-
Sporulation comprises a vital process in fungi by which the specialized cells called spores are formed. These peculiar structures which are formed during the reproduction of fungi, help in their dissemination, propagation, and survival[1,2]. Fungi reproduce via two modes (to produce spores) – sexual and asexual. Reportedly, they reproduce asexually most of the time, especially in artificial media[3,4]. Moreover, studies reveal that asexual reproduction sometimes completely overpowers the sexual mode[5]. It is because this mode of reproduction is mainly concerned with dispersal and requires less energy[2]. On the other hand, sexual reproduction predominantly acts as a survival strategy that occurs under unfavourable/stressful conditions to repair the damaged DNA, often via homologous recombination[6]. Whether asexual or sexual, spore structure analysis is the most important criterion for classifying fungi into different taxa (genera, species, and so on). Although molecular studies have gained much attention in the recent past concerning the identification of fungi, morphological characteristics are regarded as an essential and integral part of fungal taxonomy[7].
Fungal sporulation is a highly complex phenomenon influenced by both endogenous as well as exogenous factors[2, 8]. Endogenous factors may include competence, a condition in which a fungus grows and make itself competent to sporulate[9, 10]. Until the time this phase is over, a fungus cannot sporulate despite providing all the environmental factors required for its sporulation. Besides, various exogenous factors, such as nutrients, light, pH, humidity, and host tissue also influence fungal sporulation[11−13]. However due to variable requirements and complex sporulation behaviour, the influence of a particular factor/s on the sporulation of a particular fungus varies.
Endophytic fungi are a special group of mycosymbionts that are capable of residing within the plant tissues without exhibiting visible negative symptoms, at least for a part of their life cycle[14]. These symbionts improve host photosynthesis, mineral acquisition, and augment the host tolerance to biotic and abiotic stress factors[15]. Considering the sporulation in endophytic fungi, it seems more complex than in any other group to unravel the exact combination of factors responsible for the sporulation of a particular mycosymbiont. As they exhibit a wide range of lifestyles, such as latent pathogens, mutualists to saprophytes[16], the complex nature of their sporulation may be attributed to their variable colonization strategies and thereby maintenance of endophytism in planta. Since endophytic fungi are the inhabitants of plant tissues, they don't face the external stressful conditions regarding the acquisition of food and shelter[17], hence either they don't sporulate at all in planta or if they do, it is almost negligible. However, as far as in-vitro sporulation is concerned, many culturable fungal endophytes may sporulate on artificial nutrient media[18]. Studies indicate that the majority of these fungi belong to hyphomycetes exhibiting asexual mode of reproduction as the predominant mode of propagation[19]. Intriguingly, almost from all the plants explored so far, a significant fraction of endospheric mycobionts are found as sterile mycelia[20]. Many of them don’t sporulate on artificial media, several fail to do so even under nutrient stress (starvation media) or other types of stressful conditions[21]. Non-sporulating fungal endophytes mostly belong to coelomycetes[22], which either take a long time or require inductive treatments to sporulate[23]. However, in either case, they predominance sporulate via asexual mode, while a small fraction may reproduce sexually[3,9]. In general, different fungi may require varied endogenous and exogenous factors, individually or in synergy to accomplish sporulation.
The primary knowledge regarding fungal taxonomy is based on morphological aspects[7]. Induction of sporulation in fungi is essential as the phenotypic approach should be the initial step towards the identification of fungi followed by other methods viz., molecular, ecological, physiological, and chemical methods[24]. Scientists have used various methods to induce sporulation in fungi. For example, starvation media, plant tissues (leaves, stem, petioles, etc.), alternate light and dark cycles, slide culture technique, CaCO3, low-strength nutrient media, and so on[23]. However, the outcome may vary with the methods employed and the type of fungus being involved[25]. Also, it has been found that many fungi may sporulate in response to several factors while some may exhibit specificity in their response. For instance, a group of scientists successfully induced sporulation in 50% (21 out of 42) of the tested fungal isolates under various sporulation induction treatments[26]. Out of these, 14 isolates sporulated on pine needle medium, nine on 1/10 PDA medium, six on mulberry leaves, and five under near UV light. Fusarium spp. and Celoporthe spp. sporulated upon treatment with both mulberry leaves and pine needle medium whereas Diplodia scrobiculata and D. pinea sporulated in the nutrient medium supplemented with Austrian pine needles[26]. Similarly, when different isolates of sterile Alternaria spp. were investigated for their sporulation stimulus, the factors, such as, nutrient limitation, near-UV light irradiation, and temperature were found to be the key players in sporulating A. cichorii and A. solani while in A. alternata, A. dauci, and A. solani, water agar supplemented with CaCO3 stimulated sporulation[27, 28]. Likewise, Botryosphaeriaceae spp. sporulated on 2% water agar with sterilized pine needles + near-UV light irradiation[29], and Guignardia mangiferae on OMA + near-UV light irradiation[30]. Different methods have differential effects on the sporulation of a particular mycobiont. Therefore, it is imperative to understand the more influential method/ methods concerning the stimulation of endophytic fungal sporulation.
During the current investigation, endophytic fungi of Ephedra gerardiana Wall. ex Stapf., an evergreen gymnosperm belonging to the order Gnetales and family Ephedraceae were unravelled. Surprisingly, the maximum number of endophytic mycosymbionts were recovered in the form of sterile mycelia, and it was pivotal to make them sporulate for their morphological characterization. Therefore, in an attempt to make these fungi sporulate, various carbon sources and starvation media, 12 h alternate dark and light cycles, cold treatment, autoclaved host tissue, pine needles, and shaking with low strength media at different temperatures were assessed. Sporulation was indispensable in facilitating the identification and description of known and novel taxa, respectively[31]. Identification of the endophytes from extreme environmental conditions enhances our understanding regarding the evolution of plant-fungal symbiosis and reveals the cryptic ecology of these symbionts[31, 32]. Similarly, the sporulation behaviour of these symbionts may also unravel the diverse ways (strategies) by which they interact with the host plant and other endophytes in the plant endospheric microbiome.
-
Healthy root and stem samples of Ephedra gerardiana were collected from the Kargil district (34° 33' N & 76° 07' E) of Ladakh Union Territory, India. Ladakh is a cold arid desert (2,438−5,486 masl) which for the most part of the year remains covered with snow. Besides low temperature, high ultraviolet radiations, and water and nutrient scarcity in the soil are among the other oligotrophic factors. In Kargil (34.5539° N, 76.1349° E), the mean annual precipitation is 318 mm with an average annual temperature of 8.6 °C which drops to −40 °C in the winter. The collection was carried out randomly in aseptic paper bags. The samples were transported carefully to the laboratory and stored at 4 °C. Endophytic fungi were isolated from the samples within 48 h of collection.
For surface sterilization, root and stem segments were cut into 5−7 cm segments, discarding the intermittent segments. Stem segments were washed under running tap water for 5 min while root segments for 8−10 min to remove the debris. Under laminar airflow, stem segments were treated with 70% ethanol for 3 min, 5% NaOCl for 2 min, 80% ethanol for 1 min, and rinsed twice with sterile distilled water. Whereas, root segments were immersed in 70% ethanol for 4 min, 8% NaOCl for 3 min, 80% ethanol for 30 s, and rinsed twice with sterile distilled water[33].
Isolation of endophytic fungi
-
Surface sterilized segments were dried under sterile conditions and further cut into 3−4 mm segments. These segments were placed onto the pre-sterilized potato dextrose agar (PDA) (potato 200 g, dextrose 20 g, agar 15 g, water 1,000 ml; Himedia, India) medium supplemented with streptomycin (50 mg/l) contained in 90 mm petriplates (Borosil®). The petriplates were properly sealed with parafilm before incubating them at 25−28 ± 2 °C for 2−3 weeks. Inoculated tissue segments were regularly assessed for the emergence of mycelia from their cut ends. The hyphal tips, once emerged, were transferred to the fresh PDA plates for the purification of fungal isolates. Purified fungi were stored in agar slants (short-term storage) and 25% glycerol (long-term storage).
Sporulation induction treatments
Nutrient media/starvation media
-
The non-sporulating isolates were cultured on Malt Extract Agar (MEA) as it has been considered as a suitable medium for the sporulation of endophytic fungi[34]. Besides, several other carbon and nitrogen sources in the form of Czapek Dox Agar (CDA), Nutrient Agar, Oat Meal Agar (OMA), and Czapek Yeast Autolysate (CYA) were used to explore their influence on the sporulation of these fungi[35]. Apart from this, fungal isolates were grown on various starvation media, such as Water Agar (WA), Potato Sucrose Agar (PSA), Potato Carrot Agar (PCA), and Spezieller Nahrstoffarmer Agar (SNA)[4,9]. All the chemicals were procured from Himedia, India.
Light/dark rhythms and cold treatment
-
The fungal isolates that did not sporulate on the above media were incubated onto PDA medium at room temperature (23−25 °C) under 12 h alternating light and dark conditions[36,37] (Fig. 1a). Compact fluorescent lamps (CFLs) were used as light source which emits a low level of near UV radiation[38]. PDA was replaced with MEA, WA, PSA, PCA, and CDA for the isolates that remained sterile, keeping the light and dark cycles intact. The sterile mycelia were also checked for the influence of low temperature or cold shocks on their sporulation using different media (Fig. 1b). For this, they were first incubated at 25 ± 2 °C onto PDA for 10−15 d (as the fungi were extremely slow growing), before shifting the cultures at 4−6 °C. Certain isolates were also exposed to the agglomeration of cold treatment with 12 h alternate light and dark conditions to monitor the combined effect of these factors.
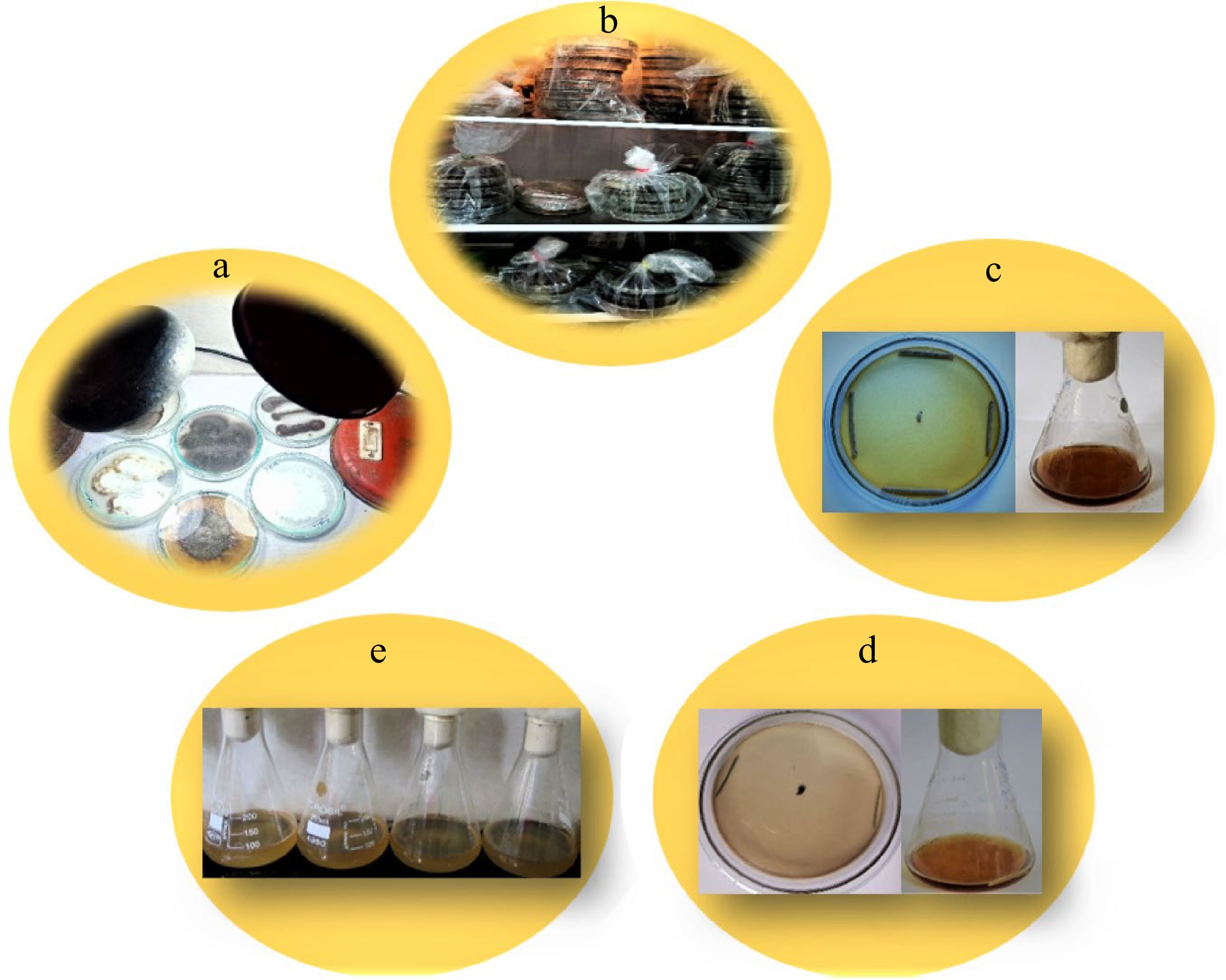
Figure 1.
Treatments for non-sporulating endophytic fungi. (a) Alternate light and dark cycles. (b) Cold treatment in a refrigerator. (c) Treatment with autoclaved host tissue on solid and liquid media. (d) Treatment with pine needles on solid and liquid media. (e) Low strength media.
Plant tissue
-
The endophytic fungi left unresponsive to the above-mentioned methods were treated with autoclaved host tissue[39−41]. Here, 90 mm petriplates with different media supplemented with the antibacterial agent were prepared, and isolates along with 6−8 cm of host stem segments were inoculated onto these media (Fig. 1c). The plates were incubated at 28 °C for 8−10 days depending upon the growth of fungi. Also, 100 ml of potato dextrose broth (200 g potato, 1,000 ml water, sliced, boiled for 30 min, filtered, sterilized at 121 °C for 15−20 min), malt extract broth (20 g malt extract, 1 g peptone, 20 g dextrose, 1,000 ml water) and Czapeck Dox broth (30 g sucrose, 2 g sodium nitrate, 1 g dipotassium phosphate, 0.5 g magnesium sulphate, 0.5 g potassium chloride, 0.01 g ferrous sulphate, 1,000 ml water) were poured into 250 ml Erlenmeyer flasks. The flasks containing broth were sterilized at 121 °C for 15 min. Concomitantly, stem segments (10−15 cm) of E. gerardiana were autoclaved for 10 min at 121 °C. Sterile mycelia were then inoculated onto both solid media and liquid broths under aseptic conditions along with one or two stem segments of the host plant and incubated at 28 °C (Fig. 1c). Similarly, sterile fungi were also inoculated onto solid and liquid media containing 2−4 segments of 5−7 cm autoclaved pine needles in aseptic conditions before incubating them at 28 °C (Fig. 1d). Regular evaluations were accomplished to monitor the changes, if any.
Low strength media
-
Generally, nutritional limitation stimulates sporulation. Keeping this in mind, certain modifications were made in artificial media by reducing their nutritional strength up to different levels[42]. Here, 100−150 ml of 1/4 potato dextrose broth (PDB; potato 50 g, water 1,000 ml, boiled for 30 min, filtered, potato infusion sterilized at 121 °C for 15 min)[28], 1/2 malt extract broth (MEB; malt extract 10 g, peptone 0.5 g, dextrose 10 g, water 1000 ml), 1/3 Czapek dox broth (CDB; sucrose 10 g, sodium nitrate 1.2 g, dipotassium phosphate 0.5 g, magnesium sulphate 0.2 g, ferrous sulphate 0.01 g, potassium chloride 0.2 g, water 1000 ml), 1/3 Oat meal broth (20 g oatmeal, 1,000 ml water), and 1/2 nutrient broth (2.5 g peptone, 2.5 g sodium chloride, 0.75 g beef extract, 1,000 ml water) were poured into the 250 ml flasks (Fig. 1e). After sterilizing the media at 121 °C for 15 min, non-sporulating endophytic fungi were inoculated into the flasks containing each type of broths before incubating them at 150 rpm at 25 °C. Here, proper aeration with slight starvation and temperature variation was assessed for their combined influence on endophytic fungal sporulation.
Statistical analysis
-
All the data procured using various methods were subjected to statistical analysis. To check the significance of variation between different methods and media, two-way analysis of variance (ANOVA) was calculated using SPSS (Statistical Package for the Social Sciences) software version 17. Furthermore, Tucky's test/Tucky's post hoc analysis was carried out at p < 0.05.
-
Six hundred and eighty one endophytic fungal isolates were obtained from 1083 surface sterilized root and stem segments of E. gerardiana. Out of these, 376 isolates were recovered from the root and 305 from that of the stem. However, 213 root isolates and 286 stem isolates failed to sporulate on commonly used lab media (Fig. 2a), thereby exhibiting the number of sterile mycelia as 56.64% and 93.7%, respectively. These mycelia sterilia were categorized into 67 different morphospecies based on their cultural and morphological characteristics. Sixty one (91.04%) morphospecies were successfully triggered to sporulate whereas six (8.95%) remained sterile upon application of these methods (Fig. 2b). The non-sporulating endophytic isolates correspond to MN1, MN13, MN26, MN48, MN54 and MN50. Intriguingly, out of 61 sporulated morphospecies, only two (3.27%) reproduced sexually while 59 (96.72%) formed asexual spores. The frequently recovered isolates from the stem were non-sporulating in nature, however, their counterparts from the roots either formed thick-walled asexual resting spores (chlamydospores) or conidia, the former being more common.

Figure 2.
(a) Scientific diagrams showing the number of sterile endophytic fungal isolates recovered from E. gerardiana. (b) Number of corresponding sterile isolates from roots and stem.
Nutrient media/starvation media
-
Considering the individual effect of media on the sporulation of these endophytic fungi, MEA showed better results. Out of the 67 morphotypes, MN5, MN9, and MN23 sporulated on MEA, MN8 on CDA while MN10 and MN34 on OMA (Fig. 3a). However, the other commonly used lab media exhibited neutrality towards the sporulation induction. Three out of four starvation media used revealed a positive effect on the sporulation of concerned endophytic fungi. Whereas PSA triggered sporulation in two morphotypes viz., MN15 and MN33; PCA and WA remained successful in sporulating MN7, MN32, and MN41, MN58, MN62, respectively (Table 1). MN33 formed resting asexual spores (chlamydospores) only. The other morphospecies, although formed dispersing asexual spores, the rate of sporulation was extremely slow. MN32 sporulated after 2 months and exhibited a longer duration requirement for sporulation. The frequency of sporulation was the highest in WA followed by PSA and PCA (Fig. 3b).
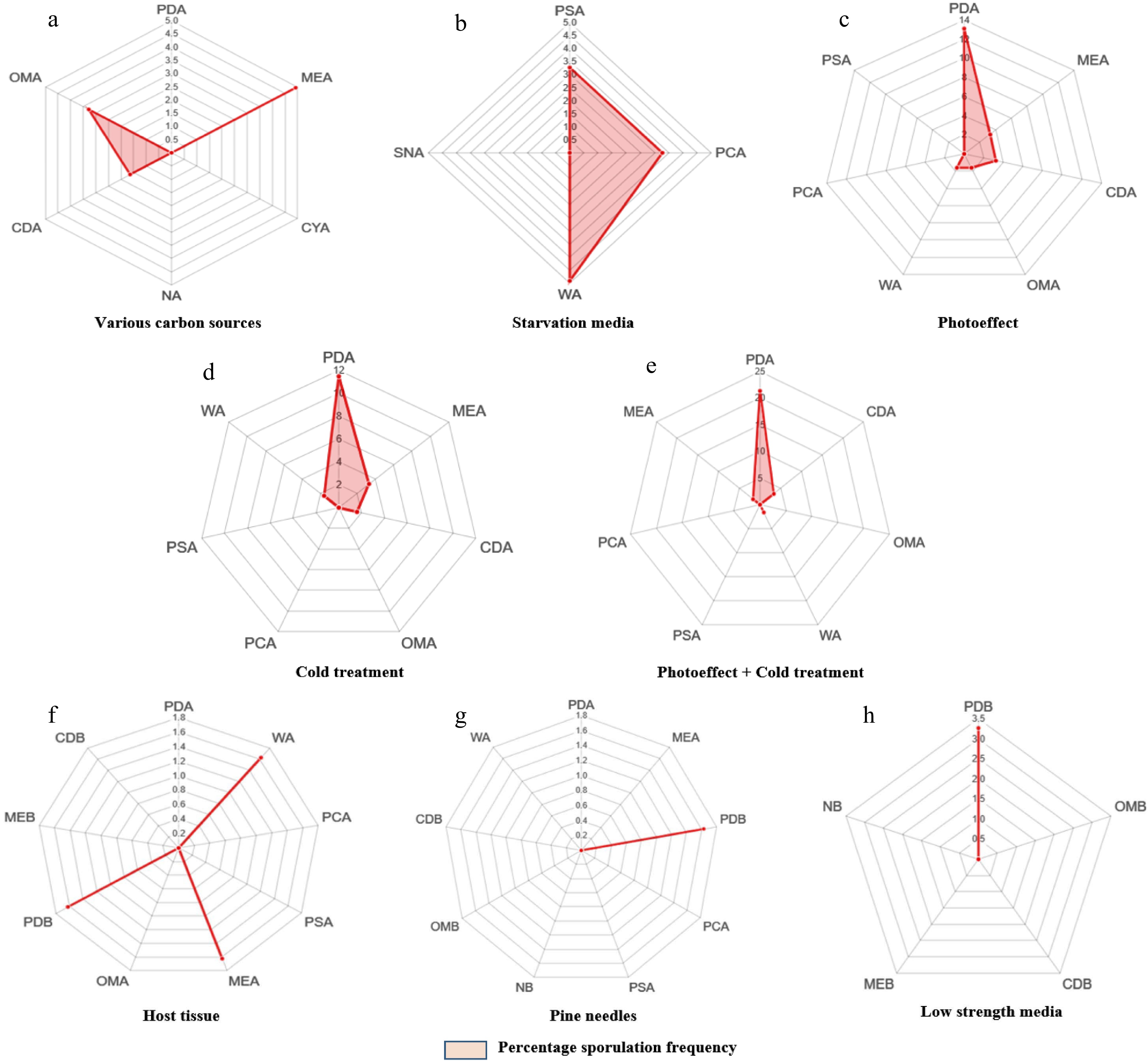
Figure 3.
(a)-(h) Radarcharts showing percentage of sporulation in various morphospecies with respect to different methods and media.
Table 1. Maximum number of morphospecies Nmax (%) sporulated after applying diverse methods.
Method Media Name of the
morphospeciesMaximum number
of morphospecies sporulated Nmax (%)Various carbon sources PDA 0 MEA MN5, MN9, MN23 4.91 CYA 0 NA 0 CDA MN8 1.63 OMA MN10, MN34 3.27 Starvation media PSA MN15, MN33 3.27 PCA MN7, MN32 3.27 WA MN41, MN58, MN62 4.91 SNA 0 Photo-effect PDA MN20, MN27, MN42, MN45, MN18, MN52, MN66, MN64 13.11 MEA MN21, MN14 3.27 CDA MN24, MN49 3.27 OMA MN65 1.63 WA MN56 1.63 PCA 0 PSA 0 Cold treatment PDA MN2, MN3, MN6, MN19, MN25, MN40, MN61 11.47 MEA MN4, MN12 3.27 CDA MN59 1.63 OMA 0 PCA 0 PSA 0 WA MN55 1.63 Cold treatment + photo-effect PDA MN11, MN16, MN28, MN29, MN30, MN31, MN36, MN44, MN46, MN47, MN35, MN37,
MN5121.31 CDA MN53, MN63 3.27 OMA 0 WA MN57 1.63 PSA 0 PCA 0 MEA MN17 1.63 Host tissue PDA 0 WA MN60 1.63 PCA 0 PSA 0 MEA MN38 1.63 OMA 0 PDB MN22 1.63 MEB 0 CDB 0 Pine needles PDA 0 MEA 0 PDB MN43 1.63 PCA 0 PSA 0 NB 0 OMB 0 CDB 0 WA 0 Low strength media PDB MN39, MN67 3.27 OMB 0 CDB 0 MEB 0 NB 0 Alternate light and dark cycles
-
The maximum number of morphospecies showed effective sporulation upon treatment with alternate light and dark cycles. A total of 14 morphospecies responded positively to this method (Table 1). However, the rate of sporulation varied in all the tested fungi with most of the isolates sporulating after 13−17 d. The maximum number of endophytic fungal isolates sporulated on PDA (Fig. 3c), thereby suggesting the influence of carbon source along with alternate light and dark cycles on sporulation. However, WA and PSA remained neutral in inducing the sporulation of these fungi under the prevailing conditions. This method showed a significant positive effect in stimulating sporulation even in the isolates that responded to other methods as well.
Cold treatment
-
As these endophytic fungi were isolated from a plant inhabiting an extremely cold habitat, we attempted to check the influence of low temperature (natural growth conditions) on the sporulation of these mycosymbionts. Surprisingly, 11 morphospecies sporulated on PDA and MEA after 1−2 cold shocks. However, 17 morphospecies showed sporulation when treated with 12 h alternate light and dark conditions after cold treatment. Therefore, this proved to be the best stimulatory combination for sporulation in endophytic fungi of E. gerardiana. Interestingly, two isolates MN2 and MN3, which responded to cold treatment after 3−4 months, were the only endophytic fungi reproducing sexually via ascospore formation. Morphospecies MN12 exclusively formed chlamydospores upon continuous exposure to combined cold treatment and light/dark cycles for 4 months. Either individually or in synergy, both treatments showed better results in the PDA medium (Fig. 3d, e).
Host tissue
-
In our assessment, three morphospecies (MN22, MN38, and MN60) sporulated upon treatment with host stem tissue onto PDB after 18 d of incubation at 28 °C (Fig. 3f).
Pine needles
-
Morphospecies MN43 sporulated on PDB-containing pine needles (Fig. 3g). The colour of the medium started changing from the 9th day onwards and significant sporulation was observed approximately after 23 d.
Low-strength media with elevated aeration
-
Fungi usually grow in conditions where suitable nutrition is provided with proper aeration. However, higher oxygen level encourages them to sporulate[43]. Here, enhanced aeration (shaking at 120 rpm) and reduced nutrient levels showed less correlation with the sporulation of tested endophytic fungi. Two morphospecies, i.e., MN39 and MN67 sporulated on 1/4 PDB after shaking at 120 rpm for 10−12 d (Fig. 3h).
ANOVA showed a higher value of F (4.15207) in comparison to that of F critical (2.714076) which suggests the differential effect of various methods along with multiple media used on endophytic fungal sporulation. In general, it highlights the significant difference between these factors. However, Tuckeys post hoc analysis revealed that nutrient media (source of carbon and nitrogen) have a more pronounced effect on sporulation. Among various media, the significant difference in inducing sporulation was speculated in combinations of PDA & PSA, PDA & PCA, PDA & CDA, and PDA & OMA (Fig. 4).
-
The majority of the endophytic fungi fail to sporulate on artificial media under normal growth conditions. If this fraction sporulates, the induction process is time consuming and influenced by complex multi-variate combinations. Many plants explored to date have revealed 11%−54% of sterile mycelia isolated from their endospheric microbiomes[44]. However, during the present investigation, so far the biggest fraction of such fungi has been recovered. This may generally be attributed either to the source type (gymnosperm) or to their survival in an extreme oligotrophic habitat.
Considering the source type, surprisingly, the highest percentage (54%) of sterile mycelia recovered as endophytic fungi so far pertain to the gymnosperm Quercus ilex[45]. Similarly, Chilean gymnosperms revealed 51.85% of isolates as sterile mycelia which remained unidentified while the lesser fraction sporulated and could be placed taxonomically[46]. Followed by this, 33% and 26% of sterile mycelia were recorded from Picea mariana and Sequoia sempervirens, respectively[47,48]. Similarly, endophytic fungi of Pinus tabulaeformis comprised 11% of sterile mycelia[49]. Although sterile mycelia can also be identified following molecular techniques, in most cases, they are unique and exhibit significantly lower sequence similarity with the already described fungi submitted in GenBank[50].
On the other hand, fungal endophytes colonizing the plants inhabiting extreme habitats are the least studied group of fungi that improve the ability of their host plants to tolerate different types of stresses[51]. Owing to this fact, these are mostly the novel, unique and diverse group of microbes[52,53]. The predominance of sterile fungi in cold environments pertains to their morphological adaptation for survival in such habitats[53]. In coherence with these studies, the majority of the recovered fungal isolates were found to be sterile and novel during our exploration[31]. Although it depicts that the sterility of fungal endophytes is correlated with the extremity of the source environment, it requires in-depth study to know the governing agencies and signal transduction pathway underlying the process.
Most of the fungi recovered from such habitats have been recognized merely based on their molecular characterization while ignoring the morphological aspects[54]. This has led to ambiguous identifications of endophytic fungi thereby emphasizing the usage of morphology and multi-locus analyses (polyphasic approach) in fungal taxonomy. For instance, in a recent breakthrough study, the exclusively endophytic genus Muscodor with sterile mycelia was studied in detail based on a polyphasic approach[55]. Despite being placed in Xylariaceae, its position was obscure. The muti-locus phylogram revealed the close affinity of Muscodor species towards the genera Emarcea and Induratia of Xylariaceae which was well depicted by producing apiospores in their corresponding sexual states. Also, the multigene analysis showed the formation of a clade by Emarcea and Induratia separate from Xylariaceae. Therefore, Muscodor is now assigned to Induratia based on morphological and phylogenetic analyses within a new family Induratiaceae.
Influence of nutrient media
-
In the current investigation, nutrient media exhibited a potent effect in inducing fungal sporulation. Previous studies have showed that fungi can regulate their metabolism for optimal consumption, growth, and reproduction after sensing the nutrient availability in the surrounding environment[56]. Besides, different carbon/nitrogen sources and starvation media revealed variable effects on sporulation. The reason being different fungi are capable of producing different enzymes which can breakdown particular carbon and nitrogen sources thereby helping in their growth and formation of peculiar sporulating structures in some specialized media[56]. A transcription factor was observed to affect the integration of carbon and nitrogen metabolism in fungi which ultimately decides the asexual sporulation initiation[56]. Also, studies indicate that carbon concentration and C:N ratio has a strong impact on fungal sporulation. It is in coherence with our study which showed more pronounced effect of nutrient media on fungal sporulation. Concomitantly, certain media exhibited their positive effect on sporulating a few morphospecies but remained neutral for others. Therefore, it can be inferred that the combination of different nutrients and other components can be sensed by the fungi, thereby integrating or influencing the concerned components via sporulation-related transcription factors. According to Kleb's law, sporulation occurs in the nutrient conditions that hamper mycelial growth[9]. During the present study, it was observed that out of the four starvation media used, three showed promising results. It indicates that nutritional stress or variable nutrient deficiencies offer an important factor to induce sporulation in fungi. Although, it is known that G protein-coupled pathway is involved in fungal sporulation which is linked with secondary metabolism[57], however, it is a matter of research at the molecular level to unravel the effect of starvation in initiating this pathway. A better understanding of these pathways may help overcome the issues regarding the sporulation of sterilia mycelia.
Influence of environmental factors (light, dark, temperature)
-
Light has been considered as an essential controlling factor in the process of sporulation in endophytic fungi[58]. Apart from affecting the ionic balances, it also influences the pH and carbohydrate metabolism of fungi involving various light-sensitive proteins[59]. Light can stimulate both sexual and asexual morph formation[60], however in the current study, it could induce asexual sporulation only. In our case, near ultraviolet light was observed to be an important factor in inducing sporulation. Amazingly, cold shocks also triggered sporulation in many fungal isolates. However, the outcome was found to be variable in different media. An interesting observation pertains to the fact that maximum morphospecies sporulated on PDA at low temperature, thereby suggesting the individual role of cold stress in sporulation induction. Previous studies also highlight the differential effect of temperature on fungal growth and sporulation[61]. Furthermore, various studies confirmed that the majority of fungi isolated from a particular habitat can thrive well in artificial media supplemented with habitat-like conditions[62]. For example, a study conducted on endophytic fungi of three bryophytes Barbilohozia hatcheri, Chorisosontium aciphyllum, and Sanionia uncinata growing in Antarctica revealed that maximum number of isolates were either psychrophilic or psychrotrophic, with a remarkable ability to grow at low temperature[53]. Similarly, endophytes isolated from submerged plants were able to grow at comparable salinities to that of seawater[63]. Intriguingly, while a marine Ascomycete Lindra thalassiae was unable to sporulate in freshwater containing media, three of its counterparts (marine ascomycetes) Halosphaeriopsis mediosetigera, Lulworthia floridana, and Torpedospora radiata sporulated well when the artificial medium was made with sea water dilutions[64]. It indicates that a major fraction of endophytic fungi from E. gerardiana require habitat-like conditions (low temperature, near ultraviolet light) for their growth and sporulation. However, the question arises whether the habitat conditions of every plant influence the sporulation of its endophytic mycosymbionts or is it only the stress factor that is playing a part. Moreover, this positive impact was enhanced after combining the light cycles with other factors, such as nutrient media and cold shocks. Interestingly, the cold shocks with photo-effect was found to be the best combination (out of the tested factors) to trigger sporulation which suggests that certain factors in agglomeration may have a cumulative effect on sporulation. It may pertain either to the enhanced sensing ability of the fungi or increased concentration of transcriptional factors which are activated during sporulation. The question here is why certain factors, individually or in combination with certain other factors, but not all, strongly induce sporulation? Particularly, why light and dark cycles with cold shocks are more effective in triggering the sporulation of these fungi? Sporulation in endophytic fungi may be mostly or partly suppressed in planta as an integral part of multivariate cross-talks involved in maintaining endophytism, by quorum sensing and quorum quenching phenomena[65]. Perhaps, the sporulation in endophytic fungi on artificial media is deeply correlated to the suppression of this phenomenon inside the host plant.
Influence of plant tissues
-
Various plant tissues are known to play an essential role in stimulating sporulation in endophytic fungi. For instance, endophytic mycelia sterilia of Livistona chinensis and banana sporulated after treatment with sterilized petiole fragments of their respective host plants[39,40]. Likewise, sporulation was induced in sterile endophytic fungi of Sophora tonkinensis after culturing them on 1/4 PDA medium containing sterile root fragments from the host[41]. Besides, other plant tissues are also known to induce sporulation in various endophytes[66]. Pine needles have also been observed to have a role in triggering sporulation in endophytic fungi[29]. Interestingly, an investigation on the comparison of growth and sporulation in Bipolaris, Curvularia, Drechslera, and Exserohilum under varied growth conditions revealed that cellulose-containing substrates could enhance sporulation by 2 to 18 times[67]. Since many endophytes are reported to produce cellulases which might help them penetrate the host cells[68,69], signalling cascades involving cellulose degradation by these fungi are believed to initiate the asexual sporulation. Here, we also assessed the role of host tissue and pine needles to demonstrate their effect on the sporulation of the recovered fungal isolates. Surprisingly, both of them could trigger sporulation in two morphospecies each. A study revealed that the leaves of 22 plant species successfully triggered the sporulation in Colletotrichum dematium[70]. It is widely expected that these plant tissues release certain chemicals/metabolites which trigger fungal sporulation. However, upon treatment with mulberry leaves, biotin was found to play a key role in inducing sporulation in Colletotrichum dematium[70]. Unveiling these chemicals will not only solve the sporulation-related issues of the fungi but also the role of these metabolites in plant-endophyte cross-talks in planta thereby enhancing our understanding of the ecological and ecophysiological behaviour of endophytes.
-
While exploring the endospheric mycobiome of a gymnosperm Ephedra gerardiana inhabiting a cold arid desert experiencing numerous oligotrophic conditions, maximum isolates were found to be sterile. In an attempt to stimulate their sporulation, diverse methods were investigated, individually as well as in synergistic combinations. Out of all the methods employed, alternate light and dark cycles and cold shocks, both individually as well as in combination exhibited the best results. There is much probability that besides being a stress factor, low temperature might be essential for the growth and sporulation of these fungi as they were recovered from a cold arid desert-dwelling plant. However, it is a matter of in-depth research to unravel the molecular mechanism involving the signal transduction pathways underlying the process of low temperature-induced sporulation. The plants growing in stressed environments harbour more percentage of endophytic fungi in the form of sterile mycelia as non-sporulating behaviour pertains to one of the morphological adaptations for survival. Interestingly, gymnosperms possess more of this percentage as compared to other plants, which suggests the influence of host type on the sterility of endophytic fungi. Also, it seems that the deficiency of a particular nutrient/s affects the sporulation of a particular fungus. This implies that the fungi have different sensors which sense the availability or non-availability of different nutrients concerning sporulation induction. A proper combination of various factors is suggested to induce sporulation in endophytic fungi instead of using them individually. It not only increases the number of isolates that respond but also decreases the time taken by them to sporulate. Since not all the fungi responded identically to various factors, it may be depicted that different mycosymbionts in the endospheric microbiome of a plant are being maintained as sterile mycelia by different strategies. The higher incidence of non-sporulating endophytic fungi in the stem as compared to that of roots along with their complex sporulation behaviour suggests that endophytic fungi colonizing E. gerardiana inhabiting extreme/harsh habitat offers an exciting frontier to be explored for understanding strategies responsible for this differential behaviour and host-endophyte interactions.
This work was supported by Council of Scientific and Industrial Research (CSIR), Grant No. 09/100(0241)/2019-EMR-I. The authors are highly thankful to the Head, Department of Botany, University of Jammu, India and SAP-DRS-II for providing laboratory facilities. Thanks are also due to Council of Scientific and Industrial Research (CSIR), India for granting Senior Research Fellowship to the first author.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mattoo AJ, Nonzom S. 2022. Investigating diverse methods for inducing sporulation in endophytic fungi. Studies in Fungi 7:16 doi: 10.48130/SIF-2022-0016
Investigating diverse methods for inducing sporulation in endophytic fungi
- Received: 24 April 2022
- Accepted: 28 November 2022
- Published online: 09 December 2022
Abstract: To date, many plants explored for their endospheric microbiomes have revealed a substantial amount of sterile mycelia, a group of fungi with pseudo-taxonomic positions. While unravelling the endophytic fungal assemblages of a plant Ephedra gerardiana Wall. ex Stapf. via culture-dependent methods, 73.2% of the total isolates failed to sporulate on the commonly used artificial media. Therefore, the current study was designed to investigate the diverse methods, either individually or in combination to check their impact in inducing fungal sporulation. The maximum number of isolates (Nmax) sporulated after individual treatments with 12 h alternate light and dark cycles and cold shocks at 4−6 °C were 22.91% and 18%, respectively. However, the combination of cold treatment with alternate light and dark cycles showed the Nmax of 27.84% and proved to be the best method of sporulation induction out of the methods investigated. To the best of our knowledge, this involves the pioneering attempt of using cold treatment as a triggering factor for sporulating endophytic fungi. Accentuating better methods for endophytic fungal sporulation would not only solve the taxonomic position of these mycosymbionts, but also lessen the time to make them sporulate along with unveiling their eco-physiological behavior inside the host plant.
-
Key words:
- Cold treatment /
- Ephedra gerardiana /
- Identification /
- Ladakh /
- Sterile mycelia













