-
Sri Lanka's food supply revolves around cereal and legume crops centered on rice, and pulses followed by fruits and vegetables. Almost 90% of the world’s food crops are grown from seeds[1]. During the second part of the 20th century, cereal and legume production in South Asian countries tripled as a result of the Green Revolution[2−4]. It has been found that the seed-borne fungi generate a considerable effect on the reduction of crop productivity as the external and internal distribution of seed-borne pathogens causes seed abortion, seed necrosis, elimination of seed germination capacity, and damage to seedlings[5]. Seed-borne pathogen prevalence has long been known to be influenced by numerous factors including abiotic factors such as temperature, humidity, and moisture content, and also biotic factors like insect damage during seed storage[6]. Different fungal pathogens and fungal like pathogens that attack cereal cultivars belong to a few of the common genera such as Alternaria, Aspergillus, Cercospora, Fusarium, Phytophthora, and Rhizoctonia[1,7]. The presence of these seed-borne pathogens causes mycotoxin production, and mycotoxin contamination of cereal grain is a worldwide issue for public health and a permanent concern for cereal-food industries[8]. For instance, aflatoxin B1 (AFB1) is generated largely by A. flavus, and A. parasiticus and it is one of the most carcinogenic, teratogenic, hepatotoxic, and mutagenic mycotoxins[9]. Fusarium species occur in several crop species, such as wheat, maize, and sorghum plants, and are also associated with the production of trichothecenes, deoxynivalenol (DON), and its toxic derivatives[10,11]. Mycotoxin production has a detrimental impact on both humans and animals, causing issues with the liver, kidneys, and intestines. Furthermore, chronic disorders are caused by long-term exposure to low levels of aflatoxins, the most common and devastating of which is cancer[12]. Therefore, control of seed-borne fungi becomes an essential aspect to reduce the impacts generated by the seed-borne fungi. In particular, controlling the seed-borne fungi can be accomplished via chemical or natural approaches[13]. However, misuse of these chemical techniques, such as synthetic fungicides has resulted in many issues around the world, including environmental, ecological, health, social, and economic sectors. Consequently, switching to natural pathogen management is a more viable and appropriate strategy for the farming community[13,14]. Considerable literature evidence showed that Aloe vera, Allium sativum, Azadirachta indica, Camellia sinensis, Chrysanthemum coccineum, Coffee arabica, Datura stramonium, and Z. officinale plant extracts have a potential to control the seed-borne fungi including Aspergillus fumigatus , A. niger, A. flavus, and Pyricularia oryzae[7,14−16]. This study was conducted to address a knowledge gap in the Sri Lankan agricultural environment and to increase public awareness of the fungal species, such as Arachis hypogea (peanut), Oryza sativa (rice), Vigna radiata (mungbean), and Vigna sinensis (cowpea). Furthermore, the study would facilitate the ability to find appropriate plant extract treatments and the feasibility of using natural plant products as fungicides to control plant diseases cause by seed-borne fungi.
-
Seeds from Arachis hypogea L. (Tissa), Oryza sativa L. (Bg251), Vigna radiata (L.) R.Wilczek (M16), and Vigna sinensis L. (Dhawala) were collected from the Seed Certification and Plant Protection Centre (SCPPC), Gannoruwa, Sri Lanka in July 2021. The Allium sativum bulbs, and Zingiber officinale rhizomes were collected from the marketplaces in the Maharagama area (Sri Lanka). Aloe vera, and Azadirachta indica leaves were collected from home gardens in the Maharagama area.
Methanol: Distilled water (4:1) extraction
-
Respective parts of each plant materials including A. sativum bulbs, A. vera, and A. indica leaves, and Z. officinale rhizome 100 g were taken and thoroughly washed with tap water to remove soil and other debris. The leaves, bulbs, and rhizomes were then separated and air-dried under the shade at 25−29 °C until they became dry and crispy. The dried plant materials were powdered using a heavy-duty blender (Panasonic MX-AC 400, India). About 25 g of each air-dried plant material was defatted with 10 mL of n-hexane (C6H14). Then the solution was extracted with methanol: distilled water (4:1) at room temperature (28 ± 2 °C) by maceration with occasional stirring for 48 h. The macerate was filtered using Whatman filter papers No. 1. Samples were concentrated by a rotary evaporator (IKA HB 10 digital, China) at 37 °C. Each sample was dissolved in Dimethyl Sulfoxide (DMSO), and DMSO-water (50%, v/v) was added to prepare the stock solutions[17,18]. Stock solutions were used to prepared the (10%, 15%, 20%) (w/v) solutions.
Aqueous extraction
-
Respective parts of each plant materials including A. sativum bulbs, A. vera, and A. indica leaves, and Z. officinale rhizome 100 g were taken, and thoroughly washed with tap water to remove soil and other debris. The leaves, bulbs and rhizomes were then separated and air-dried under the shade at 25−29 °C until they became dry and crispy. The dried plant materials were powdered using an electrical heavy-duty blender (Panasonic MX-AC 400, India). About 25 g of air-dried A. sativum, A. vera, A. indica, and Z. officinale powder were separately mixed with 75 mL of sterilized distilled water, then solutions were stored in a flask and the extracts were left standing in the dark for 3−4 d. The solutions were then filtered through two layers of muslin cloth[17,19].
Antifungal activity by agar well diffusion method
-
The antifungal activity of Allium sativum, A. vera, A. indica, and Z. officinale, plant extracts were evaluated against the identified seed-borne pathogens by the agar well diffusion method with modifications[20]. The 8−10 d old pure cultures of fungal pathogens including; Aspergillus flavus, A. niger, Orbilia foliicola, Rhizopus oryzae, and Talaromyces oumae-annae were sub-cultured onto PDA medium. Using a sterile cork borer, three 5 mm diameter wells were made on each sub-cultured plate (Fig. 1). Then 10%, 15%, 20% (w/v) of each plant extract were introduced into each well made on the medium.
Then 5 mm diameter well was created on a separate plate and it was filled with fungicide 50% (WP) Captan as the positive control. DMSO-water (50%, v/v), methanol-water (80%, v/v), sterilized distilled water, and n-hexane were introduced into another separate plate containing wells as the negative control. Then the plates were allowed to stand in an upright position for 15 min to allow for the proper diffusion of the extracts into the medium before incubation. The plates were incubated at room temperature (28 ± 2 °C) for about 7 d[21]. The experiment was performed in triplicates to minimize the error ratio. At the end of incubation period, the inhibition zones around each well was measured for 3, 7 d intervals after the inoculation to an accuracy of 0.1 mm and the effect was calculated as a mean of triplicate tests to evaluate the antifungal activity[21,22].
Antifungal activity by the poison food method
-
Plant extracts of Allium sativum, A. vera, A. indica, and Z. officinale were evaluated against the identified seed-borne pathogens including; Aspergillus flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae. Plant extracts at 10% (w/v), 15% (w/v), and 20% (w/v) concentrations were used for this study for which 10, 15, and 20 mL of stock solutions were mixed with 90, 85, and 80 mL of sterilized PDA media individually. The amended PDA medium was thoroughly shaken for uniform mixing with leaf extracts. Twenty mililitres of this mixed agar media was poured into sterile petri plates and allowed to solidify. Five millimetre diameter of agar disk of test pathogenic fungi were cut from the 8-10 d old pure cultures using a sterile cork-borer and placed in the center of each petri-plate containing different concentrations of plant extracts (Fig. 2). The experiment was carried out in triplicate[23]. The petri plates with 10 mL of each solution including; fungicide 50% (WP) Captan, DMSO-water (50%, v/v), methanol-water (80%, v/v), sterilized distilled water, and n-hexane were mixed with 90 mL of sterilized PDA media and maintained as controls. Then the plates were incubated at room temperature (28 ± 2 °C) for about 7 d[21]. At the end of the incubation period, the percentage inhibition of mycelial growth was calculated using the equation below[23].
$ {\text% } {\rm{ Inhibition\; effect}} =\frac{ {\begin{aligned}\rm Inhibition\;halo\;diameter\;in\;control-\\\rm inhibition\;halo\;diameter\;in\;treatment\end{aligned}}}{{\rm Inhibition\;halo\;diameter\;in\;control}}\times 100 $ Seed germination and seedling vigor
-
Seed quality was measured in pot experiments with soil sterilized by steam distillation. A total of 20 seeds per sample of O. sativa, V. radiata, and V. sinensis and 10 seeds of A. hypogea were soaked in plant extracts which were taken from methanol: distilled water (4:1) for 10 min. The same number of seeds were soaked in DMSO-water (50%, v/v), methanol-water (80%, v/v) and sterilized distilled water, n-hexane for 30 min as the negative control, and in fungicide 50% Captan 50% (WP) for 10 min as the positive control. The liquid was drained off and the seeds were dried under shade before use. A total of 20 seeds of O. sativa, V. radiata, and V. sinensis and 10 seeds of A. hypogea were sown per pot. The used potting mixture was Compomix potting soil with equal parts of compost, top soil, coir dust and sand which were sterilized by steam distillation. The seeds were covered with 1−3 cm deep soil layer depending on the seed size. The pots were kept in partial shade and had an average temperature of approximately 30 °C with regular watering. The germination count was recorded every day during the testing period.
Plants were carefully uprooted and gently washed with running water. The percentage of seeds germinating normally, abnormally, healthily and diseased seedlings were recorded according to International Seed Testing Association Rules[24] and infections were counted. Seedling vigor was measured using the shoot and root length[2,25,26]. Shoot lengths from the base of the shoot to the uppermost leaf tip and root length from the collar region to the end of the longest tip were measured[1]. Measurements were taken after three weeks of sowing for A. hypogea and after two weeks for the other seed varieties. The germination percentage, germination index, and vigor index were calculated using the below-mentioned equations[2,25,26]. During recording, the normal seedlings and abnormalities of germinating seeds and seedlings, were considered[27].
$\rm Germination\; percentage =\frac{\rm No.\;of\;seeds\;germinated}{\rm No.\;of\;seeds\;sown}\times100 $ $\begin{aligned}\rm Speed\; of\; germination\; index =\; & \frac{\rm No.\;of\;seeds\;germinated}{\rm day\; of\;first\;count}+\\&\frac{\rm No.\;of\;seeds\;germinated}{\rm day\;of\;final\;count}\end{aligned}$ $\begin{aligned}{\rm Vigor\; index} =\; &{\rm Germination\; percentage} \; ({\text%}) \;\times \\&({\rm mean\; root\; length + mean\; shoot\; length})\end{aligned} $ Statistical analysis
-
All experiments were performed in triplicates and all the data were expressed as mean value ± (SE). Analysis of variance (ANOVA) and Tukey’s multiple comparison were performed on all transformed data collected in respect of parameters studied on effects of plant extracts at the least significant difference (LSD) which was employed to test for significant differences between treatments at P ≤ 0.05 using Minitab (version 17).
-
Extract of Allium sativum exhibited moderate antifungal activity against the selected seed-borne pathogens; Aspergillus flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae. Following the results obtained by the agar well diffusion method, a maximum mean inhibition diameter of 2.53 cm was reported for T. oumae-annae by the A. sativum crude extract after 3 d of incubation. The lowest mean inhibition diameter of 0.40 cm was reported for A. niger by 10% (w/v) A. sativum extract after 7 d of incubation. The effect of 20% (w/v) A. sativum extracts in inhibiting the seed-borne fungal pathogens was considerably higher than the other two A. sativum concentrations (10% and 15%). Extract of A. vera exhibited low antifungal activity while the Z. officinale showed the highest antifungal activity compared with other treatments against the selected seed-borne pathogens; A. flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae. After 7 d of incubation, 10% (w/v) and 15% (w/v) A. vera extract could not exhibit inhibition against A. niger and was unable to successfully control the A. niger. Compared to the other three plant extracts, the best mean inhibition diameters of 1.50 cm and 1.33 cm for A. niger was reported by the A. indica crude extract and 20% (w/v) A. indica extract respectively after the 7 d of incubation. In this experiment Captan 50% (WP) used as the positive control, was capable of significantly arresting the growth of A. flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae compared to the negative controls (Figs 3 & 4).
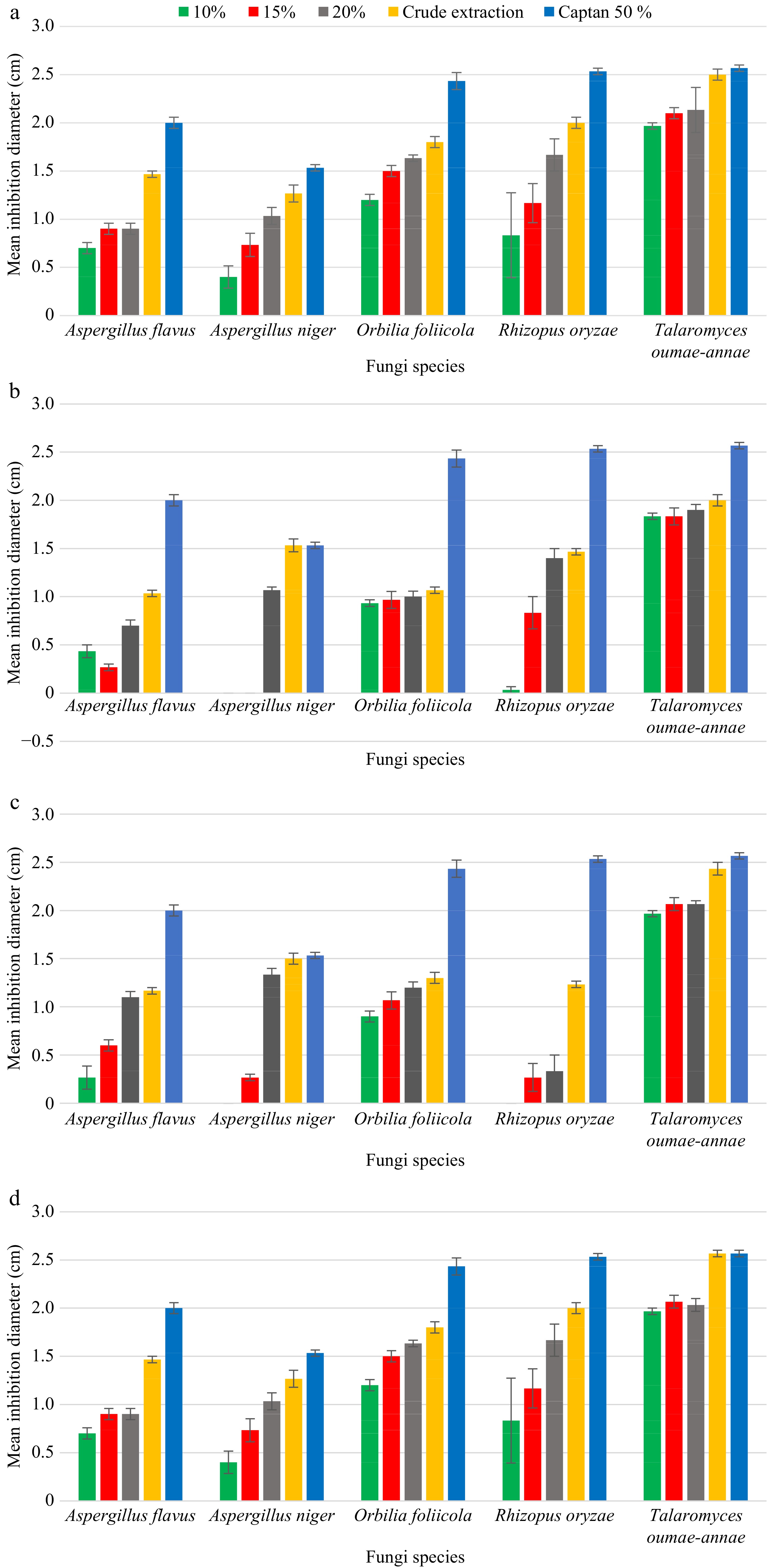
Figure 3.
Effect of different concentrations of (a) A. sativum, (b) A. vera, (c) A. indica and (d) Z. Officinale aqueous extractions against all five pathogens after 7 d of incubation.

Figure 4.
Effect of different concentrations of plant extracts against the selected seed-borne pathogens; A. niger, O. foliicola, and T. oumae-annae. (a) A. niger in negative controls; a1-Methanol 80%, a2-n-hexane, a3-DMSO 50%; (b) A. niger in A. vera (b1-10%, b2-15%, b3-20% concentrations); (c) A. niger in A. sativum (c1-10%, c2-15%, c3-20% concentrations); (d) A. niger in Z. officinale (d1-10%, d2-15%, d3-20% concentrations); (e) A. niger in Captan 50 % (WP) positive control (e1, e2, e3-Captan 50 % (WP) positive control) ; (f) O. foliicola in negative controls; f1-Methanol 80%, f2-n-hexane, f3-DMSO 50%; (g) O. foliicola in A. vera (g1-10%, g2-15%, g3-20% concentrations); (h) O. foliicola in A. sativum (h1-10%, h2-15%, h3-20% concentrations); (i) O. foliicola in A. indica (i1-10%, i2-15%, i3-20% concentrations); (j) O. foliicola in Z. officinale (j1-10%, j2-15%, j3-20% concentrations); (k) T. oumae-annae in negative controls; k1-Methanol 80%, k2-n-hexane, k3-DMSO 50%; (l) T. oumae-annae in A. vera (l1-10%, l2-15%, l3-20% concentrations); (m) T. oumae-annae in A. sativum (m1-10%, m2-15%, m3-20% concentrations); (n) T. oumae-annae in Z. officinale (n1-10%, n2-15%, n3-20% concentrations); (o) T. oumae-annae Captan 50 % (WP) positive control (o1, o2, o3-Captan 50 % (WP) positive control).
Antifungal activity by the poisoned food technique
-
Extract of Z. officinale exhibited the highest antifungal activity against the selected seed-borne pathogens and the effect of crude extract of Z. officinale in inhibiting the seed-borne fungal pathogens was considerably higher than the other concentrations (Fig. 5). The effect of crude extract and 20% (w/v) Z. officinale in controlling T. oumae-annae was significantly similar to the effect of commercial fungicide Captan (50% WP). Increasing the concentrations of plant extract caused an increase in the percentage inhibition of all tested fungi (Fig. 6). Allium sativum extract percentage inhibition ranged between 50.90% to 98.62% for all five pathogens tested. Maximum percentage inhibition (other than the positive control) of 96.81% was reported for T. oumae-annae by the A. sativum crude extract while the lowest percentage inhibition (50.90%) was reported for A. niger by 10% (w/v) A. sativum extract. Furthermore, Aloe vera extract percentage inhibition ranged between 37.43% to 98.62% for all five pathogens tested and maximum percentage inhibition (other than the positive control) of 93.61% was reported for T. oumae-annae by A. vera crude extract and 20% (w/v) A. vera extract. The lowest percentage inhibition (37.43%) was reported for A. niger by 10% (w/v) A. vera extract. The effect of crude extract of A. vera in inhibiting the seed-borne fungal pathogens was considerably higher than the other A. vera concentrations. The second most effective extraction was A. indica and maximum percentage inhibition (other than the positive control) of 96.28% was reported for O. foliicola by A. indica crude extract. The level of controlling seed-borne fungal pathogens by three different concentrations of A. sativum extract, 10% (w/v), 15% (w/v), and 20% (w/v) was statistically significant (P < 0.05) compared to the negative controls (0%) in the study.
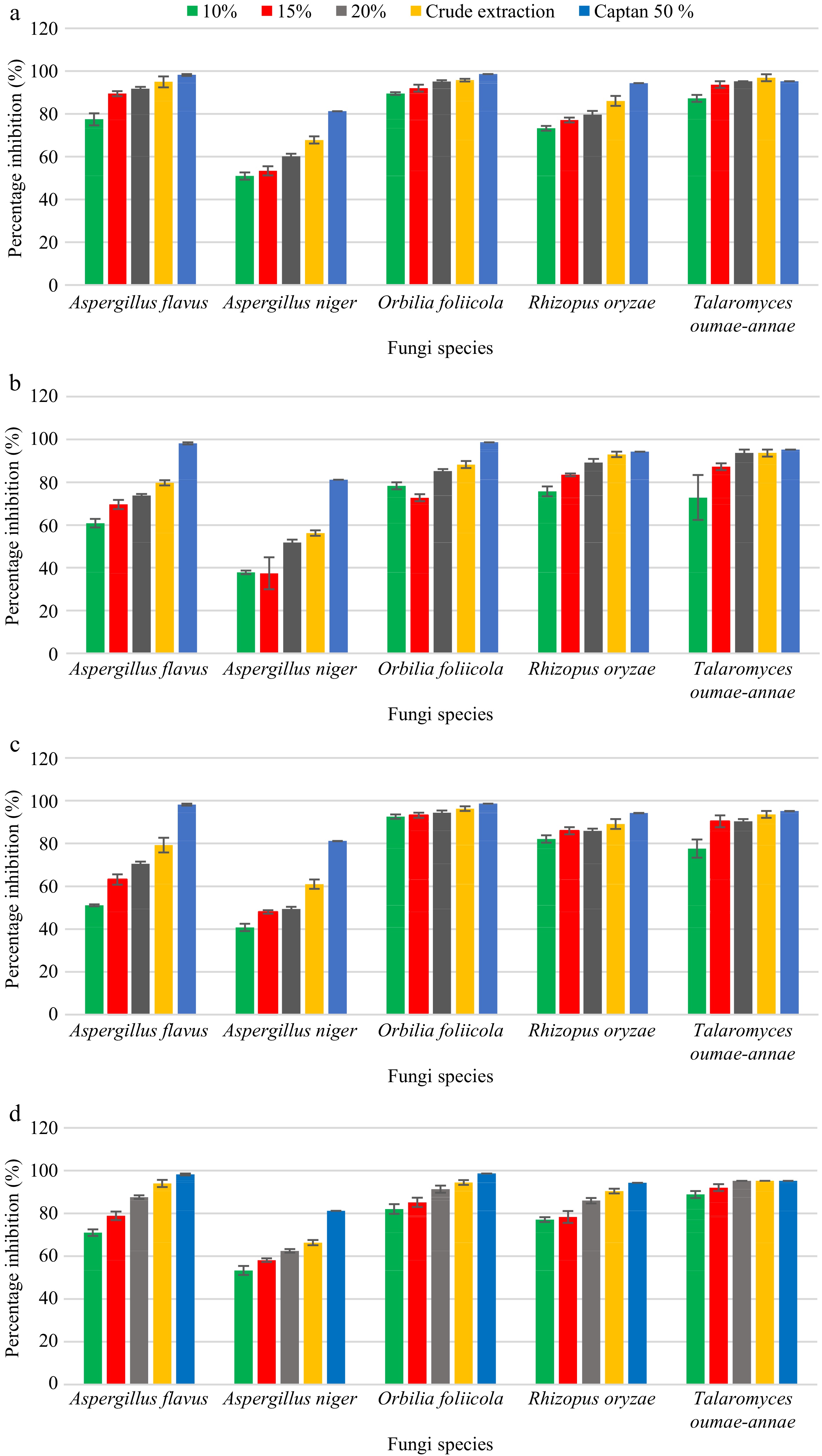
Figure 5.
Effect of different concentrations of (a) A. sativum, (b) A. vera, (c) A. indica and (d) Z. Officinale aqueous extractions against all five pathogens after 7 d of incubation.
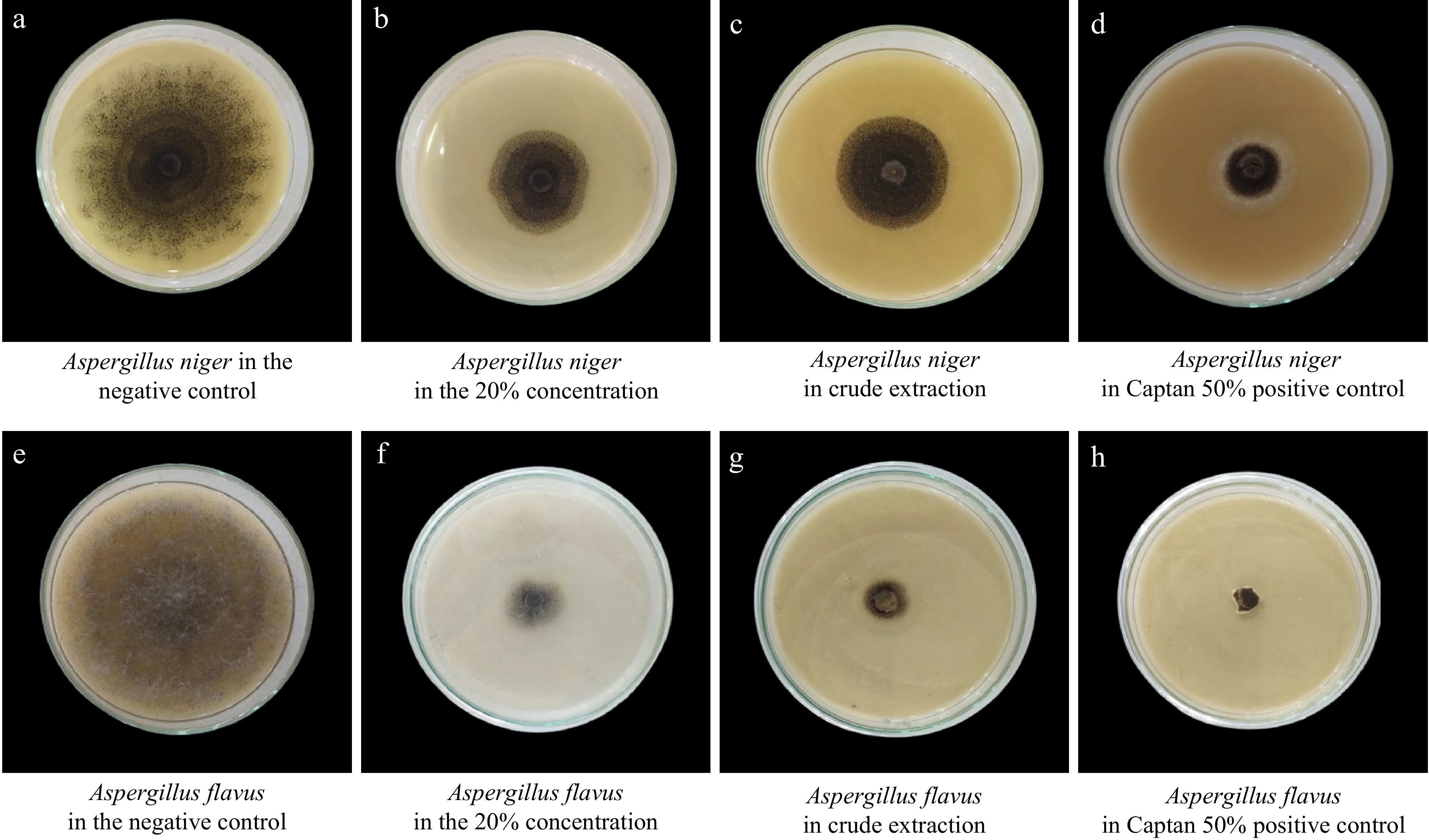
Figure 6.
Effect of different concentrations of Z. officinale extract against the selected seed-borne pathogen; A. niger and A. flavus. (a) A. niger in negative control (distilled water); (b) A. niger at 20% concentration; (c) A. niger in crude extract; (d) A. niger in Captan 50 % positive control; (e) A. flavus in negative control (distilled water); (f) A. flavus in 20% concentration; (g) A. flavus in crude extract (h) A. flavus in Captan 50 % (WP) positive control.
Seed germination and seedling vigor
-
Seed varieties including A. hypogea, O. sativa, V. radiata, and V. sinensis were treated separately with aqueous extractions (Table 1). Seed treatment with aqueous extract of A. sativum exhibited the highest (80%) germination percentage for A. hypogea similar to the germination percentage by captan (50% WP). It was higher than the germination given by all other extractions including the negative control. All three extractions including A. sativum, A. indica, and Z. officinale exhibited the highest (100%) germination percentage for V. radiata and lower germination given by A.vera extraction. Among the four plant aqueous extracts tested, the maximum vigor index of 2664 was reported from Z. officinale while the highest germination index of 5.42 was reported from A. sativum, A. indica, and Z. officinale extracts for V. radiata. Azadirachta indica and Z. officinale exhibited the highest (100%) germination percentage for O.sativa similar to the germination percentage by captan (50% WP).
Table 1. Effect of different plant extracts on seed germination data of Arachis hypogea, Oryza sativa, Vigna radiata, V. sinensis seeds.
Plant extract Seed
varietyStorage period
(months)% Germination % Abnormal
seedlingSpeed of
germination indexVigor index Allium sativum Arachis hypogea > 6 80 20 2.17 1912.8 Aloe vera > 6 60 − 0.94 2118 Azadirachta indica > 6 70 − 2.20 1710.8 Zingiber officinale > 6 50 10 1.02 1205 Distilled water > 6 30 30 0.71 713.1 Captan (50%) > 6 80 − 2.31 3329.6 Allium sativum Oryza sativa > 6 70 20 1.33 2170 Aloe vera > 6 80 − 1.66 1565.6 Azadirachta indica > 6 100 − 4.42 2824 Zingiber officinale > 6 100 30 4.72 3079 Distilled water > 6 80 − 1.73 1742 Captan (50%) > 6 100 − 4.73 4385 Allium sativum Vigna radiata > 6 100 − 5.42 2404 Aloe vera > 6 95 − 5.02 2011.2 Azadirachta indica > 6 100 10 5.42 2269 Zingiber officinale > 6 100 − 5.42 2664 Distilled water > 6 100 30 5.40 2306 Captan (50%) > 6 100 20 5.42 2238 Allium sativum Vigna sinensis > 6 20 − 1.08 509.4 Aloe vera > 6 15 − 0.81 396.4 Azadirachta indica > 6 30 − 1.42 686.4 Zingiber officinale > 6 25 − 0.96 627 Distilled water > 6 5 − 0.23 105 Captan (50%) > 6 30 10 1.09 705 The lowest germination percentage (70%) was reported for O.sativa by aqueous extract of A. sativum. The maximum vigor index of 3079 and the highest germination index of 4.72 for O.sativa were reported from Z. officinale. Seed treatment with aqueous extract of A. indica exhibited the highest (30%) germination percentage for V. sinensis which is similar to the germination percentage by Captan (50% WP). Among the four plant aqueous extracts the lowest germination percentage (15%) was reported for V. sinensis by aqueous extract of A. vera. and the maximum vigor index of 686.4 and highest germination index of 1.42 for V. sinensis were reported from A. indica. Furthermore, the lowest vigor index of 396.4 and the lowest germination index of 0.81 for V. sinensis were reported from A. vera (Fig. 7).

Figure 7.
Seed germination data of Arachis hypogea treated with (a) Azadirachta indica, (b) Aloe vera, (c) Allium sativum, and V. sinensis treated with (d) Allium sativum, (e) Aloe vera and (f) Zingiber officinale aqueous extractions.
Effect of plant methanolic extracts on seed quality
-
Seed treatment with methanolic extract of 15% (w/v),
20% (w/v) A. indica exhibited the highest (50%) germination percentage for A. hypogea while the lowest germination percentage (20%) was reported for A. hypogea by aqueous extract of 10% (w/v) A. vera and 10% (w/v) of Z. officinale. Among the four plant methanolic extracts, the lowest germination percentage (30%) and lowest vigor index of 654.9 were reported for O. sativa by methanolic extracts of 10% (w/v) A. vera extract. Seed treatment with methanolic extract of 20% (w/v) Z. officinale exhibited the highest (90%) germination percentage and maximum germination index of 3.53 for O. sativa which is lower than the germination percentage by Captan (50% WP). Also, the seed treatment with methanolic extracts of 10% (w/v), 15% (w/v), and 20% (w/v) A. indica exhibited the highest (100%) germination percentage for V. radiata while the lowest germination percentage (50%) was reported from 10% (w/v), 15% (w/v) A. vera and 10% (w/v) of Z. officinale extracts. According to the V. sinensis germination data methanolic extract of 20% (w/v) A. sativum exhibited the highest (55%) germination percentage and maximum vigor index of 1564.8 which is even higher than the germination percentage by Captan (50% WP). The lowest germination percentage (10%) was reported for V. sinensis by methanolic extract of 10% (w/v) A. vera (Table 2). Table 2. Effect of different plant extracts on seed germination data of Vigna sinensis seeds.
Plant extract (w/v) Storage period
(months)% Germination % Abnormal
seedlingSpeed of
germination indexVigor index Allium sativum 10
15
20> 6 20
35
55− 1.08
1.95
2.20705
1092.4
1564.8Aloe vera 10
15
20> 6 10
30
20− 0.42
0.94
0.94273
822.3
470Azadirachta indica 10
15
20> 6 20
30
20− 1.35
1.62
1.77670
904.8
250Zingiber officinale 10
15
20> 6 15
15
30− 1.95
2.32
2.32397.5
449.8
983.4Distilled water − > 6 5 − 0.23 105 n-Hexane − > 6 4 − 1.11 89.0 Methanol (80%) − > 6 5 − 0.94 84.6 DMSO (50%) − > 6 4 − 0.87 77.0 Captan (50%) − > 6 30 10 1.09 705 -
Seeds are the most vital input for crop production. Fungal pathogens caused by seed-borne infections are one of the most considerable risks to cereals and legume production worldwide, which cause a reduction in both quantity and quality every year[28]. Plants and other natural sources are capable of producing a wide range of complex and structurally diverse compounds, which have low to significant levels of bioactive potential[29,30]. In this present study, fungitoxicity was expressed in terms of percentage inhibition and mean inhibition diameter.
Antifungal activity of four medicinal plant extracts including; A. sativum, A. vera, A. indica, and Z. officinale were assayed by the agar well diffusion method and poisoned food technique. Our preliminary results in the current study by these two methods indicate that each of the extracts of four medicinal plants reduced the natural infection frequency of Aspergillus flavus, A. niger, Orbilia foliicola, Rhizopus oryzae and Talaromyces oumae-annae.
It was evident that A. vera extract could not exhibit significant antifungal activity against some of the selected seed-borne pathogens such as A. niger. It may be due to the low concentration of antifungal compounds in A. vera extract or may also be due to the low diffusibility of these antifungal compounds into the agar medium[31]. However, results of the poisoned food technique demonstrated a higher percentage inhibition for A. niger by A. vera extract. According to the previous literature, A. vera plant extract contains several antifungal, anti-inflammatory, antibacterial, and antiarthritic properties[32]. When comparing the two approaches (agar well diffusion and poison food technique) utilized in the current study to assess the in-vitro activity of plant extracts in reducing seed-borne fungal infections, it is noteworthy to mention that the poison food technique exhibited a rather significant effect in inhibition, which is also proven from previous studies[33]. The possible reason for this can be due to the higher distribution of antifungal compounds across the agar medium and prevents the growth of microbial strains tested. Results of the present study were aligned with previous literature data and clearly show that A. vera extracts exhibited poor antifungal efficacy against Aspergillus strains[34].
According to previous literature, considerable inhibition was reported for A. niger by Z. officinale extract[35]. Several studies also revealed that significant percentage inhibition can be exhibited for A. niger by A. sativum extract[36]. Observations from our study are in agreement with the above studies exhibiting higher level efficacies in controlling the common fungal pathogens such as Aspergillus spp. This inhibition may be due to the basic, low-molecular weight nitrogen-containing alkaloid present in A. sativum. These nitrogen-containing alkaloids have an inhibitory effect on fungal enzyme activities and it was able to degrade the chitin-made cell walls of A. niger, thus degrading the integrity of the cellular structures and causing the apoptosis of pathogenic cells. Also, the high flavonoid content in A. sativum plays a significant role in its antibacterial and antifungal effect against fungal pathogens[36].
In addition, it was found that Z. officinale crude extract showed the highest percentage inhibition against T. oumae-annae and it was just as effective as the percentage inhibition exhibited by Captan 50% (WP). Therefore, this concentration was found to have a high fungicidal effect on T. oumae-annae. However, this requires further study to confirm the exact concentration of Z. officinale crude extract which can replace the commercial fungicide Captan 50% (WP). The inhibition of A. flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae was found to increase with the concentration of plant extraction in the agar well diffusion method. Similarly, the poisoned food technique has also reflected a decrease in the fungal mycelial growth with the increase in the concentration of the plant extract.
The ultimate goal of our study was to use these different plant extractions against pathogenic fungi in order to use them as a successful natural fungicidal agent to treat seeds and increase seed quality. The search for antimicrobials from natural sources has received a lot of attention, and researchers are attempting to find compounds that can replace synthetic antimicrobials[37]. For that reason, the pot experiment was conducted to test the quality of the seeds; A. hypogea, O. sativa, V. radiata, and V. sinensis with different extracts including; A. sativum, A. vera, A. indica, and Z. officinale. According to previous literature data, A. indica in aqueous extraction enhances the germination of O. sativa at 100% strength[38]. Further studies revealed that A. indica consistently produced a considerably higher inhibitory effect on radial growth of the seed-borne fungi in O. sativa[39].
The aqueous extracts of Z. officinale had strong antifungal activity against A. niger and enhanced the germination of O. sativa[40].
Furthermore, Z. officinale was found highly effective in reducing the growth of the seed-borne fungus and enhancing seed germination[21].
According to data from the Seed Certification and Plant Protection Centre (SCPPC, Gannoruwa, Sri Lanka), V. radiata showed 75% germination percentage and the present study revealed that the aqueous extracts of A. sativum, A. indica and Z. officinale exhibited a 100% germination percentage for V. radiata. However, the germination percentage of V. radiata was reduced during methanolic extract treatments. The presence of methanol may be due to the reduction in germination by damaging the seed embryo. Though the mechanism of action of these plant components is unknown, it is apparent that the efficiency of extracts is highly dependent on the type of solvents used[41,42]. On the contrary, this study found that all aqueous and methanolic plant extracts treated A. hypogea and O. sativa showed significant activity in promoting seed germination and increasing seedling vigor compared to non-treated A. hypogea seeds and O. sativa[1]. This percentage was higher than the percentage germination reported by the Seed Certification and Plant Protection Centre (SCPPC, Gannoruwa, Sri Lanka), which is 75% for A. hypogea and 85% for O. sativa. Nevertheless, it was shown that all aqueous and methanolic plant extracts treated V. sinensis and only methanolic plant extracts treated V. radiata showed lower germination percentage compared to non-treated V. sinensis seeds and V. radiata[1].
-
The study presents the antifungal activity and seed germination potential of plant extracts. Methanolic extract of Z. officinale exhibited the highest antifungal activity while the A. vera exhibited the lowest antifungal activity against seed-borne pathogens; A. flavus, A. niger, O. foliicola, R. oryzae, and T. oumae-annae in this study compared with other treatments. Furthermore, A. indica and Z. officinale aqueous extracts are the most effective extract and A. vera extract is the least effective extract in controlling the seed-borne pathogens and promoting seed germination and seedling vigor of Arachis hypogea, Oryza sativa, Vigna radiata, V. sinensis.
-
Dr. A. Daranagama acknowledges the support provided by the grant RP/03/02/01/02/2020 from the University of Kelaniya Sri Lanka. The authors thank the Seeds and Planting Material Development Centre, Department of Agriculture, Pelwehera, Dambulla, Sri Lanka for providing the seed varieties needed for this study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Navoda H, Dinushani Anupama D. 2022. Evaluation of antifungal plant extracts against cereal and legume seed-borne pathogens for effective management. Studies in Fungi 7:9 doi: 10.48130/SIF-2022-0009
Evaluation of antifungal plant extracts against cereal and legume seed-borne pathogens for effective management
- Received: 29 July 2022
- Accepted: 20 September 2022
- Published online: 12 October 2022
Abstract: Sri Lanka as an agricultural country needs to manage the seed-borne fungal pathogens that have caused infections and diseases that result in significant crop losses and a decline in yield and productivity. Therefore, it is imperative to apply pathogen management strategies that are environmentally friendly, and economically feasible such as plant extractions, to reduce seed-borne fungi and increase the quality of the seed. This study was aimed at identifying the antifungal efficacy of Allium sativum, Aloe vera, Azadirachta indica, and Zingiber officinale extracts and their effective concentrations to control the seed-borne fungal pathogens; Aspergillus flavus, A. niger, Orbilia foliicola, Rhizopus oryzae, and Talaromyces oumae-annae isolated from Arachis hypogea, Oryza sativa, Vigna radiata, and V. sinensis respectively. Antifungal efficacy was determined by the agar well diffusion method and poisoned food technique. Plant extracts’ effectiveness for seed germination and seed quality was evaluated by pot experiments. Zingiber officinale crude extract exhibited the highest antifungal activity against the tested pathogens which was as effective as Captan 50% (WP), a positive control. Further analysis of the results from the pot experiment revealed that O. sativa, and V. radiata seeds treated with A. indica, and Z. officinale aqueous extracts showed 100% germination percentage. Azadirachta indica, and Z. officinale aqueous extracts are the most effective in promoting seed germination and seedling vigor while A. vera extract is the least effective extract. Comparing the two different extracts, aqueous extracts significantly promote seed germination and increase seedling vigor.
-
Key words:
- Allium sativum /
- Aloe vera /
- Azadirachta indica /
- seed treatments /
- Zingiber officinale