-
Heat stress induces and accelerates leaf senescence by interrupting chlorophyll metabolism, mainly by altering the rate at which chlorophyll is synthesized or degraded[1,2]. Previously, heat stress-induced leaf senescence has been positively associated with enhanced activity of key chlorophyll-degrading enzymes in Arabidopsis (Arabidopsis thaliana) and creeping bentgrass (Agrostis stolonifera)[3−7]. More specifically, the activities of chlorophyllase (CHLASE) and pheophytinase (PPH), two enzymes involved in cleaving the hydrocarbon tail from the ring structure of chlorophyll and pheophytin, respectively, were determined to have greater activity under heat stress[3−9]. When hydrogen peroxide coexists with phenols in senescent leaf tissue, chlorophyll-degrading peroxidase (CHL-PRX) causes oxidative changes to the structure of chlorophyll, and activity of this enzyme was shown to be increased in creeping bentgrass under heat stress[4,6,7,10]. Chlorophyll synthesis is also metabolically altered during heat stress, as it was previously exhibited that the enzymatic activity of porphobilinogen deaminase (PBGD), which is for catalyzing the construction of the tetrapyrrole ring of chlorophyll, was suppressed under natural senescence in pepper (Capsicum annuum) and poinsettia (Euphorbia pulcherrima), as well as under heat stress in cucumber (Cucumis sativus)[11−13]. Because plants undergo premature chlorophyll loss as a result of alterations to chlorophyll metabolism when chronically exposed to supra-optimal temperatures, developing strategies for delaying chlorophyll loss to prolong stay-green traits in leaves is important for improving plant tolerance to heat stress. Chemical priming with various small molecules is a viable approach for alleviating leaf senescence and enhancing plant tolerance to abiotic stresses, including heat stress[6,14−22].
Morphactins are plant growth regulators synthetically derived from fluorene-9-carboxylic acids that have been studied for their ability to affect the growth habit of plants[23]. Dybing & Yarrow[24] exhibited that applying the morphactin, chlorflurenol-methyl (CM), to soybean plants reduced chlorophyll loss while increasing shoot production, tissue biomass, the number of mesophyll cells, and elongation of palisade cells, suggesting that CM allowed for the maintenance of chlorophyll content by promoting cell growth and proliferation. Another study on soybean (Glycine max) demonstrated that application of a morphactin stimulated branching and increased the width of the mesophyll layer and vascular bundle[25]. Foliar application of the morphactin, 2-chloro-9-hydroxyfluoren-9-carboxylic acid (CF125), to fava bean (Vicia faba) increased leaf thickness, palisade layer width, number of mesophyll cells, and stem diameter[26]. Several studies have found that CM may delay natural leaf senescence in a variety of species, including soybean, bitter dock (Rumex obtusifolius), and radish (Raphanus sativus), as indicated by a maintenance of higher chlorophyll content in treated plants[24,27,28]. Although the findings of these studies suggest that morphactins play a role in the regulation of leaf senescence and plant growth, the metabolic factors controlling these responses have not been elucidated, and particularly, the mechanisms underlying how morphactins regulate heat-induced leaf senescence and their functions in regulating chlorophyll synthesis and degradation in plants exposed to heat stress conditions remain largely unknown.
It was hypothesized that morphactins may suppress heat-induced leaf senescence and enhance heat tolerance in cool-season plant species exposed to prolonged periods of heat stress by maintaining or enhancing chlorophyll synthesis or inhibiting chlorophyll degradation. The objectives of this study included determining whether the application of CM can control leaf senescence in creeping bentgrass under heat stress and examining the regulatory effects that CM may have on chlorophyll metabolism. To accomplish this, the authors evaluated several physiological parameters associated with leaf senescence, such as turf quality, electrolyte leakage, and chlorophyll content and quantified the rate of activity of chlorophyll-synthesizing and –degrading enzymes. Ascertaining the specific ways in which morphactins may affect chlorophyll synthesis or degradation in plants exposed to heat stress will advance our understanding of the mechanisms regulating heat-induced leaf senescence and allow us to develop effective approaches for improving plant heat tolerance.
-
Turf quality (TQ), chlorophyll content, and electrolyte leakage (EL) remained relatively unchanged during the experimental period under non-stress conditions. Application of CM had no significant effects on TQ, EL, or chlorophyll content under non-stress conditions (Figs 1a, 2a, 3a, c & e). Under heat stress, there was an overall decrease in TQ (Fig. 1b) and the content of chlorophyll a, b, and total chlorophyll content (Fig. 3b, d & f), as well as increases in EL through the duration of heat stress in untreated and CM-treated plants (Fig. 2b).
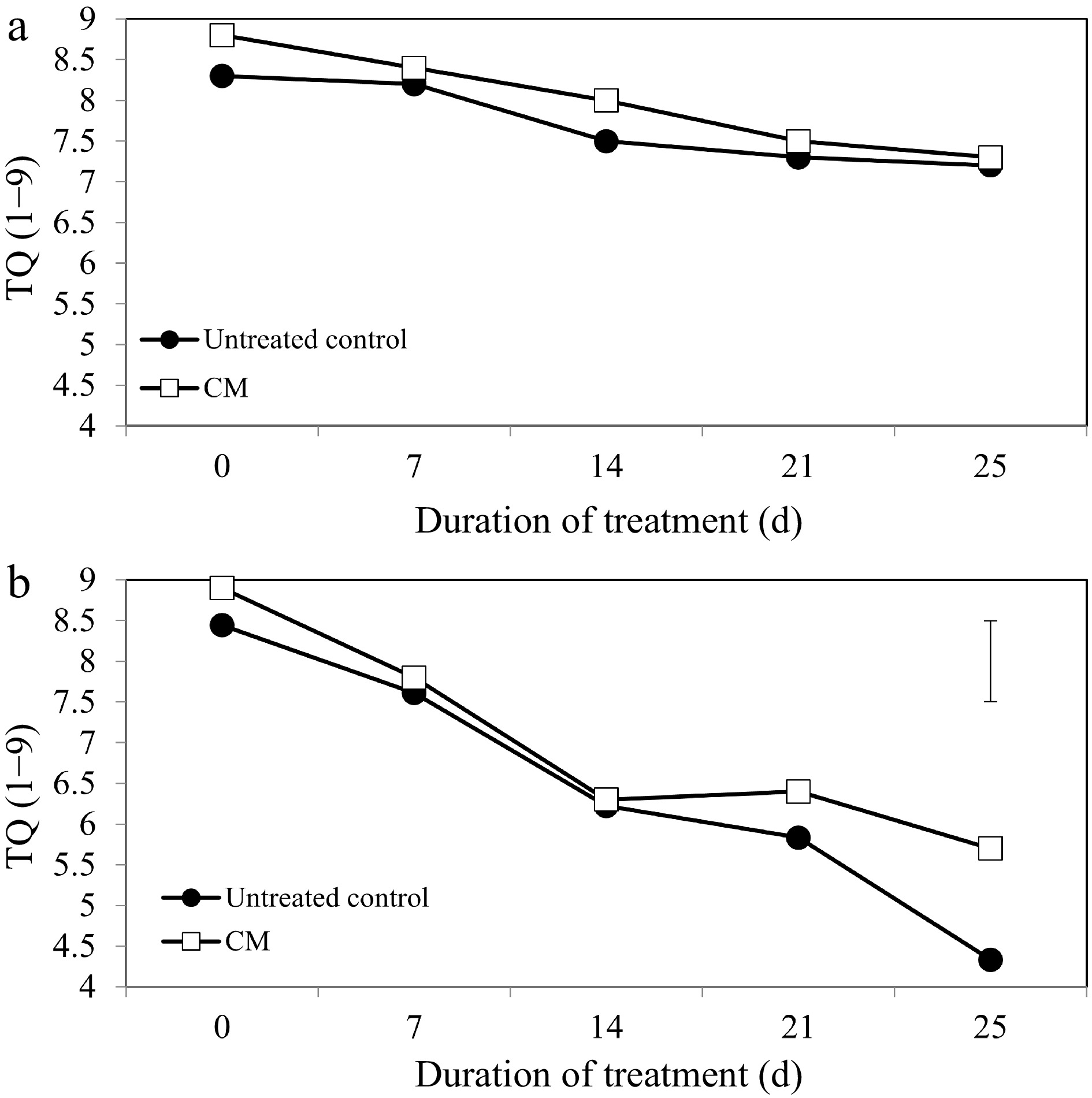
Figure 1.
Turf quality evaluations on a scale of 1 to 9 for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
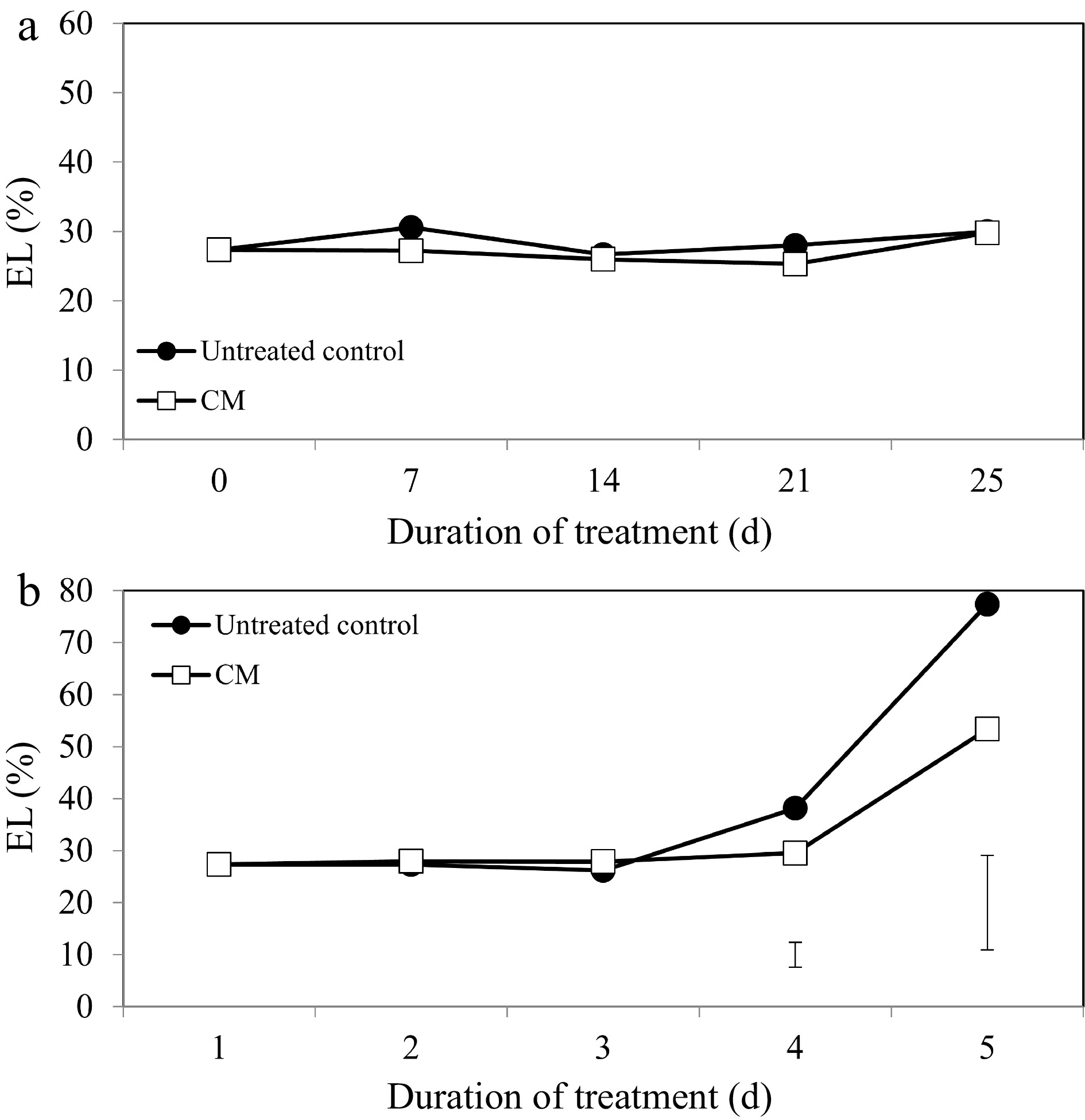
Figure 2.
Leaf electrolyte leakage for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
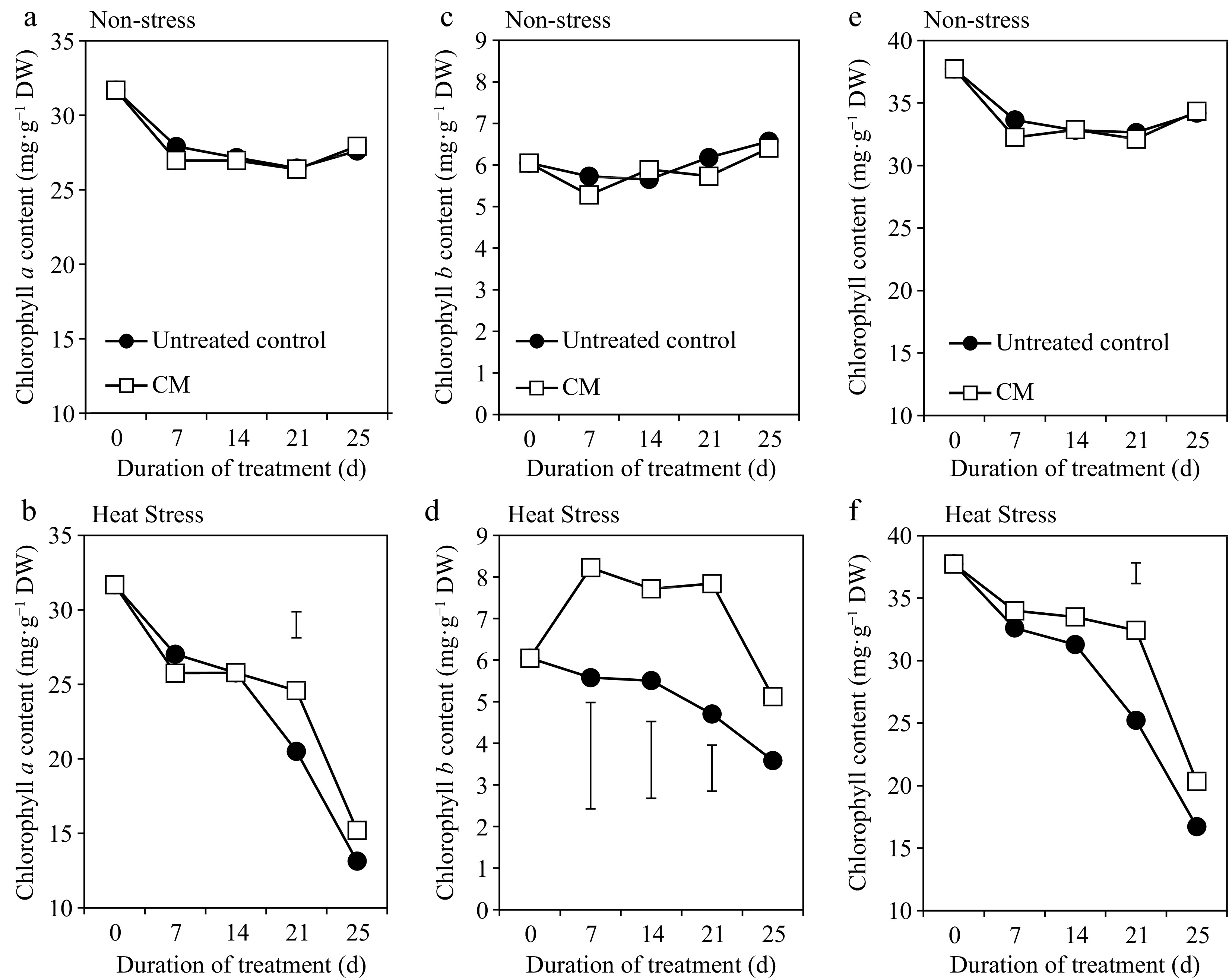
Figure 3.
Chlorophyll a [(a – non-stress), (b – heat stress)], chlorophyll b [(c – non-stress), (d – heat stress)], and total chlorophyll [(e – non-stress), (f – heat stress)] for creeping bentgrass plants treated with CM or untreated control plants under non-stress optimal temperature or heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
Heat-induced leaf senescence was suppressed by CM application as reflected by changes in TQ, EL, and chlorophyll content. Under prolonged periods (25 d) of heat stress, application of CM significantly increased TQ (Fig. 1b). Electrolyte leakage was significantly lower in CM-treated plants at 21 and 25 d of heat stress [by 22.59 and 45.06%, respectively (Fig. 2b)]. Application of CM significantly elevated chlorophyll a content at 21 d of heat stress (by 19.82%) compared with the untreated control plants (Fig. 3b). Chlorflurenol-methyl application significantly increased chlorophyll b content by 47.35, 28.61, and 66.63% at 7, 14, and 21 d, respectively (Fig. 3d). Total chlorophyll content was significantly elevated by CM treatment at 21 d of heat stress (by 28.56%) (Fig. 3f). The positive effects of CM on regulating chlorophyll content occurred earlier during the stress period and were more pronounced for chlorophyll b relative to chlorophyll a in creeping bentgrass exposed to heat stress.
For the majority of chlorophyll-synthesizing and -degrading enzymes and treatment dates, the application of CM did not significantly alter their activities under non-stress conditions (Figs 4a, 5a, 6a, 7a). The activity of the chlorophyll synthesis enzyme, PBGD, declined through the duration of heat stress; however, the decrease was not as drastic in CM-treated plants in comparison to untreated controls (Fig. 4b). Plants treated with CM had significantly higher PBGD activity at 21 and 25 d of heat stress (by 33.81 and 38.56%, respectively).
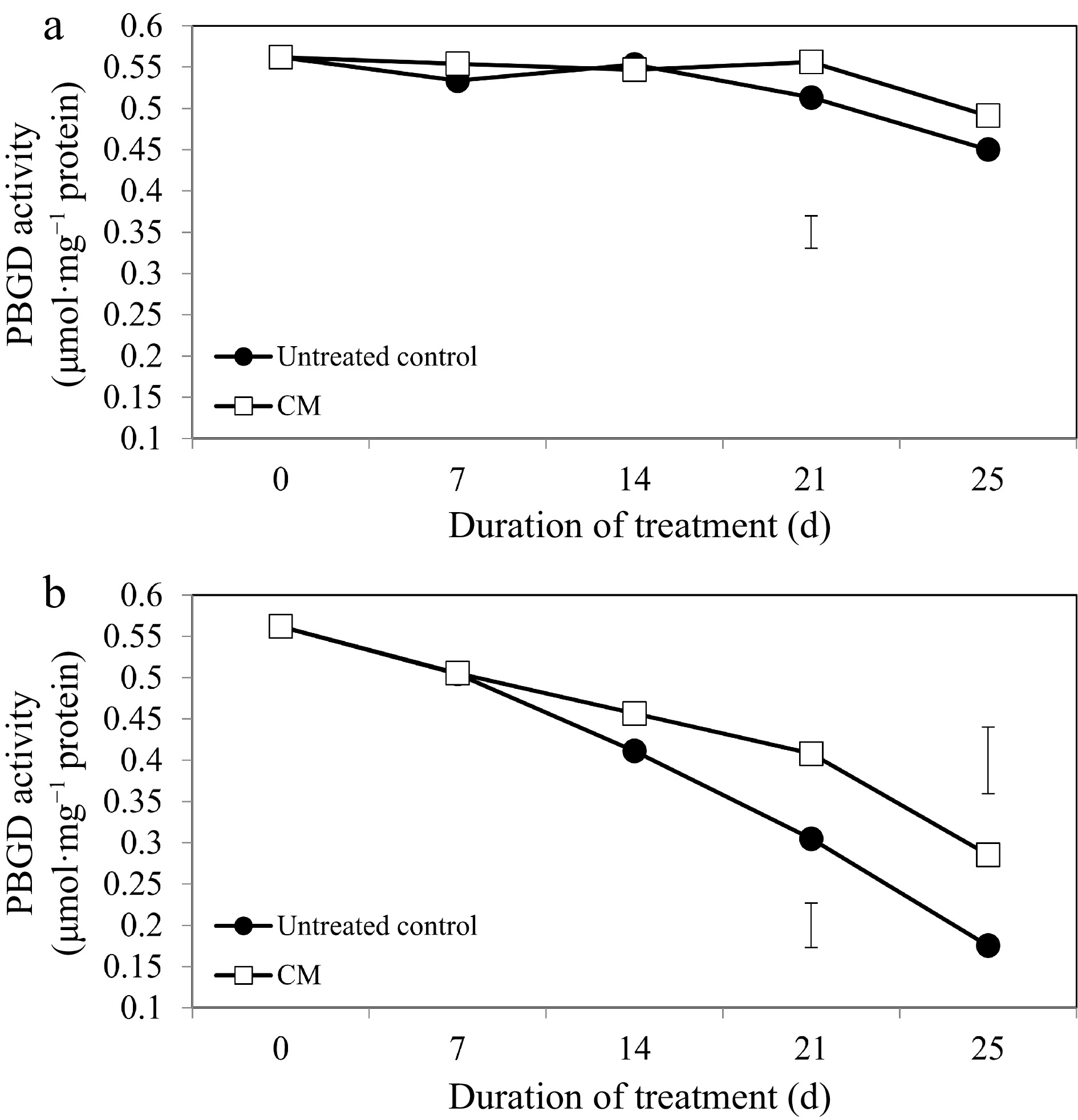
Figure 4.
Porphobilinogen deaminase enzyme activity for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
While activities of the chlorophyll degradation enzymes increased overall in response to heat stress, these alterations occurred to a smaller degree in CM-treated plants when compared with untreated controls (Figs 5b, 6b, 7b). At 14, 21, and 25 d of heat stress, CM treatment caused a significant reduction in CHLASE (by 21.07, 15.33, and 40.42%, respectively) and CHL-PRX (by 5.66, 9, and 9.35%, respectively). The activity of PPH significantly declined in response to CM treatment at 7, 21, and 25 d of heat stress (39.47, 19.04, and 49.14%, respectively) when compared to untreated controls. A pathway map showing the response of chlorophyll synthesis and degradation enzymes to CM treatment in creeping bentgrass under heat stress is provided in Fig. 8.
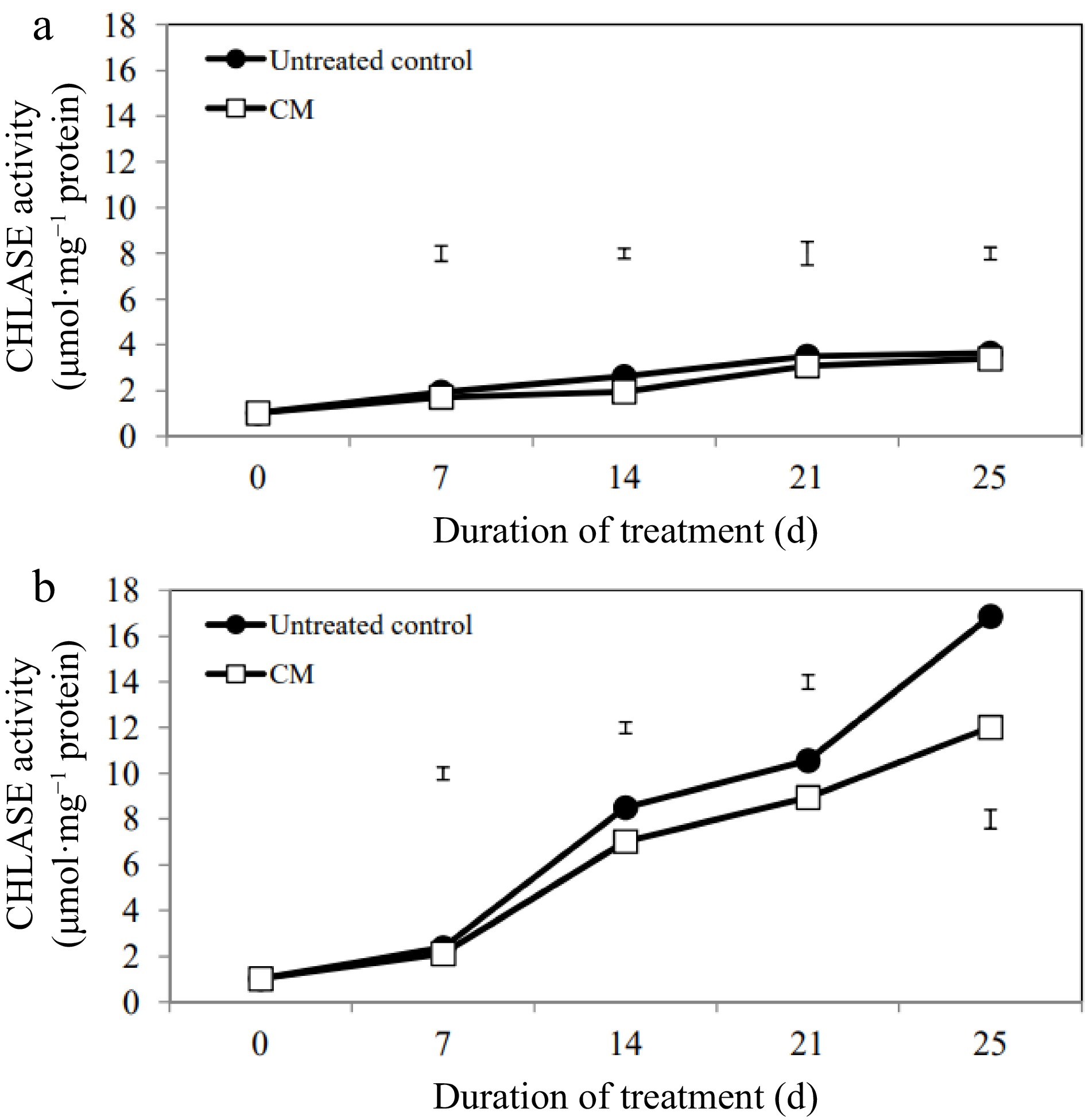
Figure 5.
Chlorophyllase enzyme activity for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
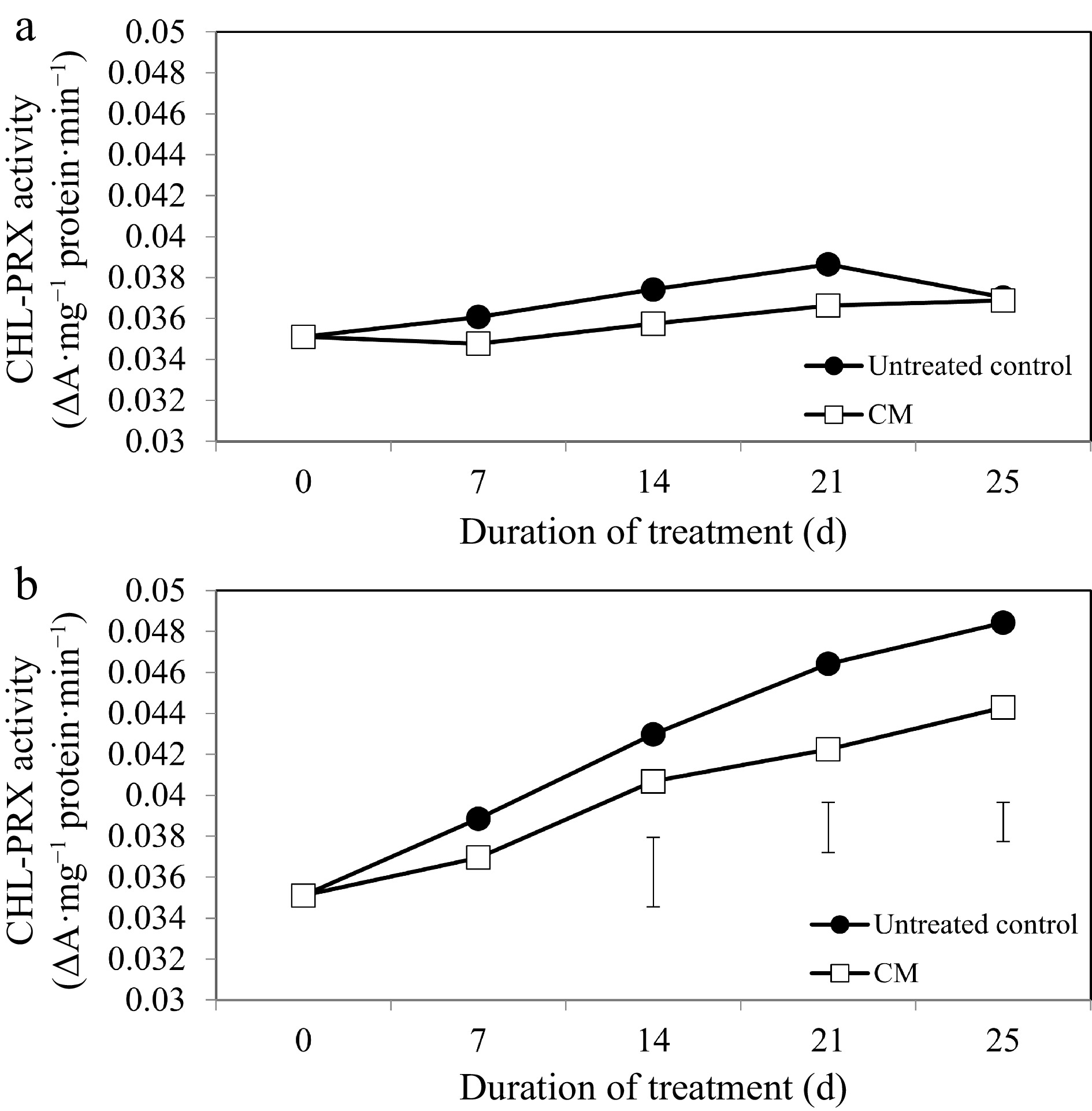
Figure 6.
Chlorophyll-degrading peroxidase enzyme activity for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
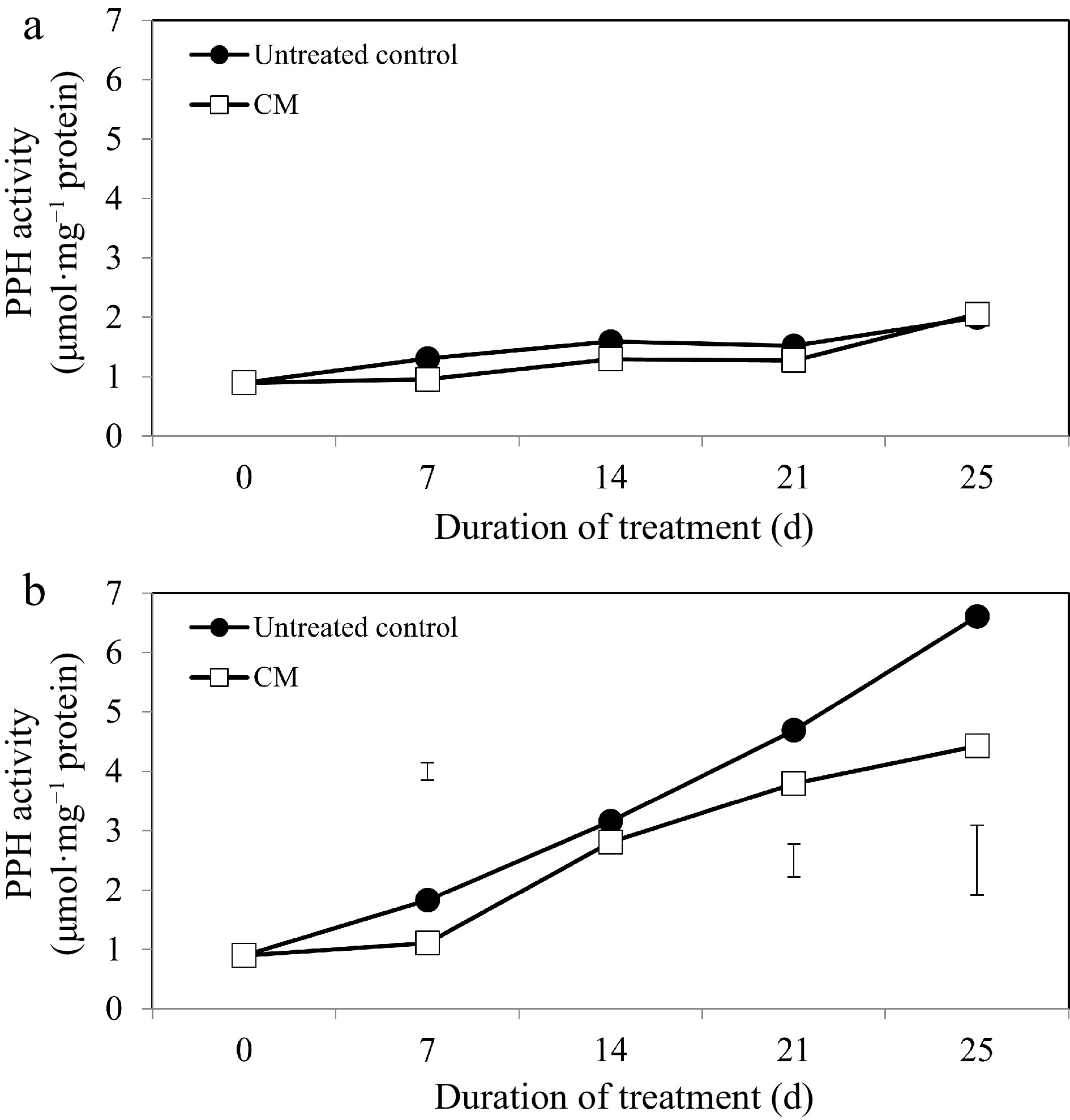
Figure 7.
Pheophytinase enzyme activity for creeping bentgrass plants treated with CM or untreated control plants under (a) non-stress optimal temperature or (b) heat stress conditions. Each vertical bar corresponds to a given LSD value (p ≤ 0.05) derived from Fisher’s protected least significant difference (LSD) test for comparison between treatments on each day of treatment.
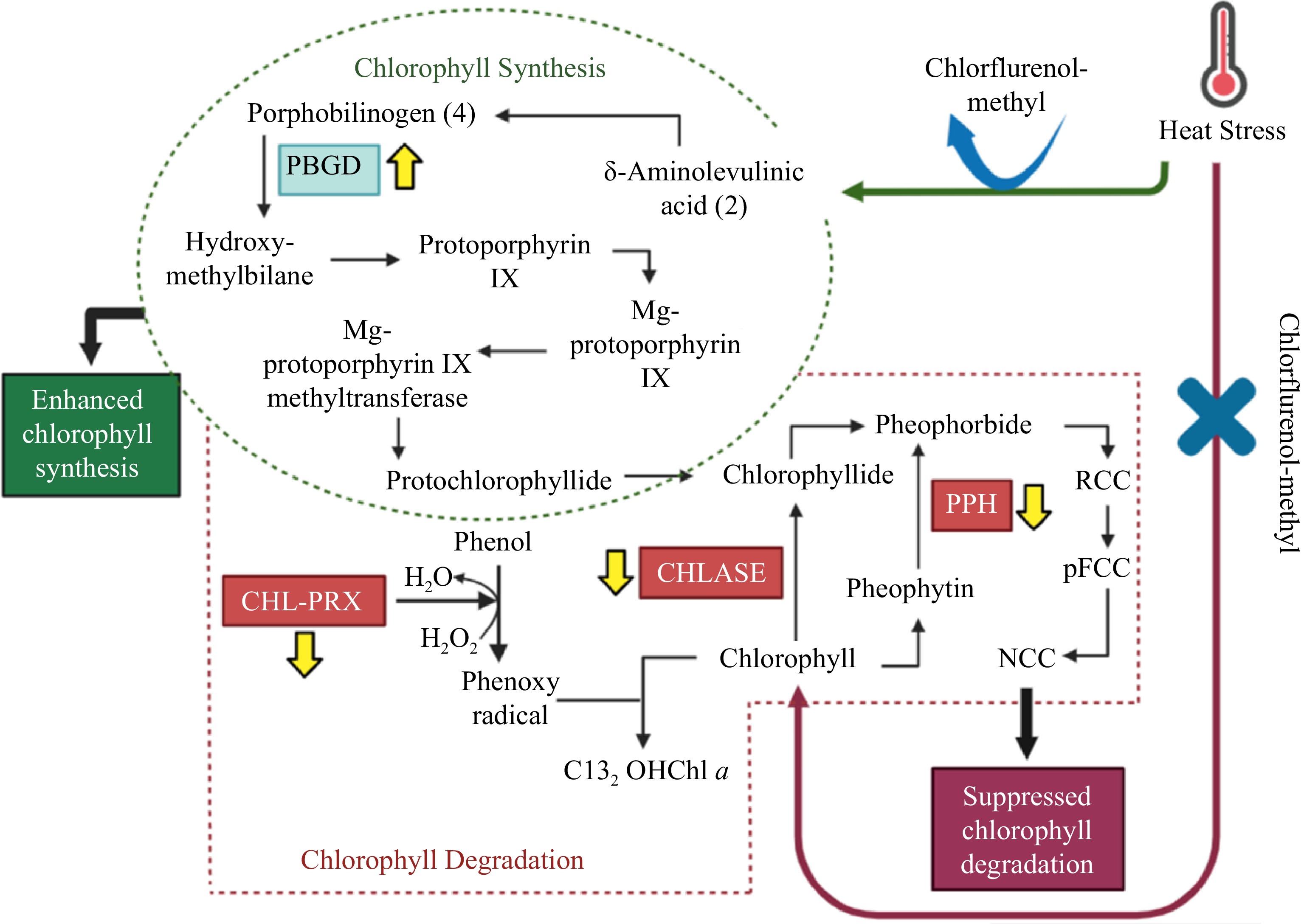
Figure 8.
Pathway map for chlorophyll synthesis and degradation in creeping bentgrass treated with CM under heat stress, showing chlorophyll enzymes enhanced or suppressed by CM treatment. Abbreviations: CHL-PRX: chlorophyll-degrading peroxidase; CHLASE: chlorophyllase; PBGD: porphobilinogen deaminase; PPH: pheophytinase.
-
Morphactins have mainly been reported to have plant growth-regulating properties on leaf chlorophyll content and shoot growth characteristics, as previously discussed in the Introduction. This study is the first to demonstrate the positive effects of morphactin on the improvement of heat tolerance in a cool-season grass species, as manifested by an increase in turf quality, chlorophyll content, and cellular membrane stability (expressed as the reduction of EL) in response to foliar application of CM in creeping bentgrass subjected to long-term stress. The CM-enhanced heat tolerance was associated with a suppression of heat-induced leaf senescence that may be attributed to the roles of CM in regulating chlorophyll metabolism.
Loss of leaf chlorophyll under stress can occur as a result of inhibited synthesis or stimulated degradation of chlorophyll. The activities of chlorophyll-synthesizing and -degrading enzymes are sensitive and responsive to abiotic stresses, including heat stress. For example, activity of the chlorophyll-synthesizing enzyme, PBGD, decreased within 2 d of heat stress in seedlings of cucumber[13] as well as in response to water and salt stress in rice (Oryza sativa) seedlings[29,30]. The activity of CHLASE and PPH was elevated by water submergence stress in perennial ryegrass (Lolium perenne)[31]. The loss of chlorophyll has been associated with an elevation in the activities of CHL-PRX and PPH, respectively, during natural leaf senescence in tobacco (Nicotiana tabacum) and broccoli (Brassica oleracea)[32,33]. Under heat stress, CHLASE activity was elevated concomitant with a reduction in leaf chlorophyll content in barley (Hordeum vulgare) and creeping bentgrass[4,34]. The present study found that the activity of the chlorophyll synthesis enzyme, PBGD, declined overall while those of the degradation enzymes, CHLASE, CHL-PRX, and PPH, increased in response to heat stress; however, foliar application of the morphactin, CM, promoted PBGD activity and reduced the activities of CHLASE, CHL-PRX, and PPH. Morphactin-mediated attenuation of the senescence process in leaf discs of radish under low light conditions was attributed to a suppression of chlorophyll breakdown[35]. The results in this study suggest that the ability of morphactin-treated plants to maintain higher leaf chlorophyll content could be attributed to the maintenance or enhancement of chlorophyll synthesis and inhibition of chlorophyll degradation under heat stress.
The mechanisms regulating how morphactin may directly or indirectly affect chlorophyll metabolism are largely unknown. During leaf senescence, the degradation of all pigments, including chlorophyll a and chlorophyll b, occurs, but they may degrade at different rates, as the ratio of chlorophyll a to chlorophyll b is altered under various senescence conditions in different plant species[36−38]. Morphactin was found to be more effective in suppressing the loss of chlorophyll a relative to chlorophyll b during low light-induced senescence in leaf discs of radish[35]. It is known that during chlorophyll breakdown, which is accelerated by stress, chlorophyll b is degraded into chlorophyll a, consequently causing the content of chlorophyll a to increase[39,40]. In the current study, morphactin treatment of heat-stressed creeping bentgrass caused plants to maintain a greater level of chlorophyll b in comparison to chlorophyll a, and the results suggest that untreated control plants may have converted chlorophyll b to chlorophyll a more rapidly, which is indicative of an accelerated rate of leaf senescence under heat stress. After chlorophyll b is degraded into chlorophyll a by the enzyme, 7-hydroxymethyl chlorophyll a reductase, CHLASE subsequently opens the porphyrin macrocycle ring of chlorophyll a and is the rate-limiting enzyme responsible for the degradation of chlorophyll into chlorophyllide[41]. The reduction of the activities of CHLASE by morphactin in this study may at least partially explain the roles of this growth regulator in controlling the loss of chlorophyll under heat-induced leaf senescence in creeping bentgrass, due to its apparent delay in the conversion of chlorophyll b to chlorophyll a, as well as its control of the successive chlorophyll a catabolism mediated by CHLASE.
In summary, foliar application of morphactin successfully alleviated heat-induced leaf senescence and enhanced the overall health of creeping bentgrass exposed to prolonged heat stress, as suggested by an improvement in TQ, cellular membrane stability, and chlorophyll content. More specifically, morphactin treatment promoted the maintenance of a greater proportion of chlorophyll b to chlorophyll a under heat stress and delayed the subsequent activity of CHLASE, which ultimately suggests that morphactins may suppress chlorophyll breakdown during heat stress in cool-season plants. This study is the first to report the ameliorative effects of a morphactin regarding the conferral of heat tolerance and repression of leaf senescence in a cool-season grass species under heat stress. Further research may investigate the biochemical and molecular mechanisms determining how morphactin may regulate the chlorophyll-degrading process in order to develop stress-tolerant cool-season grass species.
-
To obtain plant materials, 20 sod plugs of creeping bentgrass (cv. Penncross) measuring 10.16 cm in diameter were collected from a field within the Rutgers Turfgrass Science Research and Extension Farm (North Brunswick, NJ, USA). Plants were individually transplanted into pots (14 cm height, 15.2 cm width) consisting of fine (310-grit), sterile silica sand, produced according to USGA requirements (Mitchell Products, Millville, NJ, USA). Plants underwent an establishment period of 21 d in a greenhouse maintained at an average daily temperature of 24/20 °C (day/night) and photoperiod of 14-h at 750 µmol·m−2·s−1 photosynthetically active radiation (PAR), provided by natural light in addition to high-pressure sodium gas-discharge lamps. Plants were irrigated daily to canopy saturation, trimmed weekly to a canopy height of 3.0 cm, and fertilized biweekly with three-quarter-strength Hoagland’s nutrient solution[42], after which they were moved to environmentally controlled growth chambers (Environmental Growth Chambers, Chagrin Falls, OH, USA) for 7 d to acclimate to optimal conditions of 22/17 °C (day/night) at a relative humidity of 60% and a 14-h photoperiod of 750 µmol·m−2·s−1 PAR.
Experimental design and morphactin treatment
-
Plant canopies were treated with 40 mL of 10 µM chlorflurenol-methyl (CM) or water only as a fine-mist foliar spray, once canopies completely covered the sand surface. Treatments were applied beginning at the initiation of the trial and repeated every 7 d. The concentration of 10 µM CM was selected as optimal for plant growth regulation under heat stress based on foundational experimentation. Heat stress was imposed by subjecting 10 pots of plants to elevated temperatures of 35/30 °C (day/night) for a 25 d treatment period, while the remaining 10 pots of plants were maintained at 22/17 °C (day/night). The experimental design was split-plot, with temperature conditions serving as the main plots and foliar chemical treatment as the sub-plots. Each chemical treatment was replicated in five pots, and plants were exposed to either non-stress control or heat stress temperatures in four growth chambers set to each temperature regiment. Replicate pots were randomly allocated to four growth chambers, with rearrangement occurring every 3 d to avoid the potential confounding effects of variations in chamber environmental conditions on treatment effects.
Physiological parameters for assessing leaf senescence
-
Turf quality, leaf EL, and leaf chlorophyll content are three physiological parameters often examined to quantify leaf senescence, and they were measured every 7 d through the duration of the study.
Visual evaluations of TQ were made by assigning values ranging from 1 to 9 to turf based on canopy color, uniformity, texture, and density, with a 1 describing turf that is dead and brown and a 9 signifying turf that has an even canopy of a homogenous, deep green color[43].
Leaf chlorophyll content was determined through the procedure of Hiscox & Israelstam[44], with slight alterations. Approximately 0.1 g of leaf tissue was harvested from each sample plant, placed into tubes containing 10 mL dimethyl sulfoxide, and incubated at room temperature in total darkness for 3 d. The absorbance values of chlorophyll extracts were individually read via spectrophotometer (Evolution 201 model; Thermo Fisher Scientific, Waltham, MA, USA) at wavelengths of 663 and 645 nm. Extract from each sample was subsequently discarded, and leaf tissue was stored at 80 °C in a gravity convection oven (model 52412-85; Cole-Parmer, Vernon Hills, IL, USA) for 3 d so that dry weights could be taken on a mass balance (model ME204E; Mettler-Toledo Rainin, Oakland, CA, USA). Total chlorophyll content was calculated by substituting the obtained absorbance values and dry masses into the equations given in Arnon[45].
Electrolyte leakage of leaves was determined as an assessment of membrane integrity. Approximately, 0.2 g of leaves were taken from each sample, and tissue was sectioned into equal parts, submerged in 35 mL deionized water, and placed on a horizontal shaker at room temperature for a duration of 8 h. Initial conductance (Ci) was measured using a conductance meter (model 32; YSI, Yellow Springs, OH, USA). Samples were subsequently killed in an autoclave set to 121 °C for 30 min, shaken for 8 h, and maximal conductance (Cmax) was read. To calculate EL, Ci, and Cmax were substituted into the calculations presented in Blum & Ebercon[46], whereby Ci was divided by Cmax, and the value was multiplied by 100 to express EL in the form of a percentage.
Measurement of the activities of chlorophyll synthesis and degradation enzymes
-
Activities of the chlorophyll synthesis enzyme, PBGD, and chlorophyll degradation enzymes, CHLASE, PPH, and CHL-PRX, were quantified for each sample at 7 d intervals. To quantify the activities of chlorophyll-synthesizing and –degrading enzymes, 400 mg leaf tissue was excised from sample plants at 7 d intervals, flash-frozen in liquid nitrogen, and immediately stored at a temperature of −80 °C in an upright low-temperature freezer (model UXF60086A; Thermo Fisher Scientific) in preparation for subsequent measurements.
Prior to the initiation of heat stress, healthy leaf tissue was excised from plants to be used in the extraction and purification of chlorophyll necessary for subsequent enzyme measurements. To purify chlorophyll from the unrefined extract, the procedure outlined in Iriyama et al.[47] was followed. Approximately 10 g of leaves were ground in liquid nitrogen and 50 mL of iced acetone. The slurry rested for 2 h at 4 °C in dark conditions to initiate extraction, and following the incubation period, the solution was centrifuged for 5 min at 1000 gn. The supernatant was combined with 1,4-dioxane (1:7 v/v), and deionized water was added dropwise to the mixture until it became cloudy. The solution was maintained in darkness at 4 °C for 1 h, after which it was centrifuged for 5 min at 10,000 gn. The pellet derived was resuspended in 1,4-dioxane (1:7, v/v), and deionized water was added drop-wise to the solution until it became cloudy. The mixture was incubated for an additional 1 h in darkness at 4 °C, centrifuged at 10,000 gn for 5 min., and the supernatant was decanted. The resultant pellet was dissolved to a 500 µg·ml−1 concentration of chlorophyll using 50 ml of acetone.
Crude chlorophyll enzyme extract was derived from each sample and concocted into a buffer to be used in the subsequent enzyme assays by excising 0.4 g of leaf tissue from each plant, grinding it in liquid nitrogen, and combining it with deionized water, 0.1 M phenylmethanesulfonyl fluoride, 1.0% Triton X-100, and 0.5 M KH2PO4 buffer (pH 7.0). After grinding the sample an additional time with 3.0% polyvinylpyrrolidone and centrifuging at 9,000 gn for 20 min at 4 °C, the solution was maintained at −80 °C in a low-temperature freezer for use in the enzyme bioassays. The Bradford Assay was utilized to generate a standard curve[48].
Using the methods of Jones & Jordan[12] with some alterations, PBGD activity was measured. Crude enzyme extract (0.1 mL) was added to 0.5 mL 0.1 M Tris-HCl buffer (pH 7.5) containing 0.1% bovine serum albumin (BSA), 15 mM MgCl2, 2.5 mM ethylenediaminetetraacetic acid, and 2 mM porphobilinogen to make the reaction mixture. After heating the reaction at 37 °C for 1 h, 0.1% p-benzoquinone and 5 M HCl were added to stop the reactions. The absorbance of each test solution was read at a wavelength of 405 nm on a spectrophotometer.
Using the procedure outlined in Fang et al.[40], CHLASE activity was measured. To initiate the enzymatic reaction, a mixture of 200 µL pure chlorophyll, 100 µL crude enzyme extract, 300 µL acetone, 20 µL 0.1 M ascorbic acid, and 700 µL Tris-HCl buffer (pH 7) was incubated at 35 °C for 1 h. The CHLASE reaction was stopped by adding 2 mL hexane and 1 mL acetone, and each solution was vortexed and then centrifuged for 15 s at 10,000 gn so that chlorophyllide and chlorophyll would be separated into the lower aqueous and upper hexane phases, respectively. Using a spectrophotometer, the absorbance of chlorophyllide was determined at 665 nm.
CHL-PRX activity was quantified using the procedure given by Aiamla-or et al.[49] with some modifications. A solution containing 50 µL crude enzyme extract, 0.1 mL pure chlorophyll, 0.1 mL 25 mM p-coumaric acid, 1 mL 0.1 M potassium phosphate buffer (pH 7), and 0.1 mL 1% Triton X100 was mixed. To allow the rate reaction to commence, 0.1 mL 1% hydrogen peroxide was added to the reaction, and the rate of chlorophyll degradation was measured via spectrophotometer every 20 s for a total of 10 min at 668 nm. One CHL-PRX activity unit was defined as a change in absorbance by 0.1 mg−1·protein·min−1.
The procedure of Kaewsuksaeng et al.[50] was used, with some modifications, to quantify PPH activity. To obtain pheophytin, the substrate of PPH, 1 mL pure chlorophyll was mixed with 60 µL 0.1 M HCl and left to react for 5 min. To initiate the reaction, 0.1 mL pheophytin, 0.6 mL 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.75), and 0.1 mL 1% Triton X-100 were added to 0.2 mL crude enzyme extract, and the reaction commenced under dark conditions at 30 °C for a duration of 30 min. To halt the enzymatic reaction, 0.1 mL 0.5 M Tris-HCl buffer (pH 9), 1 mL acetone, 2 mL hexane, and 1 mL deionized water were added to each tube. In order to separate the pheophytin and pheophorbide into upper and lower phases, respectively, tubes were vortexed. The absorbance of pheophorbide was determined using a spectrophotometer set to 665 nm.
To determine the soluble protein content of each sample, 20% trichloroacetic acid was added to crude enzyme extract and the mixture was maintained at 4 °C for 1 h to allow the protein to precipitate out of the solution. To create stronger separation, solutions were centrifuged at 11,500 gn for 15 min. The supernatant was decanted, and the resulting pellet was exposed to the air to dry and subsequently resuspended in 1 M sodium hydroxide. Soluble protein content was calculated using the Bradford Assay, where a standard curve was created by serially diluting defined concentrations of the standard, BSA, with different volumes of Coomassie Brilliant Blue G-250 Dye (Bio-Rad Laboratories, Hercules, CA, USA)[48]. The absorbance of each soluble protein sample was read on a spectrophotometer set to 595 nm, and the values derived were substituted into the standard curve equation to calculate soluble protein content.
Statistical analysis
-
Physiological and enzymatic data were evaluated through two-way analysis of variance (ANOVA) using the general linear model (PROC GLM) procedure in SAS (version 9.2; SAS Institute, Cary, NC, USA) to define significant differences between the chemical treatment and untreated chemical control for each temperature treatment. Means between the chemical treatment and untreated control for each temperature treatment were separated by Fisher’s protected least significant difference (LSD) test at a level of p < 0.05. Only the significant differences between chemical treatments under non-stress control or heat stress conditions defined by the LSD test are presented in figures and results based on the focus of objectives for this experiment. The LSD bars in each figure indicate significant differences between chemical treatments at a given day of heat stress.
-
The authors would like to thank the Rutgers Center for Turfgrass Science and the New Jersey Agricultural Experiment Station for funding this research.
-
The authors declare that there is no conflict of interest. Bingru Huang is the Editorial Board member of Journal Grass Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of these Editorial Board members and their research groups.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Rossi S, Huang B. 2023. Regulatory roles of morphactin on suppressing chlorophyll degradation under heat stress in creeping bentgrass. Grass Research 3:11 doi: 10.48130/GR-2023-0011
Regulatory roles of morphactin on suppressing chlorophyll degradation under heat stress in creeping bentgrass
- Received: 06 March 2023
- Accepted: 17 May 2023
- Published online: 03 July 2023
Abstract: Heat stress induces and accelerates leaf senescence in cool-season plant species but may be ameliorated by chemical priming. The objectives of this study included determining whether exogenous application of chlorflurenol-methyl (CM), a morphactin with senescence-inhibiting properties, could suppress leaf senescence in creeping bentgrass (Agrostis stolonifera) exposed to heat stress and to examine its possible regulatory effects on chlorophyll metabolism. Mature creeping bentgrass plants were subjected to heat stress (35/30 °C, day/night) or non-stress control (22/18 °C, day/night) temperatures for 25 d in environment-controlled growth chambers and were foliar-treated with 10 µM CM or water every 7 d. Under heat stress, CM-treated plants had significantly enhanced turf quality at 25 d and decreased electrolyte leakage from 21 through 25 d. Under heat stress, CM application significantly elevated the content of chlorophyll a at 21 d, chlorophyll b at 7, 14, and 21 d, and total chlorophyll at 21 d. Activity of the chlorophyll synthesis enzyme, porphobilinogen deaminase, was significantly higher in CM-treated plants from 21 through 25 d of heat stress, while activities of the chlorophyll degradation enzymes, chlorophyllase and chlorophyll-degrading peroxidase, were significantly lower from 14 through 25 d, and activity of pheophytinase was significantly lower in CM-treated plants at 7, 21, and 25 d. The results of this study suggest that alleviation of heat-induced leaf senescence by foliar-applied CM could be mainly associated with its effects on suppressing chlorophyll degradation, particularly by maintaining stability of chlorophyll b during prolonged periods of heat stress in this cool-season grass species.
-
Key words:
- Creeping bentgrass /
- Heat stress /
- Morphactin /
- Leaf senescence /
- Plant growth regulator /
- Turfgrass












