the large leaves of the liver and stored in a cryopreservation tube. About 1 kg of meat sample of the longissimus dorsi muscle was collected. After quick freezing with liquid nitrogen, it was vacuum packed and transported to the laboratory with dry ice and stored at −18 °C.
Determination of lipid metabolism parameters
-
The concentrations of total triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL) and low density lipoprotein cholesterol (LDL) in the liver and serum were measured using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All procedures were conducted in accordance with the manufacturer's guidelines. The samples were then mixed with the reaction reagents in 96-well plates for designated times. The absorbance of the reaction solution was dynamically recorded at 450 nm (Thermo Fisher, Waltham, MA, USA) and the target variables were calculated.
Liver oxidative status analysis
-
For oxidative status in the liver, the concentrations of malondialdehyde (MDA) and catalase (CAT), and the activities of total superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the protocol of the manufacturer.
Determination of intramuscular fat (IMF) and liver fat content
-
Intramuscular fat and liver fat were determined by Soxhlet extraction. Meat samples (3 g) were put in petroleum ether in a Soxhlet extractor (Soxtec Avanti 2050 Auto system, Foss, Hillerød, Denmark) for 4 h. After the program finished, the receiving bottle was placed in an oven at 100 °C for drying for 40 min, and then placed in a dryer for cooling (0.5 h) before weighing. The drying process was repeated until constant flask weight (the difference between two weights should not exceed 2 mg).
Observation of liver tissue structure
-
Liver tissue was observed by the Oil Red O Stain Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Frozen liver tissue was sliced (8 μm) and dried at room temperature for 45 min, rinsed with sterile distilled water three times and dried for 5 min. The samples were then soaked in isopropyl alcohol (Sinopharm, Shanghai, China) (60%) for 30 s. After sealing treatment, samples were placed in Oil Red O solution for dyeing for 15 min. The isopropanol solution (60%) is thoroughly rinsed and then rinsed with sterile distilled water three times to remove residue. After 2 min counterstaining with hematoxylin, samples were rinsed with sterile distilled water to remove the dye. To observe slices under a light microscope (BX51, Olympus, Tokyo, Japan), the multiple of the eyepiece is 10× and the multiple of the objective lens is 40×. Fat is stained bright red, cell nuclei are stained dark blue, and other tissues are stained light blue.
Real-time polymerase chain reaction (RT-qPCR) analysis
-
RT-qPCR was applied to quantify the mRNA levels of fatty acid synthase (FAS), apolipoprotein A (APOA), apolipoprotein E (APOE), low density lipoprotein receptor (LDLR), stearoyl-CoA desaturase (SCD), lysophosphatidic acid acyltransferase beta (AGPAT2), lipin 1 (LPIN1), peroxisome proliferator-activated receptor γ (PPAR-γ), cholesterol 7α-hydroxylase (CYP7A1), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), recombinant carnitine palmitoyltransferase 1A (CPT1A) and lecithin-cholesterol acyltransferase (LCAT), which play an important role in lipid synthesis and metabolism. Total RNA was extracted from liver samples using a MiniBEST Universal RNA extraction kit (TaKaRa, Kusatsu, Shiga, Japan). And cDNA was synthesized from mRNA using the PrimeScript RT master mix (TaKaRa) according to the manufacturer's protocols. RT-qPCR was performed in a QuantStudio 6 flex real-time PCR system (Applied Biosystems, Waltham, MA, USA). The 2−ΔΔCᴛ method was used to analyze the relative gene expression[8]. The mRNA levels were normalized relative to GAPDH, and the Duroc × Landrace × Yorkshire castrate boar group was set as control for the Tibetan pigs. Primer Premier 5.0 and NCBI online software were used to design the primers, and Genscript Biotech Corporation (Nanjing, China) was commissioned to synthesize the primers. The target primers are listed in Table 1.
Table 1. Primer sequences used in quantitative RT-qPCR analyses.
Gene Forward primer Reverse primer FAS 5'> TGGGCATGGTGAACTGTCTC<3' 5'>GTCACTGCACCACTTGAGTC<3' APOE 5'>CCAATCGCAAGCCAGAAGAT<3' 5'>CATCCTGCGAGGAGGGTTAC<3' APOA1 5'>CTGGGATCGGGTGAAGGATT<3' 5'>AAAGCGGAGGCTTCAAACTG<3' LDLR 5'>CCGGCAGGAAGAACACTTTC<3' 5'>CTGACAGACAAGCAGATGGC<3' SCD 5'>CCAGCACTAGTCTACGCTCA<3' 5'>CCCAGGGATGAGACTTCAGG<3' AGPAT2 5'>GAACGGTGGAGAACATGAGC<3' 5'>ATGCTCTGGTGGTTGGAGAT<3' LPIN1 5'>GGGAGACAATGGAGAGGCAT<3' 5'>CCGATCCAGGGAGTTCCTTT<3' PPAR-γ 5'>CCTGAGAAAGCCCTTTGGTG<3' 5'>GGCGGTCTCCACTGAGAATA<3' CYP7A1 5'>TGTTCAAGACGGGCCACTAT<3' 5'>GAGCGACTTGGCTTTCTCTG<3' HMGCR 5'>AAACCCTTGGTGGCAGAAAC<3' 5'>TTCTTCATTAGGCCGAGGCT<3' CPT1A 5'>TGCAGGATACAGCTCCTCTG<3' 5'>CCAGCACATCTGCACTCAAA<3' LCAT 5'>GAGCTCAGTAACCACACACG<3' 5'>GCTTGGCTTCCAGCTGATTC<3' GAPDH 5'>TGGAAAGGCCATCACCATCT<3' 5'>ATGGTCGTGAAGACACCAGT<3' Fatty acid analysis
-
Ground meat samples (6 g) were homogenized in 20 mL chloroform-methanol (2:1, V/V) for 30 s each three times at 8,000 r/min, and the homogenate was stood for 24 h, and then filtered. The filtrate was mixed with 8 mL of normal saline, and centrifuged at 1,500 g for 15 min. The lower liquid was blow-dried with a nitrogen blower to obtain pure fat. Fat (0.1 g) was mixed with 4 mL 2-mol/L NaOH-methanol solution, and incubated in a 70 °C water bath for 10 min. Five mL 14% boron trifluoride-methanol solution was added, and incubated in a 70 °C water bath for 15 min. Seven mL n-hexane and 10 mL saturated sodium chloride solution were added and shaken vigorously for 15 s, and centrifuged at 15,000 g at 4 °C for 5 min. The supernatant was taken and injected with 0.22 μm organic filter membrane. The resulting mixtures were analyzed on a gas chromatography system (2010 plus; Shimadzu, Kyoto, Japan) with a SP2560 column (100 mm × 0.25 mm × 0.25 mm; Supelco, Bellefonte, PA, USA). Fatty acids were identified by a mixed standard containing 37 kinds of fatty acids (CEM 47885; Supelco).
According to the method of Li et al.[9], the area normalization method was adopted to obtain the content proportion of each fatty acid.
Statistical analysis
-
The measured variables were analyzed by one-way analysis of variance in which pig source was set as an independent and means were compared by t test. The statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC, USA). Figures were made using the GraphPad Prism (version 8.0.3, San Diego, CA). For all statistical tests, the level of significance was set at 0.05 and the data were presented as means ± standard deviations.
-
Circulating TG and TC concentrations are important predictors of lipid metabolism. As shown in the Fig. 1, TC and HDL in serum of DLY pigs were significantly higher than those of Tibetan pigs from three regions (P < 0.05), while there was no significant difference in TG and LDL levels between DLY pigs and Aba Tibetan pigs (P > 0.05), but significantly higher than those of Gannan and Nyingchi Tibetan pigs (P < 0.05). There were no significant differences in TG, LDL and HDL levels between Gannan and Nyingchi Tibetan pigs (P > 0.05).
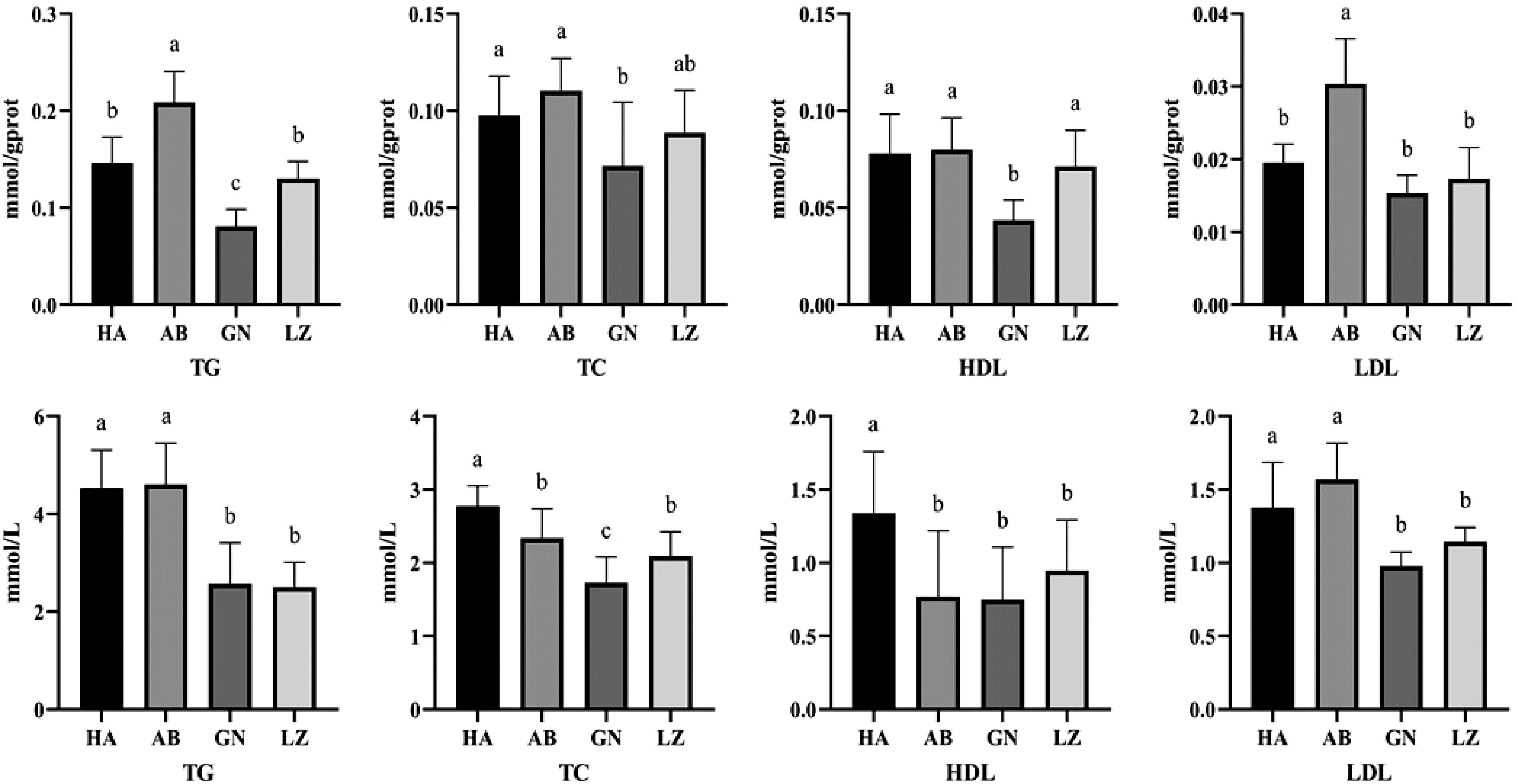
Figure 1.
Differences of liver (up) and serum (down) lipid metabolism between Duroc × Landrace × Yorkshire and Tibetan pigs. GN: Gannan Tibetan pigs; AB: Aba Tibetan pigs; LZ: Nyingchi Tibetan pigs; HA: Duroc × Landrace × Yorkshire pigs. Data are expressed as means ± SD. a, b and c indicate significant differences between different kinds of pigs (P < 0.05).
The concentration of TG and TC in liver is an important indicator of lipid metabolism in mammals. As shown in the Fig. 1, the contents of TG and HDL in liver of Gannan Tibetan pigs were significantly lower than those of DLY pigs and other Tibetan pigs (P < 0.05). There was no significant difference in LDL level between Gannan and Nyingchi Tibetan pigs (P > 0.05). The level of TG and LDL in liver of Aba Tibetan pigs was significantly higher than that of Nyingchi and Gannan Tibetan pigs (P < 0.05).
Differences of MDA, CAT, SOD and T-AOC in liver
-
Antioxidant indicators in liver of pigs are shown in Table 2. There was no significant difference in SOD activity in liver between Tibetan pigs and DLY pigs (P > 0.05). The T-AOC level in liver was significantly lower for Nyingchi Tibetan pigs than that of DLY pigs (P < 0.05), but no significant difference was observed in the T-AOC level in liver among Gannan Tibetan pigs, Aba Tibetan pigs and DLY pigs (P > 0.05). The MDA content in liver of Tibetan pigs was significantly higher than that of DLY pigs, and Aba Tibetan pigs were significantly higher than Gannan and Nyingchi Tibetan pigs (P < 0.05). The CAT activity in liver was significantly lower in DLY pigs than in Aba Tibetan pigs and Nyingchi Tibetan pigs (P < 0.05), and there was no significant difference among Tibetan pigs in the three regions (P > 0.05).
Table 2. Liver antioxidant status in different kinds of pigs.
Duroc × Landrace × Yorkshire pigs Aba Tibetan pigs Gannan Tibetan pigs Nyingchi Tibetan pigs SOD (U/mgprot) 197.8 ± 4.47a 198.7 ± 6.82a 202.5 ± 6.23a 206.0 ± 8.19a T-AOC (mmol/gprot) 0.587 ± 0.04a 0.529 ± 0.07ab 0.537 ± 0.06ab 0.488 ± 0.06b MDA (nmol/mg) 2.01 ± 0.15c 2.45 ± 0.21a 2.18 ± 0.17b 2.22 ± 0.19b CAT (U/mgprot) 198.0 ± 11.22b 221.9 ± 7.66a 211.3 ± 10.53ab 225.6 ± 6.07a Notes: The data are presented as means ± SD. The significance level is set at 0.05. a, b and c superscripts indicate significant differences between the different kinds of pigs in the same row (P < 0.05). SOD: superoxide dismutase; T-AOC: total antioxidant capacity; MDA: malondialdehyde; CAT: catalase. Comparison of IMF and liver fat content in pigs from different regions
-
Intramuscular fat, also known as marbling, is the fat that is deposited inside the muscles and has an effect on the quality of meat (tenderness, juiciness, flavor)[10]. The amount of intramuscular fat content is affected by many factors, the most important of which is animal species, while age, gender, nutrition level and other factors will also have a certain impact on intramuscular fat content. As can be seen from Table 3, there was no significant difference in intramuscular fat content of Tibetan pork among the three regions, which was significantly higher than that of DLY pigs (P < 0.05).
Table 3. Comparison of IMF and liver fat content in pigs from different regions.
Duroc × Landrace × Yorkshire pigs Aba Tibetan pigs Gannan Tibetan pigs Nyingchi Tibetan pigs IMF/% 0.97 ± 0.56b 2.83 ± 0.80a 2.79 ± 0.87a 2.15 ± 0.79a liver fat/% 2.03 ± 0.35a 2.21 ± 0.33a 1.49 ± 0.29b 2.19 ± 0.33a Notes: The data are presented as means ± SD. The significance level is set at 0.05. a and b superscripts indicate significant differences between the different kinds of pigs in the same row (P < 0.05). IMF: intramuscular fat. As shown in Table 3 and Fig. 2, the total fat content in liver of Gannan Tibetan pigs was significantly lower than that of Aba and Nyingchi Tibetan pigs (P < 0.05), but there was no significant difference among Aba Tibetan pigs, Nyingchi Tibetan pigs and DLY pigs (P > 0.05).
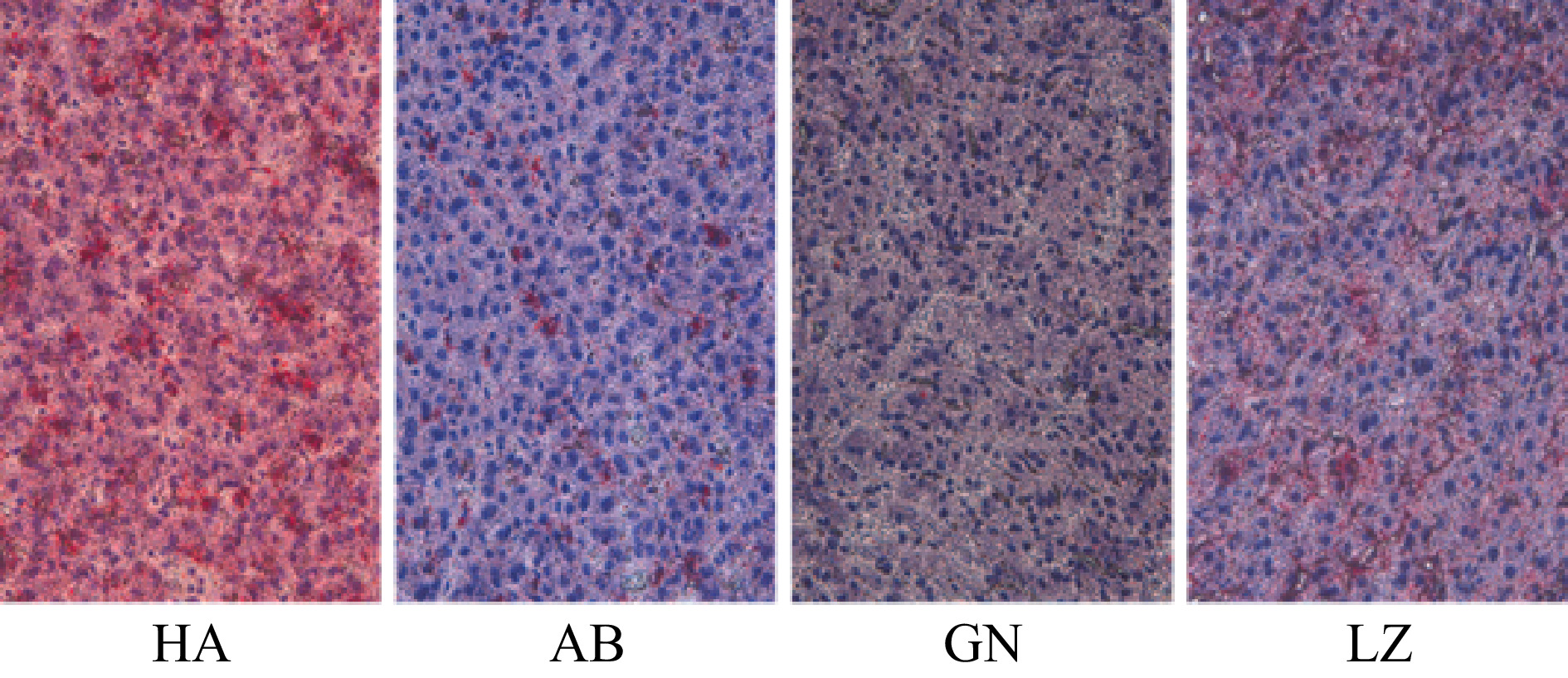
Figure 2.
Comparison of liver fat distribution of pigs from different regions. To observe slices under a light microscope, the multiple of the eyepiece is 10× and the multiple of the objective lens is 40×. The fat is stained bright red and the nucleus is stained dark blue. GN: Gannan Tibetan pigs; AB: Aba Tibetan pigs; LZ: Nyingchi Tibetan pigs; HA: Duroc × Landrace × Yorkshire pigs.
Genes related to lipid metabolism
-
Lipid metabolism includes fat formation and fat decomposition, and involves a variety of key enzymes or transcription factors, such as FAS, CYP7A1, PPAR-γ, SCD, HMGCR and CPT1A. It can be seen from Fig. 3 that the FAS mRNA levels were significantly higher in all Tibetan pigs than that of DLY pigs (P < 0.05), and the FAS expression of Aba Tibetan pigs was higher than that of the other two regions. Compared with DLY pigs, the APOA gene of Tibetan pigs in Aba, Nyingchi and Gannan was significantly down-regulated, and the APOE gene of Tibetan pigs in Aba and Nyingchi area was also significantly down-regulated (P < 0.05). In the liver, the LDLR and SCD mRNA levels of DLY pigs were significantly lower than those of Tibetan pigs in the three regions, and the AGPAT2, LPIN1 and PPAR-γ mRNA levels were significantly higher than those of Tibetan pigs in the other three regions (P < 0.05). The CYP7A1 and HMGCR mRNA levels in the liver of DLY pigs were significantly lower than that of Gannan and Nyingchi Tibetan pigs (P < 0.05). However, CPT1A mRNA level was significantly higher for the DLY pigs than that of Gannan and Nyingchi Tibetan pigs, but lower than that of Aba Tibetan pigs (P < 0.05). There was no significant difference in LCAT mRNA level among DLY pigs, Gannan Tibetan pigs and Nyingchi Tibetan pigs (P > 0.05).
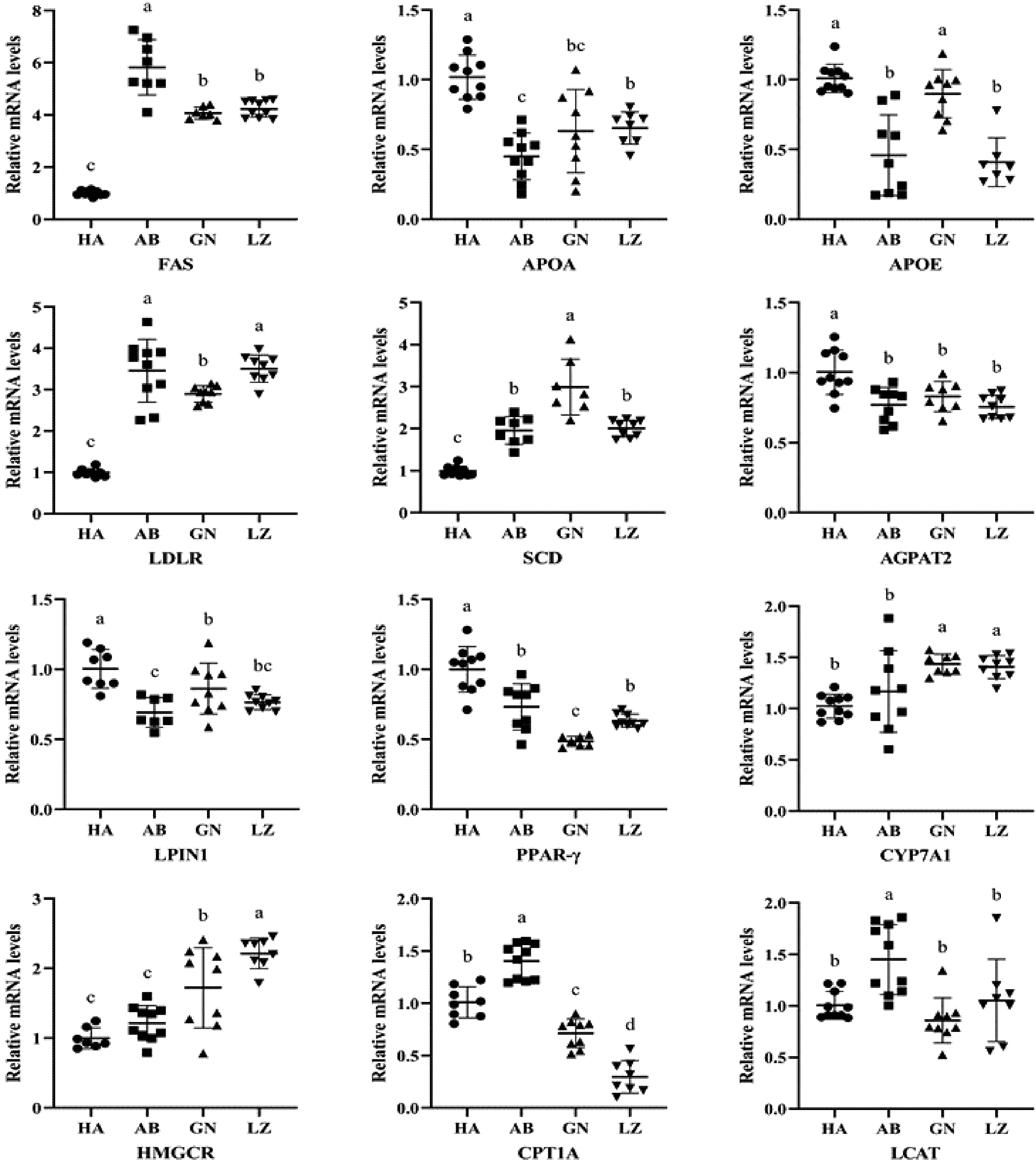
Figure 3.
Lipid metabolites mRNA expression related genes in liver of Tibetan and Duroc × Landrace × Yorkshire pigs. GN: Gannan Tibetan pigs; AB: Aba Tibetan pigs; LZ: Nyingchi Tibetan pigs; HA: Duroc × Landrace × Yorkshire pigs. Data were presented as the fold change in gene expression normalized to GADPH and relative to HA groups. Data are expressed as means ± SD. a, b and c indicate significant differences between different kinds of pigs (P < 0.05).
Fatty acid composition
-
As shown in Table 4, 26 kinds of fatty acids were detected, including 11 kinds of saturated fatty acids, seven kinds of monounsaturated fatty acids and eight kinds of polyunsaturated fatty acids. Palmitic acid and stearic acid were the main saturated fatty acids, with oleic acid for the main monounsaturated fatty acids, and linoleic acid for the main polyunsaturated fatty acids. The proportions of fatty acids varied greatly with pig breed and region. Gannan Tibetan pigs had significantly lower palmitic acid but higher eicosenoic acid in muscles than other pigs (P < 0.05). DLY pigs had the highest stearic acid (P < 0.05), and Nyingchi Tibetan pigs and DLY pigs had higher linolenic acid. It can be seen that the proportion of fatty acids in pork from different regions is very different, which may be related to their diet and lipid metabolism.
Table 4. Fatty acid composition of longissimus dorsimuscle in different kinds of pigs.
Fatty acid composition Duroc × Landrace × Yorkshire pigs Aba Tibetan pigs Gannan Tibetan pigs Nyingchi Tibetan pigs Octanoic acids C8:0 0.01 ± 0.00b 0.12 ± 0.07a 0.02 ± 0.01b − Decanoic acid C10:0 0.12 ± 0.02a 0.12 ± 0.02a 0.07 ± 0.01b 0.10 ± 0.01ab Lauric acid C12:0 0.10 ± 0.01a 0.08 ± 0.01a 0.09 ± 0.02a 0.09 ± 0.01a Myristic acid C14:0 1.57 ± 0.03a 1.36 ± 0.05a 1.36 ± 0.29a 1.55 ± 0.22a Pentadecanoic acid C15:0 0.04 ± 0.01b 0.04 ± 0.01b 0.11 ± 0.06a 0.07 ± 0.02b Palmitic acid C16:0 26.67 ± 0.48a 24.96 ± 1.39a 22.46 ± 2.21b 25.36 ± 1.49a Heptadecanoic acid C17:0 0.20 ± 0.01b 0.23 ± 0.03b 0.40 ± 0.15a 0.32 ± 0.07ab Stearic acid C18:0 13.94 ± 0.10a 11.72 ± 0.25b 12.14 ± 0.37b 11.99 ± 0.84b Arachidic acid C20:0 0.21 ± 0.02b 0.21 ± 0.02b 0.31 ± 0.05a 0.25 ± 0.04b Behenic acid C22:0 0.02 ± 0.00b 0.12 ± 0.07a 0.08 ± 0.01ab − Tetracosanoic acid C24:0 − − 0.17 ± 0.09b 0.42 ± 0.14a Saturated Fatty acid (SFA) 42.87 ± 0.51a 38.96 ± 1.22b 37.20 ± 3.19b 40.13 ± 1.89ab 9-Tetradecenoic acid C14:1 0.02 ± 0.01a 0.03 ± 0.01a 0.03 ± 0.00a 0.03 ± 0.01a 15-Tetracosenoic acid C16:1 2.45 ± 0.31b 3.85 ± 0.44a 2.10 ± 0.60b 3.75 ± 0.64a 10-Heptadecenoic acid C17:1 0.30 ± 0.18a 0.36 ± 0.18a 0.46 ± 0.15a 0.50 ± 0.21a Elaidic acid C18:1n9t − 0.20 ± 0.07b 1.20 ± 0.09a 1.36 ± 0.71a Oleic acid C18:1n9c 35.48 ± 0.13b 41.33 ± 1.72a 35.50 ± 2.70b 38.76 ± 3.82ab Eicosanoic acid C20:1 0.64 ± 0.01b 0.81 ± 0.04b 1.25 ± 0.21a 0.66 ± 0.13b Erucic acid C22:1n9 − − 0.18 ± 0.09 − Monounsaturated Fatty Acids (MUFA) 38.89 ± 0.75b 46.58 ± 1.47a 40.73 ± 2.37b 45.08 ± 3.78a Linoelaidic acid C18:2n6t 0.05 ± 0.01 − − − Linoleic acid C18:2n6c 15.27 ± 1.01a 11.13 ± 0.89b 17.06 ± 2.25a 10.93 ± 3.09b 11,14-Eicosadienoicacid C20:2 0.59 ± 0.06b 0.41 ± 0.03b 0.58 ± 0.11b 0.30 ± 0.08b Linolenic acid C18:3n3 0.90 ± 0.18b 0.57 ± 0.23b 2.21 ± 0.47a 1.94 ± 0.40a 11,14,17-Eicosatrienoicacid C20:3n6 0.16 ± 0.06a 0.24 ± 0.05a 0.23 ± 0.05a 0.20 ± 0.07a 8,11,14-Eicosatrienoicacid C20:3n3 0.11 ± 0.02b − 0.27 ± 0.05a 0.22 ± 0.04a Arachidonic acid C20:4n6AA 1.06 ± 0.45a 2.01 ± 0.35a 1.60 ± 0.59a 1.30 ± 0.64a 4,7,10,13,16,19-Docosahexaenoicacid C22:6n3DHA 0.02 ± 0.00c 0.17 ± 0.03a 0.17 ± 0.08a 0.09 ± 0.04b Polyunsaturated Fatty acids (PUFA) 18.19 ± 1.15ab 14.53 ± 0.76b 22.12 ± 2.68a 14.98 ± 4.22b SFA/UFA 0.75 ± 0.01a 0.64 ± 0.03b 0.59 ± 0.08b 0.67 ± 0.05ab PUFA/SFA 0.42 ± 0.03b 0.37 ± 0.02b 0.60 ± 0.11a 0.38 ± 0.12b Notes: The data are presented as means ± SD. The significance level is set at 0.05. a and b superscripts indicate significant differences between pork from different regions in the same row (P < 0.05). -
Lipid metabolism in the body can be reflected to some extent by analyzing the changes of lipid content in blood[11]. Pigs in different regions have different utilization capacity of various nutrients in feed[12]. After digestion and absorption, various nutrients are transported to the body tissues for metabolism, which can show the lipid metabolism of the body[13]. In this study, it was found that serum TG content of DLY pigs was significantly higher than that of Gannan and Nyingchi Tibetan pigs, TC and HDL were significantly higher than that of Tibetan pigs in other three regions. TG is the main component of adipose tissue in the body, and it combines with LDL in the blood, and LDL transport the lipids synthesized by liver to all tissues in the body[14]. The content of TG in serum of Duroc Yorkshire pigs was higher, which was consistent with the characteristics of DLY pigs under house feeding.
The results of antioxidative status in the liver tissue showed that the antioxidant capacity of Tibetan pigs' liver was higher than that of DLY pigs, which may be related to their living environment. Long-term strong ultraviolet radiation leads to the formation of more oxidation products in Tibetan pigs[15], which forms a stronger antioxidant system to remove these free radicals to protect the Tibetan pigs. In addition, diets containing high mountain plants and wild fruits rich in antioxidant components[16] (such as: highland barley, fern hemp, peach gum, sea-buckthorn and so on) may also lead to an increase in the antioxidant capacity of Tibetan pigs' liver.
Liver is a central metabolic organ, closely related to the metabolism of various nutrients, especially for the regulation of glucose, lipid, cholesterol and other content changes in the body plays a crucial role, with the function of maintaining systemic metabolic homeostasis[17, 18]. Lipid metabolism is a series of complex biochemical reactions, it refers to the process of digestion and absorption, synthesis and decomposition of fat by various enzymes. FAS is considered as a multifunctional protease, which can catalyze the synthesis of saturated fatty acids and regulate lipid metabolism. Its polymorphism can affect the cholesterol level in pig muscle, the thickness of back fat and the content of polyunsaturated fatty acids in subcutaneous adipose tissue[19]. Body fat deposition is the result of absorption, synthesis, and oxidation of lipids[20, 21] which are determined by the balance between lipogenesis and lipolysis (β-oxidation). In the present study, the FAS mRNA in liver of DLY pigs was significantly higher than that of Tibetan pigs in the three regions. FAS plays an important role in the process of fat deposition[22]. The experimental results indicated that the synthesis rate of TG in liver of DLY pig is higher than that of Tibetan pig, resulting in the lipid content of TG and TC in liver of DLY pig is higher than that of Tibetan pig. McNeel et al.[23] found that the expression levels of fatty acid binding protein, FAS, glucose transporter 4 and leptin genes in adipose tissues of fatty pigs were significantly higher than those of lean pigs, suggesting that genotype is the main factor determining fat deposition. SCD and FAS control many genes involved in cholesterol and lipid metabolism, mediating fat formation and lipid accumulation in tissues[24]. APOA is the major constituent of the protein fraction of HDL, and acts in the reverse transport process of cholesterol, from extrahepatic peripheral cells to the liver where it is metabolized[25]. According to Zhuo et al.[26], a decreased APOA gene expression may affect the formation of HDL, thereby impairing the process of cholesterol reverse transport. This suggests that decreased APOA expression could result in increased accumulation of body fat. One of the major functions of APOE is to maintain cholesterol homeostasis and lipoprotein clearance from circulation. In this study, the expression of ApoE in DLY pigs was significantly higher than that in Tibetan pigs, and the expression of ApoE was significantly higher than that in Nyingchi Tibetan pigs and Aba Tibetan pigs.
The synthesis of TC is a very complex and precise process, involving more than 30 enzyme reactions. Among them, HMGCR is one of the key enzymes in TC synthesis and regulates the catalysis of mevalonate[27]. The synthesized TC will form LDL-C with apolipoprotein, and then ingest it into cells through the LDL receptor (LDLR) pathway. In the present study, the expression of LDLR in the liver of white pigs was significantly lower than that of Tibetan pigs, while the expression of HMGCR was significantly lower than that of Gannan and Nyingchi Tibetan pigs. Studies have shown that CYP7A1 is the only rate-limiting enzyme in the classical pathway of bile acid synthesis and plays an important role in regulating cholesterol metabolism[28, 29]. In our study, the expression level of CYP7A1 in DLY pigs was lower than that in Tibetan pigs, indicating that liver cholesterol has a strong ability to be transformed into primary bile acid through the classic way under the action of CYP7A1 into small intestine along with bile and then leave the body, which is consistent with the result of low lipid level in DLY pigs measured in this experiment.
LPIN1 is a key gene that regulates fat oxidation, and CPT1A is a rate-limiting enzyme in the transport of long-chain fatty acids for β-oxidation[30]. It has been reported that fatty acid oxidation can be suppressed by downregulating the expression of CPT1A[31]. In this study, the expression of Lpin1 in DLY pigs was significantly higher than that in Tibetan pigs, while the expression of CPT1A gene was significantly higher than that in Gannan Tibetan pigs and Nyingchi Tibetan pigs, suggesting that the degree of β-oxidation in DLY pigs may be higher than that in Tibetan pigs.
The proportion of monounsaturated fatty acids in Tibetan pigs in the three regions was higher than that of DLY pigs, especially in Aba Tibetan pigs and Nyingchi Tibetan pigs, indicating that Tibetan pigs have strong antioxidant capacity, which is related to their poor growth environment and free range diversified diet. Aba and Nyingchi Tibetan pigs had similar proportions of SFA, UFA and PUFA, while Gannan Tibetan pigs and DLY pigs had similar types of fatty acids. The low proportion of polyunsaturated fatty acids in the meat of Nyingchi Tibetan pigs and Aba Tibetan pigs may be related to the strong ultraviolet radiation in the environment, leading to the oxidation of polyunsaturated fatty acids in Tibetan pigs[32]. At the same time, fatty acids as flavor precursors also play a very important role in meat flavor[33]. The composition of fatty acids in meat is the focus of attention. The content of long-chain saturated fatty acids in meat should not be too high because it is easy to solidify at low temperature, which reduces the taste of meat. Moreover, excessive intake of SFA will increase the risk of other metabolic diseases. UFA has high nutritional value and can give meat a good flavor, but high content of UFA can easily increase the risk of meat oxidation and spoilage. To ensure a healthy diet, we should eat meat with low fat content and maintain the balance between PUFA and SFA. In this study, it was found that compared with DLY pigs, Tibetan pigs had higher PUFA content in muscle and had higher nutritional value and edible value. Therefore, in the long-distance transportation of 'Tibetan pig' meat products, more attention should be paid to environmental factors such as temperature and humidity, so as to minimize the reduction of UFA content in pork during storage[34, 35].
In conclusion, the result of parameters and genes related to lipid metabolism suggested that the lipid metabolism of liver of DLY pigs may be stronger than that of Tibetan pigs, but lipid deposition in muscle is weaker than that in Tibetan pigs. The saturated fatty acid proportion of Tibetan pork is lower than that of DLY pig, while the unsaturated fatty acid proportion is higher than that of DLY pig. The high proportion of unsaturated fatty acids may have contributed to the high altitude adaptability and stress resistance of Tibetan pigs and the unique antioxidant properties of Tibetan pig muscles.
This work was funded by the Ministry of Finance and Ministry of Agriculture and Rural Affairs (CARS-35), and Jiangsu Innovative Group of Meat Nutrition, Health and Biotechnology.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yin Z, Liu F, Gu X, Zhang L, Ma Y, et al. 2022. A comparison of hepatic lipid metabolism and fatty acid composition in muscle between Duroc × Landrace × Yorkshire and Tibetan pigs from three regions. Food Materials Research 2:7 doi: 10.48130/FMR-2022-0007
A comparison of hepatic lipid metabolism and fatty acid composition in muscle between Duroc × Landrace × Yorkshire and Tibetan pigs from three regions
- Received: 07 March 2022
- Accepted: 28 April 2022
- Published online: 27 May 2022
Abstract: Qinghai-Tibet plateau with an average altitude above 4,000 m provides favorable conditions for Tibetan pigs that may have different meat quality from other pig breeds. This study was designed to compare the differences in lipid metabolism in liver and fatty acid composition in muscle between Tibetan and Duroc × Landrace × Yorkshire (DLY) pigs. Aba Tibetan pigs (n = 10), Gannan Tibetan pigs (n = 9), Nyingchi Tibetan pigs (n = 10) and DLY pigs (n = 10) were selected for the experiment. After fasting for 12 h, they were slaughtered and blood, liver and muscle samples were taken for biochemical analyses. The results showed that the intramuscular fat content was not significantly different for Tibetan pigs among the three regions (P > 0.05), which was significantly higher than that of DLY pigs (P < 0.05). However, the liver fat content of Gannan Tibetan pigs was significantly lower than those of DLY pigs and Aba and Nyingchi Tibetan pigs (P < 0.05). The hepatic lipid metabolism may be stronger in DLY pigs than in Tibetan pigs, but lipid deposition in muscle is weaker in DLY pigs than that in Tibetan pigs. Aba Tibetan pigs and Nyingchi Tibetan pigs had similar proportions of saturated, monounsaturated and polyunsaturated fatty acids, while Gannan Tibetan pigs and DLY pigs had similar types of fatty acids. The findings provide new insight into mechanisms of environmental and breed effects on pork quality.
-
Key words:
- Tibetan pig /
- Lipid metabolism /
- Fatty acid /
- Antioxidant












