-
Biomass from plantations and native forests are an important source of secondary metabolites from which new bioactive compounds can be obtained[1]. These co-products are derivatives that can be exploited as biofuels, cosmetics, food additives, pharmaceuticals, and natural products. Co-products have a high value-to-volume ratio and previous research has shown that they can increase the profitability of forest species[2]. Co-products can be obtained from forest residues. The volume of forest residues produced during forest harvesting operations varies significantly and is contingent upon forest type, harvesting techniques, and site features. For example, forest residues in Canada range from 8.6 to 59.5 million dry tonnes per year[3]. The theoretical potential of the residues composed of branches and tree tops derived from all Turkish forests has been estimated at 5 to 7 million fresh tonnes[4]. Forest waste, including litter, slash, and bark, is an important biomass resource for energy production, providing a renewable energy solution in many countries. However, these wastes can also be used to extract natural products such as antifungals. In addition, the term 'Low-Value Biomass' is used to describe this category of forest waste, which consists of non-commercial materials typically left on site after harvesting. The emergence of co-product markets has increased the motivation to harvest and use this material. Additionally, removing 'Low-Value Biomass' could reduce the risks to forest health associated with wildfire, disease, and pests[5].
Araucaria angustifolia is a striking subtropical tree growing in the Atlantic Forest of Brazil, Argentina, and Paraguay. This species has high-quality timber and is therefore harvested and cultivated for timber. It also plays an important role in the phytogeographic characterization of the Atlantic Forest and is considered a critically endangered species[6]. This species has 16,000 ha planted in Argentina alone, yet A. angustifolia has lost distribution and plantation area due to competition from other land uses, including agriculture and pinus plantations, which have a higher growth rate and a more developed international market[7]. Other factors include a lower growth rate and poor seed availability. Nevertheless, A. angustifolia shows high growth potential under good development conditions[8]. In addition, previous observations have shown that the initial application of nitrogen and phosphorus is necessary to improve the growth and quality of A. angustifolia[9].
This paper highlights the importance of co-products to increase the range of uses of A. angustifolia. In this sense, the search for new uses for A. angustifolia can revalue its plantation for productive purposes. The study of secondary metabolites in the tissues of this species has been carried out by several authors who have reported lignans and proanthocyanidins[10,11]. However, no studies were found on the evaluation of antifungal activity in soybean end-of-cycle diseases. New co-products of A. angustifolia may help to increase its profitability as a plantation tree and improve its conservation status.
In this context, the aim of this study is to investigate novel A. angustifolia co-products. These co-products can be used as botanical fungicides against Septoria glycines Hemmi. This fungus causes Septoria brown spot in soybean production areas such as the United States, Argentina, Brazil, and China, where it is a very common foliar disease[12]. Sensitivity to quinone outside inhibitor (QoI) fungicides due to single amino acid substitutions in the cytochrome b gene has been reported in soybean fungal pathogens[13,14]. In this line, fungicides with quinone outside inhibitor (QoI) active ingredients have contributed to the selection and development of QoI-resistant populations of S. glycines. Recently, the molecular mechanisms of QoI resistance in these populations were investigated by targeted analysis of the cytochrome b gene, and S. glycines isolates showed differential susceptibility to QoI fungicides. Characterization of the cytochrome b gene revealed a mutation when glycine was replaced by alanine[15] .
The results of this investigation have helped to highlight the importance of by-products of A. angustifolia and have contributed to the research of new compounds with antifungal activity against resistant fungal isolates of S. glycines.
-
The experimental design is based on bio-guided assays, which involve separating the active fractions from the inactive fractions. In this study, the ethyl acetate extract of A. angustifolia leaves was first obtained and then fractionated by column chromatography. The fractions obtained were further fractionated until the final fraction was obtained. This fraction was then injected into GC-MS and HPLC-DAD for analysis.
Isolation and structural elucidation of the antifungal compound
-
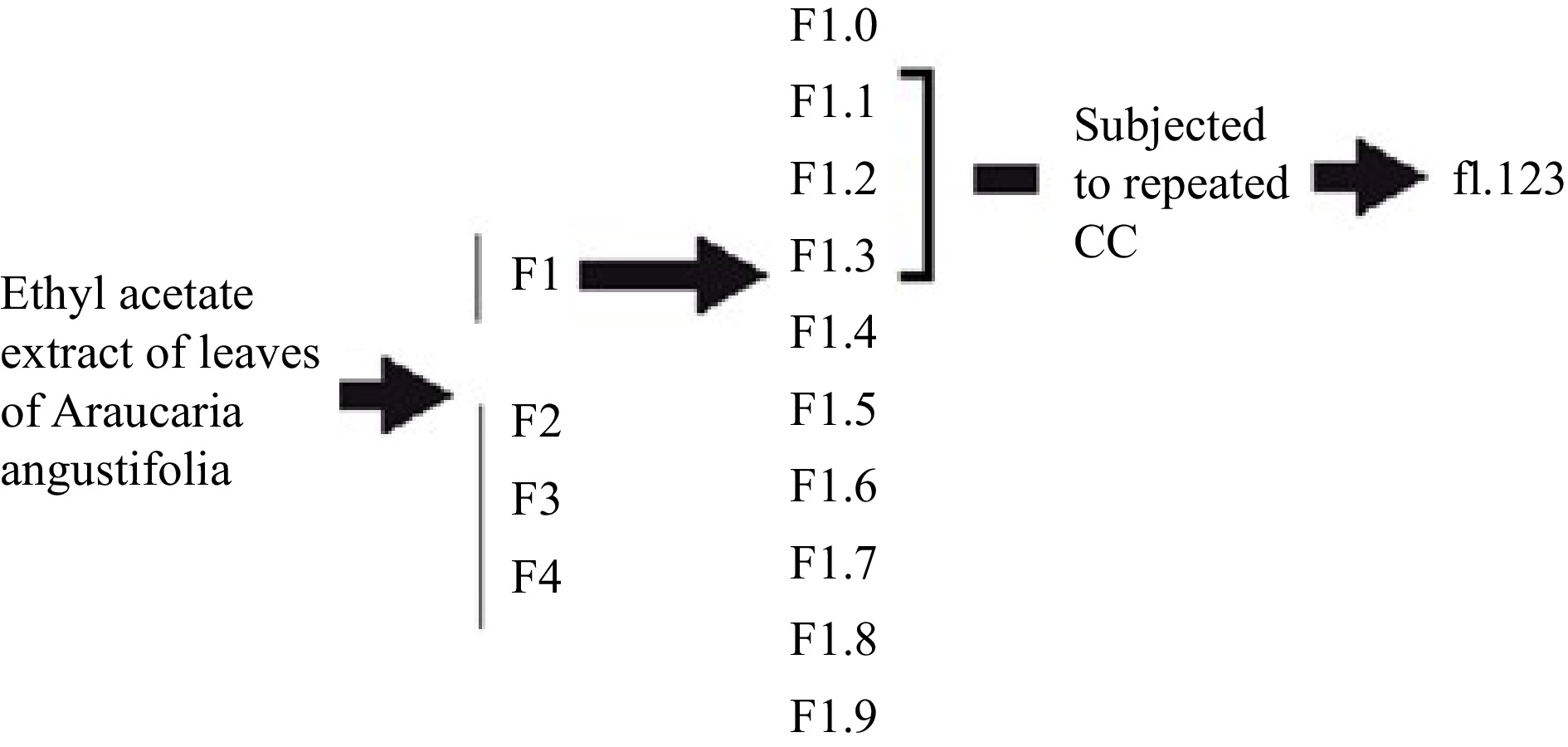
The botanical identification of A. angustifolia was based on the original descriptions. The taxonomic determination was made with the help of specific literature[16] and a specimen was deposited in the herbarium as a voucher. The leaves of A. angustifolia leaves were dried, ground in a mill, and then extracted using ethyl acetate solvent at room temperature. The plant material was immersed for 24 h, filtered, and evaporated under reduced pressure. The material was stored at 2 °C. Then, the ethyl acetate extract (4 g) was dissolved in 28 mL dichloromethane (DClM) : ethyl acetate (AcOEt), (3:1, v/v) and loaded onto a glass column packed with silica gel 60 (240−400 mesh). The column was then eluted with a series of liquid mobile phases: 200 mL DClM : AcOEt (3:1, v/v), 25 mL AcOEt and 70 mL (methanol) MeOH. Following this procedure, four fractions were obtained. These were named F1, F2, F3, and F4. Thin layer chromatograms of F1, F2, F3, and F4 were then performed on silica gel G60 F254 using DClM : MeOH (3:1, v/v) as the mobile phase. F1 was then associated with the inhibition zone observed in bioautography. Ten sub-fractions of F1 were then obtained. These were numbered F1.0 to F1.9. To obtain them, 1.9 g of F1 was introduced into the fractionation column packed with silica gel 60 (240−400 mesh) equilibrated in DClM. The column was then eluted with a series of mobile phases: 25 mL DClM, 40 mL DClM : AcOEt (3:1), 10 mL AcOEt and 30 mL MeOH. Then, F1.1, F1.2, and F1.3 fractions were pooled and 259.9 mg were injected into a column equilibrated with DClM : hexano (1:1). The column was then eluted with a series of mobile phases: 30 mL DClM : Hex (1:1), 20 mL DClM and 40 mL AcOEt. These fractions were combined and new silica gel chromatograms were run, eluted with n-hexane and ethyl acetate to give f1.123 (Scheme 1). The antifungal activity of f1.123 was tested in triplicate using the bioautography test method.
Gas chromatography–mass spectrometry (GC-MS)
-
The f1.123 fraction was dissolved in ethyl acetate and then injected into a gas chromatograph (Clarus 580-SQ8, Perkin Elmer). Data was analyzed using TurboMass 6.1.0 software. The chromatographic column used was a DB5 (30 m length, 0.25 mm internal diameter, 0.25 μm film thickness). Helium was employed as the carrier gas at a flow rate of 1 mL·min−1. The injector temperature was set at 250 °C. The temperature program began at 100 °C for 3 min, followed by a ramp from 100 to 280 °C at a rate of 5 °C per min, and then held at 280 °C for 7 min. A volume of 1 μL of the f1.123 fraction was injected. Compound identification was accomplished by comparing the mass spectra fragmentation patterns of the f1.123 fraction with those stored in NIST MS Search 2.0.
Liquid chromatography analysis (HPLC-DAD)
-
Fraction f1.123 was dissolved in methanol to obtain a concentration of 2.05 mg·ml−1. The analysis was conducted using an HPLC system consisting of a Waters 1525 pump coupled with a PDA detector (Waters 2998). Chromatograms were acquired at 220 nm, and spectra were recorded in the range of 200 to 300 nm. The chromatographic separation was performed on a C18 column (25 cm × 4.6 mm × 5 µm, Agilent). The mobile phase consisted of a mixture of water and methanol (3:7), flowing at a rate of 1.0 mL·min−1[17] ).
In vitro antifungal assay-bioautography test
-
The fungal strains Septoria glycines (EO 001) were obtained from LABIFITO (National University of Tucuman, Argentina). Thin layer chromatograms of the ethyl acetate leaf extract were obtained on silica gel G60 F254 using DClM : MeOH (3:1, v/v) as mobile phase. They were allowed to dry in a laminar flow and then each was covered with 5 mL of YMDAL medium (4 g·L−1 yeast extract, 4 g·L−1 malt extract, 10 g·L−1 dextrose, 4.5 g·L−1 agar) containing S. glycines inoculum. The bioautographies were incubated at 25 °C for 7 d. The absence of fungal growth on the bioautogram indicated the presence of antifungal agents. To identify the antifungal bands, TLC chromatograms similar to those obtained for the bioautograms were observed under 254 nm UV light. Solvent plates were used as controls[18].
Statistical analysis
-
The data obtained from the bioautography test were analysed using Rmedic software. The Chi-square test was used[19].
-
The absence of fungal growth on the bioautogram indicates the antifungal activity of the acetate extract of A. angustifolia leaves against S. glycines (Fig. 1). A statistically significant difference (p = 0.04) was found between the control and the tested fractions using the Chi-square test. The antimicrobial activity of A. angustifolia has been reported by several authors[20,21]. In the present work, it is reported for the first time the antifungal activity of A. angustifolia against S. glycines. A notable aspect of the results is the absence of fungal growth observed in the upper half of the chromatogram. This pattern suggests that the mobile phase (DClM : AcOEt; 3:1) can be utilized to separate the bioactive components.
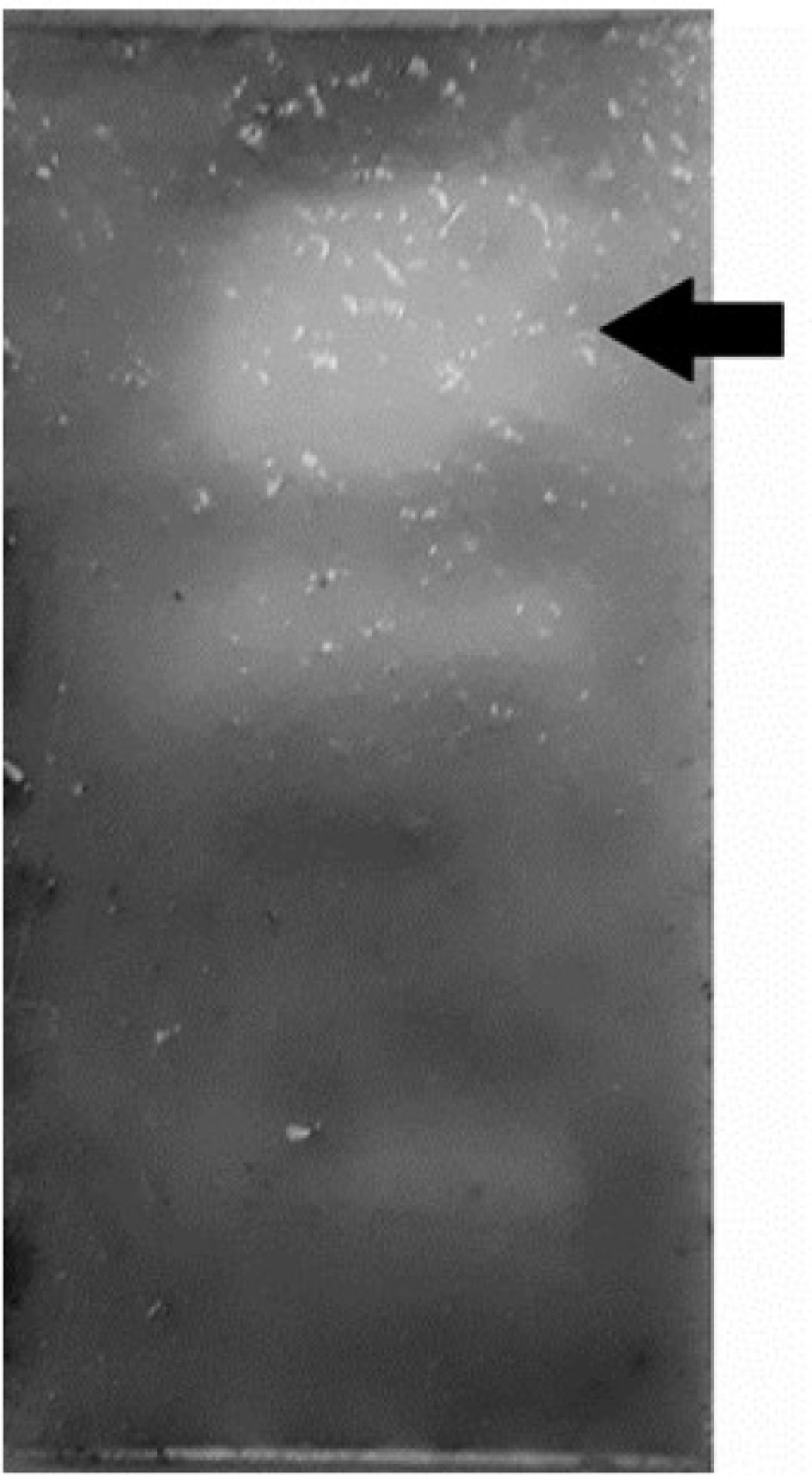
Figure 1.
Antifungal activity of ethyl acetate leaves extract of A. angustifolia against S. glycines. Arrow indicates the inhibition zone.
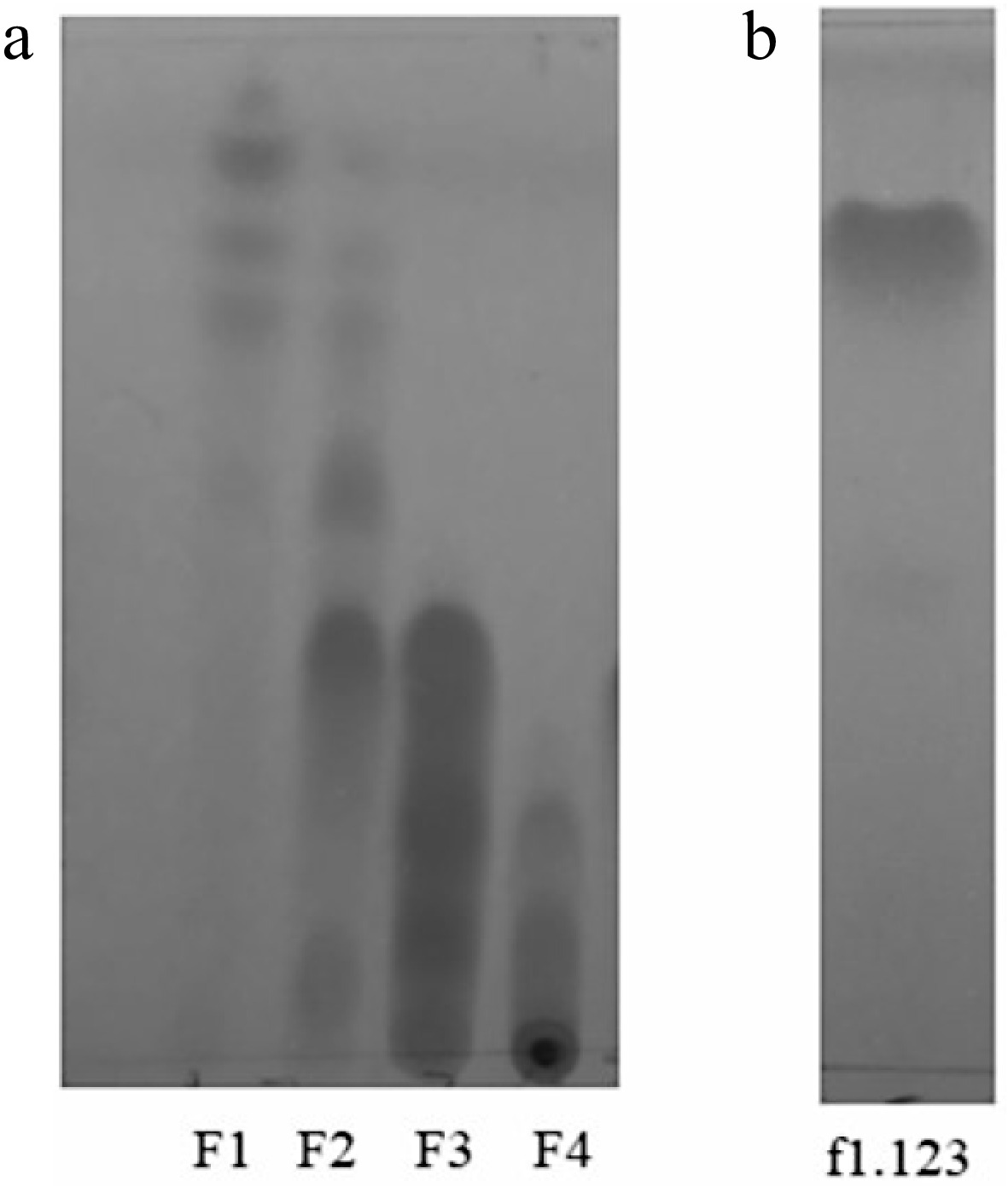
Antifungal zones were observed in the TLC chromatograms using 254nm ultraviolet light. Figure 2 compares the chromatograms obtained of initial fractions F1, F2, F3, F4, and the f1.123 fraction. As seen, the f1.123 fraction is presented as a single band that shows the degree of purification of the compound. The most interesting aspect of Fig. 2 is the location of the spot of f1.123, which coincides with the absence of fungal growth observed in Fig. 1. A similar association between bioautograms and TLC chromatograms has been reported by other authors when evaluating the antifungal activity of plant extracts against Fusarium species[22].
Phytochemical compounds of plant extracts can be identified by spray reagents. Fraction f1.123 was revealed with reagents to determine his natural chemistry. The fraction showed positive results after spraying with p-anisaldehyde reagent (to triterpene). Positive results were observed as purple spots, similar to those reported in other studies[23]. Nevertheless, the fraction f1.123 showed negative results after spraying with Dragendorff's reagent (to alkaloids) and Natural Products Reagent (to flavonoids).
Chromatogram of f1.123 is shown in Fig. 3. The analysis performed by HPLC–PDA showed the split of two compounds, one eluted at 7.90 min, and the other at 8.84 min.
Gas chromatogram of f1.123 is shown in Fig. 4. Results indicated that the fraction is enriched in two compounds. These compounds represent 76.6% of the fraction and were provisionally named compound 1 and compound 2. The difference between the peaks of compounds 1 and 2 is minor (29.928 and 30.448 respectively). It can be highlighted that the HPLC–PDA chromatogram also showed two compounds with small differences between peaks (7.901 and 8,839). These relationships may partly be explained by the similitude of compounds 1 and 2. Similar to our work, two compounds have been reported by other authors using the relationship between the chromatograms obtained by HPLC-DAD and GC-MS. This relationship has also been used by these authors to identify antifungal compounds from plant species[24].
Figure 5 displays the fragmented mass spectra and low-intensity molecular ion peaks of both compounds. Compound 1 (Fig. 5a) exhibited molecular ion peaks at m/z 374.1556, while compound 2 exhibited molecular ion peaks at m/z 302.2969 (Fig. 5b). The presence of a base peak at m/z 79.08 and strong peaks at m/z 147, 119, 91, and m/z 55 can be identified. However, neither compound can be identified by comparison with NIST MS Search 2.0.
-
New natural products are needed to complement the use of synthetic chemicals due to recent reports of phytopathogen resistance. Botanical fungicides are natural products and can be used to control phytopathogens such as S. glycines. In line with the results, the antifungal activity of ethanolic extracts of Reynoutria japonica against S. glycines has recently been reported[25]. Antifungal activity by bioautography is reported on thin layer chromatograms. These results are similar to other researchers who found antifungal activity against Septoria tritici[26]. These authors confirmed six antifungal sesquiterpenes as natural products. In this paper, two new co-products from A. angustifolia are reported for the first time. Both compounds showed antifungal activity against a widespread phytopathogen of soybean, S. glycines. These results are significant considering that recent publications have reported up to 47.5% resistance in various S. glycines isolates[15].
The low intensity of molecular ion peaks suggests the presence of a long carbon chains in compound 1 and 2. Furthermore, the base peak at m/z 79 indicates that both compounds could contain a diene or a triene in their chain[27−29]. Secondly, the mass spectrum of 1 and 2 compounds present prominent peaks at m/z 91 and 119, which had been associated with the presence of aromatic structures[30−32]. In addition to the peaks found at m/z 91 and 119, the mass spectra of both compounds exhibit an additional peak at m/z 121, which has been ascribed to the phydroxybenzoyl cation[33,34]. Finally, the presence of peaks at m/z 147 has been associated with the p-coumaroyl radical's loss[35,36]. These results support the idea of the presence of aromatic structures in compounds 1 and 2. Other researchers have associated antifungal activity with the presence of aromatic structures, which could explain the bioactivity observed in this study[37,38].
In accordance with the results obtained, we are of the opinion that it is of great importance to continue the elucidation of the bioactive compounds reported in this article.
-
Two compounds have been isolated from A. angustifolia that could potentially inhibit Septoria brown spot in soybeans. An interesting implication of this study is that the co-products from the waste of a critically endangered tree species such as A. angustifolia can be used to produce antifungal compounds. This may increase its ability to compete with other species for land use.
-
The authors acknowledge their respective contributions to the paper in the following manner: conceptualization and methodology: Sequin CJ, Trossero JA, Perusset SA; writing - original draft preparation: Sequin CJ, Aceñolaza PG; supervision: Aceñolaza PG. All authors approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study received funding from projects at the National University of Entre Ríos (PID NovelUNER 2208) and CONICET.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sequin CJ, Perusset SA, Trossero JA, Aceñolaza PG. 2024. New co-products from an endangered tree species: Araucaria angustifolia (bertol.) kuntze. Technology in Agronomy 4: e009 doi: 10.48130/tia-0024-0006
New co-products from an endangered tree species: Araucaria angustifolia (bertol.) kuntze
- Received: 23 January 2024
- Revised: 26 March 2024
- Accepted: 07 April 2024
- Published online: 08 May 2024
Abstract: Araucaria angustifolia is a notable tree species distributed in the Atlantic forest of central-eastern South America. It has been considered a critically endangered species. Due to competition from other land uses, including agriculture and Pinus plantations, A. angustifolia has lost distribution and plantation area. This study aims to investigate new co-products of A. angustifolia waste products that can be used to prevent agricultural diseases. Brown spot is the most common foliar disease of soybeans and is caused by Septoria glycines. In this work, we evaluated the antifungal activity of plant tissue-derived compounds against S. glycines. The ethyl acetate extract of A. angustifolia leaves showed antifungal activity and was analyzed by HPLC-PDA and GC-MS. The results show two compounds as the principal constituents of antifungal activity. Both compounds showed a base peak at m/z 79.08, but they could not be identified by comparison with GC-MS spectral libraries. However, peaks at m/z 91 and 119 indicate that both compounds may contain aromatic structures. As a result of this study, we found that A. angustifolia can be used to obtain reliable antifungal compounds against S. glycines.
-
Key words:
- co-products /
- Endangered /
- Species /
- Araucaria angustifolia