-
Oxalis L. is the largest genus in the Oxalidaceae family, with around 800 species. Oxalis L. is distributed all over the world, including in Brazil, the tropics of Mexico, and South Africa[1]. Oxalis L. plants are often used for ornamental purposes in gardens, flower beds, and nurseries[2]. Research on Oxalis L. plants mainly focuses on the extraction and application of chemical substances[3,4], genetic diversity[5,6], propagation[7−9], and the development and application of cash crops[10,11]. However, there is limited understanding on the interesting ornamental characteristics of leaves and flowers in most species of Oxalis. Through the study of nyctinastic movement, a dynamic and ever-changing ornamental display can be created in the garden, and the best time for herbicide application can also be provided for other invasive Oxalis L. with nyctinastic movement.
Nyctinastic movement refers to the circadian rhythmic movement of leaves or flowers in certain plant families, such as Leguminosae, Oxalidaceae, and Marantaceae[12]. The factors that determine nyctinastic movement include temperature, moisture, photoperiod, and insect damage, but none has been fully characterized in Oxalis L.[13,14]. For flowers, nyctinastic movement is related to pollination, and they open and close rhythmically responding to the activity time of their pollinators[15].
The leaves and flowers of O. triangularis 'Purpurea' exhibit nyctinastic movement. which is a convenient and ideal material for studying the nyctinastic movement of the Oxalis L. plants. Previous research has identified three important factors that influence plant nyctinastic movement: light, temperature, and circadian clock[16−19]. Light plays a significant role in the nyctinastic movement of Oxalis L. plant leaves. Red light, far-red light, blue light, and white light all regulate leaf movement, and an intense light enhances leaf unfolding[20,21]. However, no specific light intensity critically required for nyctinastic movement has been clearly determined. Warm temperature has a promoting effect on the nyctinastic movement of Oxalis L. plant leaves and flowers. Increase in temperature promotes unfolding of the closed flowers[14,22]. However, these previous studies did not examine the influence of temperature or light changes separately on flower opening and closing. Under constant environmental conditions, the circadian clock causes leaves to exhibit rhythmic movement[23]. Previous research primarily focused on the influence of individual factors on specific plant parts. It remains unclear which factor has the greatest impact on plant nyctinastic movement and how these factors differ in their influence on leaves and flowers. The opening and closing movements of Oxalis L. plant leaves and flowers in response to circadian rhythm are fascinating and ornamental. Understanding the influence of environmental and endogenous factors on their nyctinastic movement is crucial to comprehend this intriguing biological phenomenon.
To gain a deeper understanding of the mechanism underlying the nyctinastic movement of the Oxalis L. plants, this study utilized a growth chamber to precisely regulate light intensity and temperature. Movement time and angle of the leaves and flowers of O. triangularis 'Purpurea' were recorded under controlled conditions to quantify explore the impacts of light, temperature, and the endogenous circadian clock on the nyctinastic movement of Oxalis L.
-
The O. triangularis 'Purpurea' corms purchased from Taobao are asexually bred. The bulbs were uniform in size with a length of 2−3 cm and a diameter of 0.5−0.6 cm. They were planted in plastic pots containing a mix of peat soil : perlite : organic fertilizer (volume ratio = 2:1:1). Three corms were planted per pot were cultivated in the Forest Orchid Garden of Fujian Agriculture and Forestry University (located at 26°05'20" N, 119°13'45" E) in Fuzhou City, Fujian Province (China); Bulb seeding was carried out in March 2022 and experiments were carried out in May. Routine water and fertilizer management were carried out after planting. When the number of mature leaves and flowers in each pot was ≥ 10 pieces/flowers, the corresponding pot was chosen for experiment. Following each round of trial tests, the plants were placed in outdoor environment for routine management for three days before conducting subsequent tests.
Method and parameters to the track nyctinastic movement of Oxalis L.
-
Each treatment or control group consisted of 30 plants in 10 pots. The movement was recorded using a Sony CX900E camera. Three mature leaves and flowers in each pot were randomly selected, and their movement and corresponding time were recorded within a 150-min time window. For three consecutive days, a total of 30 leaves and 30 flowers were recorded in each group. To measure the angle of movement, a ruler and protractor were used to determine the angle between the midrib and petiole of the leaflet, and the angle between the central axis of flower and a line from the apex of the two opposite petals to the bottom of the calyx (Fig. 1).
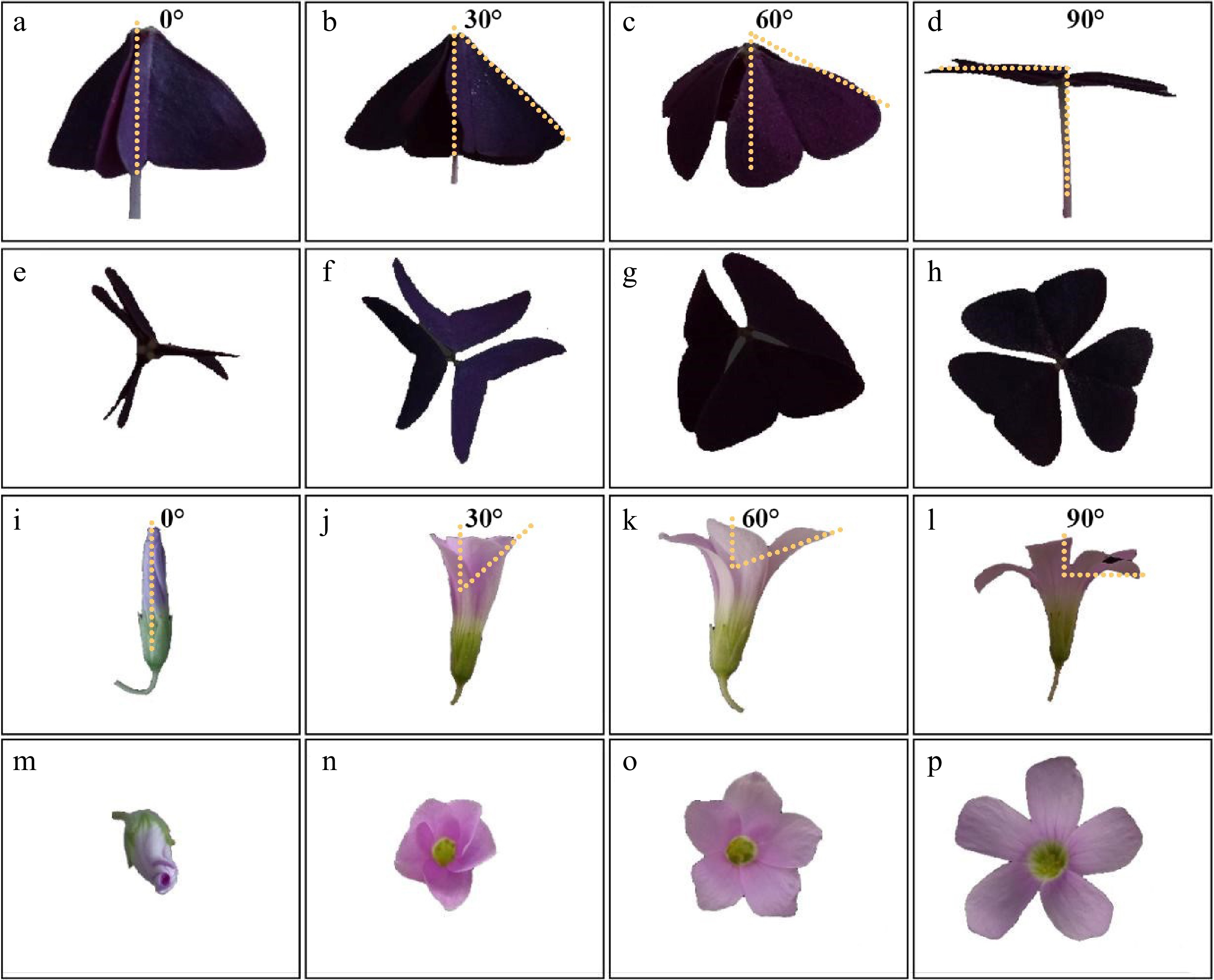
Figure 1.
Different states of nyctinastic movement of O. triangularis ‘Purpurea’ leaves and flowers. Front view of leaves from different angles: (a) 0°; (b) 30°; (c) 60°; (d) 90°; side view of leaves from different angles: (e) 0°; (f) 30°; (g) 60°; (H) 90°; front view of flowers from different angles: (i) 0°; (j) 30°; (k) 60°; (l) 90°; side view of flowers from different angles: (m) 0°; (n) 30°; (o) 60°; (p) 90°.
Statistical analysis
-
After video recording, we recorded the time, angle for each leaf and flower based on the video. We then imported the data to track the unfolding or closing of 30 leaves/30 flowers into Microsoft Excel 2016. Finally, we used one-way ANOVA in SPSS 26 software to analyze the significance of the data.
Experimental design
-
After being pre-treated for 24 h, plants were moved into a chamber growth and the light intensity and temperature, and the movement of leaves and flowers was recorded using a camera. Tests 1 and 2 were focused on the effect of light, while tests 3 to 7 were focused on the effect of temperature. Additionally, tests 8 and 9 were conducted to assess the impact of the circadian clock on the plants' movement (Table 1). The gradient light intensity was set to 0% (dark), 25% (8.34 μmol·m−2·s−1), 50% (14.72 μmol·m−2·s−1), 75% (21.52 μmol·m−2·s−1), and 100% (32.19 μmol·m−2·s−1) respectively (Note: The light is SananBio LED Grow Light (ZK3-TB14-VE03/A), PPF: 35.6 μmol·s−1, Efficacy: 2.1 μmol·J−1, Dimension: L900*W37.5*H34.6 mm, Weight: 0.27 kg, Input power: 13.5 W, Input voltage: 220−240 V, IP rating: IP65). The temperature was set to 15, 20, 25, 30, and 35 °C as indicated in different test respectively.
Table 1. Experimental design.
Test Purpose Pre-treatment Leaf/flower state
after pre-treatmentTreatment Test 1 Effects of morning light Light: 100%, 8:00−22:00, Temperature: 20 °C Leaf: closed
Flower: closedLight on: 8:00, light intensity: 0, 25%, 50%, 75%, 100%, Test 2 Effects of night light Outdoor natural lights temperature 19.9 °C Leaf: closed
Flower: closedLight on 18:00, light intensity: 0, 25%, 50%, 75%, 100% Test 3 Effects of elevated temperature in the dark Light: 100%, 8:00−22:00, temperature 15 °C Leaf: closed
Flower: closedLight on: 8:00, temperatures: 15, 20, 25, 30, 35 °C Test 4 Effect of increasing temperature in the dark Outdoor natural lights
temperature. 15 °CLeaf: unfold
Flower: closedLight on: 8:00, temperatures: 15, 20, 25, 30, 35 °C Test 5 Effect of lowering the temperature Light: 100%, 8:00−22:00, temperature: 35 °C Leaf: closed
Flower: closedLight on: 8:00, temperatures: 15, 20, 25, 30, 35 °C Test 6 Effects of increasing same temperature Light: 100%, 8:00−22:00, temperature: 15, 20, 25, 30 °C Leaf: closed
Flower: closedLight on: 8:00, temperatures: increased by 5 °C from the original Test 7 Effect of successive
different temperaturesDark, temperature: 15, 20, 25, 30, 35 °C Leaf: closed
Flower: closedLight on 18:00, 100% light Test 8 continuous dark Outdoor natural lights
temperature 20 °CLeaf: closed
Flower: closedAt 18:00, give 20 °C dark treatment for 3 consecutive days Test 9 The effect of entering darkness At 8:00, light: 100%, temperature: 20 °C Leaf: unfold
Flower: unfoldDark treatment at 16:00, 18:00, 20:00, 22:00, and 24:00, respectively Temperature: 20 °C -
Test 1 investigated the effect of morning light on the nyctinastic movement of closed leaves (flowers). The angle at which the leaves unfolded and the time it took to do so were significantly different under different light intensities. Except for the control (dark), the inner leaf could only unfold to 30° within 150 min, whereas the leaves in the other experimental groups could unfold to 90°. The time it took for the leaves to unfold to 30°, 60°, or 90° decreased with increasing light intensity, and the time from closed to fully unfolded leaves in the 100% light intensity test group was about 30 min shorter than the time of the test group at 25% light intensity. The flowers opened at 0° within 150 min under different light-intensity environments (Fig. 2a). In summary, the rate at which leaves unfolded in the morning increased with increasing light intensity, and leaves could not fully expand in dark conditions, but could reach an angle of 30°. However, the increased light intensity did not cause the flowers to unfold.
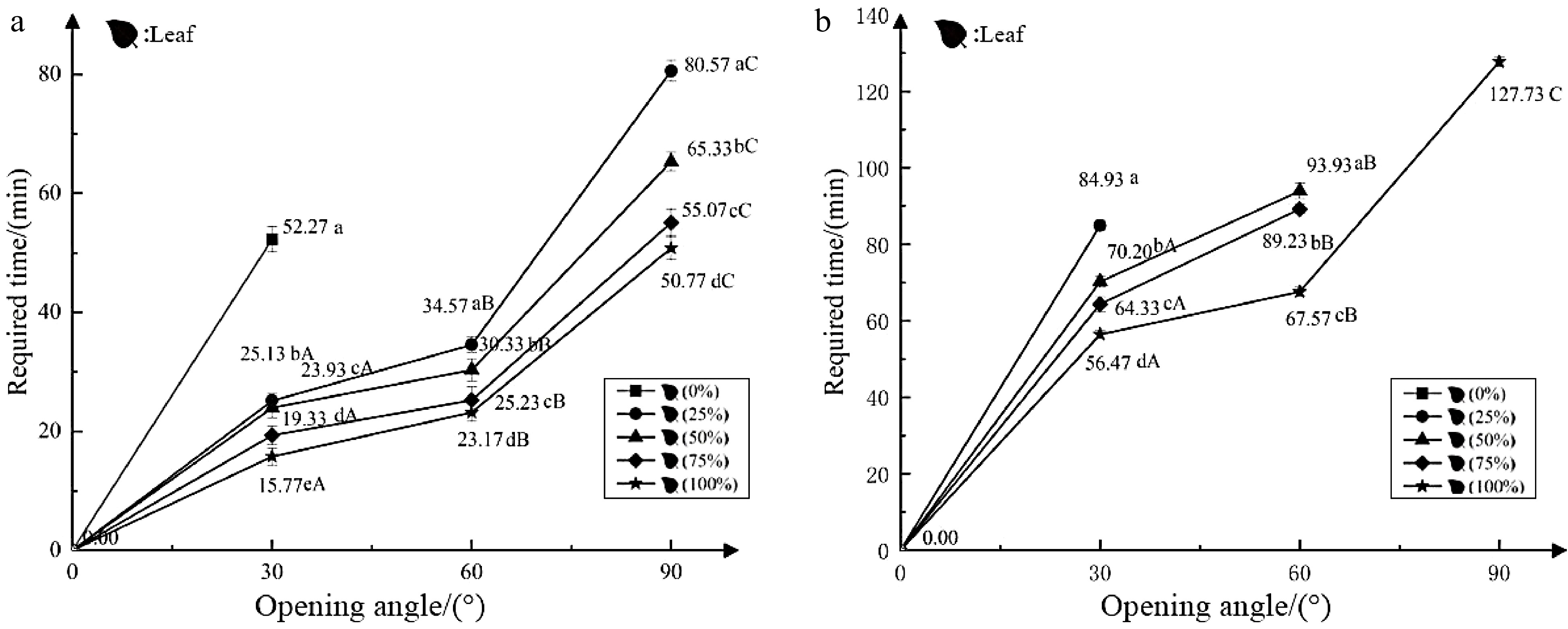
Figure 2.
Effects of light on the nyctinastic movement of O. triangularis 'Purpurea'. (a) Light in the morning, closing leaves (flowers); (b) light in the evening, closing leaves (flowers). No polyline means the state does not meet the current standard in 150 min. The small letters indicate significant differences between spreading data at the same angle (P < 0.05); the capital letters indicate significant differences between data under the same processing state (P < 0.05).
Test 2 showed the effect of evening light on the nyctinastic movement of closed leaves (flowers). Under dark conditions at night, the control group did not expand the inner leaves within 150 min, and the leaves in the 25% light intensity test group could only expand to approximately 50°. Leaves in the experimental groups under 50% and 75% light intensity could expand to around 70° and 80°, respectively, within 150 min. However, the leaves in the 100% light intensity test group could fully expand in 127.73 min. Closed flowers opened at 0° under different light-intensity environments (Fig. 2b). In summary, the angle at which the leaves could unfold at night increased with the increase of light intensity, and leaves remained closed in dark conditions, while light did not affect the unfolding of the flowers.
Effect of temperature on the nyctinastic movement of leaf and flower
-
Test 3 showed the effect of increasing temperature on the nyctinastic movement of closed leaves (flowers) under dark conditions. This group of plants kept their leaves closed at 15 °C. Exposure to 30 °C caused the leaf opening after 56.27 min, which remained open for 150 min in the dark. However, the leaves of the other test groups exposed to higher temperatures did not expand. The flowers were fully opened when the temperature was raised to 30 °C or 35 °C within 150 min, and the opening rate was faster at 35 °C compared to 30 °C. The control group at 15 °C and the test group at 20 °C remained completely closed throughout the experiment (Fig. 3a). Therefore, increasing the temperature inhibits the opening of closed leaves, but had a great impact on the opening of flowers. The greater the temperature increase, the more significant and rapid was the flower opening process.
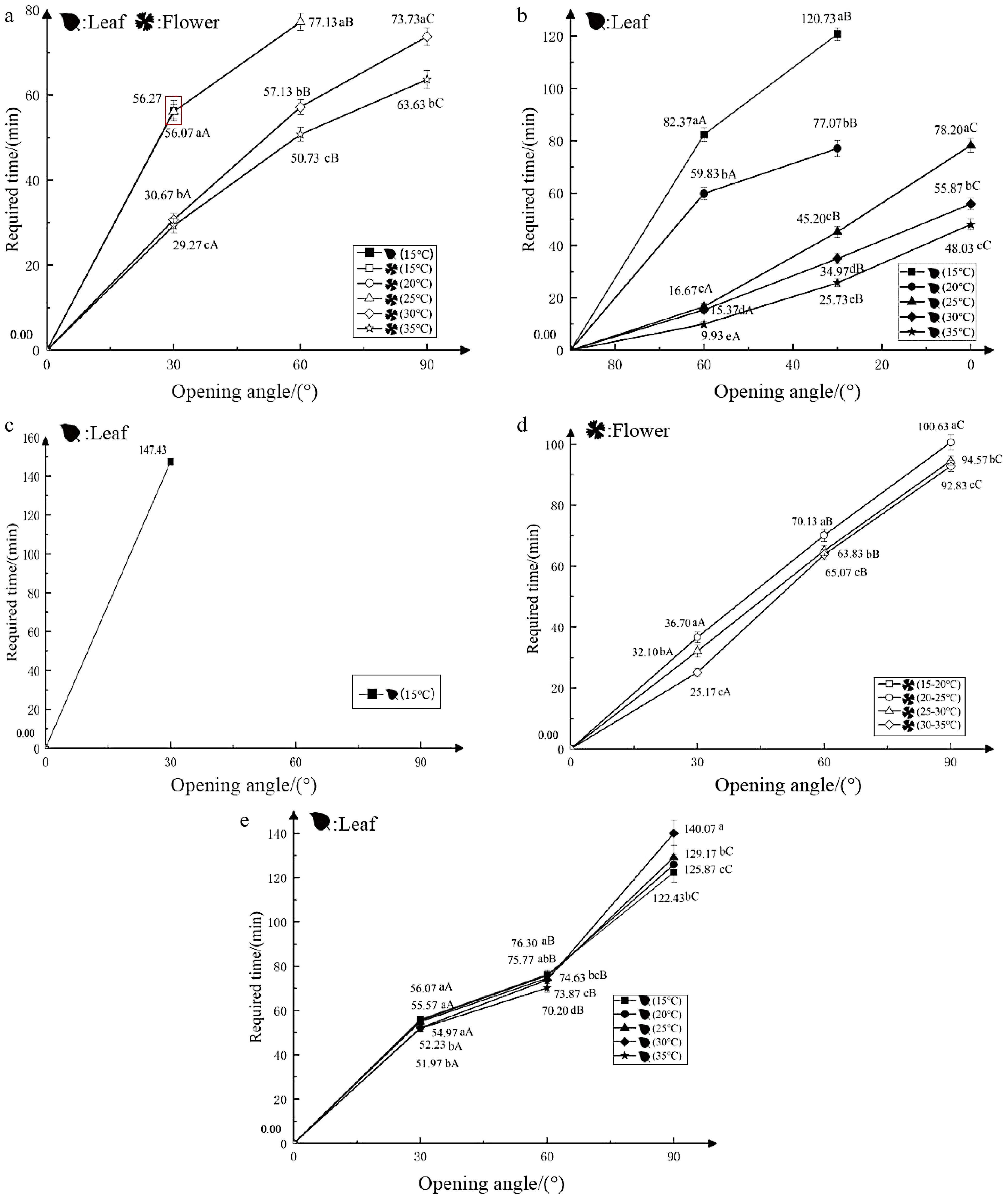
Figure 3.
Effects of temperature on the nyctinastic movement of O. triangularis ‘Purpurea’. (a) Increasing temperature in darkness, closing leaves (flowers); (b) increasing temperature in darkness, opening leaves; (c) decreasing temperature in darkness, closing leaves (flowers); (d) increasing the same temperature in darkness, closing leaves (flowers); (e) continuous different temperatures, closing leaves (flowers).
Test 4 showed the effect of increasing temperature under dark conditions on the nyctinastic movement of unfolding leaf. The leaves that had already opened were exposed to higher temperatures. The leaves from the 15 °C and 20 °C test groups could only fold up to 30° within 150 min, whereas the leaves from the 35 °C test group closed completely in only 48.03 min (Fig. 3b). Thus, a sudden increase in ambient temperature could accelerate the closure of newly opened leaves, and the greater the temperature increase, the faster the leaves closed.
Test 5 showed the effect of reducing temperature under dark conditions on the nyctinastic movement of closed leaves (flowers). Within 150 min, the closed leaves exhibited varying degrees of unfolding in the cooler environment. The largest unfolding angle (30°) in the test group was observed at 15 °C. However, it took 147.43 min to reach this angle, which was then maintained. Meanwhile, in the control group at 35 °C and the four experimental groups, the flowers did not open (Fig. 3c). In short, reducing the temperature could encourage the unfolding of closed leaves, and the maximum angle of the leaf opening increases with the degree of temperature decrease. However, lowering the temperature did not seem to have any noticeable effect on the opening of the flowers.
Test 6 showed the effect of increasing the same temperature under dark conditions on the nyctinastic movement of closed leaves (flowers). Within 150 min experimental period, there was no indication of any expansion in the closed leaves across all four trial groups. Only the closed flowers in the 15−20 °C test group were able to fully expand, while the 30−35 °C test group took the shortest time to do so, only 92.83 min (Fig. 3d). Therefore, raising the temperature has a significant impact on the expansion of flowers, and higher the baseline temperature, the faster is the rate of a flower unfolding.
Test 7 showed the effect of continuous different temperatures on the nyctinastic movement of closed leaves (flowers). When 100% light intensity was applied to the closed leaves, all the test groups except for the 35 °C group were able to fully expand. The leaf unfolding duration shortened with the decrease in temperature. For example, the 15 °C group took only 122.43 min to fully open (Fig. 3e). However, none of the closed flowers showed any tendency to expand. Therefore, the flowers were not able to unfold at a constant temperature.
Effect of the endogenous circadian clock on the nyctinastic movement of leaf and flowers
-
Test 8 showed the nyctinastic movement of closed leaves (flowers) under continuous dark conditions. Under continuous dark conditions, the closed leaves were only able to unfold to 30°, with the first unfolding occurring at 6:00 AM and then full closing at 11:30 AM. On the next day, the leaf unfolded again at 8:20 AM and fully closed at 3:00 PM. On the third day, the leaves began to unfold at 11:40 AM and closed completely at 8:30 PM. The time interval between the first and second unfolding of the leaves was like that between the second and third unfolding, which was 26.33 and 27.33 h respectively. The interval between the first and second day of leaf unfolding from the beginning to complete closure was similar, but the interval on the third day was longer. However, none of the O. triangularis 'Purpurea' flowers unfolded during the 3-day period (Table 2). In summary, under continuous dark conditions, the leaves were still able to undergo rhythmic movement with a cycle of about 26−28 h, while the flowers did not undergo any nyctinastic movement.
Table 2. Nyctinastic movement of leaves (flowers) under continuous darkness.
Tissue Leaves Flowers Number of days Day 1 Day 2 Day 3 Day 1 Day 2 Day 3 Leaf start opening time 6:00 8:20 11:40 \ \ \ The time interval between leaf opening (h) \ 26.33 27.33 \ \ \ State at 150 min 30° 30° 30° 0° 0° 0° Leaf start closing time 9:10 13:00 15:00 \ \ \ Leaf complete closing time 11:30 15:00 20:30 \ \ \ Time of start close to full close (h) 2.33 2 5.5 Time of start open to full close (h) 5.5 6.67 8.83 \ \ Test 9 showed the effect of entering darkness at different times on the nyctinastic movement of leaves (flowers). The leaves of all five test groups could only expand up to 30° within 150 min under dark conditions. The leaves of the test groups that were placed in darkness at 4 PM the previous day started to unfold at 5:30 AM the following day, with an interval of 13.5 h. On the other hand, the leaves of the test group that entered darkness at midnight began to unfold at 9:00 AM the next day, with an interval of 9 h. The earlier the leaf entered darkness, the earlier it unfolded the next day. None of the flowers in the experimental groups opened the next morning (Table 3). Moreover, flowers did not open when subjected to different times of darkness.
Table 3. The effect of entering dark conditions at different times on the nyctinastic movement of leaves (flowers).
Opening state Time to enter dark conditions 16:00 18:00 20:00 22:00 24:00 Leaves Leaf start opening time 5:30 6:30 7:30 8:30 9:00 State at 150 min 30° 30° 30° 30° 30° Time from entering dark to starting open (h) 13.5 12.5 11.5 10.5 9 Flowers Leaf start opening time \ \ \ \ \ State at 150 min 0° 0° 0° 0° 0° Time from entering dark to starting open \ \ \ \ \ -
Light is a well-studied factor that has a measurable impact on plant nyctinastic movement. The rate at which the O. triangularis 'Purpurea' leaves fully unfold from a closed state is faster at a higher light intensity. This indicates that light promotes leaf unfolding, which is consistent with previous study of O. triangularis[21]. Harshberger[24] observed that O. stricta exhibited night-time leaf unfolding from 23:00 to 7:00 for five consecutive nights in mid-August, likely due to the intense bright light of the full moon. Robinia pseudoacacia leaves maintained nyctinastic movement under 0% and 50% shading but not under 90% shading, indicating that weak light intensity is not conducive to the night movement of leaf, although the light intensity was not quantified[25]. Similarly, a study on Calathea makoyana found that the amplitude and rate of leaf unfolding decreased with stronger light intensity within the range of 0 to 10,000 lux, indicating that high light intensity could hinder leaf closure[26]. In line with this, we found that increasing light intensity promoted leaf expansion.
The results of the two light experiments indicate that leaves of O. triangularis 'Purpurea' can be stimulated to move at night in the range of 0−32.20 μmol·m−2·s−1 illumination, but the effect of this light intensity is much weaker at night compared to day. This indicates that factors other than light may also influence the nyctinastic movement of the leaves. Although the light has a significant effect on leaf movement at night, leaves cannot fully expand without light. However, regardless of the time of light application, flowers of O. triangularis 'Purpurea' did not open, indicating that light did not significantly affect the nyctinastic movement of the flowers.
Elevated temperature promotes leaf closure and flower unfolding
-
Less research has been conducted on the effect of temperature on the nyctinastic movement of plants compared to that of light. Leaves that are unfolded during the day gradually close in the absence of light, and the rate of closure is faster at higher temperatures. O. triangularis 'Purpurea' leaves cannot fully expand under dark conditions, but rapid cooling can promote leaf unfolding, and greater the decrease in temperature, faster is the rate of leaf unfolding. This is similar to the behavior of Oxalis tetraphylla, which remains unfolded at 5°C at night[27], suggesting that low temperatures are less favorable for leaf closure in Oxalis L. Similarly, leaves of the Phaseolus vulgaris remain unfolded at night at 10 °C[28]. The leaves of O. triangularis 'Purpurea' can fully close and expand within 150 min at temperatures between 15 °C and 30 °C, but cannot fully expand at excessive temperatures (35°C). This finding provides some basis for explaining why the leaves of Oxalis L. close at noon when the temperature is high[29]. Darwin called this movement phenomenon 'daytime sleep', which is not considered a type of nyctinastic movement[30]. When observing the nyctinastic movement cycle of Marchantia polymorpha leaves at different temperatures, it was found that there was no significant periodic difference in the temperature range of 18−24 °C[23]. However, there were noticeable differences in leaf movement within the range of 15−35 °C, likely due to variations in temperature perception between the two species. It can also be suggested that the impact of a wider temperature range on the nyctinastic movement of Marchantia polymorpha implants has not been investigated, and thus, the temperature range can be expanded for future studies.
Temperature affects flowers and leaves differently, and in dark conditions, the elevated temperature does not cause the leaf unfolding of O. triangularis 'Purpurea'. According to Tanaka et al.[22], increasing the temperature in darkness or light can cause the flowers of O. Martiana and O. triangularis 'Purpurea' to fully expand in a short period of time. This difference in the effects of temperature on the night-sensitive movements of leaves and flowers is likely due to different underlying mechanisms. The degree and rate of a flower unfolding in O. triangularis 'Purpurea' increases with the increase in temperature.
The circadian clock regulates the nyctinastic movement of leaf
-
The endogenous biological clock plays a certain role in the nyctinastic movement of O. triangularis 'Purpurea' leaves, but it cannot completely regulate their movement. Even in complete darkness and without changes in light and temperature, the leaves can still perform a certain degree of movement at night, unfolding up to 30° and maintaining this position for a period before closing again. This behavior is similar to that of O. triangularis, which can perform rhythmic movements for about 26 h in darkness[21]. Similar movements have been observed in Calathea makoyana, where leaves can gradually undergo transition from the unfolded state during the day to the closed state at night, demonstrating that the biological clock can independently affect the nyctinastic movements of plants without relying on light and temperature[26]. The timing of the O. triangularis 'Purpurea' nyctinastic movements varied among leaves entering darkness at different times, with no consistent pattern of leaf unfolding. This indicates that the endogenous biological clock plays a role in this process. However, the leaves only unfolded up to 30° and could fully expand, and the flowers did not exhibit any movement in the experiment. Thus, while the endogenous biological clock is involved in regulating the nyctinastic movement of O. triangularis 'Purpurea' leaves, it is not the primary factor influencing their movement.
Nyctinastic movement and garden applications
-
The night movement of O. triangularis 'Purpurea' is influenced by light, temperature, and the endogenous biological clock. Light has a significant promoting effect on leaf night movement, with higher light intensity resulting in a faster leaf unfolding rate. Rapidly increasing the temperature can cause the unfolded leaves to close quickly. Under dark conditions, the leaves can expand up to 30° due to the influence of the endogenous biological clock and their rhythmic movement lasts for 26−28 h. These three factors often interact and jointly regulate the occurrence and operation of the movement. The nyctinastic movement of O. triangularis 'Purpurea' flowers is mainly affected by temperature increase, with a less significant impact of light and the endogenous biological clock.
In summary, while selecting Oxalis L. plants with the nyctinastic movement for garden landscaping, it is important to consider the environmental conditions of the planting area. The area should have a suitable light intensity (8.34−32.30 μmol·m−2·s−1) to promote the expansion of leaves during the day. The areas with large fluctuations in daytime temperature are not suitable for using Oxalis L. plants with nyctinastic movement, whereas areas with large temperature differences between day and night are suitable for using Oxalis L. plants for flower-watching.
To enhance the ornamental value of O. triangularis 'Purpurea' plants, they should be planted in an environment with a high temperature fluctuation, allowing the quick flower unfolding in response to an instantaneous temperature increase. While both the leaves and flowers of Oxalis L. plants have night-sensitive movements, their responses to temperature differ significantly, possibly due to their distinct functions, which requires further investigation. The closure of Oxalis L. plant leaves during midday in hot weather is commonly attributed to water deprivation and sun exposure, but the sudden temperature increase during this period may also contribute to leaf closure. The impact of water on the nyctinastic movement of Oxalis L. requires further exploration.
-
In the range of 0−32.20 μmol·m−2·s−1 illumination, the stronger the light intensity, the faster the leaf unfolding rate, but the light has no obvious effect on the night movement of the flower.
In the range of 15−35 °C, increasing the temperature promotes leaf closure and flower unfolding, and the greater the amplitude of temperature increase, the faster the leaf closure (flower unfolding) rate, which will also promote the unfolding of flowers.
Under the condition of continuous darkness (constant temperature), the leaves can still be expanded for about 30° under the influence of the endogenous biological clock, and carry out rhythmic movement for nearly 26−28 h, but the flowers have been kept closed and not unfolded, and the endogenous biological clock has no obvious effect on the night movement of the flowers.
This research was supported by 2018 Fujian Agriculture and Forestry University Flower Industry Expert Guidance Service Team Project (11891008001). We would like to express our gratitude to Fuzhou Bugeng Agricultural Technology Co., Ltd. in Fujian Province, China, for offering the necessary plant resources of Oxalis L. plants.
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Le You, Wanli Tuo
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
You L, Tuo W, Dai Z, Wang H, Ahmad S, et al. 2023. Effects of light intensity, temperature, and circadian clock on the nyctinastic movement of Oxalis triangularis 'Purpurea'. Technology in Horticulture 3:11 doi: 10.48130/TIH-2023-0011
Effects of light intensity, temperature, and circadian clock on the nyctinastic movement of Oxalis triangularis 'Purpurea'
- Received: 13 March 2023
- Accepted: 05 May 2023
- Published online: 13 June 2023
Abstract: Oxalis triangularis 'Purpurea' exhibits nyctinastic movement. While the regulation of nyctinastic movement is influenced by many factors, like light, temperature, and circadian clock, there is lack of systematic research on these factors in Oxalis L. In this study, the nyctinastic motion of O. triangularis ‘Purpurea’ leaves and flowers was recorded in a growth chamber with controlled temperature and light, and. the time and angle of opening and closing of the leaves and flowers were analyzed. The results showed that the leaves could fully expand under 8.34 μmol·m−2·s−1 light intensity in the morning and 32.20 μmol·m−2·s−1 light intensity at night, taking about 150 min for the whole process. The stronger the light intensity, the lesser time it took for the leaves to fully expand after closing. However, the light intensity had no significant effect on flower movement. As the temperature increased within the range of 15−35 °C, the leaves closed quickly, and the flowers opened rapidly. Moreover, under constant darkness, the leaves still exhibited rhythmic movement, expanding about 30° possibly due to the circadian clock, lasting for approximately 26−28 h. However, the flowers remained closed and did not open. Therefore, circadian clock does not play a significant role in the nyctinastic movement of flowers. Leaf movement is influenced by light, temperature, and the circadian clock, whereas floral movement was mainly affected by temperature. This study shed new light on the regulation of nyctinastic movement and will promote better use of O. triangularis 'Purpurea' in gardens.
-
Key words:
- Oxalis triangularis 'Purpurea' /
- Nyctinastic movement /
- Light /
- Temperature /
- Circadian clock












