-
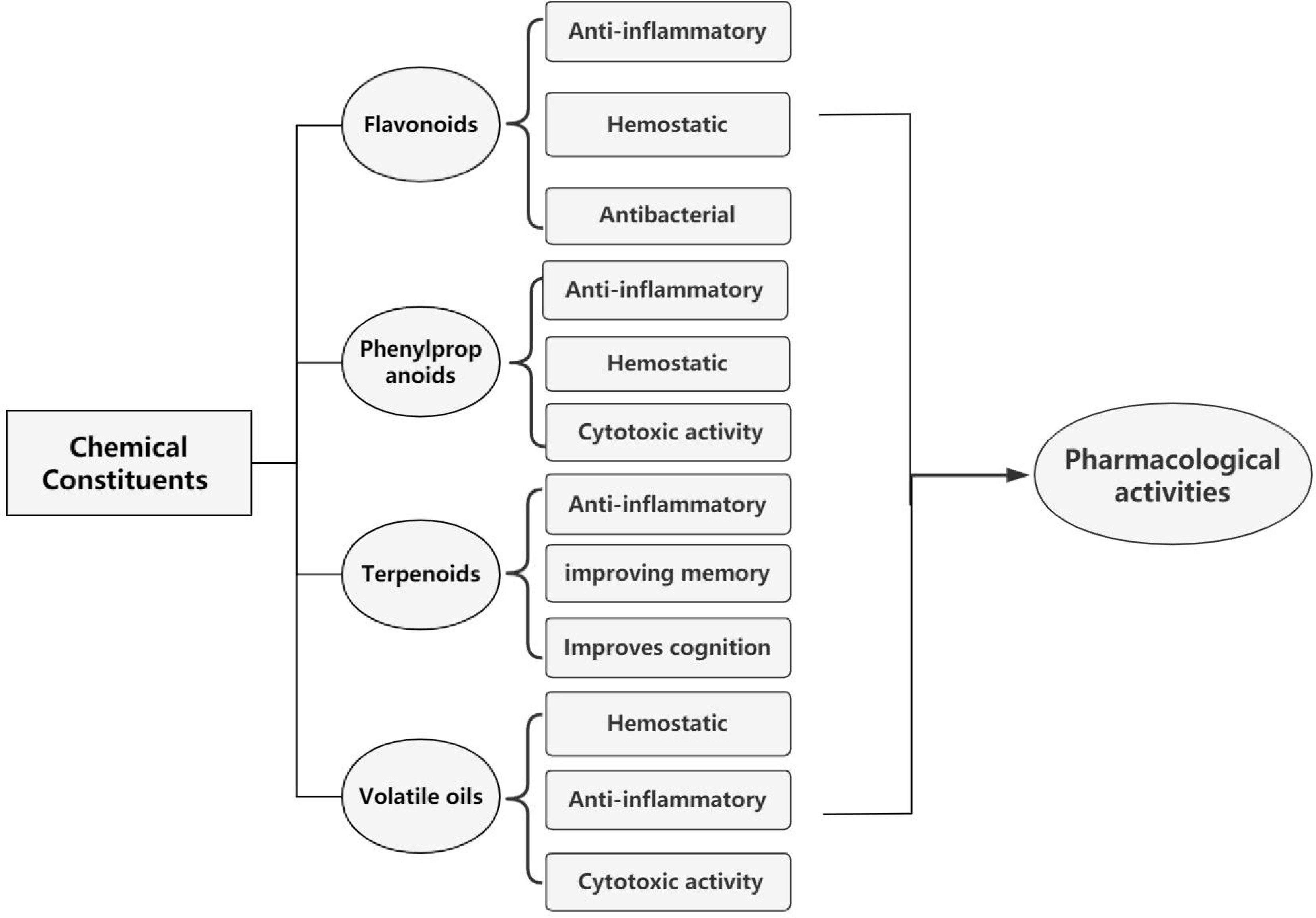
Callicarpa nudiflora is a Laminae perennial tropical rainforest-bearing herb belonging to the Taoist medicinal herbs found in Hainan Province, China. The 2020 edition of the Chinese Pharmacopoeia[1] indicates the following regarding the medicinal part of C. nudiflora: Callicarpa nudiflora folium is the dried leaf of Callicarpa nudiflora Hook. et Arn. China Flora[2] records the Latin name of C. nudiflora: Callicarpa nudiflora Hook. et Arn. Synonyms: Callicarpa reevesii,Callicarpa acuminate,Callicarpa macrophylla var. sinensis, andCallicarpa macro var. sinensis. The common names include 'deciduous purple bamboo' and 'air-dried firewood'. Nude flowers have the effects of clearing heat, detoxification, and dampness. Flavonoids, phenylpropanoids, terpenes and volatile oils, as well as phenols and sterols, are the main chemical components. C. nudiflora has anti-inflammatory, hemostatic, and antibacterial properties, and it has been clinically tested. It is used to treat tropical bacterial infections, acute infectious hepatitis, and internal and external bleeding[3]. In recent investigations, flavonoids from C. nudiflora have been demonstrated to have anti-motolity effects on breast cancer cells in vitro during the epithelial–mesenchymal transition[4].C. nudiflora extracts have been shown to inhibit breast cancer, affect colon cancer cell proliferation, migration, and invasion, and induce apoptosis sensitivity in nasopharyngeal carcinoma cells from Wuzhishan in Hainan province[5, 6]. Currently, C. nudiflora has been included in the 1977 edition of the Chinese Pharmacopoeia[7] and the 2020 edition of the Chinese Pharmacopoeia[1].
Much of the research on C. nudiflora in recent years has focused on the treatment of tropical diseases, and although there have been some reports on the chemical composition of C. nudiflora, new unknown compounds are still being discovered and their physiological effects have not been fully reported. The creation of the C. nudiflora quality criteria has been contentious. Therefore, this paper presents a detailed review of the chemical composition of C. nudiflora and its pharmacological effects, as well as the development of quality standards to provide a scientific basis for the clinical application and further development of C. nudiflora.
-
C. nudiflora originates from Hainan Province, China, and is a tropical rainforest medicinal plant, divided into wild and cultivated species. Wild species of Hainan Wuzhishan origin are the most efficacious. Recently, Wuzhishan City, with jurisdiction over Baisha and Baoting, has become the home of wild and domestic species[8]. There is also a variety of wild resources distributed throughout various regions of Jiangxi Province. Many varieties of Callicarpa are used medicinally around the world and all of the C. nudiflora types have similar effects[9]. Commonly used medicinal varieties of Callicarpa, include Callicarpa pedunculata R. Brown, Callicarpa dinhotoma (Lour.) K. koch, Callicarpa bodinieri Levi, Callicarpa bodinieri Levi. var. giraldii Rehd., Callicarpa kwangtungensis Chun, Callicarpa rubella Lindl., Callicarpa rubella Lindl. f. crenata Peih and Callicarpa cathayana H. T. Chang.
Morphology
-
Callicarpa form shrubs to small trees that are up to 7-m tall. Old branches are glabrous and lenticels conspicuous. Branchlets, petioles and inflorescences are densely gray-brown, branched, and velutinous. Leaf blades are ovate-long elliptic to lanceolate, dark green on the surface, turning black when dry, cymes spreading, and bracts linear or lanceolate. The calyxes are cup-shaped, corolla are purple or pink, anthers are elliptic. The plants flower from June to August and tiny, subglobose, red fruit appear from August to December. C. nudiflora leaves and fruit turn black when dry and can be used for identification. C. nudiflora grows on flatlands to 1,200 m above sea level on mountain slopes, in valleys, in forests by streams or in thickets. Studies have shown that C. nudiflora is more efficacious when harvested in spring, during March and April[2].
Cultivation
-
Soil factors have different effects on the content of different active ingredients in C. nudiflora, with fast-acting potassium being the dominant factor, followed by effective sulphur, effective phosphorus and organic matter[10]. C. nudiflora is usually grown from seed, cuttings and plants. It should be grown in the understory, with minimal shade and suitable light intensities of 10,100 lx, 22,000 lx, and 43,000 lx. Currently, C. nudiflora suffers from low and uneven germination, as well as low seedling establishment rates[11]. Therefore, a method for the rapid release of C. nudiflora seeds from dormancy, and to increase germination and seedling establishment, is required. At present, C. nudiflora is mostly cultivated from the wild in the home, with limited reported pests and diseases. Only locust damage has been reported, which can be controlled with highly effective and mildly toxic insecticides[12].
-
At present, studies have confirmed the following four main chemical components of C. nudiflora: flavonoids, phenylpropanoids, terpenoids and volatile oils. The two main components, flavonoids and phenylpropanoids, exert anti-inflammatory and antibacterial effects. The main chemical flavonoid component is lignan, and the main compound contained in lignan is lignoside[13]. Therefore, lignoside was the main component used to determine the flavonoid content of C. nudiflora[14]. Flavonoids inhibit breast and colon cancers, especially in tropical regions where patients are exposed to prolonged heat and humidity, and they also have in vitro antibacterial and bactericidal effects[15]. Six new compounds isolated from phenylpropanoids were identified as potential compounds for the anti-inflammatory activity of C. nudiflora and as the active substances with the greatest anti-inflammatory potential[16]. A study confirmed that six diterpenoids showed the ability to modulate the levels of inflammatory factors in vitro and confirmed the anti-cervicitis mechanism of action of C. nudiflora[17]. In addition, diterpenoids exert anti-inflammatory effects by inhibiting the production of nitric oxide[18]. Triterpenoids exert antithrombotic effects by inhibiting platelet aggregation, oleanane in pentacyclic triterpenoid compounds has a hepatoprotective activity, and iridoids are cytotoxic to tumor cells[19]. Phenylpropanoid compounds have antioxidant effects and may improve memory function and cognitive dysfunction in patients[20]. Volatile oils promote wound healing. The essential oil extracted from the leaves of C. nudiflora has insecticidal properties and is a potent insecticide[21,22]. This also confirms the mechanism of action that occurs when C. nudiflora is ground and applied externally to wounds when treating poisonous insect bites in the tropics. In addition, C. nudiflora contains small amounts of phenols and sterols[22].
Flavonoids
-
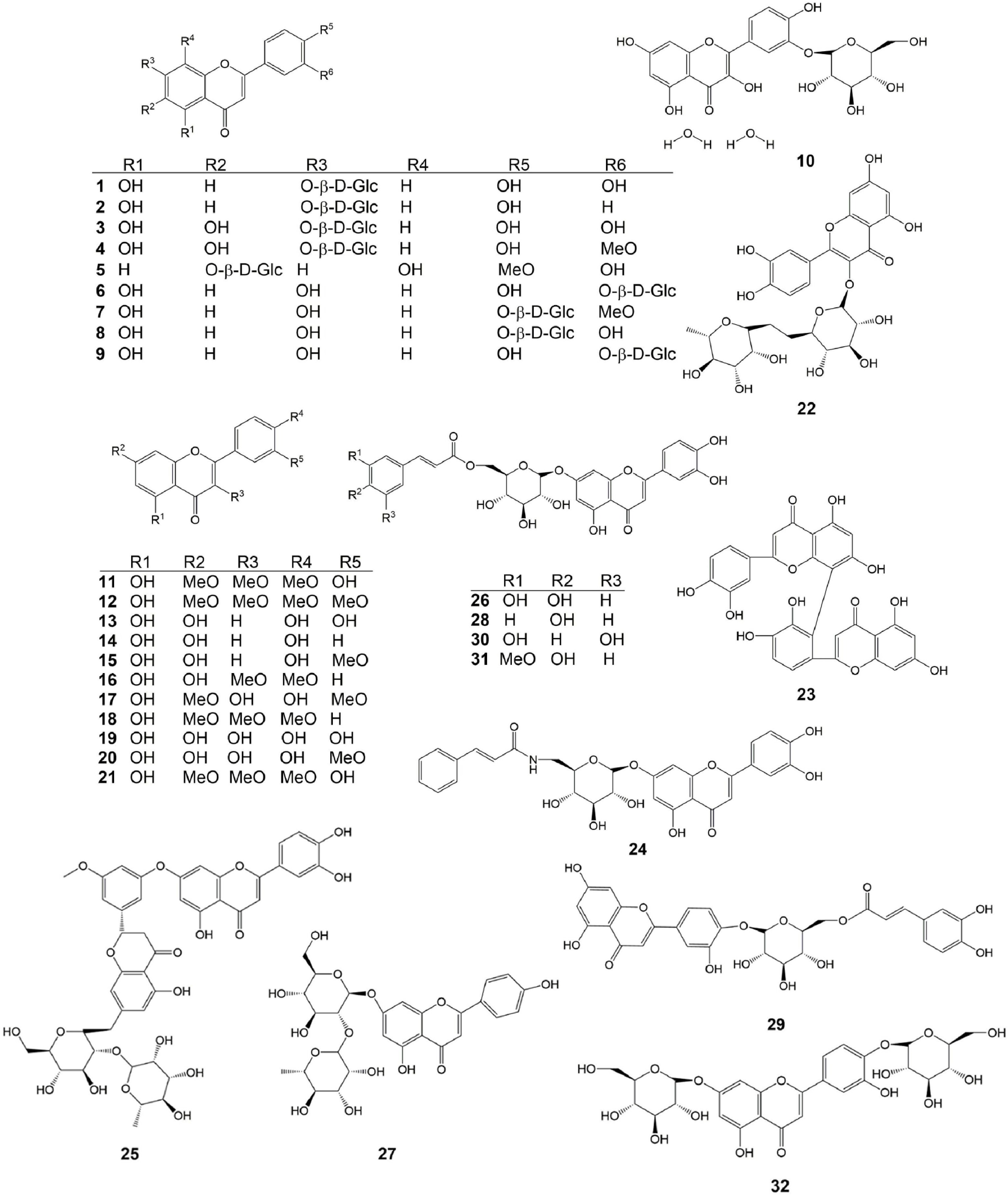
Flavonoids are the core chemical constituents of C. nudiflora and are the main components responsible for C. nudiflora's anti-inflammatory, antibacterial, hemostatic, and carcinogenic actions. Recent studies have shown that in addition to lignans, there is also a nucleoblast component in the flavonoids called apigenin[20−23]. Currently, 32 flavonoid compounds have been isolated and identified in lipid soluble, ethanol, and trichloromethane extracts of the above-ground parts of C. nudiflora. Their isolation yielded lignan, lignan-7-O-n-7-glucopyranoside, lignan-3'-O-ide, glucopyranoside, lignan-4'-O-e, glucopyranoside, and others, which were identified using the spectral data method[23]. The chemical structures of the 32 flavonoids isolated from C. nudiflora are shown in Table 1 and Fig. 1.
Table 1. Chemical constituents from Callicarpa nudiflora.
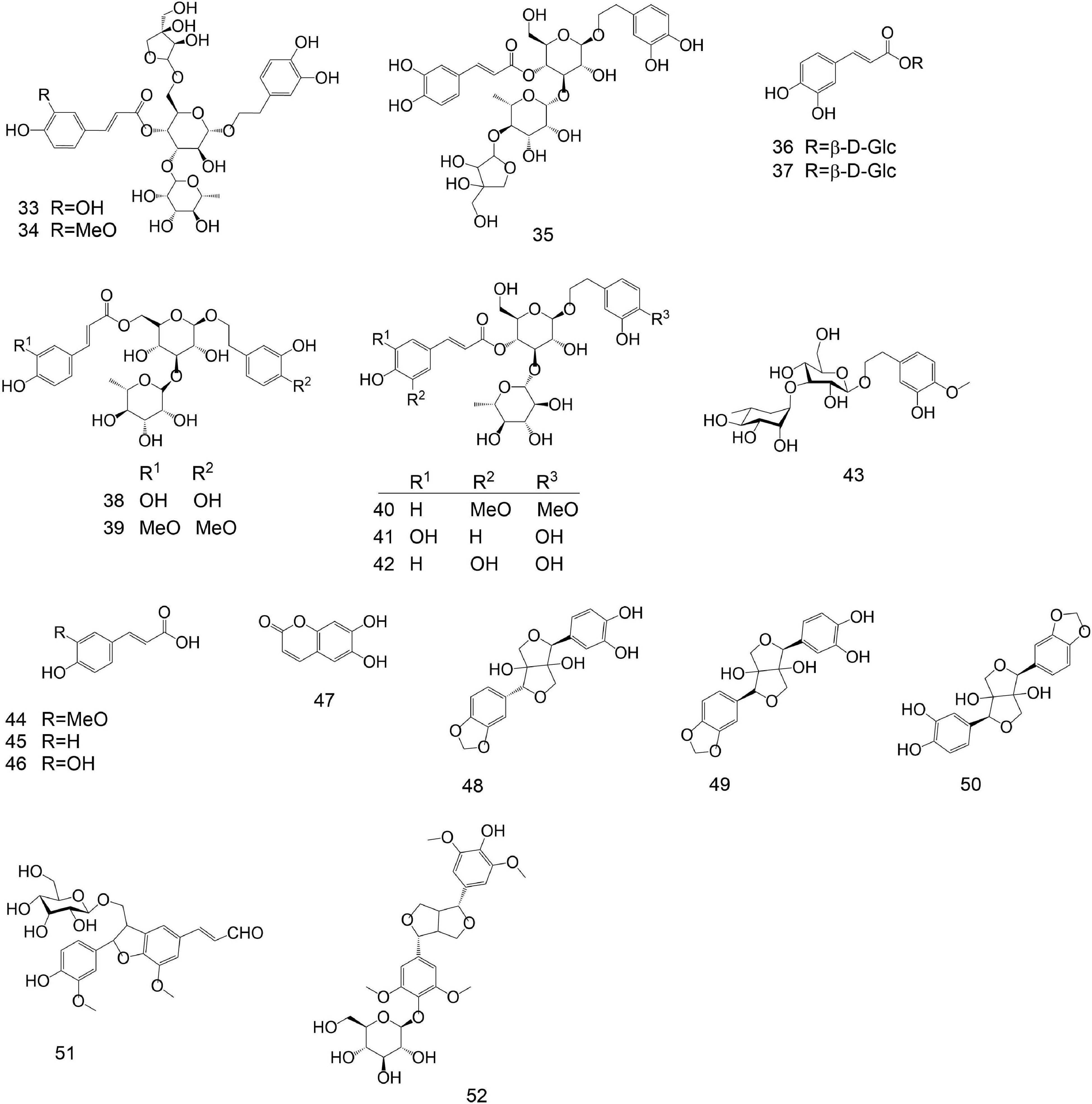
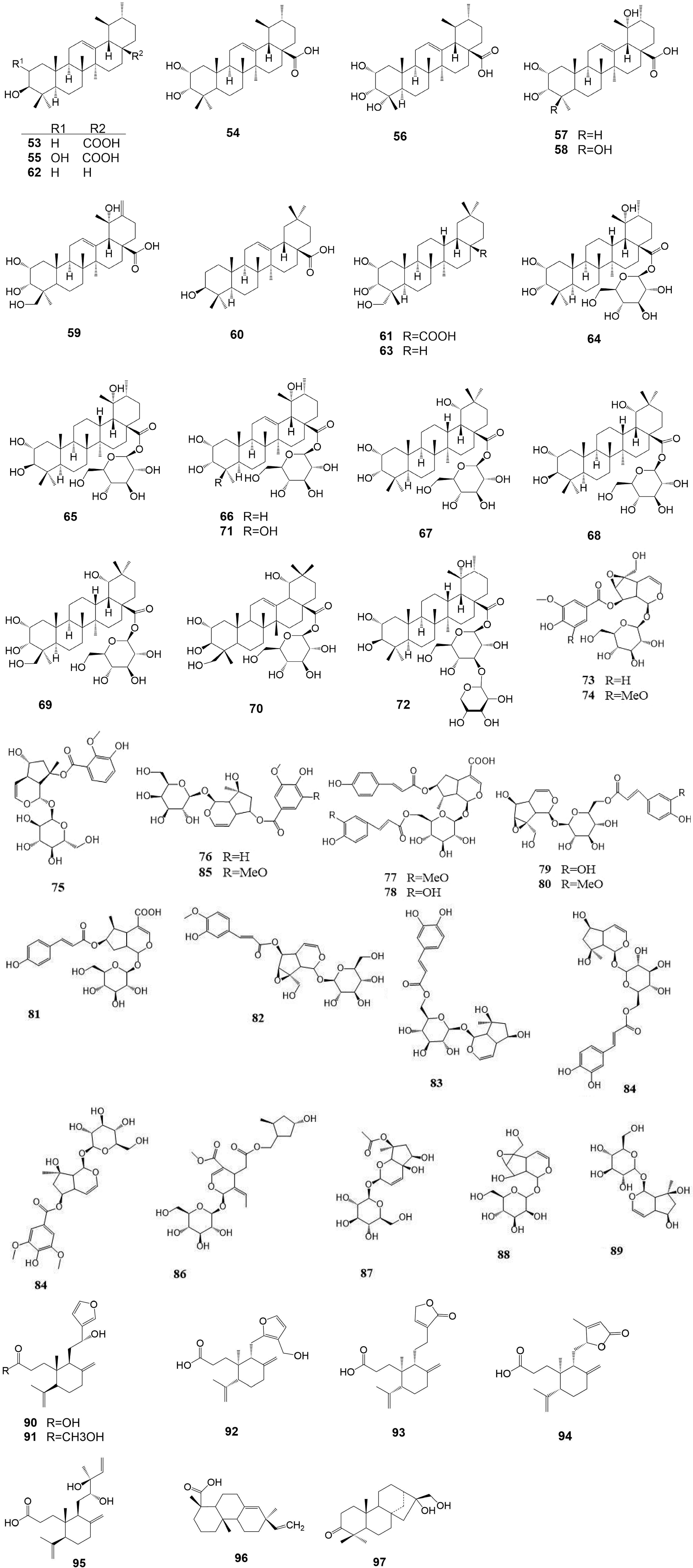
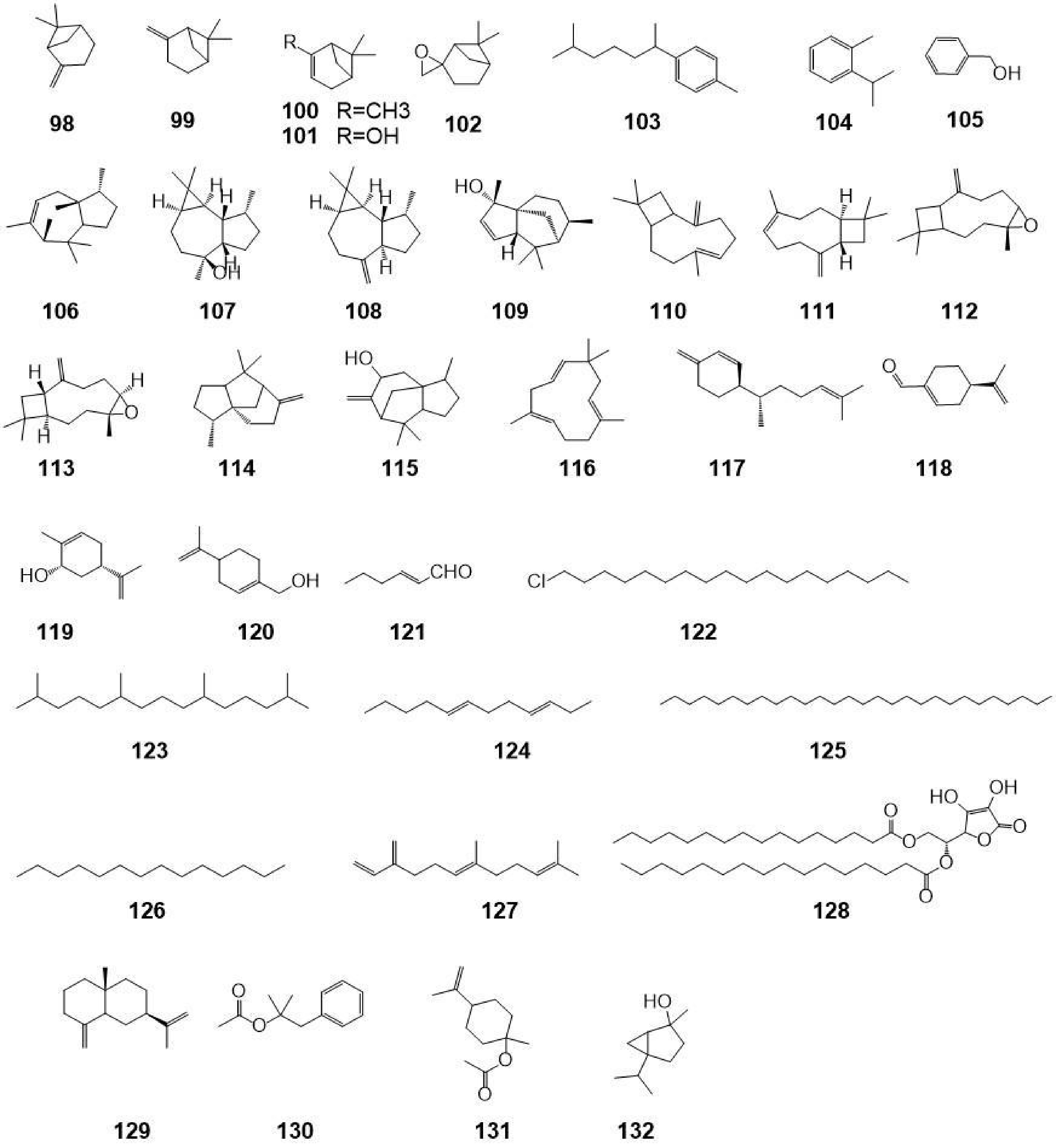
No. Category Compound name Molecular formula Molecular weight Ref. 1 Flavonoids Luteoloside C21H20O11 448.38 [22] 2 Flavonoids Pigenin-7-O-β-D-glucoside C21H20O11 432.38 [22] 3 Flavonoids 6-hydroxyluteolin-7-O-β-D- glucopyranoside C21H20O12 464.38 [22] 4 Flavonoids Luteolin-3′-methoxyl-6-hydroxy-7-O-β-D-glucopyranoside C22H22O12 478.41 [22] 5 Flavonoids Chrysoeriol-7-O-β-D-glucoside C22H22O11 462.41 [22] 6 Flavonoids Luteolin-3′-O-β-D-glucopyranoside C21H20O11 448.38 [22] 7 Flavonoids 5,7-dihydroxy -3'- methoxyflavone -4'-O-β-D- glucoside C23H24O10 460.44 [22] 8 Flavonoids Lutedin-4′-O-β-D-glucoside C21H20O11 448.38 [22] 9 Flavonoids Luteolin-3'-O-β-D-glucoside C21H20O11 448.38 [22] 10 Flavonoids Querceqin-3'-O-β-D-glucoside C21H20O11 448.38 [22] 11 Flavonoids 5,4′-dihydroxy-3,7,3′-trimethoxyflavone C18H16O7 344.32 [22] 12 Flavonoids 5-hydroxy-3,7,3′,4′-tetramethoxyflavone C19H18O7 358.35 [22] 13 Flavonoids Luteolin C15H10O6 286.24 [22] 14 Flavonoids Apigenin C15H10O5 270.24 [22] 15 Flavonoids 5,7,4′-trihydroxy-3′-methoxyflavone C16H12O6 300.27 [22] 16 Flavonoids Ermanine C17H14O6 314.08 [22] 17 Flavonoids Rhamnazin C17H14O7 330.29 [21] 18 Flavonoids 5-hydroxy-3,7,4′-trimethoxyflavone C18H16O6 328.32 [22] 19 Flavonoids Querceqin C15H10O7 302.24 [21] 20 Flavonoids Isorhamnetin C16H12O7 316.27 [21] 21 Flavonoids Ayanin C18H18O7 346.34 [22] 22 Flavonoids Rutin C29H35O15 623.58 [22] 23 Flavonoids Philonotisflavone C44H44O19 876.82 [21] 24 Flavonoids Luteolin-7-O-(6''-trans-cinnamo-yl)-β-D-glucopyranoside C30H18O12 570.46 [23] 25 Flavonoids Luteolin-7-O-neohesperidoside C27H30O14 578.52 [23] 26 Flavonoids Luteolin-7-O-(6''-trans-caffeoyl)-β-D-glucopyranoside C30H26O14 610.52 [23] 27 Flavonoids Rhoifolin C30H27NO11 577.54 [23] 28 Flavonoids Luteolin-7-O-(6''-p-coumaryl)-β-D-glucopyranoside C30H27O14 611.53 [23] 29 Flavonoids Luteolin-4′-O-(6″-trans-caffeoyl)-β-D-glucopyranoside C31H28O14 624.55 [23] 30 Flavonoids Luteolin-3'-O-(6''-E-trans-caffeoyl)-β-D-glucopyranoside C30H26O14 610.52 [23] 31 Flavonoids Luteolin-7-O-(6″-trans-feruloyl)-β-D-glucopyranoside C31H28O14 624.55 [23] 32 Flavonoids Luteolin-7,4′-di-O-glucoside C27H30O16 610.52 [23] 33 Penylpropanoids Forsythoside C34H44O19 756.71 [8] 34 Penylpropanoids Alyssonoside C35H46O19 770.73 [8] 35 Penylpropanoids Samioside C34H44O19 756.71 [13] 36 Penylpropanoids 6-O-caffeyl-β-D-glucopyranose C15H18O8 326.30 [9] 37 Penylpropanoids 6-O-caffeyl-α-D-glucopyranose C15H18O8 326.30 [9] 38 Penylpropanoids Isoacteoside C29H36O15 624.59 [15] 39 Penylpropanoids Isomartynoside C31H40O15 652.65 [7] 40 Penylpropanoids Martynoside C31H40O15 652.64 [9] 41 Penylpropanoids Acteoside C29H36O15 624.59 [11] 42 Penylpropanoids Syringalide A 3'-α-L-rhamnopyranoside C29H36O15 624.59 [11] 43 Penylpropanoids Deacylisomartynoside C21H32O12 476.47 [7] 44 Penylpropanoids Frolic acid C10H10O4 194.19 [16] 45 Penylpropanoids 4-hydroxy-cinnamic acid C9H8O3 164.16 [6] 46 Penylpropanoids Caffeic acid C9H8O4 180.16 [17] 47 Penylpropanoids Aesculetin C9H6O4 178.14 [4] 48 Penylpropanoids Nudiflorin A C19H18O8 374.36 [18] 49 Penylpropanoids Nudiflorin B C19H18O8 374.34 [18] 50 Penylpropanoids Nudiflorin C C19H18O8 374.34 [18] 51 Penylpropanoids Tortoside F C26H30O11 518.52 [9] 52 Penylpropanoids Acanthoside B C28H36O13 580.58 [12] 53 Terpenoids Ursolic acid C30H48O3 456.71 [25] 54 Terpenoids 2α,3α- dihydroxy-12-en-28-oic acid C30H48O4 472.71 [25] 55 Terpenoids 2α,3β- dihydroxy-12-en-28-oic acid C30H48O4 472.71 [25] 56 Terpenoids 2α,3α,19α,23-tetrahydroxy-olean-12-en-28-O-β-D-glucoside C29H46O5 474.33 [25] 57 Terpenoids 2α,3α,19α-trihydroxy-urs-12-en-28-oic acid C29H46O5 474.68 [25] 58 Terpenoids 2α,3α,19α,23-tetrahydroxy-urs-12-en-28-oic acid C29H46O6 490.68 [25] 59 Terpenoids 2α,3α,19α,23-tetrahydroxy-urs-12,20(30)-dien-28-oic acid C30H46O6 502.69 [25] 60 Terpenoids Oleanolic acid C30H48O3 456.71 [27] 61 Terpenoids 2α,3β,24-trihydroxy-olean-12-en-28-oic acid C30H50O5 472.71 [25] 62 Terpenoids Urs-12-en-3β-ol C29H48O 490.73 [25] 63 Terpenoids Arjunglucoside I C29H50O3 446.71 [27] 64 Terpenoids 2α,3α,19α-trihydroxy-urs -12- en -28-O-β-D- glucopyranoside C36H60O10 652.87 [25] 65 Terpenoids 2α,3β,19α-trihydroxy-urs -12- en -28-O-β-D- glucopyranoside C36H60O10 652.87 [25] 66 Terpenoids 2α,3α,19α-trihydroxy-ursane-12-en -28-O-β-D-glucoside C35H56O10 636.82 [27] 67 Terpenoids 2α,3α,19α-trihydroxy-olean-12-en-28-O-β-D-glucopyranoside C36H60O10 652.87 [25] 68 Terpenoids 2α,3β,19α-trihydroxy-olean-12-en-28-O-β-D-glucoside C36H60O10 652.87 [27] 69 Terpenoids 2α,3α,24- trihydroxy-ursane -12-en-28-oic acid C36H60O11 668.87 [27] 70 Terpenoids 2α,3α,24-trihydroxy-olean-12-en-28-oic acid C36H58O11 666.85 [27] 71 Terpenoids 2α,3α,19α,23-tetrahydroxy-ursane-12- en -28-O-β-D-glucoside C36H56O11 652.82 [27] 72 Terpenoids 2α,3α,19α-trihydroxy-urs-12-en-28-O-β-D-xylopyranosyl(1→2)-β-D-glucoside C41H68O14 784.98 [25] 73 Iridoids Ampicoside C23H28O13 512.46 [25] 74 Iridoids 5''-methoxy-ampicoside C24H30O14 542.49 [25] 75 Iridoids 6-O-vanilloyl-ajugoside C23H30O12 784.98 [25] 76 Iridoids 6-O-syringoyl-ajugol C23H30O12 498.48 [25] 77 Iridoids 3''-methoxy-agnucastoside C C24H32O13 528.51 [20] 78 Iridoids Agnucastoside C C34H36O15 684.65 [20] 79 Iridoids 6″-O-trans-caffeoylcatalpol C24H28O13 524.48 [20] 80 Iridoids 6″-O-trans-feruloylcatalpol C25H30O13 538.50 [20] 81 Iridoids linearoside C25H30O12 522.50 [20] 82 Iridoids 6-O-trans-feruloylcatalpol C25H30O13 538.50 [20] 83 Iridoids 6'-O-caffeoyl-ajugol C24H30O12 510.49 [25] 84 Iridoids 6-O-caffeoyl ajugol C24H30O12 510.49 [25] 85 Iridoids 6-O-vanilloyl-ajugol C24H32O13 528.51 [25] 86 Iridoids Nudifloside C24H36O12 516.54 [20] 87 Iridoids Catalpol C17H26O12 510.49 [20] 88 Iridoids Ajugol C15H22O10 362.33 [20] 89 Iridoids 8-acetyl-harpagide C15H24O9 348.39 [25] 90 Iridoids Callicarpic acid C20H28O4 332.44 [22] 91 Iridoids Methylcallicarpate C21H31O4 347.48 [22] 92 Iridoids Et-3,4-seco-16-hydroxy-12,15-epoxy-4(18),8(17),12,14-labdatetaen-3-oic acid C20H28O4 332.44 [22] 93 Iridoids Ent-3,4-seco-14-carbonyl-15,16-epoxy-4(18),8(17),13(14)-labdatrien-3-oic acid C20H28O4 332.44 [22] 94 Iridoids Ent-3,4-seco-12R,15-epoxy-4(18),8(17),13-labdatrien-3-oic acid C20H28O4 332.44 [22] 95 Iridoids 3,4-seco-12R,13S-dihydroxy-4(18),8(17),14(15)-labdatrien-3-oic acid C20H28O4 336.47 [22] 96 Iridoids 7α-hydroxy sandaracopimaric acid C20H30O2 302.46 [22] 97 Iridoids 16,17-dihydroxy-3-O-phyllocladane C20H32O3 320.47 [22] 98 Volatile (1S)-β-pinene C10H16 136.24 [27] 99 Volatile β-pinene C10H16 136.24 [25] 100 Volatile α-pinene C10H16 136.24 [25] 101 Volatile Myrtenol C10H16 136.24 [25] 102 Volatile β-epoxypinane C10H16O 152.24 [27] 103 Volatile Dihydrocurcumene C15H24 204.36 [25] 104 Volatile O-cymene C10H14 133.22 [25] 105 Volatile Benzylalcohol C7H8O 108.14 [27] 106 Volatile α-cedrene C16H28 220.40 [27] 107 Volatile Viridiflorol C15H26O 222.37 [26] 108 Volatile Aromadendrene C14H21 189.322 [26] 109 Volatile Cedrenol C14H24O 220.37 [25] 110 Volatile (E)-β-farnesene C15H24 204.36 [27] 111 Volatile 1-caryophyllene C15H24 204.36 [25] 112 Volatile Caryophyllene oxide C15H21O· 205.32 [25] 113 Volatile Epoxycaryophyllene C15H24O 220.36 [25] 114 Volatile β-cedrene C15H24 204.36 [27] 115 Volatile α-cedrenol C15H24O 220.37 [25] 116 Volatile α-caryophyllene C15H24 204.36 [26] 117 Volatile β-sesquiphellandrene C15H24 204.36 [27] 118 Volatile Perillaldehyde C10H14O 150.22 [27] 119 Volatile Cis-carveol C10H16O 152.24 [27] 120 Volatile L-perillyl alcohol C10H16O 152.24 [27] 121 Volatile Trans-2-Hexenal C6H10O 98.15 [27] 122 Volatile 1-Chloroctadecane C18H37CI 288.94 [27] 123 Volatile 2,6,10,14-tetramethylpentadecane C19H40 152.24 [27] 124 Volatile Dodecane-3,7-diene C12H22 166.31 [26] 125 Volatile Octacosane C28H58 394.78 [27] 126 Volatile Tetradecane C14H30 198.39 [27] 127 Volatile α-curcumene C15H24 204.36 [27] 128 Volatile L-Ascorbyl dipalmitate C38H68O8 652.95 [27] 129 Volatile β-selinene C15H24 204.36 [27] 130 Volatile Dimethylbenzylcarbinyl acetate C12H16O2 192.26 [27] 131 Volatile β-terpinyl acetate C12H20O2 196.30 [27] 132 Volatile Trans-4- thujylalcohol C10H18O 154.25 [25] Phenylpropanoids
-
Phenylpropanoids are another major active ingredient of C. nudiflora and are the main components of C. nudiflora responsible for anti-inflammatory and antibacterial activities. Currently, 20 phenylpropanoids substances have been reported, with lignans, 3-phenylpropanoic acid, and phenylpropanoid glycosides being the most numerous. Verbascoside and forsythoside B are often selected as indicator components when evaluating the quality of C. nudiflora using high-performance and ultra-performance liquid chromatography for the detection of multiple active ingredients[24]. The chemical structures of the 20 phenylpropanoids compounds isolated from C. nudiflora are shown in Table 1 and Fig. 2.
Terpenoids
-
To date, as many as 45 species of terpenoids have been identified in C. nudiflora, including 20 triterpenes, 17 iridoids, and eight diterpenes, Sixteen of the iridoids are considered to be the main monoterpenoids in C. nudiflora[25,26], and diterpenes, mainly callicarpic acid and methylcallicarpate, are the focus of current research on terpenoids in C. nudiflora herbs[22]. The triterpenes mainly include oleanane triterpenoid and ursane triterpenoid, and small amounts of lupane triterpenoid have also been identified[27]. In recent years, limited new structures of sesquiterpenes have been isolated from C. nudiflora; therefore, they will not be described here. The structures and names of the above confirmed terpenoids are shown in Table 1 and Fig. 3.
Volatile oils
-
Volatile oils are also a major component of C. nudiflora, and 36 components have been isolated and identified in volatile oils, namely monoterpenes and their oxygenated derivatives, as well as alkanes. Although the leaves and flowers of C. nudiflora contain volatile oils having different chemical compositions, most of them are terpenoids[26]. In addition, studies have confirmed that the leaves of C. nudiflora contain more volatile oils than the flowers. Most of the research on volatile oils has focused on their chemical composition[19, 28]. At present, the constituents in volatile oils have not been considered as markers for qualitative and quantitative examination. The names and structures of the 36 chemical components mentioned above are shown in Table 1 and Fig. 4.
-
Anti-inflammatory and hemostatic effects are the two main pharmacological actions of C. nudiflora[26,28]. C. nudiflora also has antibacterial effects, particularly against Staphylococcus aureus, and it is considered a potential drug for S. aureus infections[29]. There have been studies on the mechanisms by which C. nudiflora improves cognitive dysfunction and improves memory in Alzheimer's disease patients[20]. Recent studies have found that C. nudiflora inhibits the production of cervical and ovarian cancer cells, as well as the growth of breast and colon cancers, in humans, confirming the anti-tumor effects of C. nudiflora[6, 15, 17, 30]. C. nudiflora essential oil has a good insecticidal effect and is a potent insecticide[21]. In addition, C. nudiflora has recently been found to have antioxidant properties, anti-diabetic and anti-psoriasis effects, and to treat alcoholism[31−33].
The combined pharmacological activity of two or more compounds inC. nudiflora is greater than that of individual compounds, with flavonoids and phenylpropanoids dominating, although terpenoids and volatile oils also have some pharmacological activities. Platelet aggregation is inhibited by oleanolic acid and ursolic acid from C. nudiflora. Thus, triterpenes appear to have an anti-platelet coagulation effect[27]; therefore, C. nudiflora not only has an hemostatic effect but also has an activating effect on removing the phlegm and turbid urine[34−36]. At present, many biologically active compounds have been isolated from C. nudiflora by domestic and foreign scholars, but the research has not fully investigated the chemical composition and biological activities of the compounds; therefore, there is still the need for continued exploration.
Anti-inflammatory activity
-
Chen et al. conducted experiments on C. nudiflora treated mice and C. nudiflora treated cells. The cell experiments showed that extracts from different parts of C. nudiflora produced good anti-inflammatory effects, with those of the flower extracts and flower isolates of C. nudiflora being more robust. In mice, exposure to C. nudiflora reduced xylene-induced auricular swelling and significantly inhibited auricular swelling and toe swelling, which are indicators of its immune-enhancing effects[36]. In addition, ethanol extracts of C. nudiflora: CNE-p, CNE-d, and CNE-b, have the ability to regulate inflammatory factors in vivo and in vitro, and the strongest anti-inflammatory activity of CNE-p was found in in vitro tests[17]. Wang et al. isolated four new compounds from the leaves of C. nudiflora using a comprehensive spectroscopic analysis and confirmed the significant inhibitory effects of these compounds, comparable to that of the positive control dexamethasone, on nitric oxide production[37]. The anti-inflammatory effects of Compounds 2–4 from C. nudiflora were confirmed through the inhibition of superoxide anion production and elastase release by Lin et al[38]. In addition, C. nudiflora cell experiments revealed that diterpene and 5-oxhydryl-3,7,3',4'-tetramethoxyflavone have some anti-inflammatory effects, and 5-oxhydryl-3,7,3',4'-tetramethoxyflavone inhibits inflammatory factors. In addition, nitric oxide production was only slightly inhibited[39]. Wu et al. isolated new compounds from the flowers of C. nudiflora that showed anti-inflammatory activities and had significant NLRP3 inflammasome inhibitory activities[40]. In addition, flavonoids and diterpenoids from C. nudiflora were found to inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 cells[41]. These are the most recent studies on the anti-inflammatory effects of C. nudiflora available. Chen et al. found that C. nudiflora tablets alleviated chronic pelvic inflammatory disease by decreasing inflammatory cytokines, such as IL-1β, MCP-1, and GM-CSF[42].
Hemostatic activity
-
The two main classes of pharmacological components among the chemical constituents of C. nudiflora that exert haemostatic activity are phenylpropanoids and flavonoids. Virtually all parts of C. nudiflora can be used and extracts from its roots, stems and leaves have been confirmed to have hemostatic effects[27]. There are two main ways for C. nudiflora to exert the hemostatic effect, one is through the intrinsic coagulation pathway, and the other is through both the intrinsic and extrinsic coagulation pathways. C. nudiflora extract acts on endogenous coagulation by affecting the timing of prothrombin and fibrinogen content[43, 44]. A study about C. nudiflora acting on the intrinsic and extrinsic coagulation pathways at the same time confirmed that its ethyl acetate and n-butanol extracts could shorten the bleeding and coagulation times and reduce the activated partial thromboplastin time, indicating that it has hemostatic activity; Both can increase the number of platelets and stimulate platelet activation, indicating that it has a hemostatic effect. In addition, it has been found that extraction of C. nudiflora with ethyl acetate can significantly enhance the endogenous platelet activating substance adenosine diphosphate, which can promote hemostasis. In C. nudiflora, phenylpropanoid glycosides and pentacyclic triterpenoids produce the main hemostatic effect and can reduce the activation time for partial thromboplastin. They are followed by flavonoids, which also have a significant effect on fibrinogen content[44, 45]. Wang et al. investigated the mechanism of the haemostatic effects of butanolic extracts of C. nudiflora and showed that these extracts had significant haemostatic activities, which may be stimulated by platelet activation, associated with the activation of the PI3K-PKB signaling pathway in platelets[46]. Zhou et al. isolated two new triterpenoids, 2α,3α,19α,23-tetrahydroxyurs-12,20(30)-dien-28-oic acid and 2α,3α,19α-trihydroxyurs-12-en-28-oic acid-28-O-β-D-xylopyranosyl (1→2)-β-D-glucopyranoside, and the anti-platelet aggregation activities of these two compounds in vitro indicated their inhibitory effects on adenosine diphosphate-induced platelet aggregation[27]. In the future, researchers will likely isolate new compounds from C. nudiflora that will reveal new mechanisms by which it exerts hemostatic effects.
Antibacterial activity
-
Studies have shown that C. nudiflora has antibacterial activity against a wide range of bacteria. Wang et al. used six polar solvents to extract C. nudiflora and completed antibacterial tests of C. nudiflora against eight bacteria, showing that the ethanol extract of C. nudiflora had good antibacterial activity against Escherichia coli and Bacillus subtilis[47]. A study by Liu et al. showed that C. nudiflora has an in vitro inhibitory effect on five indicator bacteria: E. coli, Pseudomonas, Candida albicans, B. subtilis, and S. aureus[48]. In addition, C. nudiflora also shows good antibacterial activity against streptomycin-resistant and streptomycin-sensitive mycobacterium tuberculosis, and this study suggests that the antibacterial activity of C. nudiflora may be related to protein expression[49]. Three compounds in C. nudiflora, alpha-borneol, betulinic acid, and betulin aldehyde, may exhibit specificity activities against specific bacteria through pathways that are absent in the human host, and, therefore, these compounds are referred to as lead compounds. In addition, C. nudiflora in combination with vancomycin hydrochloride has a synergistic antibacterial effect against Methicillin-resistant S. aureus (MRSA)[50]. Currently, C. nudiflora extracts are commercially available as ointment preparations for trauma-related wound healing.
Cytotoxic activity
-
The cytotoxicity levels of compounds in C. nudiflora were evaluated using the MTT assay and revealed that C. nudiflora has monomeric proliferative inhibitory activity against Hela, A549, and MCF-7 cell lines, in which the main acting compound is phenylethanoid. However, no cellular activity was found against cancer cell lines. An extremely subtle cytotoxic activity was also expressed by C. nudiflora glycoside[51].
Anti-cancer activity
-
Several in vitro studies have shown that rhoifolin in C. nudiflora extracts has antimotility properties against breast cancer cells[4, 15]. This property is attributed to its downregulation of the podocalyxin–ezrin interaction during the epithelial mesenchymal transition. BALB/c Nu mice experiments have shown that ethyl acetate extracts from C. nudiflora significantly inhibit intracellular p-Snail and increase E-cadherin expression in breast cancer cells[52]. Cellular experiments have shown that C. nudiflora from Wuzhishan, Hainan Province (China) can inhibit the proliferation and growth of intestinal cancer cells by activating the Wnt/β-catenin signaling pathway[53].C. nudiflora affects the epithelial mesenchymal transition, proliferation, and migration of colon cancer cells in vivo by inhibiting the activation of the TGF-β1/P38/PAI-1 pathway. In vitro experiments have shown that C. nudiflora reverses the drug resistance of colon cancer cells in vitro by activating the IL-6/STAT3 signaling pathway[6].
Others
-
Pharmacological studies have shown that C. nudiflora has the functions of improving memory, anti- herpes simplex virus-1, and anti-abdominal adhesion after abdominal surgery[54]. Zhou et al. studied the extraction of C. nudiflora using three different solvents (petroleum ether, n-butanol, and ethyl acetate) and observed the effects of the three different solvent extracts against herpes simplex virus-1. The ethyl acetate extract was found to be the most effective[55, 56]. Ba Li'na determines the anti-psoriasis effect of C. nudiflora extract. Sixty KM mice were selected and grouped according to the random number table method. They were given normal saline, C. nudiflora extract, and 1 mg/kg methotrexate by gastric perfusion for seven days, respectively. Conclusion showed that the C. nudiflora administration group was significantly higher than the other groups. The C. nudiflora extract can exert an anti-psoriatic effect by inducing apoptosis[57,58]. The C. nudiflora alcohol test reduces mortality and serum alanine aminotransferase and glutamate pyruvic transaminase levels in mice with acute alcoholic liver injury and has a protective effect against alcoholic liver injury[33]. Phenyl ethanol glycosides have neuroprotective and memory-improving effects; therefore, they have great potential in the treatment of diseases such as Alzheimer's disease[59]. C. nudiflora extracts reduce serum insulin, total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels. They can improve insulin resistance and reverse liver and pancreatic damage caused by diabetes[60]. In addition, studies have shown that C. nudiflora can promote angiogenesis in human microvascular endothelial cells, reduce the body's inflammatory response, and improve urinary bacterial clearance[61, 62] (Fig. 5).
-
Current research on C. nudiflora focuses on the chemical composition and pharmacological activity of the plant. Its chemical components include flavonoids, phenylpropanoids, terpenes, and volatiles. According to previous studies, flavonoids and phenylpropanoids are the two main components of C. nudiflora and the basis for its medicinal effects. Therefore, these two components became the marker compounds for the quality control of C. nudiflora. Significant amounts of verbascosides have been found in C. nudiflora[63−65], and these compounds can also act as markers for C. nudiflora quality standards. The main components of C. nudiflora can change owing to various factors, including origin, harvesting, distribution[66,67], storage, and forgery, leading to great variations[68]. In C. nudiflora produced in Hainan Province (China), the coefficient of variation exceeded 50% for seven different active ingredients. This data indicates the importance of developing quality standards for C. nudiflora. Owing to the complexity and uncertainty of the pharmacological mechanisms of C. nudiflora, coupled with the gradual identification of more and more compounds, one or a few markers in C. nudiflora may not be enough to set an overall quality standard for the herb. Therefore, some researchers have suggested that in the future, phytofingerprinting technology may be used, because it is already widely accepted as the basis for the effective identification of herbal medicines[69]. C. nudiflora samples harvested at different times and under different weather conditions have significant variations and specificities. Researchers have also confirmed this by testing fingerprints with other Verbenaceae plants, such as C. kwangtungensis, C. macrophylla, C. formosana, and C. kochiana[70]. At present, there is a gap in research on the changes in pharmacological activities caused by the changes in chemical components of C. nudiflora plants. Thus, there is a need to strengthen the research on the mechanism of action between the variability in the chemical composition and the pharmacological activities of C. nudiflora to improve better clinical applications.
The pharmacological activity of C. nudiflora is greater when used clinically in combination
-
Studies have confirmed the rich pharmacological activity of C. nudiflora. C. nudiflora tablets have been widely used clinically with good results. However, only a few individual isolates from C. nudiflora have been reported in the literature to have good anti-inflammatory activities. Because of the limited formulation development capacity of C. nudiflora, only a few pharmaceutical companies are focused on developing C. nudiflora raw materials. Soup made from C. nudiflora is mostly given to patients in tropical regions, and its efficacy is limited. Recent studies have shown that C. nudiflora has better activity when taken together with another herb than when taken alone[71,72]. C. nudiflora granules combined with entecavir in the treatment of cirrhosis upper gastro-intestinal bleeding can effectively improve patients' liver function and enhance patients' coagulation function. C. nudiflora granules combined with montelukast retention enema in the treatment of chronic radiation colitis can effectively improve clinical symptoms without the occurrence of serious adverse reactions. C. nudiflora capsules or tablets combined with antibiotics can effectively relieve symptoms and reduce inflammation in the treatment of pelvic inflammatory disease, sequelae of qi stagnation, and blood stasis[73]. C. nudiflora tablets combined with indocin therapy can effectively prevent vaginal bleeding and shorten the time of menstrual return in patients after medication-induced abortions[74,75]. C. nudiflora capsules combined with amikacin sulfate injections in the treatment of urinary tract infection can reduce the inflammatory response and improve the clearance of urinary bacteria[62]. Studies have shown that combinations of drugs can be more stable and significant than a single drug and can effectively reduce the adverse effects caused by Western drugs.
C. nudiflora has good development prospects
-
At present, research on C. nudiflora is mainly focused on ethanol extraction, and the compounds isolated are mostly terpenoids, flavonoids, phenylethanol glycosides, and phenylpropanoids. The pharmacological effects, including antisepsis, anti-inflammatory, and hemostasis, possessed by C. nudiflora have been tested, and more products are being developed based on these efficacies. A new toothpaste made from an extract of C. nudiflora improves the symptoms of gingivitis[76,77]. A new hemostatic disinfectant containing an extract of C. nudiflora leaves was developed and tested to be effective[77]. C. nudiflora is no longer confined to the treatment of insect and snake bites or trauma in tropical rainforests, it has also been discovered to aid in rheumatic paralysis, tropical diseases, internal injuries, and tumor-based diseases. Preparations of C. nudiflora are currently being developed for the market. Anti-uterine inflammatory tablets and capsules containing C. nudiflora as well as C. nudiflora-containing tablets, dispersible tablets, suppositories, and granules are being developed. Some contain a combination of C. nudiflora and other herbs. C. nudiflora can act as an insecticide owing to its insecticidal properties[21].C. nudiflora has a variety of pharmacological effects, and there are still many chemical constituents and biological activities to be further studied.
In recent years, a number of additional potent and new medicinal resources have been discovered in the tropics, with 137 different types of plants producing anti-oncogenic medicinal ingredients. In addition, plants required to make a number of drugs that previously had to be imported have been found in Hainan Province (China). These resources need to be developed.
This work was supported by Hainan Province Science and Technology Special Fund of China (No. ZDKJ2021034) and the Hainan Province Natural Science Foundation of China (No. 822RC697).
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Yan Ma, Licheng Guo
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ma Y, Guo L, Hou W, Zhuo J, Zhao M, et al. 2022. Phytochemistry, bioactivities, and future prospects of Callicarpa nudiflora: A review. Medicinal Plant Biology 1:5 doi: 10.48130/MPB-2022-0005
Phytochemistry, bioactivities, and future prospects of Callicarpa nudiflora: A review
- Received: 26 July 2022
- Accepted: 26 October 2022
- Published online: 09 December 2022
Abstract: Callicarpa nudiflora is a regional medicinal herb in China. A typical tropical rainforest medicinal plant, it is processed in China's Hainan province, Guangdong province and Guangxi Zhuang Autonomous Region, as well as India, Vietnam, Malaysia and Singapore. C. nudiflora has the functions of clearing away heat, detoxification and dampness. Flavonoids, phenylpropanes, terpenoids and volatile oils as well as phenols and sterols are the main chemical constituents. Modern pharmacology shows that C. nudiflora has anti-inflammatory, hemostatic, antibacterial, and hepatoprotective effects, treats breast and colon cancer, and is clinically used to treat tropical bacterial infections, acute infectious hepatitis, and internal and external bleeding. C. nudiflora is different from traditional herbal medicines because it has significant anti-inflammatory and hemostatic effects and is widely used as a single prescription medicine. This review evaluates recent advances in fully determining the chemical composition and pharmacological effects of C. nudiflora, thereby laying the foundation for future pharmaceutical research.
-
Key words:
- Callicarpa nudiflora /
- Flavonoids /
- Anti-inflammatory action /
- Hemostatic action