-
Vegetables are the foundation of a healthy diet because they contain essential nutrients and bioactive compounds. The 2022 Chinese Dietary Guidelines[1] recommend a daily intake of > 300 g of fresh vegetables. Rapid urbanization has allowed for improvements in the standard of living and changes in lifestyle, which have fueled the demand for high-quality vegetable products. Vegetable quality is a dynamic combination of physicochemical properties and evolving consumer perceptions, including organoleptic, nutritional, and bioactive components[2]. Overall quality is a key factor in fresh vegetable consumption, which influences consumer purchasing[3,4]. Previous studies have highlighted the protective roles of vegetable consumption against many chronic diseases, such as ischemic and hemorrhagic strokes, ischemic heart disease, and certain cancers[5−8]. Therefore, greater effort is needed to improve the appearance, taste, aroma, texture, and nutritional value of vegetable products to meet the increasing demand for high-quality vegetables.
Two important and effective approaches exist for improving the quality of vegetables: (1) breeding for high-quality traits and (2) cultivating them in suitable environments to promote the accumulation of nutritional compounds[9]. Breeding is the most commonly used method for improving vegetable quality. The goal of breeding is to develop varieties with exceptional and distinctive characteristics that can influence purchasing decisions, such as size, shape, color, taste, nutrient concentrations (e.g., proteins, sugars, lipids, and fiber), levels of health-promoting compounds (e.g., polyphenols, sesquiterpene lactones, and carotenoids), and ease of processing (e.g., harvesting, milling, baking, malting, and blending)[10]. Recently, rapid advancements in cost-effective and high-throughput sequencing technology has greatly contributed to vegetable breeding, enriching our understanding of the genetic basis and regulatory network of improved vegetable quality and further promoting the development of vegetable breeding[11]. Accurate phenotypic measurements and quality screening of large genotypic materials are at the core of these programs[12,13]. Most quality traits in vegetables (mainly primary and secondary metabolites) are controlled by multiple quantitative trait loci (QTLs), and their expression is influenced by both genotype and environmental factors[14−17]. In particular, a stable environment is essential for identifying metabotypes, which is the most time-consuming step in breeding[14−17]. In contrast, cultivation involves the creation of ideal conditions to achieve the optimal phenotype from selected genotypes through breeding.
Environmental factors and agronomic practices can affect the quality of vegetables, especially their color, taste, aroma, and the accumulation of primary and secondary metabolites[17−20]. However, in open and natural environments, such factors are dynamic and variable, which can result in unstable vegetable quality[17,21]. Additionally, environmental and agronomic influences are interrelated and highly unpredictable across different growing trials, which makes it difficult to replicate and produce robust phenotypes and metabotypes under different climatic conditions. Trials under interclimatic conditions are required to test and certify new varieties. Therefore, high-precision, controllable, and stable environments are important for achieving replicable and robust metabotypes and stably high-quality vegetables[17].
HTML
-
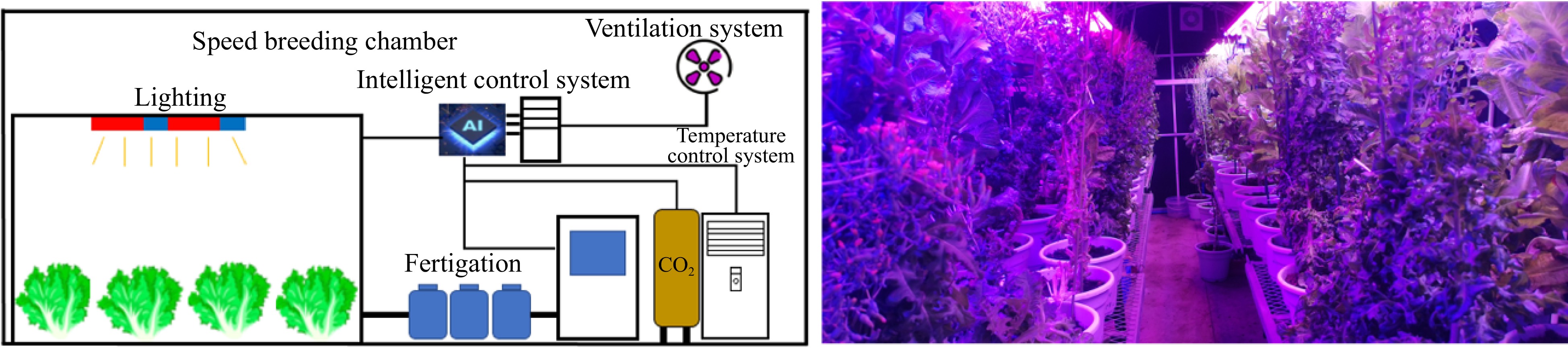
Plant factory technology (PFT) provides a sustainable vegetable cultivation approach and is one of the most advanced agricultural systems that allows for the precise control of environmental factors and fertigation[21−24]. To achieve stable vegetable quality, a powerful strategy of breeding and cultivation practices using PFT was developed in this study (Fig. 1). A lettuce-based model was employed as a case study to improve the quality of leafy vegetables by applying PFT.
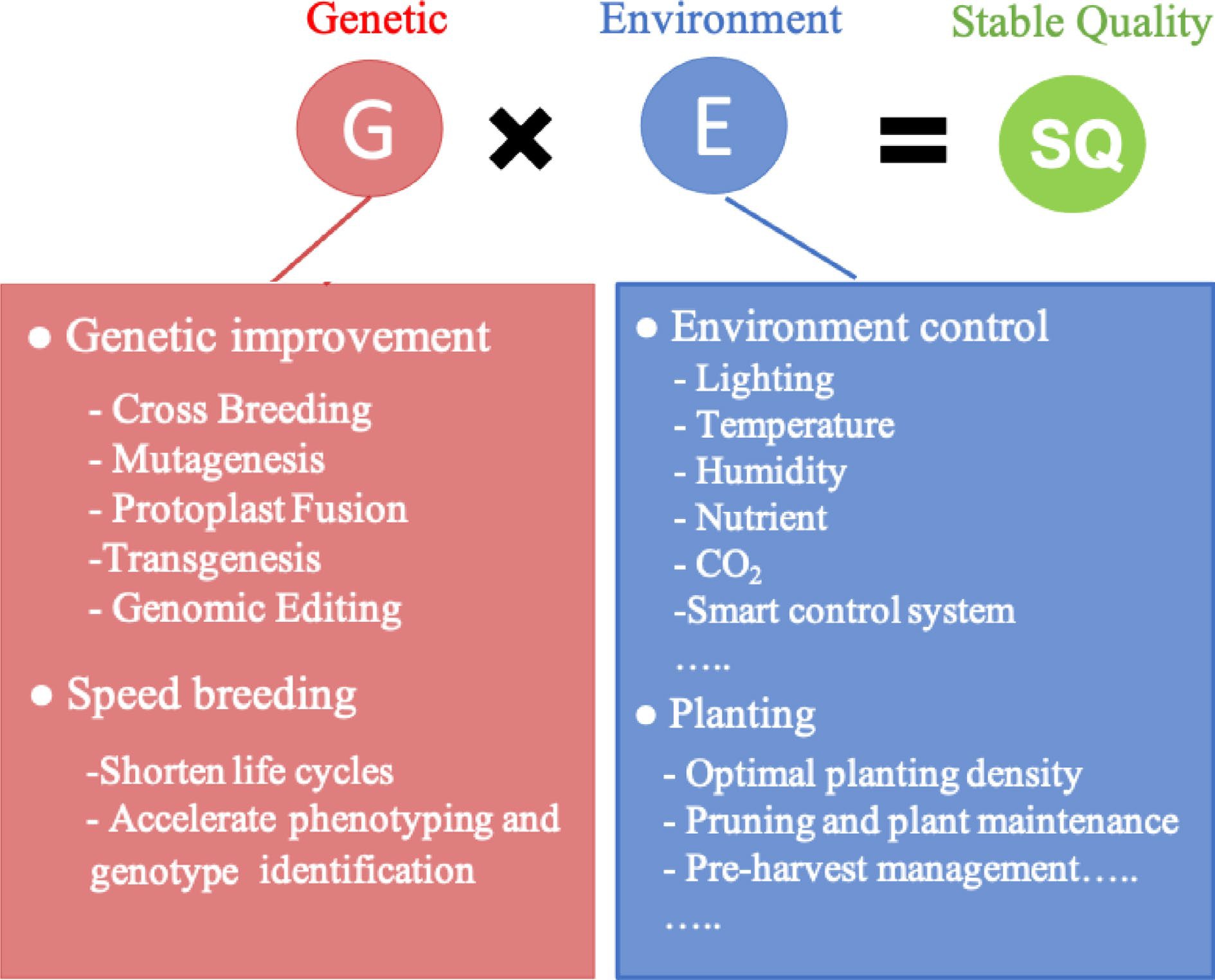
Figure 1.
Stable vegetable quality. PFT promotes vegetable breeding and helps obtain new varieties. Growing new cultivars using PFT can improve quality.
PFT contributes to vegetable breeding by facilitating breeding and selection. For example, plant factories can be used as a speed-breeding facility to shorten the life cycle and induce early flowering and maturity by manipulating the optimal day length, light intensity, temperature, and photoperiod to reduce the time required to produce each breeding generation[25−27]. Hot pepper plants blossom earlier under a photosynthetic photon flux density of 420 µmol/m2/s with a 12-h photoperiod, and this reduces reproductive cycles and produces up to four generations per year[28]. The parallel integration of plant factory-based vegetable speed-breeding facilities with other technologies, such as crossbreeding, mutagenesis, transgenic breeding, genome editing, and genomic selection can greatly accelerate vegetable improvement[27,29]. During the selection period, it is convenient and efficient to grow breeding candidates in a plant factory-based facility, as this allows for the precise phenotyping of specific traits through high-throughput phenotyping, candidate-gene screening, and candidate-genotype screening. This accelerates the breeding process by shortening generations and producing robust phenotypes in a stable environment[27,30]. For example, PFT has been used in the molecular breeding of tomatoes by crossing a transgenic tomato line expressing the miraculin gene driven by the CaMV 35S promoter with a dwarf tomato to develop cultivars suitable for miraculin production in a closed cultivation environment[31]. Overall, PFT reduces breeding time and provides a stable environment for phenotype and metabolite identification, which aids in stably obtaining high-quality vegetables.
Environmental factors are also vital in the production of high-quality vegetables. Several studies have demonstrated a positive correlation among light intensity, spectral quality, and phytochemical production, particularly in microgreens and vegetables grown under controlled conditions[32,33]. Recent studies have reported that ultraviolet (UV)-A, UV-B, and blue light activates protective pathways in plants, leading to an increased accumulation of health-promoting compounds, such as flavonoids and carotenoids[34,35]. Chowdhury et al.[19] revealed that low temperature is one of the most important environmental variables affecting kale development and glucosinolate content; the optimum growth temperature for kale was found to range from 20–23 °C, while glucosinolate concentration was high during the early stages of growth at low temperatures, from 14–17 °C. Relative humidity (RH) is another important environmental factor that contributes to vegetable quality. In lettuce, low RH treatment has improved vegetable quality, while low nighttime temperatures have increased total polyphenol and anthocyanin levels, as well as antioxidant activity[36]. Lettuce grown at 41% RH yielded higher levels of phytochemicals than those grown at 77%[37]. Similarly, peppers grown at 38% RH exhibited higher carotenoid concentrations than those grown under higher RH conditions[38]. Meanwhile, biostimulant-based nutrient solutions, such as fulvic acid and seaweed extracts have improved the biomass, quality, and nutrient uptake of hydroponically cultivated lettuce[39].
In addition to environmental factors, agronomic practices are important factors that influence plant growth and development. Wu et al.[40] demonstrated that planting 1,450 plants/m2 led to a higher yield, increased generation of reactive oxygen species, enhanced total chlorophyll and net photosynthetic rates, and reduced malondialdehyde content in perilla sprouts compared to other groups. Managing vegetable growth is important. Pruning and plant maintenance enhance photosynthesis, flowering, and fruiting, thereby promoting the production of high-quality vegetables[41]. Pre-harvest measurements are also useful for obtaining high-quality vegetables. For example, pre-harvest nitrogen limitation and continuous lighting have been shown to effectively produce lettuce with reduced bitterness and higher sugar content[42]. Short-term exposure to low temperatures and high light intensity can further enhance the quality and flavor of lettuce at harvest (unpublished data). Thus, the combination of appropriate environmental settings and agronomic measures, together with new high-quality vegetable varieties, can lead to stable vegetable production.
-
Compared to wild lettuce, Lactuca serriola, cultivated lettuce displays substantial phenotypic variation in leaf shape, nodulation, and stem enlargement, as well as in chlorophyll and anthocyanin content and other traits, owing to continuous cultivation and domestication[43−45]. The yield and productivity of lettuce varieties have greatly increased in recent years due to the efforts of breeders (e.g., the 'Haonong 1' cultivar bred by the Shanghai Agrobiological Gene Center, which has a fresh weight of 0.43 kg/plant that results in a yield of 30,000 kg/hm2)[46]. However, improving the nutritional value and overall appearance of lettuce to meet the new and more demanding requirements of consumers is still a major challenge that requires us to consider the complex genetic and physiological bases and lengthy process required to develop new lettuce varieties. Here, lettuce was used in a case study of high-quality breeding for improving the quality of vegetables treated with PFT. As shown in Fig. 2, PFT helped in the breeding process and greatly shortened the time required to obtain a new variety. In this case study, lettuce required 90 d for one life cycle, whereas 180 d were required under open-field conditions. Lettuce lines were crossed with other lines and hybrid offspring were selected from PFT-based breeding and open fields. Using this approach, the breeding cycle was reduced by more 50%. As PFT-based facilities are suitable for both transgenic and mutagenic breeding, they were used to obtain the next generation.
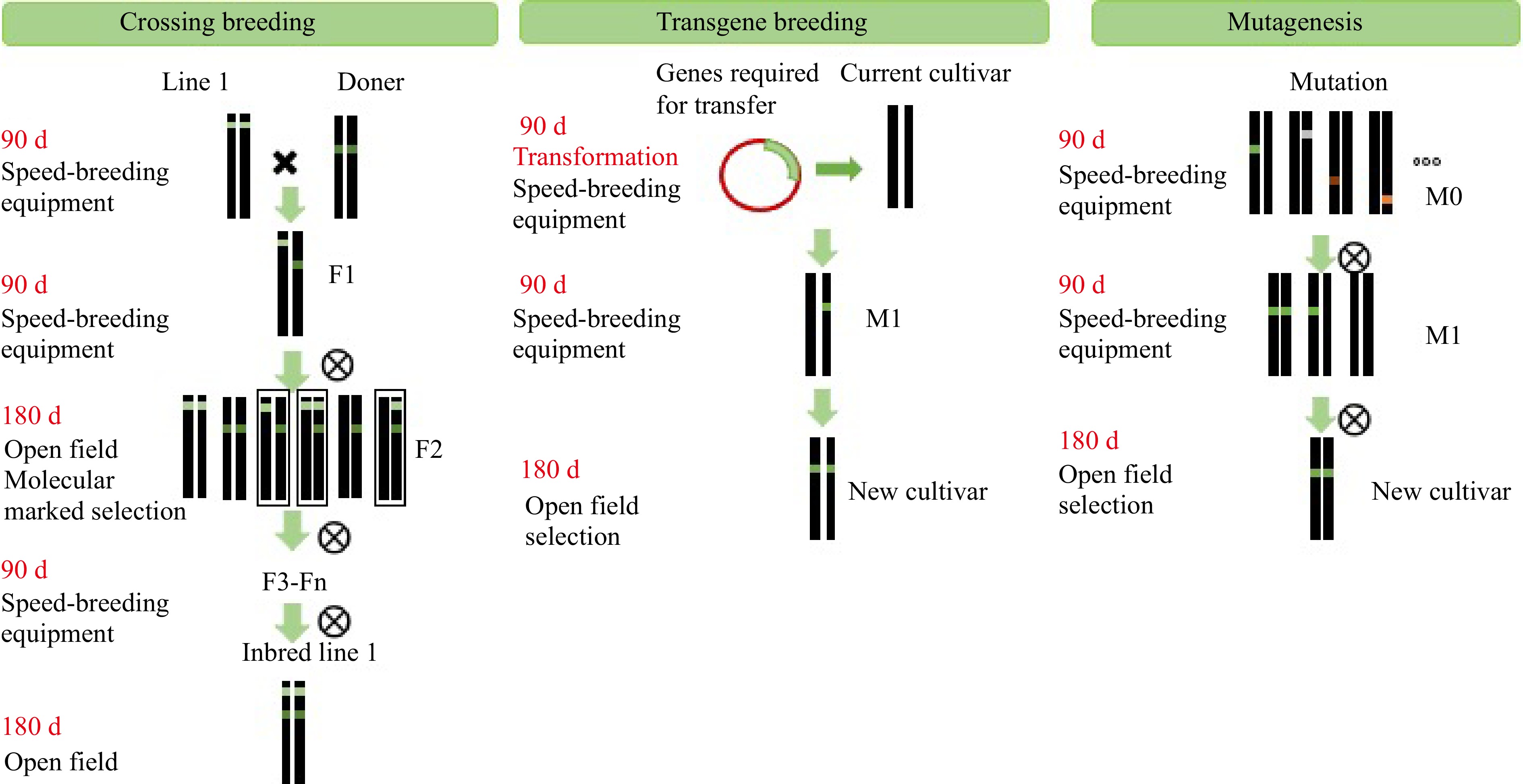
Figure 2.
Proposed strategy to improve the quality and breeding of lettuce based on PFT. Parent plants and lettuce materials were cultivated in a PF environment to accelerate the growth cycle and promote the development of a new lettuce cultivar.
In this study, a PFT-based breeding process was used to develop a new lettuce cultivar, 'Zhongsheng No. 1', which has high vitamin C content. This cultivar was developed by purifying a line from a natural variant with high ascorbic acid content. In this case, the natural variant was grown in an open field for selection and in a speed-breeding chamber equipped with a phenotypic platform for seed harvesting. The environmental conditions in the speed-breeding chamber were established as follows: LED lighting was used within the photosynthetically active radiation band (400–700 nm); the photoperiod was set to 22 h at 22 °C, with 2 h of darkness at 17 °C; RH was set to 60%–70% (Fig. 3). Due to practical considerations in the experimental design, two generations were cultivated in a PFT-based speed-breeding apparatus capable of theoretically producing three or four generations. After six generations spanning 3 years, 'Zhongsheng No. 1' was developed. The ascorbic acid content (24.1 mg/100 g fresh weight) of 'Zhongsheng No. 1' lettuce was higher than most crisphead, loose-leaf, and butterhead types[7].
Growing new lettuce varieties using PFT via environmental manipulation and pre-harvest management can improve lettuce quality is many ways. A 1:1 red-to-blue-light mixture, combined with nitrate deficiency, can induce anthocyanin accumulation in lettuce[47], as can pre-harvest nitrogen limitation and continuous light[42]. We used these pre-harvest approaches and environmental-control measurements to build a quality-based model by practically optimizing for high-yield and high-quality objectives. A predictive model of phenotypic (e.g., red-green-blue, hyperspectral, and infrared data) and quality-related (e.g., chlorophyll, flavonoids, and anthocyanins) data was developed, and the accuracy of the predictive model was optimized through machine learning to achieve high-precision predictions. A model that considers the influence of environmental factors on the phenotype and accumulation of quality components (starting with individual factors and gradually introducing multiple coupled factors) was developed. Artificial intelligence techniques were then utilized to iteratively optimize the simulation accuracy and directly predict lettuce quality from environmental factors.
-
Vegetable breeding is characterized by continuous innovation and the development of new cultivars that meet the requirements of growers and consumers. Nevertheless, breeding new vegetable cultivars is time-consuming and requires large amounts of human and material resources. Breeders and seed companies aim to achieve highly efficient and short breeding periods with new cultivars. PFT is a novel tool to obtain stably high-quality vegetables by accelerating breeding and cultivating plants effectively. Here, we highlight the potential of PFT in improving the quality of vegetables, focusing on appearance, health-promoting phytonutrients, and bioactive compounds associated with flavor and taste.
PFT can be used as a breeding device to assist in speed-breeding and candidate selection across different generations owing to its stable environmental control. PFT also enables breeders to develop crops with traits influenced by environmental factors. Traits such as nutrient efficiency, space utilization rate, and productivity can be targeted through molecular breeding programs and by selecting and developing crops with desirable characteristics, breeders can contribute to the high-quality development of the vegetable breeding industry, thereby helping to ensure food security for a growing global population. Moreover, within a PFT environment, breeders have the flexibility to create traits that meet consumer demands, such as a visually appealing appearance, enhanced nutrient content, and diverse and enjoyable flavors. Thus, studying the effects of specific environmental factors on the appearance and quality of vegetables when using PFT may provide insight into the pathways of gene transcription factors and metabolites[17]. It is also important to note that PFT-based speed-breeding chambers are increasingly used to accelerate parts of breeding or research programs that would benefit from accelerated generational progress (e.g., wheat, rice, and soybean)[25,27], and are now being employed for vegetable breeding. Breeders use speed-breeding chambers to continuously breed generations every year, regardless of environmental or geographical conditions. Integrating PFT with other breeding technologies could greatly shorten breeding progress. Combining modern breeding techniques, such as genetic engineering and marker-assisted selection, with rapid breeding allows for the efficient selection of elite genotypes with novel features, including higher yields, superior qualitative traits, and biotic/abiotic stress resistance.
Multiple studies have highlighted the impact of environmental factors, in addition to breeding, on the primary and secondary metabolites of vegetables, and have provided guidance for enhancing vegetable quality using PFT. For instance, by studying the relationships among agricultural practices, coupled environmental effects, and phytochemical concentrations in vegetables, the quality of vegetables growing using PFT can be improved by determining the influence of agricultural practices and environmental changes on the accumulation of specific phytochemicals. In this study, an accurate quality model was developed based on input resources (variety, environment, and farming practices) and trained the model using deep learning algorithms and then optimized the model iteratively through cultivation practices using PFT to generate data on specific varieties, environmental factors, and fertigation conditions to achieve stable and high-quality lettuce cultivation. Customers and breeders can support the development of vegetable quality models by providing verified data and feedback on their prediction accuracy (Fig. 4).
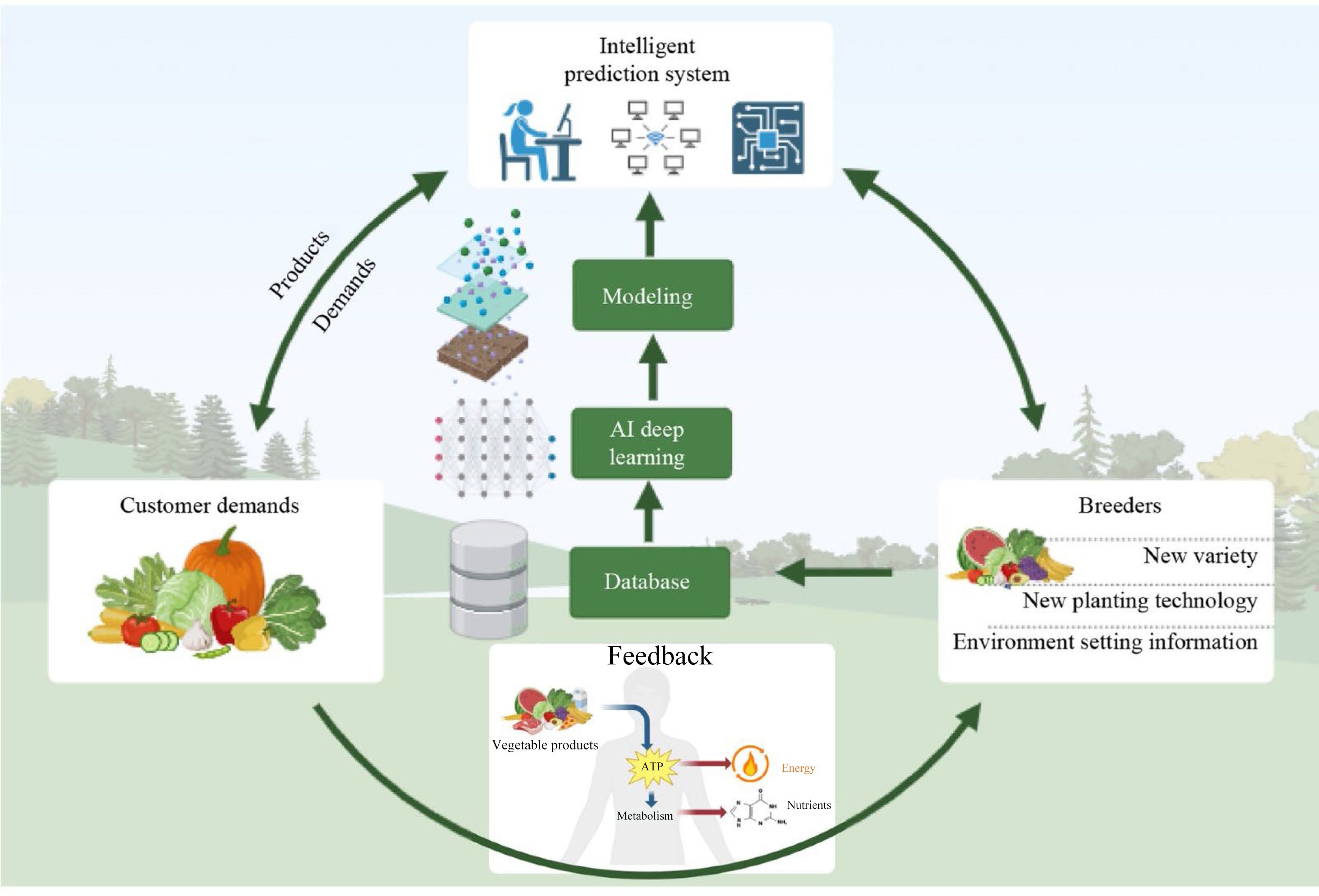
Figure 4.
Precise vegetable quality prediction system for precise control of nutritional contents in vegetables.
PFT-based strategies for enhancing vegetable quality have some notable limitations that influence the economic feasibility and scalability of PFTs in horticultural practice. A major obstacle to the use of PFT-based speed-breeding chambers is their high initial cost, which includes expenses for building construction, equipment installation, cultivation, lighting systems, environmental control, fertigation, and smart control systems. In this case, government support and policy incentives could be provided to encourage large-scale production of plant factories, thereby reducing unit production costs by scaling up production. Also, government capital or venture investors could invest directly, as plant factories used for high-quality vegetable production are forward-looking with public benefits and long-term market value. In addition, multiple agricultural producers can collaborate and share PFT facilities, which would allow construction and operational costs to be shared and increase resource utilization efficiency. In terms of sustainability, the PFT strategy faces substantial challenges owing to its high operating costs and energy consumption. However, with the development of clean energy, these limitations will eventually be overcome through the widespread adoption of renewable energy sources, including nuclear, solar power, wind energy, and hydrogen power[21]. Also, energy consumption costs could be reduced through the use of energy-saving technologies, such as LED lighting, smart environmental control, and fertigation systems[21]. Moreover, implementing closed-loop agriculture systems could also reduce operating costs.
Managing a PFT facility requires expertise in horticulture, engineering, and automation, as well as the continuous monitoring and adjustment of environmental parameters, which can be challenging for inexperienced growers. Solutions to overcome the complexity of PFT include training and education, technical support, data monitoring and analysis, automation, and collaboration, which will enable growers to better manage and operate PFT facilities. Despite these challenges, ongoing advancements in technology and operational practices are aimed at addressing these issues and making PFT more efficient, cost-effective, and environmentally sustainable. Thus, PFT has great potential to overcome these limitations and promote the development of a high-quality vegetable production.
-
The authors confirm contribution to the paper as follows: study conception and design, draft of the manuscript: Song B, Li Y, Yang Q, Yang X; data collection, statistical analyses, manuscript revisions: Zhang Q, We S, Peng J; manuscript review and editing: Zhang L, Huang T, Escalona Contreras VH. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
We appreciate the constructive suggestions of our colleague, Dr. Fang Wang. This work was supported by the Sichuan Science and Technology Program (2022NSFSC1719, 2023NSFSC0164 and 2023NSFSC0168), the Local Financial Project of the National Agricultural Science and Technology Center (NASC2023TD01, NASC2023ST07, NASC2023TD10 and NASC2024TD03), and by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (ASTIP-IUA-2023002).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Li Zhang, Tao Huang
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Zhang L, Huang T, Zhang Q, Wei S, Escalona Contreras VH, et al. 2024. Plant factory technology as a powerful tool for improving vegetable quality: lettuce as an application example. Vegetable Research 4: e017 doi: 10.48130/vegres-0024-0015 |