-
As a tropical crop, coffee contributes significantly to the livelihoods of smallholder farmers, especially in rural communities. The coffee species belongs to the genus Coffea in the family Rubiaceae and is grown mainly in the tropics[1]. The genus Coffea comprises 124 species[2], but commercial production depends mainly on two species, C. arabica L. and C. canephora Pierre and Froehner each accounting for approximately 70% and 30% of the global coffee market, respectively. According to the World Coffee Organization, by geographical distribution and quality group, the top five coffee-producing countries are Brazil, Vietnam, Ethiopia, Colombia and Indonesia[3,4]. Ethiopia is the 5th largest exporter of C. arabica in the world after Brazil, Vietnam, Colombia and Indonesia and the largest coffee producer in Africa. The C. arabica is the only allopolyploid species (2n = 4x = 44) grown commercially. Studies of its origin have revealed that the primary center of origin of C. arabica is the highlands of southwestern Ethiopia and the Boma plateau of South Sudan[5]. However, other species including C. canephora grown commercially are diploid (2n = 2x = 22) but are self-sterile except for C. heterocalyx and C. moloundou, which are diploid and self-compatible[6,7].
In terms of international trade, coffee has been a valuable commercial commodity since the 1800s and is of great economic importance to developing countries, including least developed countries (LDCs)[8]. Furthermore, it is of considerable social and economic importance for the consumer countries, where the coffee industry contributes multi-billion dollar to the economy. According to Fairtrade Foundation[9], more than 125 million smallholder farmers in coffee growing regions around the world earn direct or indirect income along the value chain of growing, processing, transporting, and trading of coffee[8, 10].
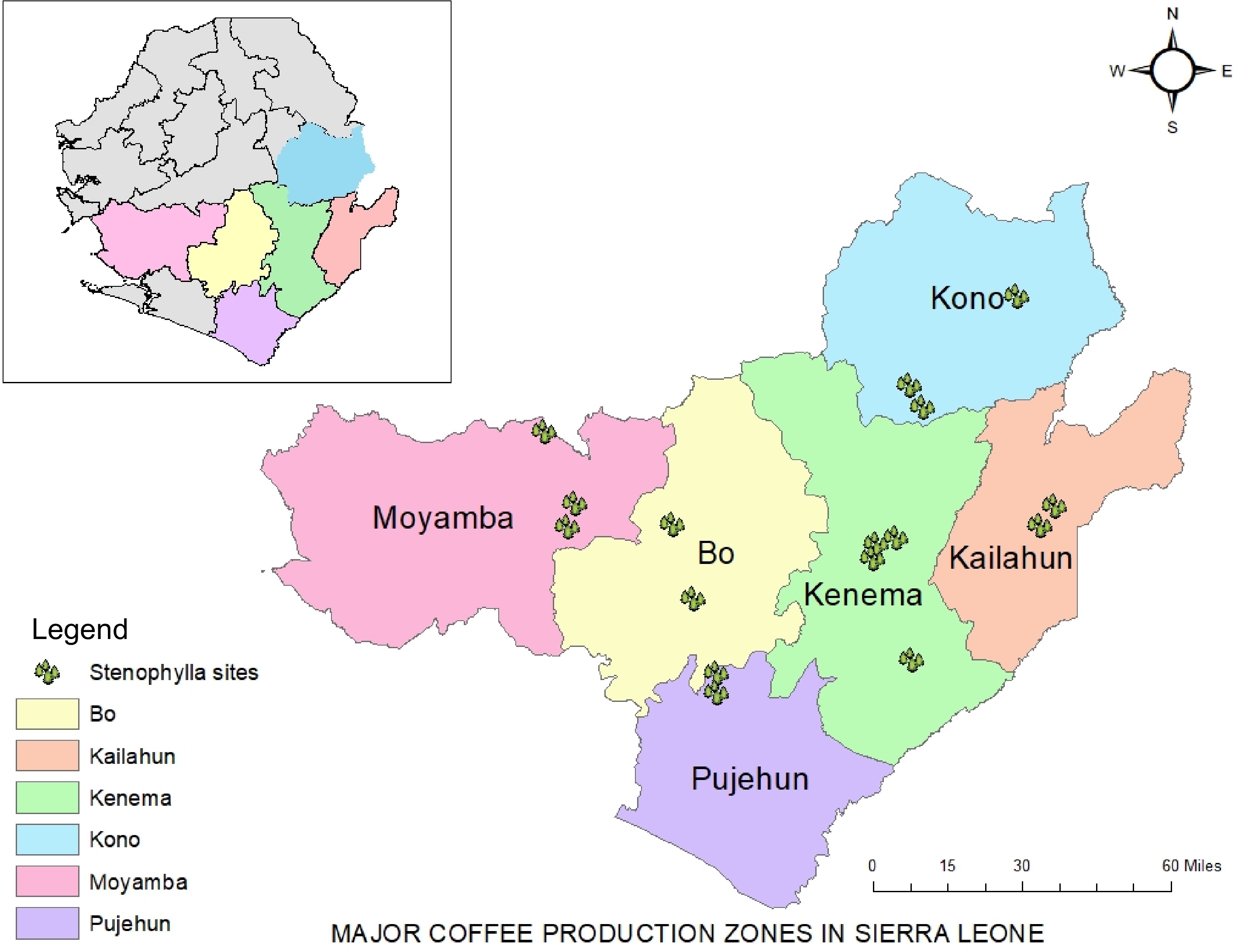
Sierra Leone is a globally recognized biodiversity hotspot (of genetic diversity concern) in the forests of Upper Guinea with a rich native flora and fauna including important endemic and rare species at local and international levels[11]. However, dramatic changes in forest cover have taken place over the past century due to urbanization, population growth and expansion of arable land (agricultural/plantation). In Sierra Leone, the continued depletion of natural resources, especially flora and fauna, has led the government to develop policies aimed at protecting areas such as the Kambui and Kasewe hills, which serve as hot spots for substantial populations of the wild C. stenophylla. The C. stenophylla is critically endangered due to the exponential increase in deforestation, mainly logging and charcoal production.
To some extent, the coffee research programs in Sierra Leone have not yet undertaken full-time research to assess the genetic diversity of coffee for further development of new varieties. Despite the foregone, Sierra Leone is one of the places in the world that hosts a diversity of coffee wild relative species and thus potentially allows them to withstand varying environmental factors.
-
The cultivation and production of coffee in Sierra Leone on a commercial scale date back to the 1950s, when the first group of coffee varieties were imported from Uganda, Côte D'Ivoire, and Nigeria, aimed at improvingthe viability of the economy of the country's agriculture through the formation of the coffee industry. Upon establishing a formidable coffee sector between 1960 and 1980, production volume had already reached 20,000 tons per year[12]. However, after the 1991 interregnum of the civil conflict, most of the coffee plantations were abandoned and growers had to flee production areas which resulted to a sharp decline in production. Although the conflict ended in the early 2000s, production has never reached the level of previous decades.
Prior to the civil conflict, the world market price of coffee was very attractive which led to massive coffee plantation establishment in Sierra Leone, but the plantations are now too old, and can no longer reach their full production potential. However, the government of Sierra Leone and development partners have developed initiatives to help farmers create new farms and increase production with the available coffee varieties. For instance, in 2018, a European Union project was launched to restore abandoned coffee plantations in Sierra Leone. Implementation of this initiative involved intensive pruning of aging coffee trees as well as providing smallholder farmers with more agricultural inputs such as fertilizer.
The eastern region of Sierra Leone which consists of Kenema, Kono and Kailahun serves as the hub for coffee cultivation in the country (Fig. 1). Other areas of cultivation include Southern Province in the districts of Pujehun, Moyamba and Bo, while Northern districts include Tonkolili and Koinadugu.
Much of the coffee production in the country is carried out by youths who are in their prime and face challenges such as maintenance of plantations, especially clearing, pruning, and harvesting, most of which is done through local means. According to the International Coffee Organization[3], the average size of small coffee farms is four hectares, so the contribution of each small farm to the GDP of the country is negligible, unless aggregated. The climate of the main coffee-producing provinces of Sierra Leone could be described as seasonal having six-months of rainy season lasting from May to October and six months of dry season lasting from November to April. The weather is characterised by hot temperature with an average monthly temperature of 26 to 28 °C from June to October and a maximum temperature of 32 °C[13]. The vegetation in this area is mostly classified as evergreen with six months of continuous rainfall. However, due to the erratic rainfall at the end of March in the production areas, coffee cherries harvested during this period often do not achieve the optimal moisture content (10%−12%) as required for export.
Cleaning of coffee plantations in Sierra Leone is usually done twice during the growing season, i.e., before flowering and around the time of fruit ripening. On the other hand, pruning is immediately done after harvest and consists of pruning old plants and removing young shoots (leaving only four buds or stems per basal tree).
Harvesting is usually carried out December through March with the labour force consisting of mostly youths and the aged. After harvesting, the cherries are dried on a high or raised platform that had been installed in notable coffee growing communities through the help of the Robusta Coffee Development Project. Other communities usually dry their berries on a drying floor that is protected by mesh, preventing domestic animals from entering the area. In addition to the lack of a domestic cooperative function, most smallholder farmers grow coffee on small areas of land, resulting in low productivity. Labour shortage and lack of advanced tools or implements are the two major constraints to coffee production in Sierra Leone. It is worthy to note that most of the young people who see coffee farming as a lucrative business have migrated to cities and now ride motorbikes as an alternative means of survival.
-
Germplasm of different cultivated Coffea species exist in Sierra Leone, including C. canephora, C. liberica, and C. arabica. In addition to the cultivated species, two wild species – C. stenophylla (also known as highland coffee) and C. affinis, are indigenous to Sierra Leone (Fig. 2).
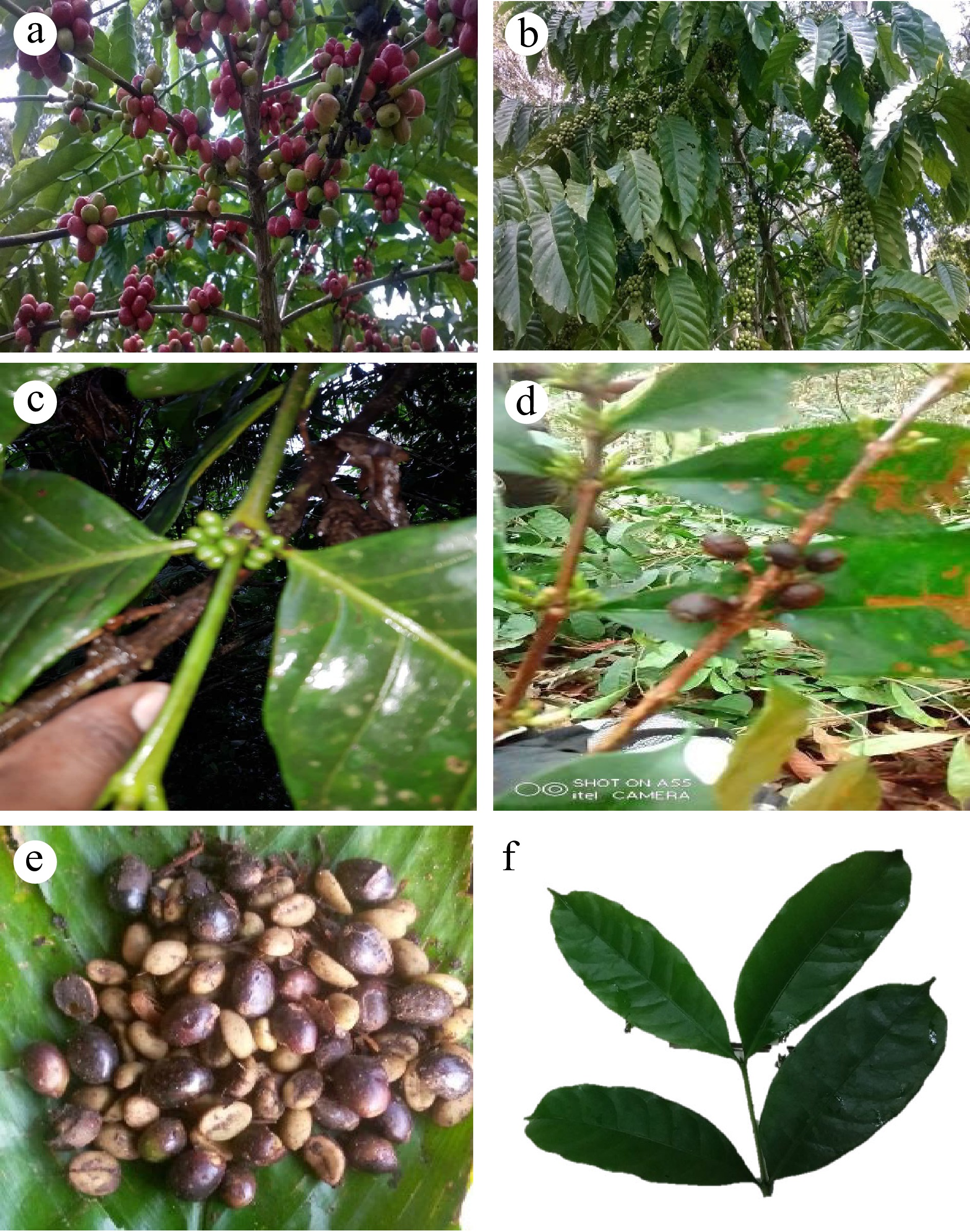
Figure 2.
(a), (b) High yielding G-98 and Unripe A-2 C. canephora. (c) Branch of C. liberica with young fruit bunches. (d) Relatively low yielding C. stenophylla branch. (e) Ripe fruits of C. stenophylla. (f) Secondary branch with leaves of C. stenophylla.
Since the 11-year interregnum full of civil strife, unrest and political instability, there has been little or no research effort on tree crops in the country. However, the Sierra Leone Agricultural Research Institute (SLARI) is geared up through its donor partners to boost the tree crop sector in terms of research and infrastructure. In the late 1950s, the Ministry of Agriculture introduced coffee clones from Uganda, Côte D'Ivoire and the Coffee Research Institute of Nigeria as part of its germplasm collection. The introduced germplasm included 'C115', 'A2', 'G98', 'C47', 'C182', 'C107', 'C461', 'C126' and 'C181'. As recent as 2019, the Boosting Agriculture and Food Security (BAFS) project introduced ten varieties of C. arabica, including 'Mgscatigua' 3', 'Catuai amarelo' (2SL), 'IAC 125 RN', 'Catuai Amarelo' (IAC 62), 'Paraiso 2', 'Paraiso' (H 419-1), 'Topazio Mg 1190', 'Mundo Novo' (IAC 379-19), 'Catuai Vermelho' (IAC 144) and 'Oeiras Mg 6851' from the Brazilian Agricultural Research Corporation (Embrapa). The morphology traits of the different varieties that are conserved at SLARI research station at Pendembu reveals considerable variability within this pool of germplasm (Fig. 2a, b & c).
The two wild species C. stenophylla and C. affinis, both found in the hills of Kambui and Kasewe, at an altitude of at least 400 m above sea level, are potential coffee species that the country can utilize in coffee breeding programs through seed or cutting propagation.
Coffee beans cannot be stored for a long time in the gene bank because of their recalcitrant nature. Although they are resistant to desiccation when they are at a moisture content of 6%–8% fresh weight, they are still unorthodox due to their sensitivity to cold and desiccation[14]. The maximum storage time achieved when fully hydrated seeds were stored at 19 °C and 100% relative humidity was 36 months for C. arabica and 15 months for C. canephora and C. stenophylla. Although ex situ gene bank in the field is the most common alternative for conserving wild seeds, this approach can still be difficult to preserve seeds of primary interest. However, this method of seed preservation provides easy access to planting materials for characterization, evaluation, and subsequent use for the intended purpose. However, the number of genotypes that a field gene bank can hold is limited with the constraints of available land and financial resources. Therefore, only limited genetic diversity can be conserved in the gene bank[5]. Ex situ conservation of coffee genetic resources in the field can be characterized by the risk of loss of valuable genetic material due to pest invasion, disease infestation and poor adaptation to the environment.
Unlike the ex situ method, in situ conservation preserves genetic resources in their natural habitat by protecting the area from human interference. This method allows the dynamic evolution of the species to be carried out in its natural habitat, including the creation of new plant varieties thought to be resistant to pests and disease, thus remains an important factor for long-term conservation of coffee genetic resources. However, the in situ conservation often faces risk when the natural habitat is subject to destruction due to natural disasters and climate changes, resulting in genetic erosion. For example, the hills of Kambui and Kasewe, known as the source of C. stenophylla and the recently discovered variant C. affinis, are under serious threat from loggers, charcoal-burners, farmers, and mining activities from the surrounding communities.
The phenotypic diversity of C. stenophylla was evaluated by Lahai et al.[15] in which a total of 203 samples from Kenema and Moyamba districts were evaluated according to the IPGRI morphological descriptors[16]. Data analysis using the Shannon-Weaver diversity index (H') revealed inter-sample differences for the 13 observed morphological traits, including stalk strength, growth habit, seed size, color of young leaves, stem habits, fruit shape, seed shape, leaflets, leafshape, angle of primary branches on the main stem. Significant variation was observed at the species, population, and individual levels[15]. The presence of C. arabica, C. excelsa, C. canephora, C. stenophylla, C. affinis and possibly other wild relatives suggest that Sierra Leone is also part of the primary gene pool of Coffea species.
Various researchers[17−19] have reported that the species C. canephora and C. liberica have higher genetic diversity than C. arabica because C. canephora and C. liberica are native to different geographical regions while cultivated C. arabica is thought to have a narrow genetic base and geographical origin. Variations have been shown to exist within and between C. stenophylla, C. congenesis, C. racemose and C. eugenioides species[19]. These reports therefore indicate that these species possess many valuable genetic variations that can be exploited for the development of new commercial varieties, using traditional breeding approaches and advanced breeding methods.
Over the years, assessing genetic identity and diversity of coffee genetic resources in Sierra Leone has been a major challenge. To address the problem of genetic integrity in coffee germplasm, leaf samples of C. stenophylla, C. canephora, C. liberica and C. arabica were collected in Sierra Leone and sent to the USDA ARS, Sustainable Perennial Crop laboratory (SPCL) for DNA fingerprinting. The single nucleotide polymorphisms (SNP) panels developed by USDA-ARS[20, 21] were used for the characterization of C. stenophylla. Since there is no molecular marker for this species, efforts are being made to develop SNP markers using genotyping by sequencing (GBS) technology. A total of 143 C. stenophylla samples were sequenced using dual digestibility restriction site-associated sequencing (ddRAD-seq), which is a cost-effective strategy to generate SNP data. The variants were mapped onto the C. canephora genome and pruned based on linkage disequilibrium (LD).
After further filtering with call rate and minor allele frequency, a total of 7,464 high-quality SNP markers were selected for C. stenophylla. The information index values of this selected SNP panel ranged from 0.184 to 0.693 with a mean of 0.446. The mean observed heterozygosity was 0.351, ranging from 0.05 to 1.00, while the mean expected heterozygosity was 0.285, ranging from 0.056 to 0.500. Clustering and division analysis of the population structure showed that there is a clear substructure in cluster of C. stenophylla, suggesting that genetically distinct populations exist at the origin of this species (Lahai et al., unpublished data).
The results therefore suggest that geographic representation is an important factor for sampling the C. stenophylla gene pool, as well as for the design of an in situ conservation strategy in Serra Leone. In addition, a subset of 192 SNPs was selected (out of 7,467 SNPs) for larger-scale genotyping of the C. stenophylla germplasm collected from different geographic regions of Serra Leone. After determining the genetic identity and diversity of these coffee plants, the cuttings of the selected trees will be propagated and planted at the SLARI field trials for evaluation. Promising clones will be selected and used as parent clones for developing new varieties of C. stenophylla and C. canephora.
-
Despite the economic importance of coffee production in Sierra Leone, to date no systematic breeding effort has been undertaken to develop coffee varieties in the country. The planting materials of Robusta, Liberica and Arabica coffees used by farmers are all based on introduced varieties. The C. stenophylla is native but it’s not currently grown commercially because the low yield and small fruit make it inferior to other species. However, there are large phenotypic differences in the genetic material of C. stenophylla[15], justifying efforts to enhance C. stenophylla genetic resources and develop improved varieties.
-
Different coffee breeding methods have been used in various coffee producing countries of the world with the ultimate goal of improving the yield, quality and diseases resistance of the resulting varieties. However, the application of these breeding methods may vary depending on the amount of genetic variation that exists in the gene pool, ecological conditions, breeding goals, and production issues[22−25]. The goal of coffee breeding in Sierra Leone is like many other coffee producing countries in Africa. Methods such as climate adaptability, crossbreeding, developing F1 hybrid and genotypes × environment (G × E) testing have been used with the aim of developing improved varieties through breeding for resistance, yield, quality, and adaptability to different agro-ecological regions[26]. Efforts by the Sierra Leone Agricultural Research Institute (SLARI) to develop coffee varieties included the use of clonal materials (cuttings) and seeds introduced from prominent coffee producing countries. As an outcrossing diploidy species, the breeding method for C. stenophylla may not be the same as those designed for C. arabica in which high yielding inbreeding genotypes with high degree of homozygosity are selected and the "pure line" then is used in production. Instead, C. stenophylla would likely adopt the breeding method for Robusta varietal development, in which specific parental combinations were crossed and their F1 generation was evaluated for the traits of interest. Then, hybrids were selected and distributed either as clones or as seeds (produced by crossing the two parental lines in a seed garden)[27]. During the development of F1 hybrids, the appropriate selection of parents with known genetic differences from each other and the identification of promising heterozygous combinations is important[28] as this has implications important for progeny performance at the end of the selection cycle.
Mating between clones of unrelated parents can produce varieties with minimal inbreeding risk[29]. In other crops, hybrid breeding usually involves the crossing of inbreeding lines developed through multiple rounds of autogenous reproduction. However, like Robusta coffee, C. stenophylla is a species that does not readily self-pollinate. Therefore, the parental clones used in hybrid development in C. stenophylla are heterozygous. However, progenies developed by mating between clones of heterozygous parents can be considered as hybrids capable of providing superior performance (hybrid vigor). The combination of yield and hybrid vigor are the two most important factors to consider when selecting a superior F1 hybrid for C. stenophylla due to the good combining ability of the parents.
Breeding objectives for C. stenophylla
-
The goal of the C. stenophylla coffee breeding program in Sierra Leone is to develop high yield, good quality, resistance to diseases and pests (mainly coffee berry diseases), high planting densities, and adaptability to all coffee growing districts in the country. To achieve these goals, breeding strategies will focus on identifying high-yielding wild populations to develop improved purebreds and crosses between more productive plants to produce hybrids.
Breeding for productivity/yield
-
The coffee breeding program in Sierra Leone is based on the identification of superior parental lines with high yield potential and desirable characteristics. Several coffee breeding centers are currently focusing on hybrid coffee varieties as the best strategy to rapidly increase crop yield. Hybrid coffee varieties have more consistent yields over location and time (less genotype × environmental interaction effect). The chances of achieving significant hybrid vigor will be increased by bringing together parents selected from genetically diverse populations. Since the traits of yield components (e.g., bean size, bean weight, and number of fruits per branch) typically have moderately high heritability, selection on these traits may achieve larger genetic gain than direct selecting on yield, which usually have low heritability and larger G × E interaction.
Breeding for disease resistance
-
Since C. stenophylla was reported to be susceptible to coffee leaf rust (CLR) in Sao Tome and Principe[30], CLR resistance needs to be included as one of the main breeding objectives. CLR, together with coffee berry wilt disease (CBD), are the two main diseases of Arabica coffee. Unlike the CBD, which is endemic to the African continent, especially in the highlands of East Africa, CLR is a widespread disease affecting most of the world's coffee-growing regions. The variability of pathogens in Hemileia vastatrix is great, making the development of resistant coffee varieties complicated. To date, more than 30 physiological strains of H. vastatrix have been identified and are associated with CLR disease. According to Carvalho[31], at least seven major dominant genes are associated with resistance and seven dominant genes have been found for resistance to this pathogen, thereby negating the corresponding genetic predisposition of coffee for this resistance. So far there has been no information on breeding efforts of CLR resistance in C. stenophylla. Herein we use the information of CLR, CBD and CWD on Arabica coffee to illustrate the potential breeding needs of disease resistance in C. stenophylla.
The economic impact of coffee leaf rust on global Arabica coffee production, estimated at 1−2 billion USD per year due to crop failure (20%−25%) and the need to implement control and cultural measures (10% of production costs), can be deductible. According to Guzzo[32], more than 75% of the coffee grown in the world is susceptible to different pathogenic strains. Most of the breeding initiatives focus on creating disease-resistant varieties. For example, in Ethiopia, a high degree of horizontal (non-specific) resistance to this disease, partial disease resistance, and genetic diversity of Arabica coffee all contribute to the coffee's ability to be protected against disease as part of the CLR prevention program under the current environmental conditions. The presence of such a wide variety of CLR resistance in wild forest populations provides an opportunity for the development and use of resistant materials to control coffee leaf rust.
CBD is another fungal disease in Arabica coffee caused by Colletotrichum kahawae. The pathogen causes anthracnose disease in green and ripe fruit. It was first discovered in 1922 in newly established imported Arabica coffee farms with a limited genetic base in the Sotik region of western Kenya, south of Mount Elgon. CBD is a major risk for the coffee production of Tanzania, Kenya, Ethiopia, and other African countries. The Ethiopian coffee breeding operations, which began about 30 years ago, have succeeded in creating new varieties with long-term CBD resistance[33]. It’s unknown whether Colletotrichum kahawae attacks C. stenophylla. However, CBD is considered as a potential threat to the production of C. stenophylla in Sierra Leone.
In addition, CWD is a potential biotic constraint to produce C. stenophylla. CWD is a vascular disease that can result in complete death of infected coffee trees. This fungal pathogen is known by its teleomorph Gibberella xylarioides (Fusarium xylarioides). Hosts of CWD include C. arabica, C. canephora, and C. liberica[34]. Resistance to wilt depends on the genetic potential of the coffee plant for virulence in the pathogen population, inoculum concentration and host genetic potential[34]. Efforts to control CWD are based on the selection of disease-resistant plants, environmental management, and the use of synthetic fungicides[35]. Twelve Arabica coffee genotypes were tested in different agro-ecological regions with different resistant responses based on artificial inoculation trials on CWD-infected natural soil to verify previous results to select promising resistant genotypes[36]. The breeding method of CWD resistance is potentially useful to other coffee species, including C. stenophylla.
Breeding for quality attributes
-
The selection of Arabica coffee has attracted positive attention in terms of bean and cup quality, especially in light (washed) coffee producing countries. Most coffee quality parameters show significant (additive) genetic variation, while environmental factors also have an impact[37]. Among the genotypes of Arabica coffee, three types of plants can be distinguished: wild genotypes from the Sudano-Ethiopia region; non-invasive cultivation lines (Typica and Bourbon); and invasive variants, mainly consisting of Timor hybrid genotypes. A study on the diversity and correlation between quality and biochemical properties of different potential genetic resources of C. arabica in southwestern Ethiopia was performed by Abeyot et al.[38]. The results showed that, compared to coffee collections from other origins, the collections from Sheko, Dizi and Meanit exhibited significantly different organoleptic and biochemical characteristics. In addition, they found that at the phenotypic level, caffeine, bitterness, and astringency were inversely related to measures of high cup quality.
Combining ability
-
The concept of combining ability analysis identifies parents who are likely to pass on their desired traits to their offspring by following a recommended breeding strategy. Furthermore, it suggests the best hybrid combinations and provides genetic information highlighting different agronomic traits. Hybridization in coffee usually refers to the behavioural pattern of the parent clones during the crossbreeding process, either directly or back and forth to obtain hybrids. The general composability (GCA) of a clone refers to the behavior of the clone in a series of crosses based on the mean of the obtained F1 hybrids. From the results of Allard[39], the deviation from the mean of the GCA due to the performance of a single clone is called the specific combining ability (SCA). So far, studies on the combining ability of coffee have only been done on C. arabica. The results showed that both additive and non-additive types of types of gene actions are important in the inheritance of these traits[40−43]. However, for most of the interesting characteristics of Arabica coffee, the non-additive genetic impact proved more interesting than the additive ingredients. Using crop yield data obtained over 14 years, Cilas et al.[44] estimated the genetic parameters of several bean traits and the yield of Robusta coffee using two mating alternatives of 18 parents. The authors found that the general combining ability (GCA) was the main cause of significant variation for the studied traits. This result enabled the identification of superior parents with good GCA in terms of yield and bean trait. Montagnon & Bouharmont[45] analyzed the between population crosses (Congolese vs Guinean), combining yield and susceptibility to coffee leaf rust (SCLR), using other heterozygous assays. Their findings showed no interaction between tested genotypes and testers, although the correlation between test values obtained from different testers was significant. In addition, test values of tested genotypes can be used to predict the yield and SCLR of cross-population hybrids.
Heterosis
-
The phenomenon known as heterozygosity refers to F1 hybrids that are robust or stronger than their parents. Therefore, the terms hybrid dominance and hybrid vigor are often used interchangeably[46]. This is usually manifested by an increase in vigor, size, growth rate, yield, or some other characteristics. Information on the hybrid superiority of coffee is generally scanty compared to other crops as crossbreeding studies have begun quite recently[47]. The perennial nature of the crop poses a challenge as it takes several years to achieve significant results[44]. Since hybrid performance depends on parental selection, the efficiency of hybridization can be improved by predicting hybrid performance from parental genetic distance (GD). Therefore, it is suggested that such predictions of relevance are possible if there is a positive relationship between GD and hybrid performance[48]. The hybrid vigor of coffee can be useful for imparting establishment like early growth and high early yield. However, prediction of hybrid yield using genetic distance has been under-investigated in coffee than in other economically valuable species.
In C. arabica, hybrid vigor was exploited, and its existence was demonstrated by hybridization between different genetic groups[44,49−53]. Likewise, in C. canephora, combinations of different parents exhibit a greater heterozygous effect, and it is suggested that parental selection in this species should consider both genetic differences and superior agricultural value[54]. Berthaud[55] and Montagnon et al.[56] also observed that the crossing of genotypes with high genetic variation produced progeny that were 20% to 50% more productive than the average for the clones. As evident in C. canephora, Leroy et al.[57] and Ferrao et al.[58] also reported the heterozygous potential of different combinations. In addition, Leroy et al.[59] and Montagnon et al.[60] showed that hybrids between Guinean and Congolese species (the two major genetic groups of C. canephora) as identified by Berthaud[61] exhibit heterozygosity due to the presence of high genetic variability in the species. DNA markers were used to estimate genetic distance and assess its relationship with hybridization performance. The SNP marker is more suitable for high throughput applications at a lower cost than other marker systems[62,63]. Recently, the SNP was used to estimate the genetic distance between the C. canephora genotypes and observed the highest genetic distance between the Conilon (Coffea arabica) and Robusta genotypes. These markers have been shown to be effective in assessing the genetic diversity and population structure of C. canephora. Selection was performed within and between cultivar groups and hybrids with greater genetic distance were selected as they were considered important for C. canephora breeding programs.
Akpertey et al.[21] used SNP markers to determine the genetic distance between the parental clones of C. canephora and evaluate the relationship between genetic distance and hybrid yield. The experiment included 64 parental clones and 56 crosses that were evaluated over 11 years. Their results showed a significant correlation (r = 0.351, p < 0.01) between the genetic distance of the parental clones and the yield of their hybrids. Subsequent selection of hybrids with the highest genetic distance showed an increase in yield relative to the overall average as the genetic distance between the parent clones of the hybrids increased. Inter-group hybrids outperformed intra-group hybrids in cumulative yield, according to cross-population and inter-population comparisons determined by Bayesian clustering. The results support the hypothesis that genetic distance is related to the yield of hybrid Robusta coffee varieties and is an effective predictor of hybrid yield. The information from this study will help coffee breeders identify promising parental genotypes and confirm whether predicting hybrid yield based on SNP genetic distance is feasible. The established method can be applied to the development of C. stenophylla cultivars. Given the availability of recently developed SNP markers and information on the genetic diversity of C. stenophylla, parental clonal selection can be performed based on known relationships to explore hybrid predominance.
In addition, the recently developed SNP markers (Lahai et al., unpublished data) will allow research and application of advanced molecular selection methods on C. stenophylla. Among these technologies, genomic selection is increasingly being adopted by plant breeders, and the benefits are even more significant for perennial crops such as coffee, which have a prolonged juvenile phase. Genomic selection aims to predict the breeding value of selected individuals based on information provided by genome-wide molecular markers. The SNP marker is the most used molecular marker for genomic selection. Unlike other molecular breeding methods such as genome-wide association analysis (GWAS), which use only markers that are significantly associated with phenotypic features, genomic selection uses all genotyped SNP markers. Therefore, the selection is based on the complete genotype, not the phenotype. This type of method can improve the accuracy in the selection of young coffee trees and shorten the long coffee growth cycle, which requires a minimum of 15–20 years to complete the development of a new variety[21]. Ferrao et al.[58] investigated the effectiveness of gene prediction models in Robusta coffee, using data from two periodic breeding populations evaluated at two sites. The performance of 13 statistical methods was evaluated based on their ability to predict three traits such as coffee bean yield, leaf rust incidence and green bean yield. Their results showed that the difference in predictive accuracy of competing models is small. In addition, the prediction accuracy of the analysis in the environment was on average higher than those predicted between sites and between populations. Their results support the potential of genomic selection to accelerate the breeding program of C. stenophyllaand C. canephora.
-
The wild coffee species such as C. stenophylla, C. affinis and other related species are native to Sierra Leone. These species have great potential as a heat stress tolerant coffee for the West African lowland environments, such as the eastern and southern Sierra Leone. Compared to C. arabica, C. stenophylla has unique characteristics of flavor and is described as complex and naturally sweet with medium-high acidity, fruity and palatability. The organoleptic properties may complement locally grown Robusta and Arabica coffees. Their ecological adaptability and natural resistance to some of the major pests and diseases of Robusta and Arabica coffee makes them ideal for lowlands and rapidly changing climatic conditions. However, this species is still in its primitive stage, with low productivity. Investment in the research of these species is insignificant in Sierra Leone. Thus, a breeding program for C. stenophylla is needed for Sierra Leone. Germplasm is available from original native populations and potentially introduced from neighbouring countries. However, understanding local adaptation and genotypes × environment (G × E) will be essential for a new crop such as C. stenophylla. Robusta coffee breeding methods can be used as examples for breeding hybrid type varieties of C. stenophylla, based on the knowledge gained from the combination of fitness and hybrid superiority. A new generation of molecular markers such as SNPs can be applied to predict the hybrid viability of the F1 generation. Like Robusta coffee, cuttings can be taken from the best plants and then propagated by continuous vegetative propagation as an elite clone. Furthermore, with the availability of recently developed SNP markers on C. stenophylla, new molecular breeding technologies, such as genomic selection, could play an important role in increasing breeding accuracy, thereby speeding up breeding and providing improved varieties with high yield, good adaptability, and good disease resistance.
-
The authors confirm contribution to the paper as follows: conceptualization and writing of manuscript: Lahai PM; proof reading and technical advice: Bah MA; advice on the design of the manuscript and data collection: Bah MT; proof reading and structural arrangement of manuscript: Aikpokpodion PO; aided in providing relevant literature and data collection: Johnson RAB. All the authors read and approved the final manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Paul Musa Lahai, Mohamed Alieu Bah
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lahai PM, Bah MA, Lahai MT, Aikpokpodion PO, Johnson RAB. 2023. Genetic diversity of coffee germplasm in Sierra Leone: implications for conservation and breeding programs. Beverage Plant Research 3:26 doi: 10.48130/BPR-2023-0026
Genetic diversity of coffee germplasm in Sierra Leone: implications for conservation and breeding programs
- Received: 30 May 2023
- Revised: 07 September 2023
- Accepted: 17 September 2023
- Published online: 20 October 2023
Abstract: Global coffee production is dominated by Coffea arabica (Arabica coffee) and C. canephora (Robusta coffee) due to their relatively high-yielding and quality attributes as opposed to other coffee species. Despite these advantages, production of Arabica and Robusta coffee is facing mounting challenges though not limited to increasing prevalence and severity of biotic and abiotic stresses. These challenges bring forth an indication that the global coffee crop portfolio requires diversification to ensure resilience to the key challenges for sustainable production. Sierra Leone is in the center of genetic diversity of genus Coffea, and the country hosts rich coffee genetic resources. The C. stenophylla, C. affinis and possibly other wild relative species are indigenous to Sierra Leone and these species offer great potential for a new coffee market and income generation. However, more efforts of conservation and genetic improvement on these species, are needed to realize these opportunities. The objective of this paper is to review the coffee genetic resources in Sierra Leone with an emphasis on the wild coffee species including their conservation status, and the phenotypic and molecular characterization. We also present perspectives for future genetic improvement of C. stenophylla, and discuss breeding methods, combining ability, and molecular marker-assisted prediction of hybrid vigor. Moreover, with the availability of recently developed single nucleotide polymorphisms (SNP) markers on C. stenophylla, we suggest that new technologies of molecular breeding, such as genomic selection can significantly accelerate the breeding progress and deliver improved varieties with high yield, good adaptability, and disease resistance.