-
Losses by chilling may happen in all fruit-producing regions. On the other hand, low temperature is an environmental factor that restricts the planting, cultivation and production of horticultural crops. For example, the growing season of an annual crop is dictated by chilling-free time. Similarly, some limiting factors of producing permanent horticultural crops are low winter temperatures, early chilling in autumns, and late chilling in spring[1]. Adverse climatic conditions, especially winter frosts and spring chilling, are the most important parameters determining the distribution of species and, of course, the most important indicator of site selection for the construction of fruit orchards[2].
Persimmon (Diospyros kaki L.), which belongs to the family Ebenaceae, is native to Sri Lanka and Southeast Asia. There are approximately 200 species in the genus Diospyros. Kaki, or the so-called oriental persimmon, is mainly grown for fruit production[3]. Among the various varieties of persimmon, only a few are important for commercial fruit production. The most important species of persimmon for commercial fruit production is Japanese persimmon (Diospyros kaki L.). In Iran there are genetic variations of 22 species of kaki and seven species of lotus and virginia (locally known as date-plum). Another persimmon cultivar in Iran is 'Fuyu kaki'[4]. Persimmon resembles pomegranate in winter chilling resistance so that it can resist the temperatures as low as −10 °C. The chilling requirement of the buds of this tree is 100−400 h of < 7 °C temperature. Some persimmon cultivars can tolerate the temperatures of as low as −18 °C[5].
According Yu & Lee[6] chilling stress is a main ecological stress controlling the geographical distribution, growth, and development of temperate fruits. The range of freezing injury in the trees depends on the reduction rate of temperature, the lowest temperature reached, and the period of the stress conditions. They reported that freezing must be evaluated to predict the winter survival and productivity of the trees in particular regions, to monitor for tolerant genotypes, and to develop cultivation approaches that reduce chilling stress. Various methods are used to evaluate freezing injury in trees under field and laboratorial conditions, including estimate of tissue discoloration, thermal analysis, and determination of ion leakage. Low temperature reduces biosynthetic and physiological activity of plants and imposes permanent injuries and eventually death. Cold resistance can be defined as the ability of plants to tolerate freezing temperatures without incurring significant damages, and this is an important indicator for assessing the potential of planting species and cultivars[7]. Phenological stage can be vital for chilling injuries. Trees are more sensitive to low temperatures at flowering and petal shedding stages[8]. Low temperatures disrupt the balance between energy absorption and its use by target metabolites, so enzymatic activities are reduced to a greater extent than photophysical and photochemical processes involved in light absorption, energy transfer and transformation[9].
According to Miranda et al.[10], the species of the genus Prunus, such as almonds, are resistant to chilling before flowering, but they become more susceptible at full-bloom and next stages than pre-flowering stage (deep dormancy of floral bud), but under identical phenological conditions, genotype is the determinant factor. In a study on the frost tolerance of eight olive cultivars, Barranco & Ruiz[11] concluded that electrolyte leakage was increased as the temperature was decreased to as low as −22 °C. Interestingly, this increase in electrolyte leakage was more pronounced in the susceptible olive cultivars. Electrolyte leakage method has been used to evaluate chilling injury in grape organs[12], raspberry branches[13], blueberry shoots[14], and peach tissues[15].
Because of the importance of chilling resistance for permanent plants to pass through winters, there is a great interest in finding ways to determine the cold resistance of plants. Most of these methods have been designed to test controlled frosts and assess the resulting chilling injuries. But, researchers have long been looking for indirect ways to measure non-frosting cold[7]. Since persimmon has turned into an economic base for some orchard owners, including those in Guilan province (north of Iran), and given the economic significance of its wood, fruits, and its medicinal properties, it is imperative to explore factors limiting it's planting[4]. Understanding low-temperature hardiness as one of the environmental factors in order to find coping strategies to alleviate the damage caused by this phenomenon is one of the important research goals for horticulturalists and plant physiologists. As such, some persimmon genotypes were evaluated for cold resistance of dormant bud, swelled bud, and flower.
-
The experiment was conducted on three important persimmon commercial genotypes, including date-plum, 'Fuyu kaki', and Japanese persimmon in Talesh County (Guilan province, North of Iran) from mid-December to mid-March 2017. Persimmon trees in the same age of 10 years in the region were used in the experiment. The experiment was designed as factorial with three factors on the basis of a randomized complete block design with three replications. The first factor was assigned to persimmon genotype at three levels of date-plum, 'Fuyu', and Japanese persimmon. The second factor was assigned to chilling treatment at two levels of 4 °C and −16 °C. The third factor was assigned to three phenological stages including bud dormancy phase, bud swelling phase, and anthesis phase. The traits evaluated in this experiment included electrolyte leakage, electrical conductivity (EC), and proline.
The change in EC of treated tissue minus the change in EC of the control at each temperature, assuming that the differential increase in ion leakage is due to the chilling temperature[14, 15]. The total capacity to leak can be determined by autoclaving the tissues and measuring the electrical conductivity of the aliquot[6]. To apply the experimental treatments, the selected tissues (branches and buds) were first sprayed with distilled water. Then, they were placed in an incubator and were cooled to 2 °C at a freezing rate of 10 °C/h. Then, they were cooled to the desired temperature at a freezing rate of 5 °C/ha and were kept at that temperature for 3 h. To this end, 0.5 g of the buds from each experimental plot was placed in closed container containing twice-distilled water and it was placed at the laboratory temperature for 24 h. Then, its EC was measured with an EC-meter to give EC1. To measure EC2, 0.5 g of the buds was frozen at −20 °C for 24 h. Then, they were placed at room temperature for 24 h. Then, EC2 was read and the electrolyte leakage (EL) was calculated by the following equation:
$ {\text{EL }} = {\text{ }}\frac{{{\text{E}}{{\text{C}}_{\text{1}}}}}{{{\text{E}}{{\text{C}}_{\text{2}}}}} \times 100 $ Proline was measured by Bates et al.'s[16] procedure. Fresh tissue (0.1 g) was ground in 10 mL of 3% sulfosalicylic acid to yield a uniform mixture. The resulting extract was centrifuged at 10,000 rpm for 5 min. Then, 2 mL of supernatant was mixed with 2 mg of ninhydrin reagent and 2 mL of pure acetic acid and was placed in a warm water bath at 100 °C for 1 h. The tubes containing the mixture were then cooled down in an ice bath to stop all reactions. Then, 4 mL of toluene was added to the mixture and the tubes were well shaken. After the tubes were held still for 15−20 s, two complete separate layers were formed in them. The colorful upper layer that contained toluene and proline was used to measure proline concentration. Then, its absorption was read at 520 nm and proline content of the samples was found out using a standard curve.
$\rm Y = 0.129X + 0.009 $ in which Y is the absorption read and X is proline concentration in mM/L. Data were analyzed by MSTATC statistical software and the means were compared by the LSD test.
-
Based on the results, proline content was significantly influenced by the simple effect of phenological stage (bud dormancy, bud swelling, and anthesis) and the trilateral interaction of 'genotype × chilling × phenological stage' at the p < 0.01 level and the interaction of 'genotype × phenological stage' at the p < 0.05 level. But, the impact of genotype, chilling, 'genotype × chilling', and 'chilling × phenological stage' was insignificant on this trait (Table 1).
Table 1. Analysis of variance for the effect of experimental factors on proline content.
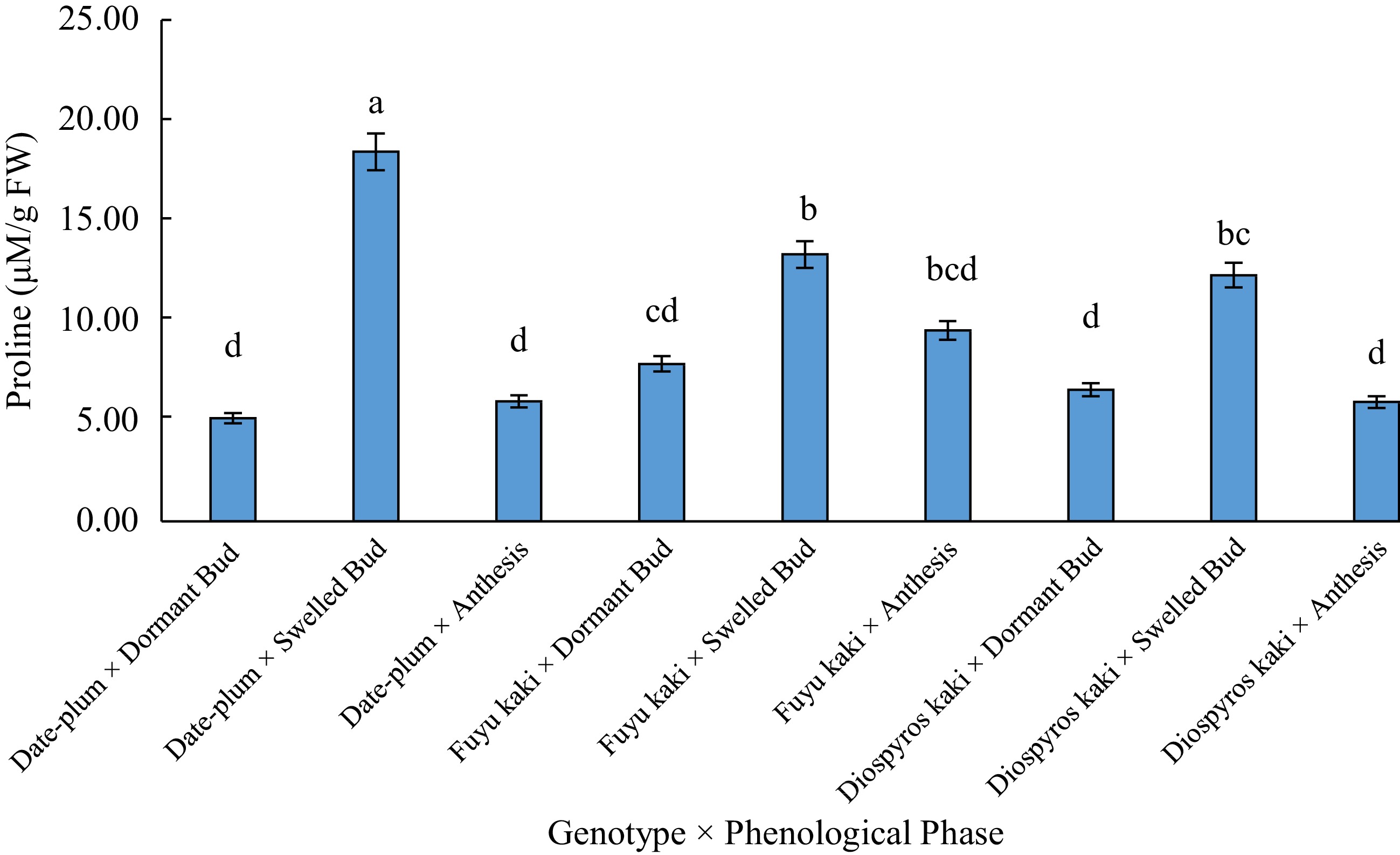
Sources of variations df Means of squares Proline content Electrolyte leakage EC Replication 2 88.74** 89.39ns 32.14ns Genotype (A) 2 19.48ns 71.32ns 117.79ns Chilling (B) 1 21.37ns 234.04* 284.97* AB 2 29.13ns 138.85* 461.82** Phenological phase (C) 2 369.23** 6673.85** 7819.65** AC 4 40.96* 26.31* 115.24ns BC 2 10.59ns 143.87* 285.73* ABC 4 65.57** 85.79+ 464.13** Error 34 14.42 33.59 59.83 ns: insignificant difference; **: significant difference at 0.01; *: significant difference at 0.05; +: significant difference at 0.10. The results of means comparison (Table 2) revealed that the highest proline content was obtained at bud swelling phase, but it was lower at bud dormancy and anthesis phases among which there was not a significant difference. Means comparison of the data for the interactive effect of 'genotype × phenological stage' on proline content indicated that the highest proline content was related to the treatment of 'date-plum × bud swelling' and the lowest proline was obtained in 'date-plum × bud dormancy', 'date-plum × anthesis', 'Japanese persimmon × anthesis', and 'Japanese persimmon × bud dormancy' (Fig. 1).
Table 2. Means comparison for the effect of phenological stage on the studied traits.
Treatment Proline
(μmol/g FW)Electrolyte
leakage (%)EC
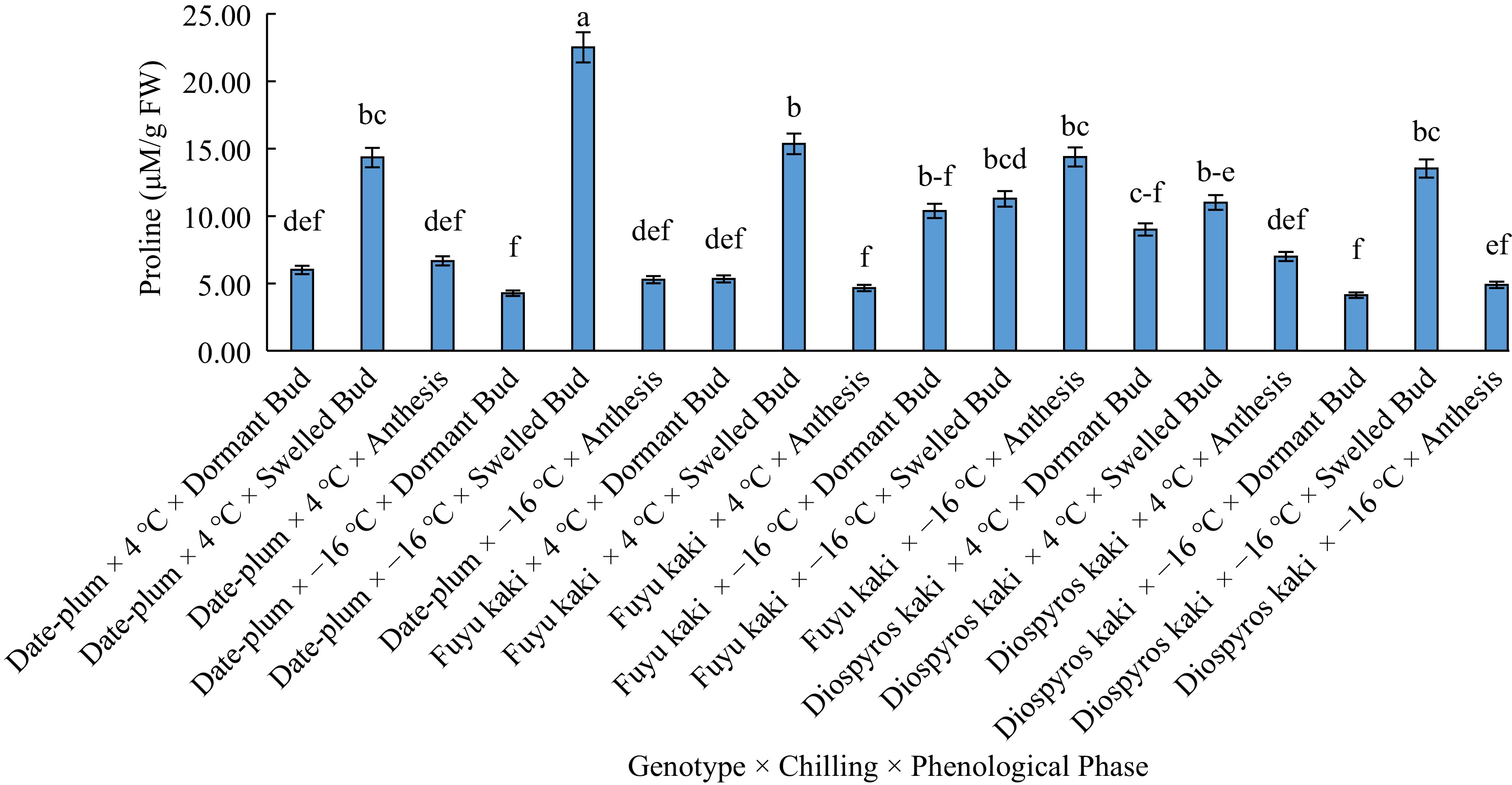
(μS/cm)Bud dormancy 6.52 b 2.54 b 0.1 b Bud swelling 14.66 a 34.64 a 36.23 a Anthesis 7.15 b 0.16 b 0.16 b Similar letters in each column shows insignificant differences at the p < 0.05 level. With respect to the trilateral effect of 'genotype × chilling × phenological phase' on proline content, the highest proline content was obtained from 'date-plum × −16 °C × bud swelling' and the lowest from 'date-plum × −16 °C × bud dormancy', 'Fuyu kaki × 4 °C × anthesis', and 'Japanese persimmon × −16 °C × bud dormancy' (Fig. 2).
It is argued that proline accumulation is an indicator of environmental stresses and plays a vital role in this respect[17]. By interacting with enzymes, proline contributes to protecting the structure and sustainability of their activity[18]. In a study on variations of injury level and proline content in the buds of some commercial genotypes of apricot at different phenological stages, the highest proline content was obtained at bud swelling phase and the lowest at anthesis phase[19], which is consistent with our findings.
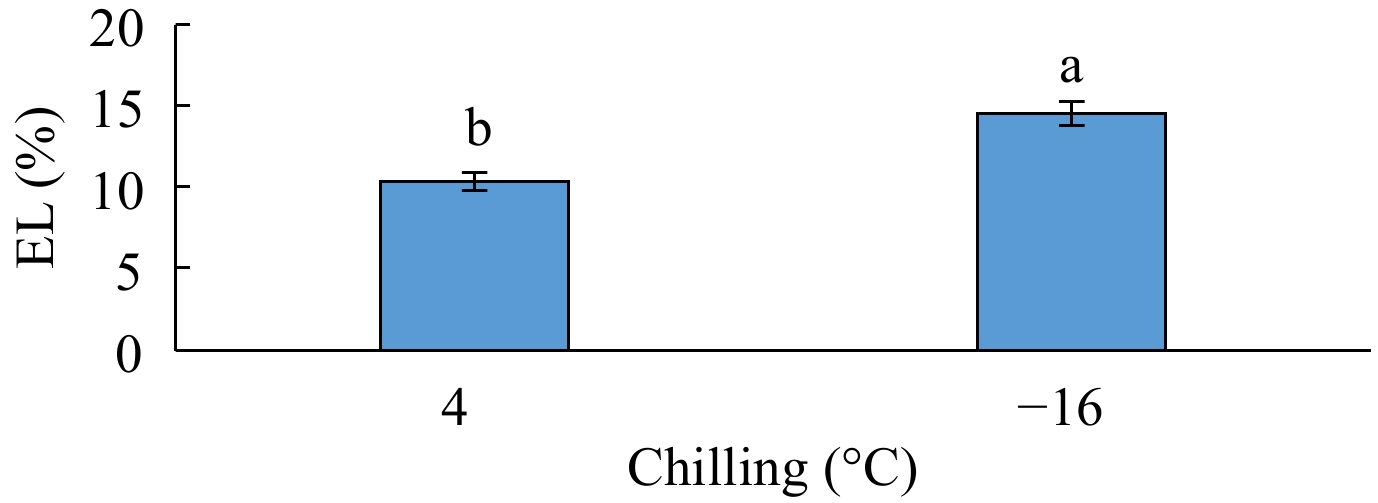
The analysis of variance for electrolyte leakage showed that the simple effect of genotype and the interactive effect of 'genotype × phenological phase' was not significant on this trait, but this trait was significantly influenced by the simple effect of phenological phase at the p < 0.01 level, by the simple effect of chilling and the interactive effects of 'genotype × chilling' and 'chilling × phenological phase' at the p < 0.05 level, and by the interactive effect of 'genotype × chilling × phenological phase' at the p < 0.10 level (Table 1). According to the results of means comparison, the highest electrolyte leakage was obtained from the fruits exposed to −16 °C and the lowest from those exposed to 4 °C (Fig. 3). Among different phenological phases, swelling had the highest electrolyte leakage and anthesis had the lowest but not differing from bud dormancy significantly (Table 2).
Means comparison for the interactive effect of 'genotype × chilling' on electrolyte leakage (Table 2) indicated that the highest and lowest rate of electrolyte leakage were obtained from 'date-plum × −16 °C' and 'Japanese persimmon × 4 °C' respectively, but showing insignificant differences with that of the other treatments. With respect to the interactive effect of 'chilling × phenological stage', it was revealed that '−16 °C × bud swelling' yielded the highest leakage and '4 °C or −16 °C × anthesis or bud dormancy' yielded the lowest (Table 3).
Table 3. Means comparison for the effect of 'chilling × phenological phase' on the studied traits.
Treatment Ion leakage
(%)EC
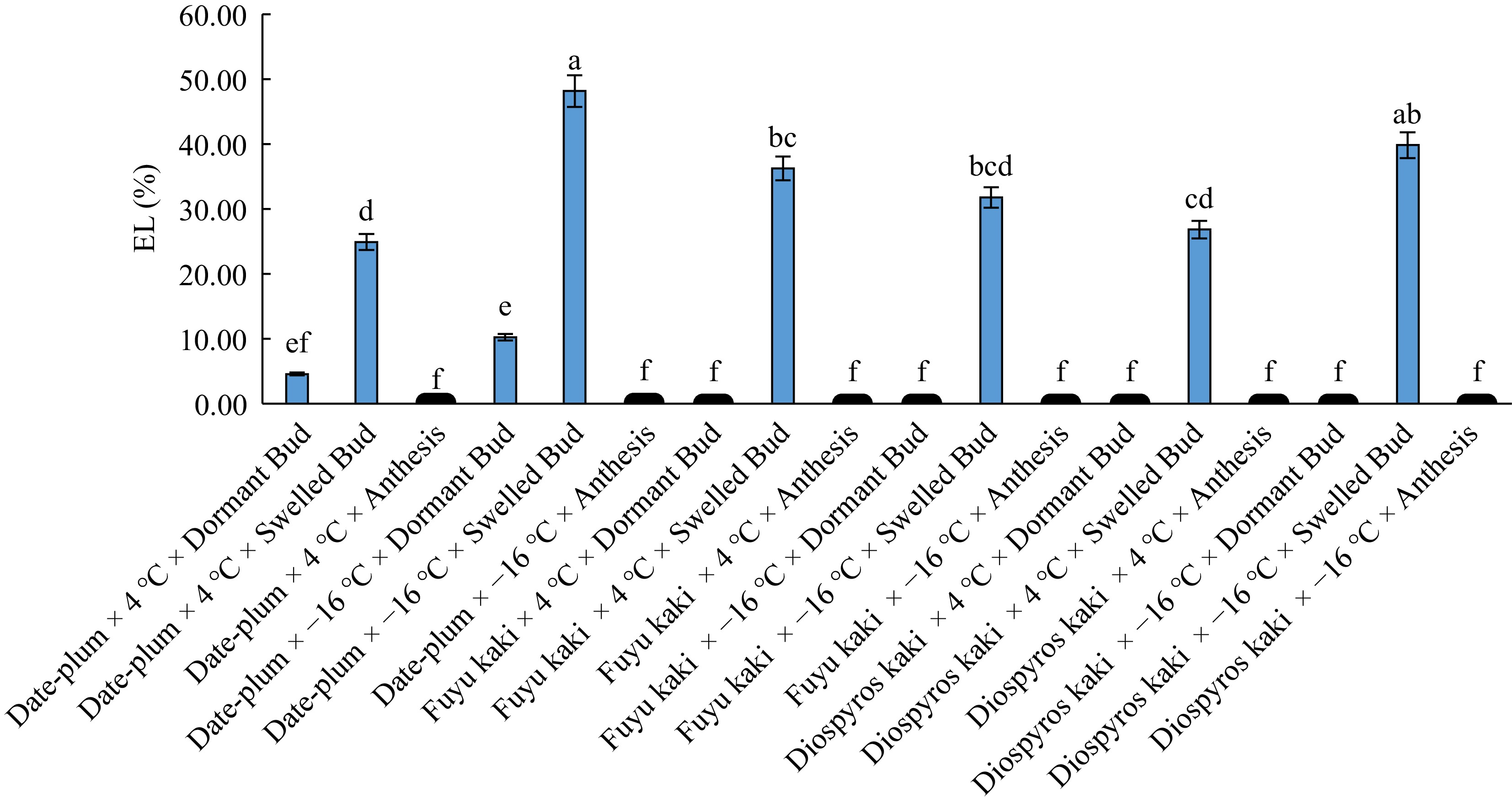
(μS/cm)4 °C × bud dormancy 1.60 c 0.10 c 4 °C × bud swelling 29.34 b 29.33 b 4 °C × anthesis 0.16 c 0.16 c -16 °C × bud dormancy 3.48 c 0.11 c -16 °C × bud swelling 39.94 a 43.13 a -16 °C × anthesis 0.16 c 0.15 c Similar letters in each column shows insignificant differences at the p < 0.01 and p < 0.05 levels. The results for the interactive effect of 'genotype × chilling × phenological stage' (Fig. 4) indicated that the highest electrolyte leakage was related to 'date-plum × −16 °C × bud swelling' and all studied genotype had the lowest electrolyte leakage at 4 °C and −16 °C both at bud dormancy and anthesis phases.
These results mean that electrolyte leakage is the highest at the early stage of dormancy breaking of trees in late winter (bud swelling phase). High electrolyte leakage reflects the inability of membrane in maintaining intra-cellular compounds and the outflow of ions from membrane, which injures cell membrane[20]. Therefore, it can be said that date-plum at −16 °C and bud swelling stage incurred the highest injury in cell membrane whereas all genotypes at bud dormancy and anthesis phase had the lowest electrolyte leakage and membrane injury, thereby showing hardiness to chilling-induced injury to their membrane. Since electrolyte leakage index is calculated on the basis of injury to cell membranes and this injury causes electrolyte leakage (especially K+) from cell[7], it can be inferred that the tissues of persimmons are damaged more at −16 °C than at 4 °C. In a study on frost tolerance of eight olive cultivars, Barranco & Ruiz[11] observed that electrolyte leakage was increased by applying colder temperature until the temperature of −22 °C, which is consistent with our findings.
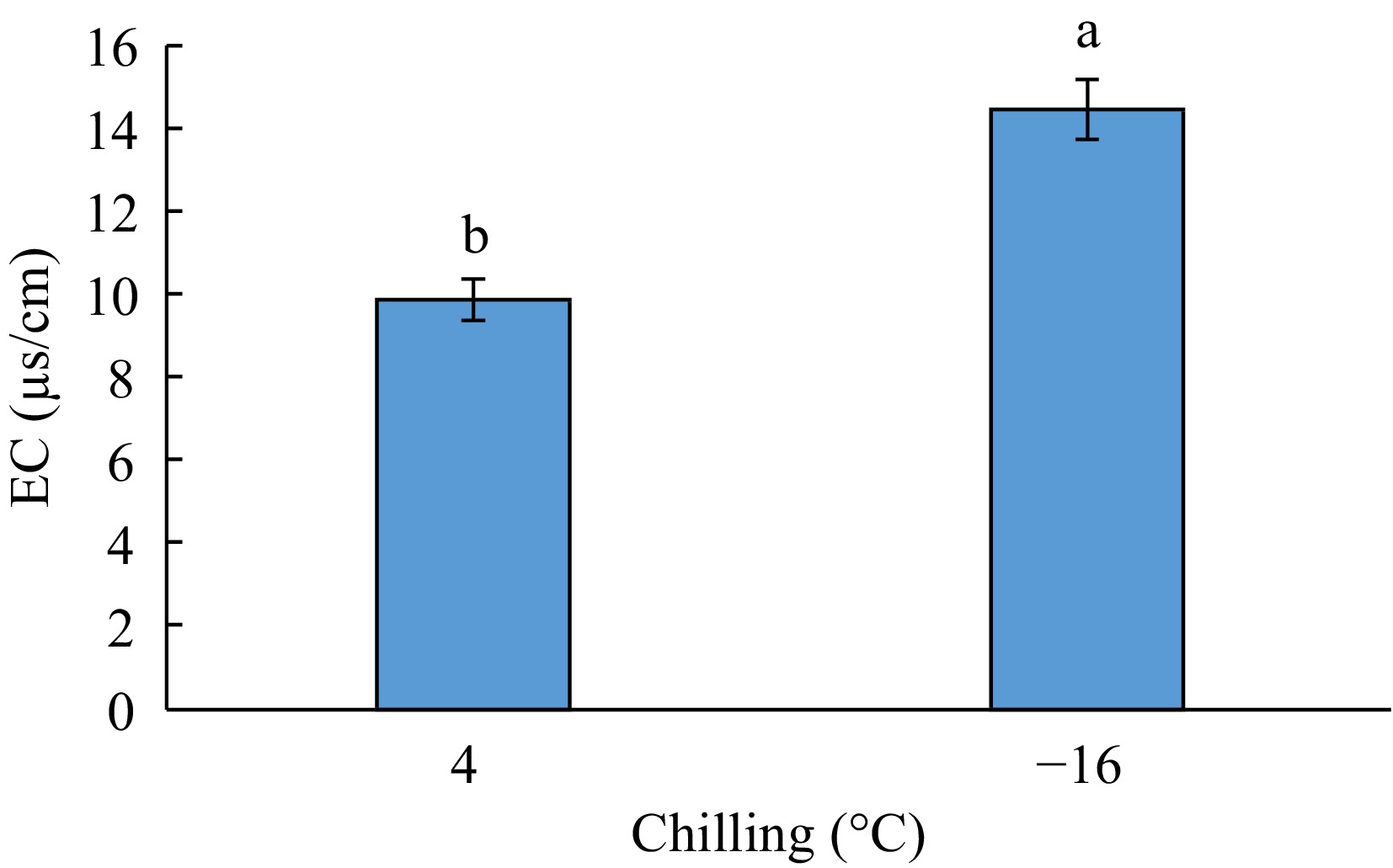
The results of variance analysis for the effect of experimental factors on electrical conductivity (EC) showed that the simple effect of the phenological stage (bud dormancy, bud swelling, and anthesis) and the interactive effect of 'genotype × chilling' and 'genotype × chilling × phenological stage' was significant on this trait at the p < 0.01 level and the effect of chilling and 'chilling × phenological stage' was significant at the p < 0.05 level. But, this trait was not affected by the simple effect of genotype and the interactive effect of 'genotype × phenological stage' (Table 1). The results of means comparison indicated that the highest EC was obtained from −16 °C and the lowest from 4 °C (Figs 5 and 6).
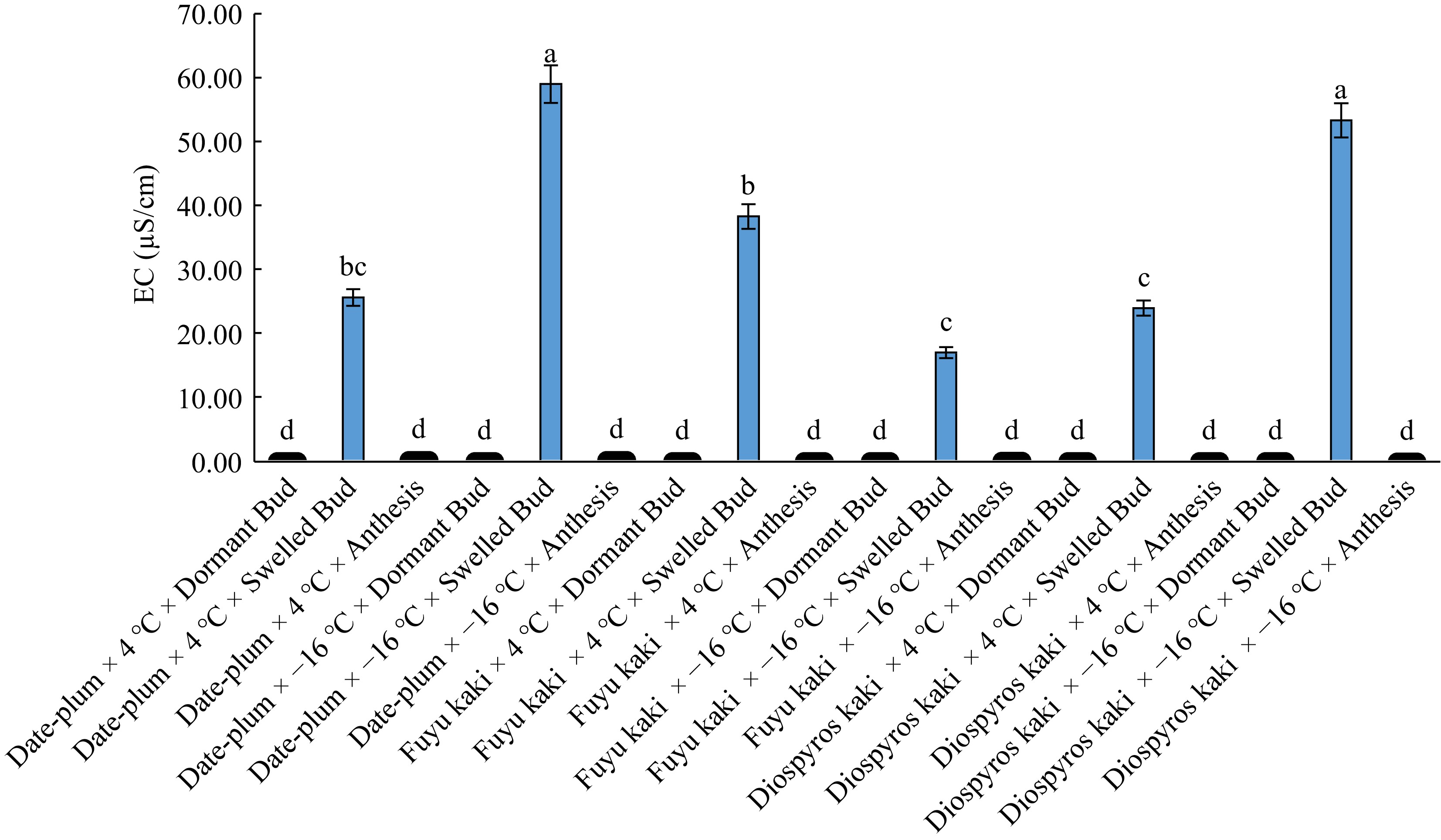
The plants had the highest EC at bud swelling phase and the lowest at bud dormancy phase, but this latter did not differ from that at anthesis stage significantly (Table 2). Means comparison for the interactive effect of 'genotype × chilling' on EC (Table 3) revealed that the highest EC was related to 'date-plum × −16 °C' and 'Japanese persimmon × −16 °C' and the lowest to 'kaki Fuku × −16 °C' showing insignificant difference with the treatments of 'Japanese persimmon × 4 °C' and 'date-plum × 4 °C'. Means comparison for the effect of 'chilling × phenological stage' on the EC of samples indicated that '−16 °C × bud swelling' had the highest EC while the lowest EC was related to bud dormancy and anthesis at both 4 °C and −16 °C (Table 4). The highest EC was obtained from 'date-plum × −16 °C × bud swelling' and 'Japanese persimmon × −16 °C × bud swelling' and the lowest from all studied genotypes at both 4 °C and −16 °C at bud dormancy and anthesis (Fig. 6).
Table 4. Means comparison for the effect of 'genotype × chilling' on the studied traits.
Treatment Ion leakage
(%)EC
(μS/cm)Date-plum × 4 °C 9.92 b 8.68 b Date-plum × -16 °C 19.56 a 19.80 a Kaki Fuyu × 4 °C 12.15 b 12.84 ab Kaki Fuyu × -16 °C 10.68 b 5.77 b Japanese × 4 °C 9.02 b 8.07 b Japanese × -16 °C 13.35 b 17.82 a Similar letter(s) in each column shows insignificance differences at the p < 0.01 and p < 0.05 levels. In most trees, the EC of the solution leaking from chilling-exposed samples is a reliable parameter to evaluate chilling-induced injuries. To put it another way, when studying the injuries of chilling to plant tissue by electrolyte leakage, EC is a more reliable parameter than pH so that EC of samples (directly or indirectly) is used in the relevant formula to calculate both relative leakage at a certain temperature and injury index at a certain temperature. Chilling injuries to trees are assessed and measured by visual observations and electrolyte leakage[7]. Likewise, it was found that as chilling was intensified, EC was increased. The assessment of freezing resistance of fennel by measuring electrolyte leakage indicated that as freezing temperature was decreased, electrolyte leakage was significantly influenced in different parts and roots and leaves showed the highest and lowest electrolyte leakage percentage, respectively[21].
-
The results indicated that proline content, electrolyte leakage index, and EC of the samples was the highest in late winter when the dormancy of the trees started to break (bud swelling phase), but they were not statistically different between bud dormancy and anthesis phases. Since phenological phase can be crucial for chilling injuries, the recognition of phenological stages to find out the stage of chilling resistance the trees are at can be very helpful in avoiding chilling injuries. Also, it was revealed that these indices were increased as chilling was intensified, but the simple effect of genotype was not significant on any recorded traits. It may be said that chilling hardiness was similar across the studied genotypes. The results indicated that date-plum exhibited the highest membrane injury due to chilling (ion leakage) at −16 °C and bud swelling phase. Thus, it is more susceptible than other genotypes and the temperature of −16 °C at bud swelling stage can impose heavy damages to this genotype.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sedaghathoor S, Baladeh MK, Piri S. 2023. A study on chilling hardiness of three persimmon genotypes by electrolyte leakage parameter. Technology in Horticulture 3:10 doi: 10.48130/TIH-2023-0010
A study on chilling hardiness of three persimmon genotypes by electrolyte leakage parameter
- Received: 17 February 2023
- Accepted: 11 April 2023
- Published online: 13 June 2023
Abstract: Most fruit trees in temperate regions are exposed to chilling, which causes extensive economic losses. Low temperatures reduce biosynthesis activity of plants and the functioning of physiological processes, impose irreparable injuries, and finally, destroy the plants. So, to study chilling tolerance of three genotype of persimmon by electrolyte leakage parameter, a factorial experiment was carried out in a randomized complete block design with three replications. The first factor was assigned to persimmon cultivar at three levels of date-plum, 'Fuyu kaki', and Japanese persimmon. The second factor was assigned to chilling treatment at two levels of 4 °C and −16 °C. The third factor was assigned to three phenological stages including bud dormancy phase, bud swelling phase, and anthesis phase. The recorded traits included electrolyte leakage, electrical conductivity (EC), and proline content. The results showed that proline content, electrolyte leakage and EC of all samples were the highest at bud swelling phase, but they did not differ significantly between bud swelling and anthesis stages. The highest electrolyte leakage and EC was observed in plants exposed to −16 °C and the lowest in those exposed to 4 °C. The membrane of date-plum at −16 °C and bud swelling phase was most heavily injured by chilling. Among the trilateral effects of studied factors on the recorded traits, 'date-plum × −16 °C × bud swelling phase' showed the highest proline content, electrolyte leakage, and EC, and 'date-plum × −16 °C × bud dormancy' and 'Japanese persimmon × −16 °C × bud dormancy stage' exhibited the lowest proline content, electrolyte leakage, and EC.
-
Key words:
- Proline /
- Date-plum /
- Fuyu kaki /
- Electrolyte leakage /
- Electrical conductivity