-
Microplastics (MPs; < 5 mm) are ubiquitous in different environmental compartments, including soil, water, air, mountain catchments and sediments[1−4]. Among different environmental compartments, soils are one of the largest sinks of these high molecular polymer particles[5]. The concentrations of MPs in agricultural soils and natural soils have reached 50–18,760 particles kg−1 and 50–130 particles kg−1, respectively[6,7]. Because of the underdevelopment of accurate quantification methods, these figures for MPs concentration in soils might be underestimated. In agricultural soils, MPs enter directly through soil additives (such as sewage sludge and organic fertilizers) or fragmentation of large plastic materials such as films, creating MPs indirectly in soils[8]. After entering into soils, MPs change soil physicochemical properties (such as aggregates, organic matter, pH and nutrient content), alter microbial abundance (such as Ruminiclostridium, Mobilitalea and Xylanophilum), and impact plant growth[9−13]. These changes ultimately lead to adverse effects on crop yield and quality[14,15]. More importantly, MPs traverse the food web by entering into many crop plants (e.g. wheat, carrot and beans), threatening human and animal health[14,16−19]. Consequently, MPs pollution in soil has received increased research attention[20]. Given that this material is found in different parts of plants, research on microplastic uptake by crops is now well underway. The initial results show that MPs infiltrate into casparian strips during root formation and subsequently translocate into aerial parts via the apoplast pathway[14]. An initial focus on crop productivity/toxicity and soil properties under MPs pollution is a very reasonable starting point but the effects of MPs interaction with other pollutants are unclear.
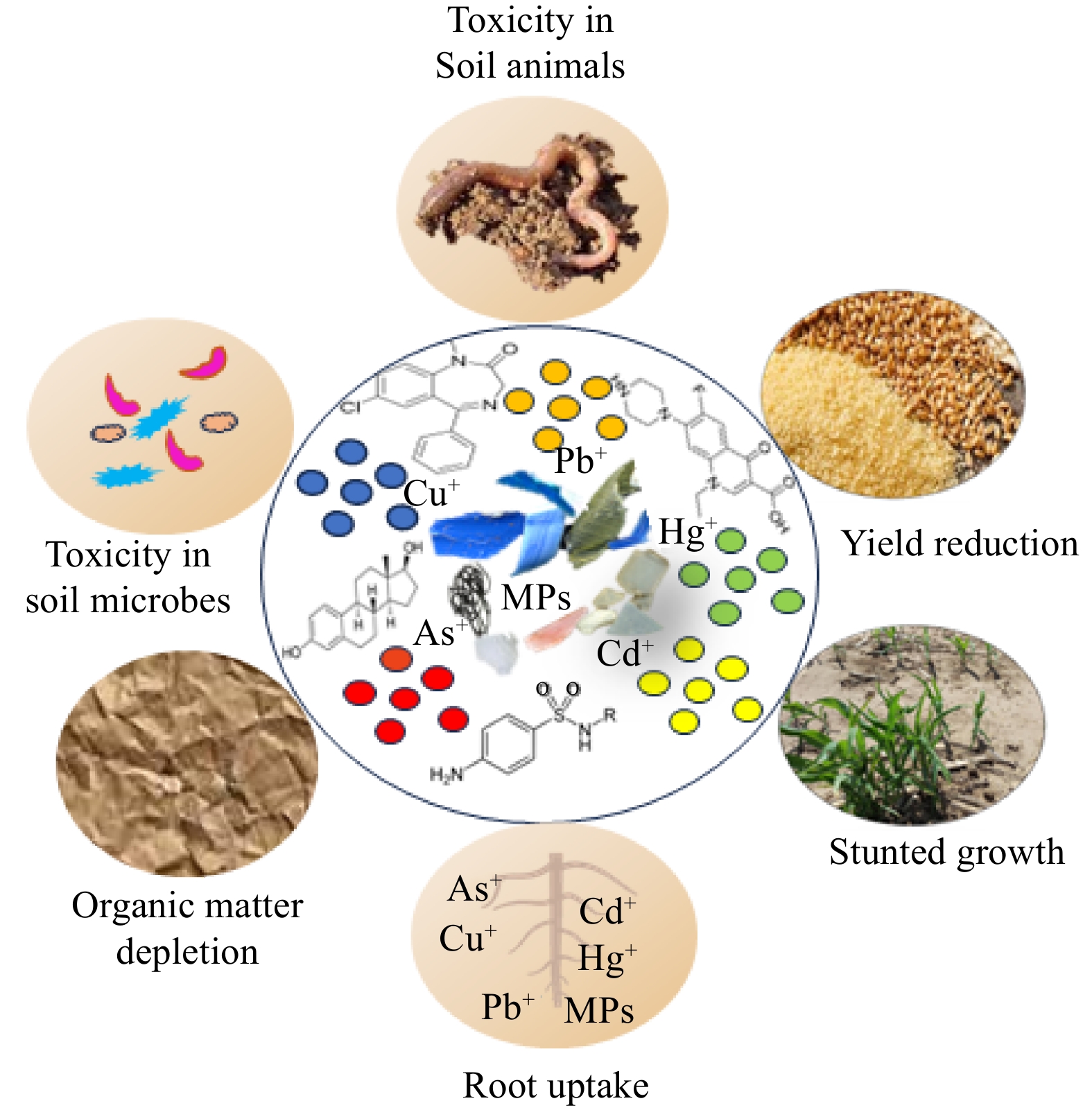
With the increase in soil MP pollution and their interactions with other pollutants, a new set of issues that are particularly relevant to soil and plant toxicity may emerge. MPs have a large surface area; thus, they can adsorb other pollutant materials (e.g. heavy metals and pharmaceuticals; Fig. 1) on their surface and serve as vectors[21−23]. The mechanisms of MPs adsorption include strong electrostatic and hydrophobic interactions[24,25]. These interactions are mainly linked to functional groups (such as hydroxyl and carboxyl groups and benzene rings) on the surface of microplastics as well as the soil solution pH and ionic strength[26−28]. Some studies claim that the adsorption capacity of MPs is much lower than soil[29,30]. This is because soil contains a large number of reactive minerals such as iron oxides, clays and carbonates[31]. Other studies show that due to hydrophobicity, MPs exhibit stronger adsorption over soil[32,33]. In addition to acting as vectors, MPs influence the bioavailability of pollutants (such as heavy metals) by altering the soil properties such as aggregates and dissolved organic carbon (DOC)[34,35]. In conclusion, MPs with other pollutants can bring more diverse risks compared to MPs alone that are associated with increasing the bioaccumulation and ecotoxicities of coexisting pollutants by serving as vectors and governing the speciation of pollutants[15,23,24,36−38]. Thus, studies on interactions between MPs and other pollutant materials are important.
The combined effects of MPs and other soil pollutants (e.g. heavy metals) on the crop-soil-microbe system are of diverse nature (Fig. 1). In particular, interactions between MPs and other contaminants may alter their environmental behaviours, bioavailability and toxicity, leading to varied risks to soil ecosystems[15]. Such effects are directly inferred from the depletion of organic matter, organo-mineral complexes, or aggregation in soil by MPs[39,40]. Nevertheless, MPs type has varied effects on soil properties (including aggregate stability and organic matter composition) and microbes (e.g. Firmicutes and Proteobacteria), and thus on the bioavailability of the pollutants[39,41]. The altered bioavailability may lead to increased uptake of heavy metals by plants or root toxicity causing stunted growth, and yield reduction. Any change to the bioavailability of heavy metals by MPs is also likely to affect the symbiotic relationship between plants and microbes (e.g. Arbuscular mycorrhizal fungi (AMF)) by negatively affecting their abundance and diversity[15]. Thus, it is highly likely that the co-occurrence of pollutants may produce combined toxic effects on crops[20]. Higher plants are the primary basis of many food chains, and the adverse impacts of the bioaccumulation of MPs and associated contaminants on higher plants have been primarily reported separately. However, it is unknown if MPs with other pollutants can coexist in the plant body, and what will be its consequences? Given that MPs adsorb many pollutants on their surface, it is highly likely that the impacts of such coexistence on crop growth, yield, and food safety will be catastrophic. In addition, MPs presence with other pollutants in the soil can facilitate its uptake by plant, which ultimately negatively affect crop productivity[14].
Notably, soil has a mixed cocktail of pollutants (Fig. 1), and there is a paucity of knowledge on how MPs coexistence with different soil pollutants affects the soil-plant-microbe system[15,20]. Although such interactive studies are limited, it is pertinent to discuss possible risks to soil-plant-microbe system. Indeed, reviews based on the available literature provide sound bases for further investigating the impacts of MPs interactions with other soil pollutants, and highlighting the knowledge gaps. With this review, we intend to evaluate the interaction of MPs with heavy metals and organic pollutants commonly found in agricultural soils and investigate the effects on soil quality characteristics, microbial life, and plant growth.
-
Since both MPs and heavy metals are persistent in soils, the interactions among these pollutants are receiving increased attention[20,40]. However, there are an insufficient numbers of studies as research has just started to embrace such interactions, having initially focused on MP toxicity alone (Fig. 2). There are mixed effects of MPs on the bioavailability of heavy metals (such as Cd), soil microbes, and yield (Table 1); however, the mechanistic understanding of such effects is lacking.
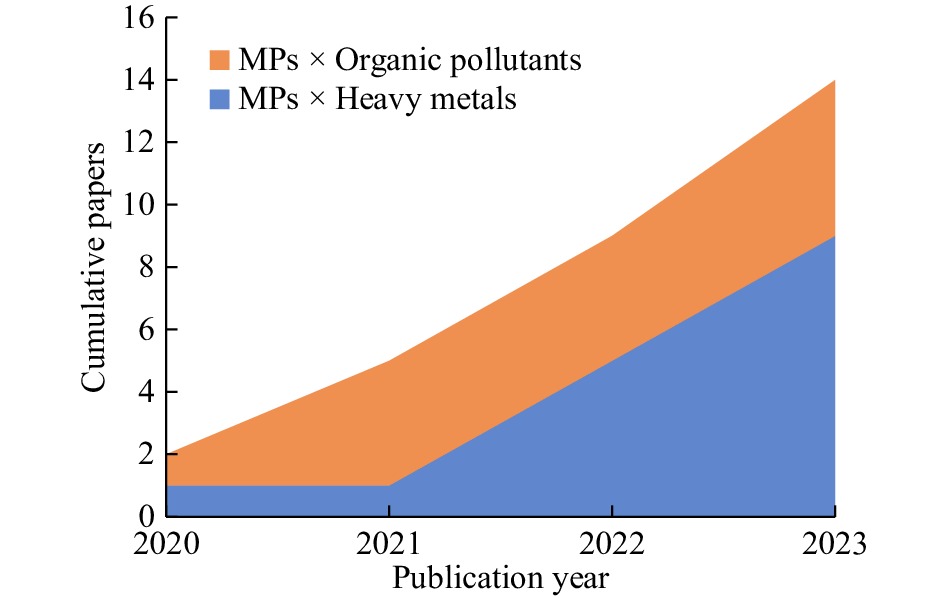
Figure 2.
Cumulative papers on interactions between microplastics (MPs) and heavy metals or organic pollutants.
Table 1. Effects of microplastics (MPs) and heavy metal interactions on the soil and plant properties.
Variables MPs × Cd MPs × Cu MPs × Pb MPs × As MPs × Hg Heavy metal solubility ↑↓ − − − ↓ Organic matter ↓ − − − − Microbes/animals ↓ − ↓ ↑↓ Heavy metal uptake − ↑ ↑ − − Root biomass ↓ − − ↓ − Shoot biomass ↓ ↓ − − − Yield − ↓ − − − Cd: Cadmium; Cu: Copper; Pb: Lead; As: Arsenic; Hg: Mercury; ↑: Increase; ↓: Decrease; ↑↓: Increase or Decrease; −: not known. Microplastics × cadmium (Cd)
-
Cd is a highly toxic heavy metal introduced in soil through anthropogenic activities[42]. After entering soils, Cd is distributed within different compartments through adsorption, complexation, and precipitation[43,44]. Although MPs may interact with Cd and affect its bioavailability, the resultant environmental risks are unclear[40]. The presence of polypropylene MPs in soil has been shown to increase Cd bioavailability by the decomposition of organic matter and organo-mineral complexes. This also results in an increase in soil DOC content[40]. Similarly, both polyethylene and polylactic acid MPs increase the bioavailability of Cd with a greater extent by polylactic acid. Thus, MPs can increase or decrease Cd bioavailability depending on their type (Table 1). The interaction between polyethylene and Cd reduces root biomass in maize. Both MPs with Cd reduced AMF symbiosis by affecting the abundance of AMF[15]. In wheat crops, the combination of MPs polyethylene and Cd appeared to decrease phenological indices, leaf gas exchange, and belowground root traits[45]. Such bioavailable and subsequent toxic effects may depend on soil types. In sandy and clay soils, polypropylene and polyurethane MPs decrease the availability of Cd. The strong reduction of Cd bioavailability occurs in clay soil. This could be due to the influence on adsorption and precipitation of Cd resulting from the formation of CdCO3, mineral adsorption, and iron oxide formation by affecting bacterial communities related to carbon and iron cycles[46]. Moreover, aged MPs (such as polyethylene and butyleneadipate-co-terephthalate biodegradable) have more reactive functional groups on the surface that strongly interact with Cd and reduce bioavailability[28]. Thus, it is implied that aged MPs could reduce the toxicity of Cd for crops and soil microorganisms.
Despite MPs affect Cd bioavailability, the information on the uptake of Cd by crops and subsequently effects on yield are not available (Table 1). The initial focus has been on polypropylene, polyethylene, and polylactic acid. Other types of MPs are not yet explored, for example, polyester, polyurethane, and polystyrene. In addition, different shapes of these MPs will also have very different effects on Cd availability, and might also present different challenges for crop production and food safety. All these research aspects are quite relevant, and we currently do not know if such interactions can affect our agricultural systems.
Microplastics × copper (Cu)
-
Cu is one of the essential micronutrients for plant growth as it is required for balancing nutrition during protein synthesis. In soils, excessive amounts of Cu cause phytotoxicity such as stunted growth and decreased metabolite activity and photosynthesis[47−49]. Few studies have investigated the combined effects of MPs and Cu on plant performance. Therefore, the information on soil and plant variables are limited (Table 1). The chronic exposure to polystyrene MPs and Cu has shown to decrease the yield of pea (Pisum sativum) crops. Surprisingly, nutrient content increased in beans (proteins and amino acids), but Cu accumulation in plants did not change. Polystyrene particles are able to penetrate into incomplete Casparian strips during root formation and translocate into aerial parts via the apoplast pathway[14]. Thus, the coexistence of polystyrene and Cu could be a new threat to other crops; however, further investigations are required to confirm it.
Nonetheless, short-term exposure to polystyrene MPs in Cu-contaminated soils could reduce the accumulation of Cu in wheat seedlings, thus increasing chlorophyll content and photosynthesis while reducing the accumulation of reactive oxygen species[50]. These results imply the role of chronic and short-term exposure of MPs in mediating the interaction effects with Cu on crop growth and yield. In rapes (Brassica napus L.), MPs facilitate Cu uptake. Subsequently, this alters malondialdehyde content, and activities of superoxide dismutase and guaiacol peroxidase in rape plants, suggesting server damage to the quality of the crop[51]. Although an initial focus on Cu uptake, crop growth, and yield is a very reasonable starting point, we lack information on soil quality characteristics (Table 1). Such information is important to mechanistically understand the interactions between different MPs and Cu.
Microplastics × lead (Pb)
-
Pb is the second most toxic heavy metal after As that decreases seed germination and plant growth and yield[52]. Since MPs have a strong sorption capacity of Pb (Fig. 1), this could lead to serious ecological concerns and environmental risks[35,39]. However, sorption of Pb depends upon the type of MP as they cause varied physical changes in soil e.g. aggregates[39]. The coexistence of MPs with Pb appeared to facilitate Pb uptake in rapes (Brassica napus L.). Subsequently, oxidative stress occurs in plants. In particular, Pb uptake alters malondialdehyde content and superoxide dismutase and guaiacol peroxidase activities in rapes plants[51]. These facts suggest that the co-toxicity of MPs and Pb could seriously reduce crop growth and yield.
Many fundamental questions related to the effects of MPs and Pb are not yet investigated. For example, microfibers, micofilms, and microbeads have varied effects on soil structure[53], and it is unknown how they will affect Pb bioavailability, soil microbial life, and crop performance. It is also reasonable to assume that MPs size has substantial effects on these soil and plant variables as different sizes of MPs cause significant changes in soil aggregates and organic matter[54].
Microplastics × arsenic (As)
-
In agricultural soils, As pollution is widespread and increasingly severe. The knowledge on co-toxicity of MPs and As for soil and plant toxicity is in its infancy (Table 1). Due to the sorption of As on MPs[28], the contaminated effects on gut bacteria in earthworms could be reduced[55]. This implies that MP pollution saves earthworms from As toxicity, so they can perform important soil ecological processes. However, we lack empirical evidence of such benefits in soil-plant system. On the other hand, the presence of polystyrene and polytetrafluoroethylene with As decreased root biomass, photosynthesis rate, chlorophyll fluorescence, and the Chl content in rice. Moreover, oxidative stress is induced in roots and leaves with the destruction of antioxidant enzymes, induction of lipid peroxidation and destruction of cell membranes[56]. Thus, it can be concluded that the co-toxicity of MPs and As represents a serious concern for plant growth and yield.
Currently, there is considerable uncertainty about the effects of cotoxicity of MPs and As on the soil-plant system (Table 1). It is unknown, whether the toxicity is related to chemical, physical, or both. Thus, research exploring the possible effects of MPs and As interactions are crucial.
Microplastics × mercury (Hg)
-
The contamination of Hg in our food is a global concern[57]. Dietary consumption of contaminated crops such as rice represents an important route of human exposure to Hg[58]. Still, there is not enough research available to explain the interactions between MPs and Hg (Table 1). An initial study in paddy soil showed that exposure to PVC MPs could decrease the bioavailability of MeHg concentrations both in red and alkaline soil. These effects are related to differences in DOM composition, sulfate and dissolved Fe concentrations, and the abundance of Proteobacteria, Firmicutes, and the hgcA gene[41]. However, we suggest that these bioavailable effects may be different in the case of other crops, MPs type and soil type. Therefore, future studies should consider more crops and MPs. In addition, measuring diverse soil characteristics and plant growth parameters will be important to understand the joint pollution effects.
-
Soils are also sinks of organic pollutants. These pollutants are potentially toxic, persistent and interact with MPs which could negatively impact soil ecosystem functioning. So far, studies that consider the interactive effects of MPs and organic pollutants are limited (Fig. 2).
Sulfonamides (e.g. sulfamethazine and sulfadiazine) are the antimicrobial chemicals used for livestock. Approximately 30-90% of applied dose discharge into the soil environment causes severe risks to the food chain[59,60]. Polystyrene MPs have strong affinities to sulfonamides because of polar and π–π interactions[61,62]. Research that discloses the effects of these interactions on soil microbial life and plant growth is however scarce. An early study shows that polystyrene MPs decrease the negative impacts of sulfamethazine on bacterial diversity, composition and structure in the soil[63]. Similarly, another study found that co-contamination of polystyrene MPs and sulfadiazine or norfloxacin decreased the nutrient contents in plants and impacted leaf metabolites[64]. These results suggest potential ecological risks to the soil-plant nexus from contamination of MPs and antibiotics. Therefore, future studies should intensively check the interactions of different MPs with antibiotics.
High levels of steroids are also detected in soil environments due to extensive application in livestock[65,66]. In particular, estrogens 17β-estradiol contamination has received growing attention[67]. The environmental behaviour of both MPs and 17β-estradiol in combination should be clear in the Anthropocene. Among MPs, polyethylene, polyvinyl chloride and polystyrene have strong adsorption capacity for 17β-estradiol[66]. This might result in the control of 17β-estradiol pollution in soil; nonetheless, it needs to be studied carefully.
Diazepam and phenanthrene contamination in the environment represent another growing concern[68,69]. Due to the potential role of MPs in the distribution of such organic contaminants[70], it is important to understand the interactions of MPs with diazepam and phenanthrene from the perspective of soil and plant health. So far, studies on such interactions are scarce. It is noted in a study that at different concentrations (0.1%, 1%, and 10%) of polyethylene, polypropylene, and polystyrene, the sorption of diazepam decreases only with the highest dose of MPs. This shows that diazepam has a low affinity with MPs. The sorption of phenanthrene to polyethylene is the highest as compared to polypropylene, and polystyrene[71]. Such varied behaviours of diazepam and phenanthrene with MPs suggest that soil and plant may experience different levels of toxicity.
-
Overall, the interactive effects of MPs and heavy metals on soil and plant are toxic. These toxic effects are linked to certain factors: MP type, MP exposure time and soil type. The main pathway through which MPs alter the toxicity of heavy metals is an increase or decrease in bioaccumulation. Co-existance of MPs polypropylene, polyethylene and polylactic acid with Cd, and MPs polystyrene with Cu and As, are able to negatively affect plant growth and soil properties. However, positive effects of MPs and heavy metals are also possible. MPs PVC can decrease Hg bioavailability and alter soil bacterial abundances. A strong affinity of MPs with organic pollutants such as sulfamethazine, 17β-estradiol and diazepam can reduce their toxicity for bacteria and subsequently on plants. Future research should consider the following: 1) effects of more properties of MPs, including size and shape, on the interactions with other pollutants; 2) long-term studies are needed to check these effects with the prospectives of plant and soil health; and 3) the impacts of aged and fresh MPs in determining the interactions with pollutants are important.
Authors gratefully acknowledge funding from the Postdoctoral Directional Training Foundation of Yunnan (Grant No. EO3A581261), CAS-President’s International Fellowship Initiative (2021PB00094), and National Natural Science Foundation of China (Grant No. 31861143002). Prof. Jianchu Xu acknowledges funding from Yunnan Department of Sciences and Technology of China (Grant Nos 202101AS070045, 202205AM070007, 202302AE090023, 202303AP140001).
-
The authors confirm contribution to the paper as follows: study conception and design: Iqbal S; draft manuscript preparation: Gui H, Khan S, Nadir S; Supervision and Editing: Xu J, Bu D, Alharbi SA. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated during the current study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Iqbal S, Xu J, Gui H, BU D, Alharbi SA, et al. 2024. Interactive effects of microplastics and typical pollutants on the soil-plant system: a mini-review. Circular Agricultural Systems 4: e007 doi: 10.48130/cas-0024-0008
Interactive effects of microplastics and typical pollutants on the soil-plant system: a mini-review
- Received: 20 August 2023
- Revised: 24 January 2024
- Accepted: 31 January 2024
- Published online: 16 April 2024
Abstract: Although research on microplastics (MPs) interactions with other soil pollutants is increasingly becoming available, most studies do not consider risks to soil fertility or plant growth. This review aims: 1) to summarize the results of current studies on interactions between MPs, heavy metals, and organic pollutants; and 2) subsequently evaluate risks to the soil-plant nexus. Available-literature shows that polypropylene, polyethylene and polylactic acid increase cadmium (Cd) bioavailability and subsequently reduce root growth. Such effects are not evident in sandy or clay soils due to the formation of CdCO3 and iron-oxide by altered bacterial communities that stabilize Cd contamination. Chronic instead of short-term exposure to polystyrene in copper (Cu) - polluted soils decreases crop yield. With coexistence of MPs and lead (Pb) in soil, the uptake of Pb in crops increases, causing altered malondialdehyde content and superoxide dismutase and guaiacol peroxidase activities. Moreover, co-toxicity of polystyrene or polytetrafluoroethylene with arsenic (As) decreases root biomass, photosynthesis rate and the chlorophyll-a content. In alkaline soil, polyvinyl-chloride could decrease the bioavailability of MeHg due to changes in the abundance of Proteobacteria, and Firmicutes. We also found strong interactions between MPs and organic pollutants. Polystyrene decreases negative impacts of sulfamethazine on bacterial diversity, and structure in soil. Polyethylene, polyvinyl-chloride and polystyrene have a strong adsorption capacity for 17β-estradiol. This implies that 17β-estradiol toxicity can be reduced by these MPs. At low concentrations, polyethylene, polypropylene, and polystyrene have low affinity to diazepam. In conclusion, serious ecological risks are associated with MPs and other pollutants' interactions to soil-plant system.
-
Key words:
- Heavy metals /
- Crops /
- Organic pollutants /
- Soil toxicity /
- Plastic fragments