-
Ethiopia is a country with a rich and diverse flora, estimated to have about 6,000 species of higher plants, of which 10% are considered to be endemic[1, 2]. The diverse physiographic features have contributed to the formation of different ecosystems characterized by variations in species diversity, vegetation types, soil types, and diverse climatic conditions. However, a significant number of species are threatened as a result of direct drivers such as habitat loss and change, over-harvesting, pollution, agricultural expansion, and climate change and indirect drivers such as population growth, changes in economic activities, socio-political factors, cultural factors and technological changes[3]. One of the threatened species found in Ethiopia is Senegalia venosa, also known as Acacia venosa, which belongs to the family Fabaceae[1]. The species is an endemic species to Ethiopia. The plant is only found in the Tigray and Gonder lowlands of Ethiopia, while it may exist in the escarpments of western Eritrea near to the Ethiopian border. It grows at altitudes ranging from 1200 to 2400 m. In the areas where the Senegalia venosa is located, it was found under poor regeneration status and regeneration potential. This is mostly because this plant species as well as other Acacia species are vital in sustaining the livelihood of the local population in firewood, charcoal manufacture, and fencing. The country's western escarpment and lowlands have a high ecological affinity for Gash-Barka (west Eritrean lowland)[1,4].
Senegalia venosa is listed as endangered (EN) according to the International Union for Conservation of Nature (IUCN) criteria of threat level[1]. According to certain documented studies on the species, it has been utilized locally for fuel and medicinal purposes. Consequently, human disturbance, and grazing currently pose a high threat pressure on the species locally[4]. Therefore, the presence of Senegalia venosa in the IUCN red data list of Ethiopia highlights the need for conservation efforts to protect this species from further decline and extinction[3].
This plant has an important role in the Ethiopian ecosystem and economy. Therefore, conservation efforts to protect the plant are not only important for the survival of this species but also for the preservation of the Ethiopian flora and the livelihoods of local communities. The objectives of the study were: (1) to assess the regeneration status of S. venosa; (2) to record the occurrence points of the species; and (3) to document the major threats to the species.
-
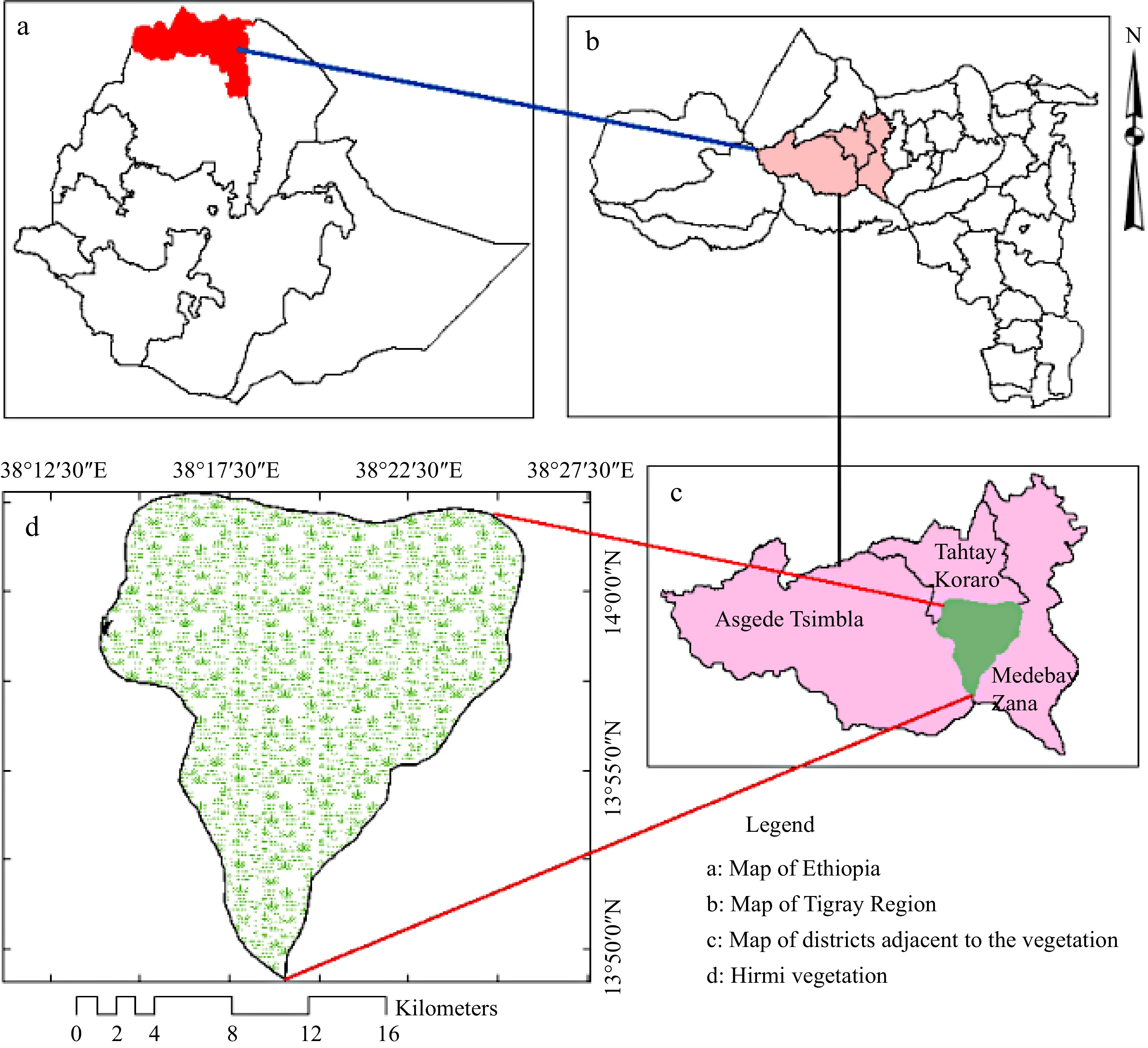
The study was conducted in Hirmi woodland vegetation and its environs. Hirmi woodland vegetation is stretched in the Northwest Zone of Tigray National Regional Administration, Ethiopia, over 30,900 hectares of area coverage (Fig. 1). The vegetation ecosystem is the home of several wildlife species, especially birds, reptiles and invertebrates, as well as being critical for the production of natural gum and incense[4].
Hirmi's vegetation is classified as Acacia-Comiphora, Combretum-Terminalia, and Dry-evergreen Afromontane forest type[4, 5]. Species including Senegalia venosa, Albizia malacophylla, Aloe elegans, Bidens macroptera, Lippia adoensis, Pennisetum glaucifolium, Phragmanthera macrosolen and Urtica simensis were among the dominant endemics species. Species such as Albizia malacophylla, Combretum hartmannianum and Combretum rochetianum as well as Senegalia venosa among the species that have scant populations and are found sparsely in Hirmi dryland ecosystem[6,7]. Currently, the study area is dominated by native remains and a few cultivated species. This is due to human disturbance and livestock interference in the forest.
Data collection
-
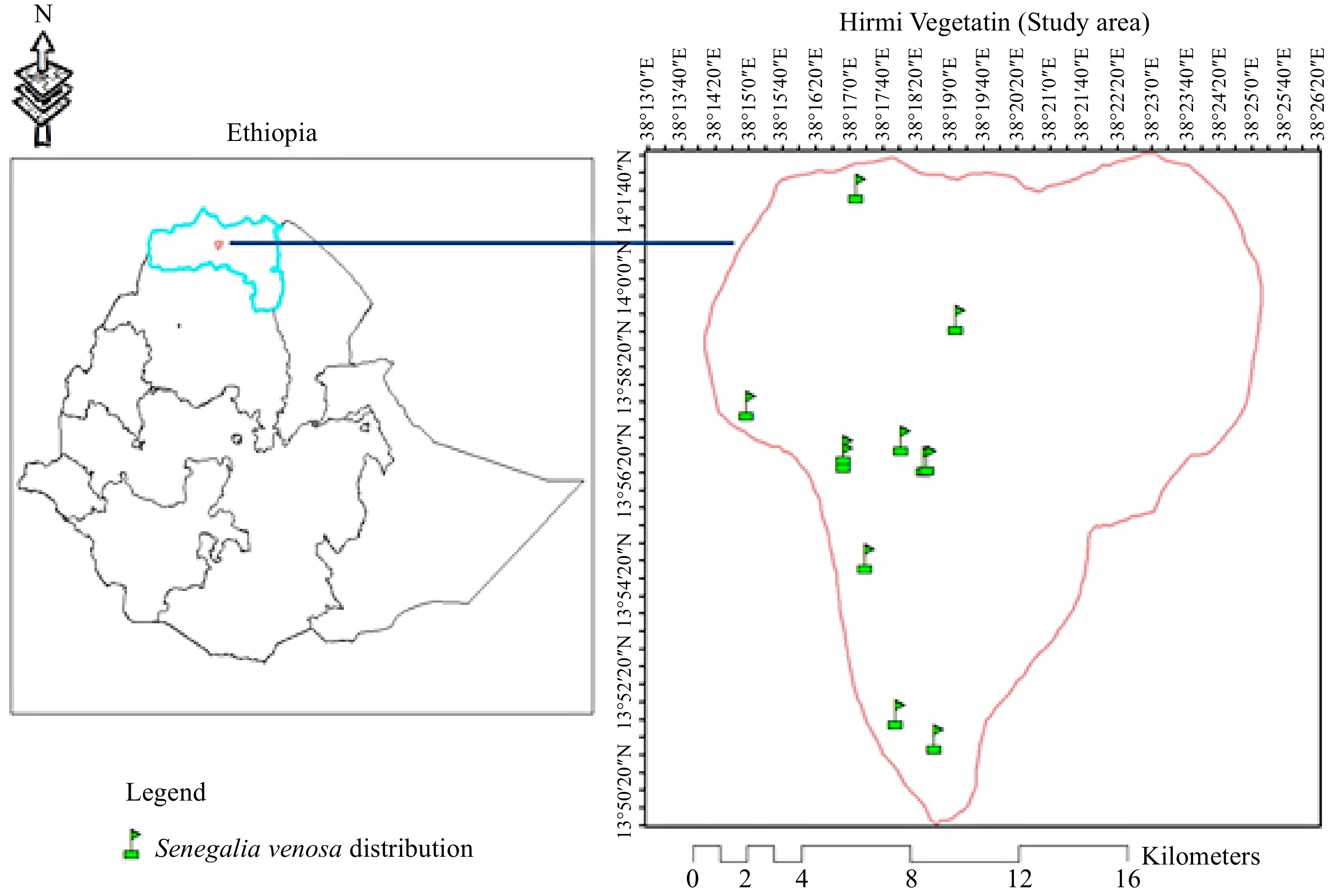
To collect the required data for this study, a thorough survey which helps to identify the location of the species was conducted in the 30,900 hectares of the study area. At every site where the species is located, GPS points were tracked. Accordingly, a total of 11 plots with a size of 10 m × 10 m were laid in the site where the species is found following a purposive sampling approach. At every sampling plot, data related to the species abundance and growth habit were computed. The species growth habit with a height of ≥ 2 m and DBH ≥ 2 cm was considered as mature Senegalia venosa whereas those with a height of 1−2 m and a height ≤ 1 m were considered as saplings and seedlings, respectively[3, 8]. Environmental or ecological information, including disturbance signs, geographical coordinates (longitude and latitude) and altitude were recorded in each sample plot.
Data analysis
-
The collected data were analyzed in different approaches. ArcGIS version 10.5 was used to track the geographical distribution and map the sites where the species was located. Following that, a map was plotted that shows the locations of the 11 spatial coordinate points of the sample plots. Vis-à-vis the disturbances factors, it was measured and scaled from 0 to 4 based on visible signs of vegetation disturbance parameters including cutting, debarking, grazing, fire and charcoal production signs following[3]. Accordingly, values were coded: 0 − for plots with no any disturbance, 1 − for sample plots with one disturbance factor, 2 − for sample plots with two disturbance factors, 3 − for sample plots with three disturbance factors, and 4 − for sample plots that have more than three disturbance factors.
The regeneration status of Senegalia venosa, was assessed by employing the total count of all seedlings, saplings and mature plant in each plot where the species were found following the techniques in[2]. Accordingly, the regeneration status was analyzed by calculating the density of seedlings, saplings and mature plants per the sample area as follows:
$\begin{aligned}&\rm Density\;of\; mature/seedling/sapling = \\&\rm\quad\dfrac{The\; number\; of\; mature/seedling/sapling}{Area\; of\; the\; sample \;in\; a\; hectare}\end{aligned}$ -
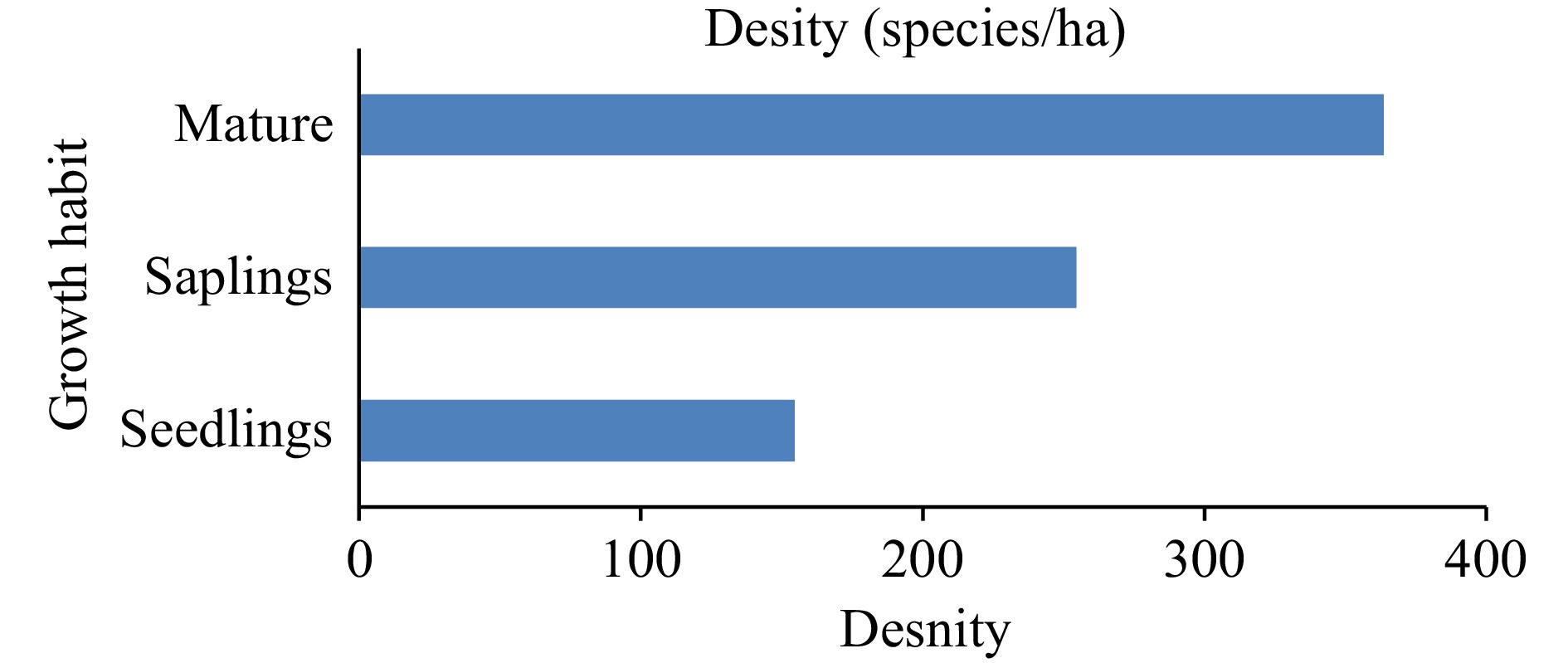
The research findings indicate that the distribution of Senegalia venosa was restricted to specific locations within the Hirmi forest vegetation ecosystem. It was located in just 11 sites (Fig. 2). Even in the sample plots where the species was found, it was sparsely distributed between altitudes of 1,110−1,900 meters above sea level. This was associated typically with the existing topography and anthropic impacts. The species appeared (Fig. 3) in most of the plots at elevations between 1,700 and 1,900. Out of the 0.11 hectare sample plot, 40 mature, 19 saplings and 14 seedlings of Senegalia venosa were recorded. Accordingly, the mature Senegalia venosa has the highest density (363.6 species/ha) followed by saplings (172.7 species/ha) and seedlings (127.3 species/ha).
Regeneration status of Senegalia venosa
-
The regeneration status of Senegalia venosa was characterized by computing the density of seedlings, saplings and maturity of the plant. A total of 40 mature Senegalia venosa as well as 28 saplings and 17 seedlings, were recorded in all sampled plots. In other words, the mature shrub of Senegalia venosa has the highest density followed by saplings and seedlings of the species (Fig. 4).
Major threats to the species
-
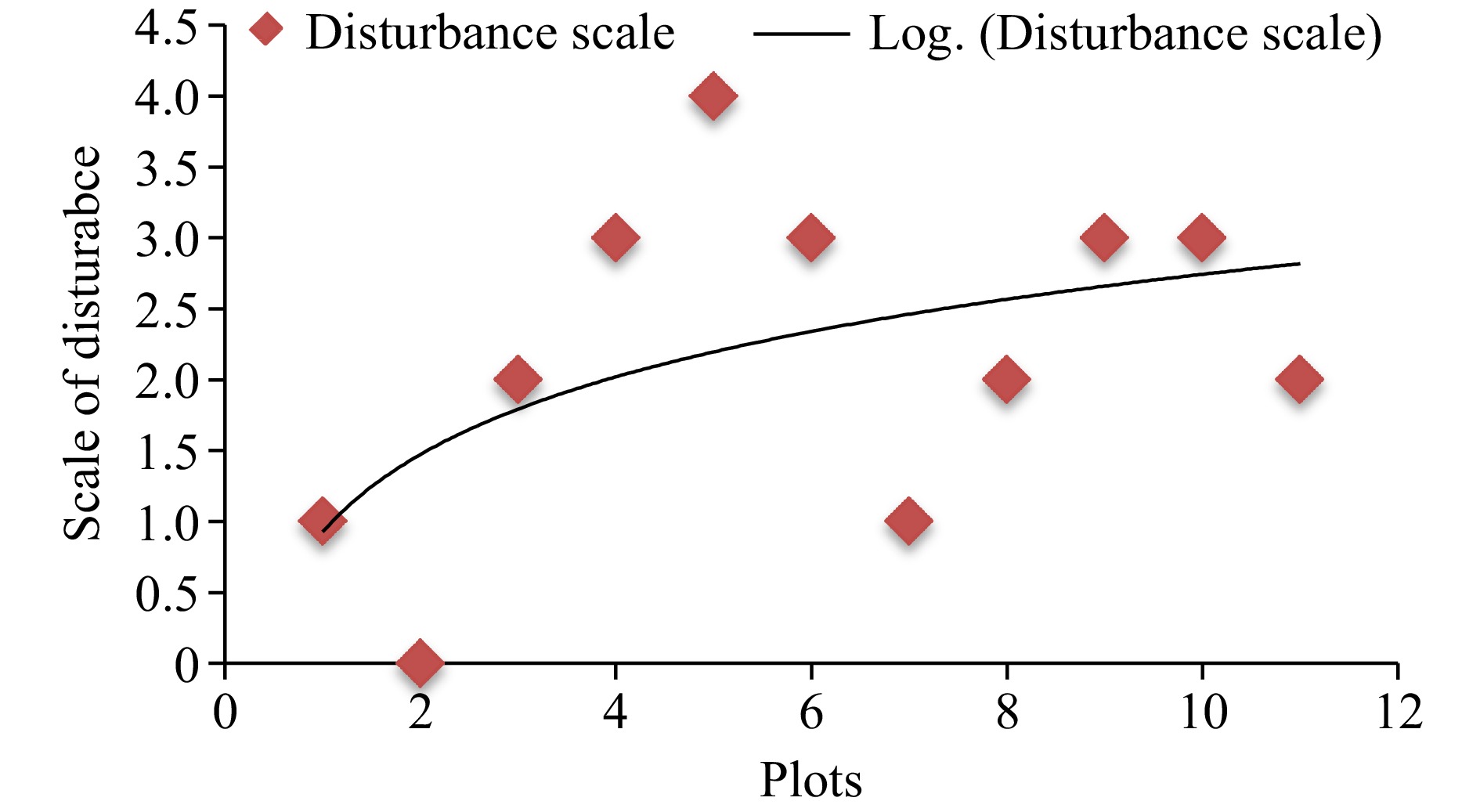
According to the interviews made with the local communities in the study area as well as the researchers' field observation, anthropogenic disturbance signs such as charcoal production and cutting were frequently revealed in around the sampled area. Besides, livestock grazing and trampling were additional threats to the study species seedlings and saplings. The second plot has no visible disturbance whereas plot 5 has the highest rate of disturbance (Fig. 5).
Regarding the types of disturbances (Table 1), there was a high intensity of livestock disturbances such as grazing and trampling in plots 3, 4, 7, 9, and 11. Anthropogenic disturbances such as cutting and charcoal production were revealed in the 1st, 4th, 5th, 6th, and 10th plots.
Table 1. Locations and types of disturbance in sampled plots.
Plot Latitude Longitude Types of disturbance 1 13.85504 38.31161 Cutting 2 13.86265 38.29898 No 3 13.91186 38.28886 Grazing 4 13.94372 38.2816 Cutting and grazing 5 13.94241 38.30817 Charcoal production & cutting 6 13.94287 38.30928 Charcoal production & cutting 7 13.94889 38.30065 Trampling 8 13.98725 38.31893 Cutting 9 13.981472 38.26925 Grazing & trampling 10 13.996350 38.326788 Cutting and cutting 11 14.02838 38.2858 Grazing -
The vegetation of the Hirmi woodland is dominated by species of Acacia, Terminalia, Combretum, Albiza, Ficus, and Oxythenanthera abyssinica. It is one of the few habitats in the country where the plant Senegalia venosa is found[4]. The potential vegetation of Ethiopia studies by Vivero et al. and Kelbessa & Demissew[9, 10] also shows that ecosystems such as the Hirmi dryland can harbour Senegalia venosa due to favourable geographic conditions. However, the result of the study shows that the distribution of the species is restricted in a few sites (11 sites) out of the 30,900 hectares of Hirmi woodland vegetation. This indicates that the species are under critically endangered status either due to the existing anthropogenic threats[4, 11] or climate changes[3]. In line with this study, the poor regeneration status of species is mainly associated with climate change, habitat suitability and anthropogenic disturbances[12].
Information related to the density ratio of seedlings, saplings and mature plants is used to determine the status of the regeneration of a given species and take pertinent conservation measures accordingly[13,14]. In this study density ratio density of Senegalia venosa was decreased from the mature of the plant to sapling and seedlings respectively. Based on the assessment criteria established by Khan et al.[2] & Khumbongmayum et al.[13], the vegetation's regeneration status is thus classified as poor. This outcome might be a result of the existing various disturbance factors, the demand of the species for medicinal value and the land use land cover dynamics due to agricultural expansions[4,15,16]. Additionally, because of the steep terrain where the study plant species is located, the soil is shallow, which could be difficult for seedling germinations. These findings were in line with a study by Wang et al.[17].
Different disturbances were recorded in the majority of the sampled plots. However, the plots found near the center of the study area were relatively less disturbed since they are less accessible by humans and animals relative to these plots found on the edge of the vegetation area. Moreover, it was shown that heavy livestock grazing and trampling had a detrimental effect on Senegalia venosa seedling and sapling distribution. A similar result was reported in the study area by Girmay et al.[4,11].
-
The Senegalia venosa's poor regeneration status and limited ecological distribution as a result of various factors. This implies the requirement of further investigation on its propagation, and creating favourable niche and microclimate for better establishment of the species. Besides, avoiding unwanted anthropogenic disturbances, livestock pressures and other potential threats are some of the pertinent techniques to overcome the existing challenges. Establishing nursery sites to grow the seedlings and replanting in the study area to improve the poor population structure and recruitment succession of the species is prioritized. To do this, thorough research and surveys should be undertaken to determine the species' seed-producing and ripening phase so that it can be harvested appropriately for both in-situ and ex-situ conservation through nursery establishment. Additionally, this study underscores the necessity of raising the local community's understanding of how to manage and utilize plant species including Senegalia venosa in the study region sustainably.
-
The authors confirm their contribution to the paper as follows: study conceptualization, data curation, formal analysis and Writing of original draft: Girmay M, Gebrehiwot K; supervision, review & editing: Negussie A, Warkineh B; resources: Alemu S, Gidey T; methodology and software: Wondimu D, Kahsay G. All authors have reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We gratefully acknowledge the financial support from Mohamed bin Zayed Species Conservation to conduct this study.
-
The authors declare that they have no conflict of interest.
-
Received 15 December 2023; Accepted 18 April 2024; Published online 4 June 2024
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Girmay M, Gebrehiwot K, Negussie A, Warkineh B, Alemu S, et al. 2024. Distribution, regeneration status and threats to Senegalia venosa (Hochst. ex Benth.) Kyal. & Boatwr. in Hirmi Dryland areas of Northern Ethiopia. Tropical Plants 3: e018 doi: 10.48130/tp-0024-0017
Distribution, regeneration status and threats to Senegalia venosa (Hochst. ex Benth.) Kyal. & Boatwr. in Hirmi Dryland areas of Northern Ethiopia
- Received: 15 December 2023
- Revised: 17 April 2024
- Accepted: 18 April 2024
- Published online: 04 June 2024
Abstract: Based on the IUCN threat level, Senegalia venosa is one of the species that has endangered status and is restricted in Tigray and Gonder drylands. The purpose of this study was to identify the distribution of the Senegalia venosa species, assess its regeneration status, and map its location for potential future conservation efforts. Plant attributes and environmental data (including disturbance factors) were collected from plots (10 m × 10 m) where the species was found. Accordingly, Senegalia venosa was found in 11 sites in the study area. The number of mature, sapling, and seedling individuals in the sampled area was counted and density was computed. The mature plant of the species had the highest density (363.6 stems/ha) in terms of the species' regeneration status, followed by the sapling (254.5 stems/ha) and seedling (154.5 stems/ha). This implies Senegalia venosa has poor regeneration status, which could be associated with revealed disturbance factors including charcoal production, cutting and grazing.
-
Key words:
- Conservation /
- Drylands /
- Endangered species /
- IUCN /
- Senegalia venosa