-
With its significant humidity, temperature, and precipitation, the Philippines is recognized as providing an ideal growing environment for many organisms, including animals, plants, and even fungal species. The Philippines' biodiversity comprises about 70%−80% flora and fauna and is considered one of the most biodiverse countries in the world[1]. Additionally, available reports from 2001 to 2021 show that there is also a rich mycological flora in the Philippines, with 193 genera found in the country, with the genus Trametes having the highest number documented of species[2].
Trametes, an agaric mushroom belonging to the order Polyporales, is a saprophytic fungus that grows on decaying logs or wood substrates and is characterized by having a thin-walled (non-amyloid) basidiospores, a pore-like hymenophore, three kinds of hyphae, and having both pileus and basidioma[3]. Trametes are globally distributed and known to thrive in a wide range of temperatures with most records found in tropical regions[4]. According to Species Fungorum, there are 195 species of Trametes recorded worldwide. In the Philippines, a total of 18 Trametes species, including Trametes cinnabarina (Jacq.: Fr.) Fr., Trametes aspera (Jungh.) Bres., Trametes coccinea (Fr.) Hai J. Li and S.H. He., Trametes corrugata (Pers.) Bres., Trametes elegans (Spreng.) Fr., Trametes ellipsospora Ryvarden, Trametes flavida (Lév.) Zmitr., Wasser and Ezhov., Trametes gibbosa (Pers.) Fr., Trametes hirsuta (Wulfen) Lloyd., Trametes membranacea (Sw.) Kreisel., Trametes ochracea (Pers.) Gilb. and Ryvarden., Trametes pubescens (Schumach.) Pilát, Trametes polyzona (Pers.) Justo, Trametes sanguinea (L.) Lloyd, Trametes suaveolens (L.) Fr., Trametes trogii Berk., Trametes villosa (Sw.) Kreisel, and Trametes versicolor (L.) Lloyd have been recorded[5].
Numerous studies have been published on Trametes, such as ethnomycological and biodiversity studies[3,6,7], molecular and phylogenetic studies[8,9], and identification of bioactivity and bioactive compounds[10,11]. However, despite abundant records of Trametes worldwide, limited optimization studies have been conducted. Establishing the optimum culture condition of a specific mushroom is an important step in the domestication of wild mushrooms prior to industrial utilization. Mushroom mycelial growth requires nutrients from a culture media. For mycelia to grow effectively in pure culture, pH, temperature, light, and oxygen have to be optimal. Establishing mushroom nutritional needs is required to provide optimal growth conditions for mass production and enable the mushroom to generate the necessary biological compounds and end products.
T. versicolor (SJ18) collected in Central Luzon State University (CLSU), San Juan Circle, Science City of Muñoz (SCM), Nueva Ecija, Luzon, Philippines (PHL) was the mushroom used in this study (Fig. 1). T. versicolor, locally known as Turkey tail mushrooms, is known for its medicinal and industrial applications. T. versicolor possesses abundant mycochemical compounds such as flavonoids, alkaloids, triterpenes, and steroids[12]. In addition, T. versicolor secretes enzymes such as lignin peroxidase, manganese peroxidases, H2O2 generating enzymes, and laccase, which generate potent oxidants that break down the lignin framework[13]. Aside from bioactive compounds, T. versicolor also exhibits different bioactivities such as anticancer[14] antitumor[15], antimicrobial[16], antioxidant, and anti-inflammatory[17] activity. In addition, T. versicolor was also utilized in the industry as an aflatoxin control[18], decolorization of textile dyes[19], and biosorption of heavy metals[20]. In the present study, the culture conditions of both intrinsic and extrinsic factors for the mycelial growth of T. versicolor were established. Additionally, the mycochemical and cytotoxic activity of the different extracts of the fruiting body of this mushroom was determined to utilize these natural resources in the industry.
-
Trametes versicolor (SJ18) used in this study were collected from CLSU, San Juan Circle, SCM, PHL, 15°44'22.6" N 120°56'43.0" E. The mushroom was brought to the Center for Tropical Mushroom Research and Development (CTMRD), CLSU, SCM, PHL and was morphologically verified as T. versicolor by a local mycotaxonomist and was added to the culture collection coded as SJ18. The T. versicolor was aseptically tissue cultured in a sterile potato dextrose agar (PDA) prior to the optimization study. Incubation was carried out at 30 °C for 7 d. Afterwards, 10-mm mycelial disc isolates were prepared using a 10-mm cork borer from the 7-day-old culture.
Mycelial optimization of intrinsic and extrinsic factors of T. versicolor
-
The intrinsic (culture medium and pH) and extrinsic (aeration, illumination, and temperature) factors were optimized for the mycelial growth of T. versicolor. Following the study by Dulay & Garcia[21], optimization of the culture conditions of T. versicolor was carried out, from media preparation, sterilization, inoculation, and incubation with slight modification. Three commercial media, namely Potato Dextrose Agar (PDA), Malt Extract Agar (MHA), and Saboraud Dextrose Agar (SDA), and four locally modified media, namely potato sucrose gulaman (PSG), coconut water gulaman (CWG), rice bran decoction gulaman (RBDG), and corn grit decoction gulaman (CGDG) were used and adapted from the study of Fabros et al.[22]. The optimum pH condition was determined using four sets of pH conditions (pH 5, 6, 7, and 8). Incubation was carried out at room temperature (30 °C) and the mycelial growth diameter was measured and recorded. Mycelial density was visually evaluated.
After establishing the intrinsic factor for the mycelial growth of T. versicolor, the extrinsic factors (aeration, illumination, and temperature) were evaluated. Using the optimal culture medium and pH, two sets of aeration (sealed and unsealed) and illumination (light and dark) were determined, respectively. Afterwards, three sets of temperatures (6−9 °C, 23−25 °C, and 30−32 °C) were evaluated. Similar to the intrinsic factors, incubation was carried out at room temperature, except for the temperature condition, and both mycelial growth and density were measured and recorded.
Spawning preparation
-
Spawn of T. versicolor was prepared using the unmilled rice prior to the fructification. The study of Dulay & Garcia[21] was followed by this study on the preparation, sterilization, inoculation, and incubation of grains. Once the full mycelial ramification of T. versicolor had been achieved, the grain spawn was used as an inoculant for the fruiting of the mushroom.
Fruiting body production
-
The mushroom's fruitification performance was assessed after determining T. versicolor's optimal culture conditions for intrinsic and extrinsic parameters. Following the study of Kalaw et al.[23], the fruiting bag was formulated using (7:3 rice straw-sawdust formulation), sterilization, inoculation, incubation, and determination of biological efficiency (BE).
Preparation of extracts
-
Following Dulay et al.[24], Lin et al.[25], and Eguchi et al.[26], T. versicolor was extracted in ethanol, methanol, and hot water. Twenty grams of T. versicolor powdered fruiting body were extracted in 500 mL of 95% ethanol while 10 g of T. versicolor fruiting body were extracted in 100 ml of methanol for 48 h. Both extracts were incubated in a shaking incubator at 30 °C. Whatman filter paper No. 2 was then used to filter both the mushroom extracts. After filtration, rotary evaporators condensed extracts at 40 °C. The aqueous extract used 20 g of T. versicolor fruiting bodies. Powdered fruiting bodies were diluted in 500 mL of distilled water and heated using a double boiler (80−90 °C) before filtration. All the extracts were refrigerated after extraction prior to bioactivity assessment.
Qualitative mycochemical analysis
-
The methods of Guevarra & Recio[27] were used for qualitative analysis of T. versicolor fruiting bodies for mycochemicals. The findings were compared to distilled water as a control and interpreted as (+) if the chemicals are present, and (−) for no chemicals.
Brine Shrimp Lethality Assay (BSLA)
-
Following the study of Olowa & Nuñeza[3], the cytotoxic effect of ethanolic, methanolic, and aqueous extract of T. versicolor against the brine shrimp (Artemia salina) was performed. The brine shrimp was acquired from the College of Fisheries, CLSU, PHL. Triplicate vials containing the extracts at different concentrations (µg/ml) were prepared. Exposure for 24 h was carried out in each vial containing 10 nauplii and the mortality rate was recorded. The study of Mendoza et al.[28] toxicity index was used to generate the LC50 values determining the cytotoxic level.
Statistical analysis
-
Treatments followed a completely randomized design (CRD). ANOVA and Tukey's Honestly Significant Difference were used to compare treatment means at a 5% level of significance. A paired t-test compared both lighted and agitation conditions.
-
The effect of culture media and pH on the mycelial growth of Trametes versicolor was determined (Table 1). Commercial medium, malt extract agar (MEA), and potato dextrose agar (PDA) were determined to be optimal for the luxuriant mycelial growth of T. versicolor with an average mycelial growth rate of 10.27 mm d−1 and 8.64 mm d−1, respectively. In addition, coconut water gulaman (CWG), an indigenous culture medium also favored the excellent mycelial growth of this mushroom (8.36 mm d−1). These three-culture media (MEA, PDA, CWG) show high mycelial growth with thick mycelial density.
Table 1. Mycelial growth of Trametes versicolor with different intrinsic and extrinsic factors.
Growth rate of
mycelia (mm d−1)Mycelial
densityIntrinsic factors Culture media CWG 8.36 ± 0.94ab +++ PSG 3.80 ± 0.57d ++ CGDG 3.93 ± 0.60d ++ RBDG 5.91 ± 0.73cd +++ PDA 8.64 ± 1.18ab +++ MEA 10.27 ± 0.61a +++ SDA 6.64 ± 0.10bc +++ pH 5.0 7.69 ± 0.21b ++++ 6.0 11.91 ± 4.52a ++++ 7.0 14.26 ± 2.11a ++++ 8.0 14.18 ± 2.19a ++++ Aeration Extrinsic factors Sealed 17.45 ± 0.6a ++++ Unsealed 11.51 ± 0.23b ++++ Illumination Lighted 16.97 ± 1.79a ++++ Dark 13.36 ± 6.76b ++++ Temperature Refrigerated 2.00 ± 0.00b No growth Air-conditioned 17.36 ± 1.12a ++++ Room temperature 18.00 ± 0.00a ++++ Means with similar superscripts are statistically comparable from each other using Tukeys HSD and t-test at 5% level of significance. Coconut water Gulaman (CWG), Potato Sucrose Gulaman (PSG), Corn Grits Decoction Gulaman (CGDG), Rice Bran Decoction Gulaman (RBDG), Potato Dextrose Agar (PDA), Malt Extract Agar (MEA), Saboraud Dextrose Agar (SDA). Refrigerated condition (9 °C), Air-conditioned (25 °C), Room Temperature (32 °C). (+) very thin, (++) thin, (+++) thick, (++++) very thick. After establishing the optimum culture medium, MEA was used to determine the optimum pH for the mycelial growth of T. versicolor. Based on the data obtained, a wide range of pH from 6.0 to 8.0 were determined to be optimal for the mycelial growth of T. versicolor with an average mycelial growth of 11.91, 14.26, and 14.18-mm d−1, respectively. In addition, a very thick mycelial density was also evaluated.
Influence of extrinsic factors
-
Aeration, illumination, and temperature conditions for the growth of T. versicolor were also determined in this study. Using the optimal intrinsic factors MEA adjusted to pH 7, the aeration condition was determined. Two sets of aeration conditions unsealed and sealed (with parafilm) conditions are shown in Table 1. With an average mycelial growth rate of 17.45 mm per d−1, it was determined that T. versicolor grew best under sealed conditions. Moreover, two sets of illumination conditions (light and dark) were also evaluated in this study using the optimum medium, pH, and aeration condition (MEA, pH 7, sealed). Based on the data obtained, lighted conditions (16.97 mm d−1) were found to be more suitable for the luxuriant mycelial growth of T. versicolor compared to dark conditions (13.36 mm d−1). However, a very thick mycelial density was evaluated for both sets of aeration and illumination conditions.
Temperature was the last extrinsic factor determined in this study. Three sets of temperatures namely refrigerated (9 °C), air-conditioned (25 °C), and room temperature (32 °C) conditions were evaluated. T. versicolor exhibits a luxuriant mycelial growth in a wide range of temperatures from 32 °C. Both air-conditioned (17.36 mm d−1) and room temperature (18.0 mm d−1) show excellent mycelial growth and very thick mycelial density. Hence, the optimal culture conditions for both intrinsic and extrinsic factors of T. versicolor were established in this study. Mycelial growth of T. versicolor on a different culture condition is shown in Fig. 2.
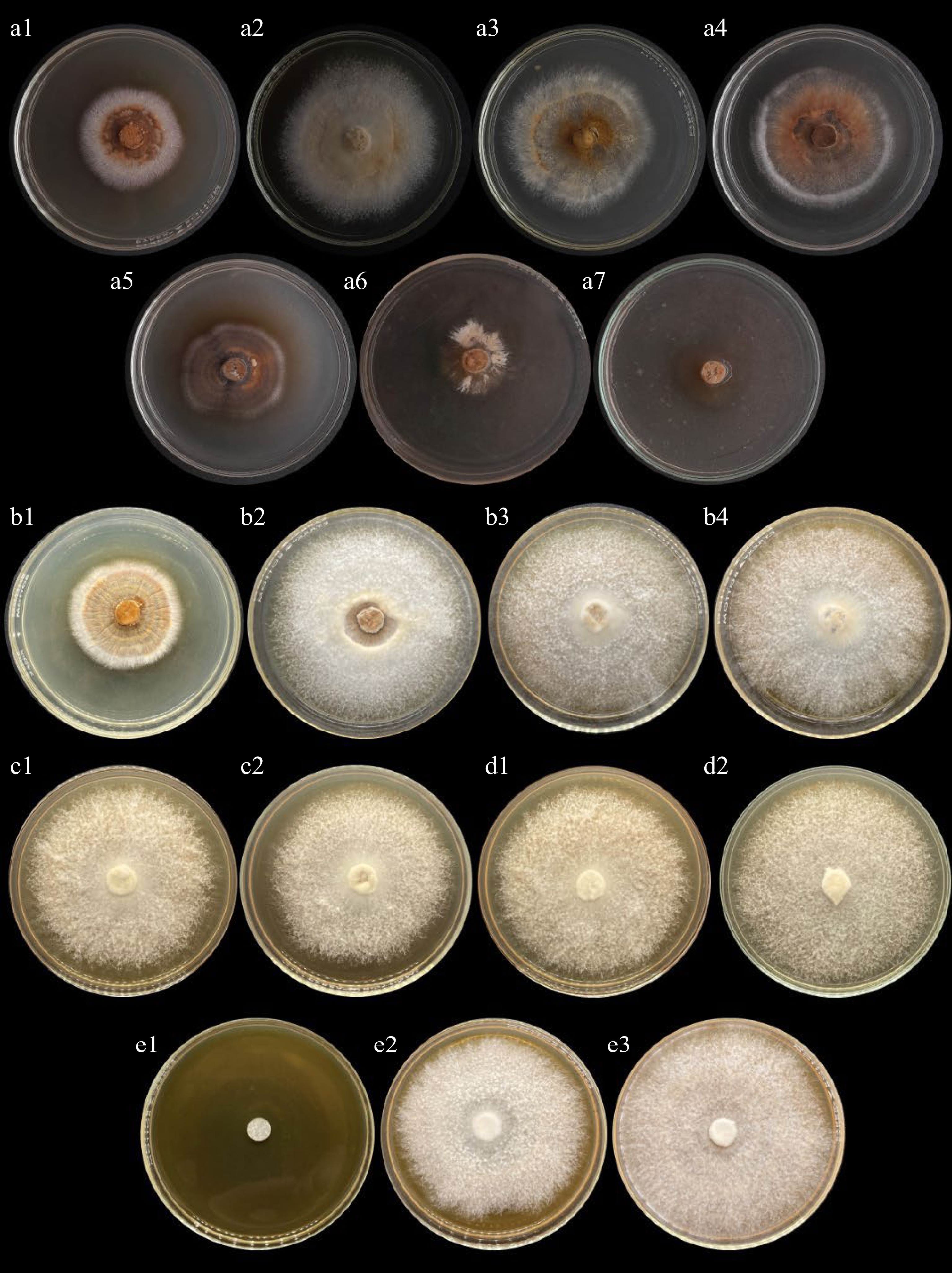
Figure 2.
Mycelial growth of Trametes versicolor on different culture conditions. (a) Culture media (a1, SDA; a2, MEA; a3, PDA, a4, CWG; a5, RBDG, a6, PSG; a7, CGDG). (b) pH condition (b1, pH 5; b2, pH 6; b3, pH 7; b4, pH 8). (c) Aeration conditions (c1, Sealed; c2, Unsealed). (d) Illumination conditions (d1, Light; d2, Dark). (e) Temperature conditions (e1, Refrigerated; e2, Air conditioned; e3, Room temperature).
Evaluation of the fruiting body production of T. versicolor
-
After evaluating the nutritional and physical conditions of T. versicolor mycelial growth, this mushroom was subjected to fructification using the Center for Tropical Mushroom Research and Development (CTMRD) formulation of rice straw and sawdust (Fig. 3). Table 2 shows the parameters of fructification for T. versicolor. Under favorable environmental conditions, incubation days for mycelial colonization of fruiting bags were observed. The duration of incubation, days of primordia initiation, cap diameter, and yield per bag were determined. In addition, biological efficiency was determined.

Figure 3.
Fruiting bodies of T. versicolor grown in 70% rice straw and 30% sawdust substrate formulation.
Table 2. Fructification parameters of Trametes versicolor.
Incubation period (d) Days of primordia initiation (d) Cap diameter (mm) Yield per bag (g bag−1) Biological efficiency (%) 19.70 ± 0.48 36.90 ± 14.76 22.11 ± 3.59 37.68 ± 15.70 7.54 ± 3.14 Mycochemicals found in T. versicolor
-
Mycochemical screening was carried out to determine the presence of secondary metabolites such as steroids, terpenoids, flavonoids, glycosidase, tannins, saponins, and alkaloids present in T. versicolor. Table 3 presents the mycochemical components present in the aqueous extract of the fruiting body of T. versicolor. Among the seven mycochemicals tested, terpenoids, flavonoids, tannins, saponins, and alkaloids were found present in T. versicolor.
Table 3. Mycochemical analysis of an aqueous extract of the fruiting body of T. versicolor.
Mycochemicals Reaction Findings Alkaloids Turbidity was formed + Flavonoids Lighter brown coloration* + Glycosidase No reaction − Steroids No reaction − Saponins Frothing formation + Tannins Brownish with lighter green coloration + Terpenoids Brown coloration interface + * Initial color of the extract was brown, thus positive yellow coloration results in a lighter brown. Note: (+) present; (−) absent. Cytotoxic effect of T. versicolor
-
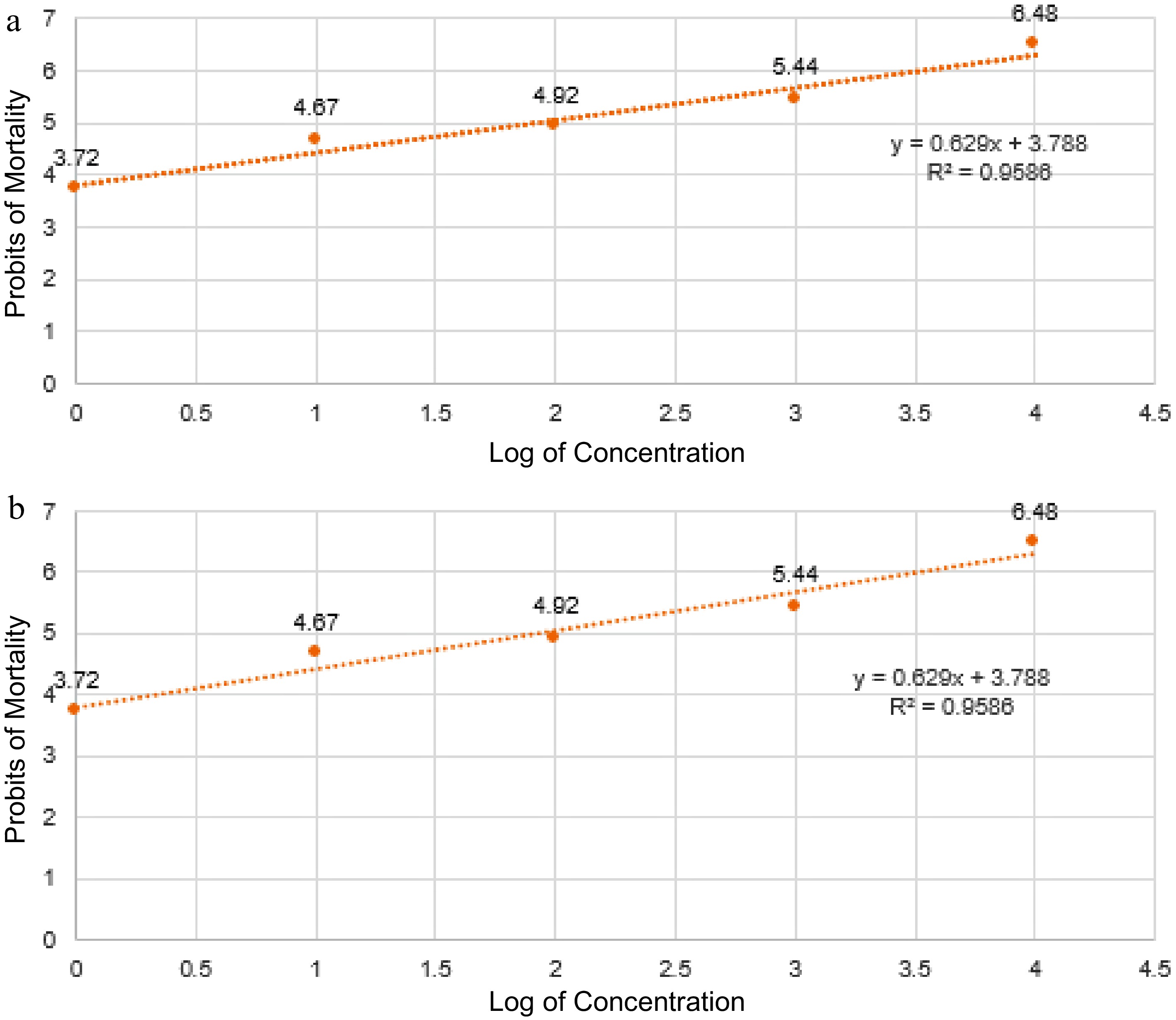
T. versicolor's cytotoxic activity was evaluated using the brine shrimp lethality assay. After being exposed to various concentrations of T. versicolor ethanolic and methanolic extract for 24 h, the mean percent mortality and LC50 values of A. salina nauplii are shown in Table 4. It can be noticed that at 10,000 ug/ml concentrations of both ethanolic and methanolic extract, the highest mortality rate of 96.67% and 93.3%, respectively was recorded. Moreover, the lowest mortality rate was recorded at 1 µg/ml accounting for 16.67% and 10.0% mortality for the ethanolic and methanolic extracts, respectively. Based on the data obtained, the cytotoxic effect of ethanolic extract is higher than methanolic extract. Analysis of variance shows that there are no significant differences in the mortality rate for 10,000 µg/ml and 1,000 µg/ml for ethanolic extract. However, significant differences were observed at 10,000 µg/ml and 1,000 µg/ml for methanolic extract. Additionally, both ethanolic and methanolic extracts of T. versicolor exhibited high toxicity levels with LC50 values of 70.93 µg/ml and methanolic extract of 74.43 µg/ml (Fig. 4). The data consist of corrected %mortality values that have been probit converted and are compared to the log of treatment concentration. For each concentration, the average percent mortality was calculated from three (3) replicates.
Table 4. Cytotoxic effect and LC50 values of ethanolic and methanolic extract of T. versicolor on brine shrimp nauplii after 24 h of exposure.
Extract Concentration
(µg/ml)Mortality
(%)LC50
(µg/ml)Toxicity level* Ethanol 0 0.00e 70.93 Highly toxic 1 16.67de 10 40.00cd 100 53.33bc 1,000 73.33ab 10,000 96.67a Methanol 0 0.00d 74.43 Highly toxic 1 10.00d 10 36.67c 100 46.67bc 1,000 66.67b 10,000 93.33a Means with similar superscripts are statistically comparable from each other using Tukey's HSD and t-test at 5% level of significance.
* Toxicity level was based on the study of Mendoza et al.[28]. -
Agaricomycetous represent a diverse class of fungi that gained significant attention in various fields, including the medical and the pharmaceutical industries, due to their therapeutic applications and nutraceutical properties. In addition, Agaricomycetes mushrooms are known to possess bioactive compounds with potential medicinal properties. These compounds include polysaccharides, terpenoids, phenolic compounds, and proteins that exhibit various therapeutic effects[29−32].
In this present study, Trametes versicolor (SJ18) an agaricomycetous fungi characterized by its fan-shaped or shelf-like fruiting bodies with a thin leathery and velvety upper surface that display concentric bands of brown-black and white color and undersurface cap features numerous tiny spores. Domestication of this mushroom and morphological identification were carried out and this mushroom was classified as T. versicolor according to the local mycologist and mycotaxonomist. After collection, this mushroom was domesticated through tissue culture, and the optimum culture condition was established.
The determination of nutritional requirements for specific mushroom species is necessary in order to establish optimal growing conditions for mass production and to facilitate the production of industrial products. According to De Leon et al.[33], the growth of mycelia is influenced by the nutritional content of the organic matter present in the substrates. Therefore, it is vital to perform a nutritional evaluation in order to determine the optimal medium that promotes the luxuriant growth of mushroom species. In this present study, the T. versicolor grows best on both commercial agar, MEA, and PDA with mycelial growth rates of 10.27 mm d−1 and 8.64 mm d−1, respectively. In addition to indigenous media, CWG also favored the luxuriant mycelial growth of T. versicolor with a mycelial growth rate of 8.36 mm d−1. Similarly, four wild agarics, Coprinus vericillata, Leucoagaricus americanus, Leucoagaricus meleagris, and Leucocoprinus cretaceous also show positive growth in MEA[22]. Meanwhile, in the study of del Rosario et al.[34], five Ganoderma species, Ganoderma applanatum, Ganoderma gibbosum, Ganoderma australe, Ganoderma lucidum strain 1, G. lucidum strain 2, and Ganoderma weberianum preferred CWG as their basal culture medium. Likewise, CWG favored the luxuriant mycelial growth of Lentinus sajor-caju, Pleurotus cystidiosus, and Coprinopsis cinerea[35]. For the commercial medium, both the malt extract and mycological peptone of MEA effectively support the luxuriant mycelial growth of T. versicolor. Moreover, the nutritional content of coconut water such as carbohydrates, sugar, calcium, magnesium, phosphorus, and potassium also effectively support the mycelial growth of T. versicolor[36].
Another intrinsic factor was also optimized as mycelial cultivation depends on the culture medium pH. Based on the data obtained, the T. versicolor has a wide optimum pH requirement of pH 6.0 to 8.0. This is similar to the Lentinus swartzii (pH 6 to 8), Lentinus squarrosulus (pH 5 to 8), and Chlorophyllum molybdites (pH 4.0 to 8) with a wide range of optimal pH requirements for luxuriant mycelial growth[23,37]. The wide optimal pH only indicates the ability of T. versicolor to thrive in a slightly acidic and basic condition with minimal or no significant differences. According to Dulay et al.[5], the pH affects the medium ionic state, physiology, and morphology of mushrooms, as well as product development. Therefore, it is recommended to assess the mycelial growth of T. versicolor specifically into higher basicity to determine the exact range of pH optima for the growth of this mushroom.
Aside from the culture medium and pH, the extrinsic factors, aeration, illumination, and temperature also affect the mycelial growth of mushrooms in a culture medium. Based on the data obtained, T. versicolor shows luxuriant mycelial growth under sealed conditions. The sealed condition was performed by securing the plates with parafilm to ensure anaerobic conditions. The absence of oxygen is ideal for the mycelial growth of T. versicolor as it shows a 17.45 mm d−1 mycelial diameter, compared with unsealed condition (11.51 mm d−1). Similar findings were observed with Formitopsis feei which grows more luxuriantly under sealed conditions with a mycelial diameter of 85.00 mm in comparison with unsealed conditions (80.97 mm)[38]. However, some agaricomycetes are more favorable under aerobic conditions. In the case of Paneolus antillarium and Paneolus cyanescens, both mushrooms exhibit higher mycelial growth of 80.88 and 78.50 mm, under sealed conditions[39]. Moreover, aeration conditions were not a major factor in the growth of five strains of Pleurotus djamor, as they luxuriantly grow best under both conditions[40].
In terms of illumination conditions, T. versicolor preferred the lit condition with a mycelial density of 16.97 mm d−1 for its mycelial growth. This is similar to L. sajor-caju and L. squarrosulus which also favored lit conditions[33,23]. Moreover, in the study of Abon et al.[41], different strains of Volvariela volvacea shows different reaction in illumination condition. V. volvacea La Clementina strain shows no significant differences in their mycelial growth for both light and dark conditions, while V. volvacea Vinces strain favored dark conditions and V. volvacea Montalvo strain favored lighted conditions. According to Idnurm & Heitman[42], light exposure can regulate the growth as well as the production of certain secondary metabolites, pigments, and enzymes in fungi. Additionally, light is an important aspect of the reproduction, survival, and dissemination of fungal species. Therefore, it is also important to determine the optimal illumination of specific mushrooms as different mushrooms show specificity when it comes to light requirements.
Lastly, for the temperature requirements both air-conditioned (17.36 mm d−1) and room temperature (18.00 mm d−1) conditions favored the luxuriant mycelial growth of T. versicolor as it shows no significant differences between the conditions. In the optimization of the mycelial growth of Xylaria papulis, no significant differences were also observed for both air-conditioned and room-temperature conditions[43]. However, in the case of G. lucidum, the best radial mycelia growth was recorded in room temperature conditions with a 50.67 mm mycelial diameter[44]. Temperature preferences influence the geographic distribution of mushrooms. Some species are adapted to colder climates and can be found in temperate regions, while others are more suited to warmer climates that can be found in tropical regions. The data obtained in this study which regards the temperature requirement of T. versicolor are a possible indicator for the distribution of these mushrooms in the tropical regions, the Philippines.
After establishing the optimal culture condition for the mycelial growth of T. versicolor, the yield performance of T. versicolor was evaluated. Using the substrate formulation of 70% rice straw and 30% sawdust the fructification of T. versicolor was evaluated. After 36.90 d of incubation, the initiation of primordia was observed. Based on the data obtained, the yield performance of T. versicolor accounts for 37.68 g bag−1 which is equivalent to 7.65% biological efficiency (BE). The data obtained is comparable to the study of Dulay et al.[45] on Trametes elegans which shows lesser yield and BE using different substrate formulations. However, data obtained in this study only focuses on one substrate formulation. Therefore, it is highly recommended for future studies to conduct optimization studies focusing on the substrate formulation to further determined the optimum substrate formulation for higher yield and BE.
This study also evaluated the mycochemical compound present in T. versicolor. According to Bustillos et al.[39], the term mycochemical refers to a classification of mycological chemicals, and these mycochemicals are produced mostly from mushrooms to perform metabolic functions and serve as their mode of protection. Based on the data obtained, mycochemicals such as terpenoids, flavonoids, tannins, saponins, and alkaloids were present in the aqueous extract of T. versicolor. Similarly, in the study by Herawati et al.[46], mychochemicals such as flavonoids, triterpenes, saponins, tannins, and coumarin were also found present in the ethanol extract of T. versicolor. Moreover, Habibi et al.[47] elucidated a more comprehensive analysis of the chemical properties of n-hexane, chloroform, and ethyl acetate extract of the fruiting body of T. versicolor. Based on their findings, chromatography of these extracts yielded 10 compounds, such as Ergosterol, Trilinolein, Ergosterol Peroxide, Ergosta-7,22-dien-3β-ol, 4-Isobutoxyphenyl Palmitate, Betulin, N-D-2′-Hydroxyheptanoic-1-O-β-DGlucopyranosyl-9-Methyl-4,8-Sphinga-Dienine, Betulinic Acid, 3β-Linoleyloxyergosta-7-ene, and 3β-Linoleyloxyergosta-7,22-Diene with the description of the pharmacological effects for each compound. According to Jakovljevic et al.[9], the presence of primary metabolites such as carbohydrates, and proteins is ideal for nutritional supplements, while the presence of mycochemical compounds such as phenolics, alkaloids, terpenoids, and flavonoids are known to perform different bioactivities such as antimutagenic, antitumor, anti-malarial, cancer preventive, anti-inflammatory, among others which are beneficial to the pharmaceutical industry[8,7,48].
Furthermore, both ethanolic and methanolic extracts of T. versicolor were subjected to cytotoxicity using a brine shrimp lethality assay. Considering several anticancer drugs are known to be cytotoxic, this serves as the preliminary assessment for the anticancer potential of T. versicolor. Data shows that the highest dosage (10,000 µg/ml) also shows the highest percent mortality for both extracts, thus mortality rate is directly proportional to the concentration of the extract. Additionally, based on their LC50 values, the ethanolic and methanolic extracts of T. versicolor both show high toxicity levels. In a comprehensive study of the three Trametes species' cytotoxic potential, it was found that Trametes gibbosa, Trametes hirsuta, and T. versicolor had less potent cytotoxic effects on the human cervix adenocarcinoma (HeLa), colon cancer (LS174), and lung adenocarcinoma (A549) cancer cell lines compared to the control cis-DDP and doxorubicin[49]. On the other hand, a study conducted by Jakovljevic et al.[9] found that MCF-7 and HepG2 tumor cell lines were cytotoxic to the ethanolic extract of T. versicolor's fruiting bodies. It was claimed that the presence of gentisic, syringic, and protocatechuic acids was what caused the cytotoxic effect. In addition, T. versicolor polysaccharide-rich extracts demonstrated showed cytotoxic effects in LoVo and HT-29 human colon cancer cells[50]. Therefore, it is evident from the results of this research and the supporting studies that T. versicolor contains bioactive substances with cytotoxic properties.
-
The current study established the optimal culture conditions for the mycelial growth of T. versicolor. Both intrinsic and extrinsic factors play an important role in the mycelial growth of this mushroom. Substrate formulation containing rice straw and sawdust effectively supports the fructification of T. versicolor. Moreover, qualitative mycochemical analysis revealed the presence of some secondary metabolites which are known to exhibit different bioactivities. The cytotoxic effect of both the ethanolic and methanolic extract of T. versicolor also shows promising results. Therefore, further studies are recommended to explore the more detailed compound analysis, and more comprehensive cytotoxicity assessments to establish the full therapeutic potential of this agaricomycetous fungus.
-
The author confirms contribution to the paper as follows: study concept and Design: Fabros JA, Dulay RMR, Kalaw SP, and Reyes RG; data collection: Fabros JA, Lazo MKM, Magpantay JES, and Abon MD; analysis and interpretation of results: Fabros JA, Dulay RMR, Lazo MKM, and Magpantay JES; Draft manuscript preparation: Fabros JA, Dulay RMR. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
The authors are very grateful to the Philippine Council for Health Research and Development, Department of Science and Technology for the funding support.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Fabros JA, Lazo MKM, Magpantay JES, Abon MD, Dulay RMR, et al. 2023. The effect of nutritional and physical factors on the growth of Trametes versicolor (L.) Lloyd and its mycochemical and cytotoxic properties. Studies in Fungi 8:18 doi: 10.48130/SIF-2023-0018
The effect of nutritional and physical factors on the growth of Trametes versicolor (L.) Lloyd and its mycochemical and cytotoxic properties
- Received: 14 August 2023
- Accepted: 04 October 2023
- Published online: 22 November 2023
Abstract: Trametes versicolor (L.) Lloyd is an agaricomycetous fungi characterized by its fan-shaped or shelf-like fruiting bodies with a thin leathery and velvety upper surface that displays concentric bands of brown-black and white color and undersurface cap features with numerous tiny spores. In this study, the culture conditions for the mycelial growth as well as fruiting body production was established. The mycochemical compositions and the cytotoxic activity were also elucidated. Optimization study of the secondary mycelia shows that T. versicolor grew well on malt extract agar (MEA), potato dextrose agar (PDA), and coconut water gulaman (CWG) culture media. In terms of pH of the medium, pH 6.0 to 8.0 supports the best mycelial growth. On the other hand, sealed, lighted conditions incubated at 25 to 32 °C were the requirements for the optimum growth of T. versicolor. After 37 d incubation, the fruiting body production of T. versicolor was determined. T. versicolor produced 37.68 g bag−1 which is equivalent to 7.65% biological efficiency (BE) on a substrate consisting of rice straw and sawdust at a 7:3 ratio by volume. Moreover, qualitative mycochemical analysis of the aqueous extract of T. versicolor revealed the presence of different mycochemicals such as terpenoids, flavonoids, tannins, saponins, and alkaloids. In terms of the cytotoxic effect of T. versicolor, the LC50 values of ethanol and methanol extracts were calculated, showing that they had a high toxicity level of 70.93 and 74.43 µg/ml, respectively against brine shrimp nauplii after 24 h of incubation. Overall, the optimum culture condition for mycelial growth and fruiting body production, mycochemical compounds, and cytotoxic effect of T. versicolor tabulated in this study provide significant data that elucidated the value of this mushroom in the pharmaceutical industries.
-
Key words:
- Mycochemical /
- Cytotoxicity /
- Biological efficiency /
- Trametes versicolor /
- Optimization