-
Tomato (Solanum lycopersicum) is a major horticultural model crop worldwide for studying fleshy fruit development, ripening, and the molecular basis of commercially important traits, such as flavor, aroma, and nutritional quality[1]. Sugar is one of the important components of fruit quality (especially sweetness), flavor and aroma[2]. In addition, sugar is also regarded as an important source of energy storage, osmolytes, signals, carbon skeletons, and transport molecules[3]. In most higher plants, sucrose functioned as the major form of photoassimilates is produced in source leaves and transported to non-photosynthetic sink organs via the phloem, such as the fruits and seeds[4,5]. Therefore, the sugar unloading from source leaves to sink organs is important to the organ growth and development of plant organs, especially for fruits with high level of sugars[6].
Sugar allocation from source leaves to sink organs is realized through three key steps: namely phloem loading, phloem transport, and phloem unloading[7]. During the early development of tomato fruits, the symplasmic pathway is employed. While in the hexose accumulation, the symplasmic pathway switches to the apoplasmic pathway[8]. Many important sugar transporters have been identified in tomato[9].
In tomato, LeSUT2 may play a role in the retrieval of sucrose from sieve element/companion cell (SE/CC) rather than the release of sucrose to the fruit apoplast. LeSUT2 (located in phloem SE, encoding sucrose transporter) antisense lines produced the reduction of unloaded sugar, which suggests LeSUT2 is directly or indirectly involved in phloem unloading[10,11]. Moreover, three hexose transporters (LeHT1, LeHT2, and LeHT3) are mainly expressed in storage parenchyma cells, and play a key role in hexose accumulation of storage parenchyma cells[12].
In plants, SWEETs function as bidirectional uniporters that mediate the influx and efflux of sugars across cell membranes[13]. It has been shown that SWEET transporters are involved in the release of sugars to the apoplast[14]. Besides key roles in phloem-loading, SWEETs also participate in the unloading of sugars in sinks, as observed in fleshy sink fruits. PuSWEET15 functions as a sucrose transporter and overexpression of PuSWEET15 increased sucrose content of pear fruit[15]. MdSWEET15a and MdSWEET9b present in the locus of QTLs for sugar content are two possible candidates regulating apple fruit sugar accumulation[13]. ClSWEET3 is highly induced during watermelon fruit development and overexpression of ClSWEET3 increases the sugar contents of fruit[16]. CsSWEET7a is localized in companion cells and involved in phloem unloading of cucumber fruit by releasing hexoses to the apoplasmic space[7]. Recently, several studies have reported the functions of SlSWEETs family members. SlSWEET1a regulates the fructose:glucose ratio in the development fruit[6]. During tomato fruit development, SlSWEET7a interacted with SlSWEET14 to regulate sugar storage and transport[17]. In red ripening tomato fruits, SlSWEET12c is highly expressed and takes part in sucrose effusing[18]. SlSWEET15 is highly expressed in developing fruits and may mediate sucrose efflux from phloem and seed coat to the development fruit and embryo[19]. These findings suggest SWEET genes play crucial roles in sugar unloading in sink organs.
Sucrose metabolism is also vital to sugar accumulation in tomato fruit. Upon unloading in sinks, sucrose is hydrolyzed into hexose or their derivatives by invertase or sucrose synthase (SS)[20]. Invertase is composed of three different subgroups, named cell wall invertase (CWIN), vacuolar invertase (VIN), and cytoplasmic invertase (CIN). Among them, CWIN is likely as a functional component of phloem unloading[5]. Therefore, CWIN and SWEET transporters are both crucial in sugar allocation, however the relationship between CWIN and SWEETs during sugar allocation have hardly been reported[21]. Compelling evidence has revealed the regulation of SWEET is closely related with CWIN activities, silencing of CWIN could lead to the downregulation of SWEET gene expression (such as SWEET8) during ovule development[22]. The expression of SlSWEET12c was enhanced by the elevated CWIN activity during tomato fruit development[21]. In some fruits (such as grapes, kiwifruit, and citrus), sucrose synthase has higher enzyme activity than invertase and more important than invertase for sugar accumulation[23]. Moreover, previous reports also show coordinate expression of sucrose synthase (VvSS3) and SWEET (VvSWEET15) transporter promotes the hexose accumulation in grape berries[23]. Based on the above review, a synergistically regulatory network composed of sugar metabolism-related enzymes (CWIN and SS) and SWEET transporters have been established, which determine the sugar allocation of fruits. SWEETs have been revealed recently that can regulate the CWIN activity in tomato fruit[17]. However, it remains unclear whether or how SWEETs regulate SS activity in tomato fruits.
In our previous study, SlSWEET14 was shown to function in regulating sugar transport and storage in tomato fruits[17]. Therefore, we have constructed a yeast cDNA library of tomato fruits at different periods with a split-ubiquitin membrane system. By using SlSWEET14 as the bait protein, SlSWEET10a was identified as the potential interaction protein. In this study, we report a new plasma membrane-localized sucrose transporter SlSWEET10a, belonging to clade III. Function analysis of SlSWEET10a, we found it involved in sucrose unloading, as well as sucrose metabolism by affecting sucrose metabolism-related enzymes (CWIN and SS) in tomato fruits. What's more, SlSWEET10a interacted with SlSWEET14, which might provide a molecular basis for better understanding the roles of SWEET transporters in sugar allocation in fruit crops.
-
Wild-type tomato 'Solanum lycopersicum Micro-Tom' (MT) was used and grown at 25 °C with a 16 h/8 h photoperiod. Roots, stems, young leaves (the terminal leaflet < 2 cm), mature leaves (the terminal leaflet > 4 cm), and flowers (the first day of anthesis) from 6-week-old plants, as well as sepals, fruit stalks, mature green (MG) fruits (after 35 d of flowering), red ripe (RR) fruits (after 50 d of flowering), and the seeds of RR fruits from 3-month-old plants were collected from MT for expression analysis. For each tissue type, one sample was collected and pooled from at least three plants. Fruits and leaves from at least five plants were collected at the mature green (MG) and red ripe (RR) stages for sugar, starch, and enzyme activity analysis, and at least two fruits of each plant were collected and pooled, samples from each plant as one biological replicate. All samples were immediately frozen in liquid nitrogen, and stored at −80 °C for further use.
Expression analysis
-
Various tissues samples (0.2 g) were ground into powder to extract total RNA. A Trizol reagent (Tiangen, Beijing, China) was used according to the manufacture's introductions. The cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara, Dalian, China) following the manufacturer's instructions. Quantitative real-time PCR assays were performed as described previously[24]. The tomato housekeeping gene ACTIN was used as an internal control[25].
GUS assay
-
The SlSWEET10a promoter sequence of (1,839 bp upstream to ATG) was cloned into pBGWES7.0 to generate pSlSWEET10a: GUS construct. The recombination vector was transformed into to 'Micro-Tom' to generate transgenic plants. Various tissues were examined using a GUS assay kit (Real-times, Beijing, China) as described by the manufacturer's instructions. The GUS activity was observed and imaged by a Nikon SMZ800 stereomicroscope.
Yeast two-hybrid assay
-
The CDS sequences of SlSWEET10a and SlSWEET14 without/with ATG codon and stop codon were cloned into the pBT3-STE bait vector and pPR3-N prey vector using the SfiI site, respectively. Recombinant vectors were introduced into yeast strain NMY51. The assays were performed as described previously[26].
BiFC assay
-
As described previously, the CDS sequences (without stop codons) of SlSWEET10a and SlSWEET14 were introduced into pXNGW and pXCGW vectors[27]. The fusion proteins were transformed into Agrobacterium tumefaciens strain GV3101, which were used for Agrobacterium-mediated transient transformation of N. benthamiana leaves. The fluorescence signals were detected 2 d later with a confocal laser scanning microscope (Leica SP8, Germany) at 488 nm for excitation wavelength and 500−572 nm for emission wavelength.
Subcellular localization assay
-
The CDS sequences of SlSWEET10a and SlSWEET14 without stop codons were cloned into pCAMBIA1302-GFP and pCAMBIA1302-mCherry vector with KpnI site, respectively, to produce the SlSWEET10a-GFP and SlSWEET14-mcherry fusion protein. Recombinant vectors were introduced into A. tumefaciens strain GV3101 and further used to be transiently expressed in N. benthamiana leaves. A known plasma-localized aquaporin AtPIPA2 was used as positive control[28]. The fluorescence signals were detected with a confocal laser scanning microscope (Leica SP8, Germany). The argon laser excitation wavelength was 488 or 561 nm, and the emission wavelength was 500−572 or 605−635 nm.
Firefly luciferase complementation imaging assays
-
The CDS of SlSWEET10a and SlSWEET14 was cloned into pCAMBIA1300-nLUC and pCAMBIA1300-cLUC, repectively. The infused vectored were transformed into Agrobacterium tumefasciens GV3101, respectively. Then the infused stains were co-transformed tobacco plant leaves. After 3 d, the leaves were treated with 0.1 mmol/L potassium luciferin and were used to capture the firefly luciferase (LUC) image with the plant fluorescence image system.
Heterologous expression of SlSWEET10a in yeast
-
The yeast (Saccharomyces cerevisiae) mutant strain EBY.VW4000 and SUSY7/ura3 were used to test the hexose transport activity and sucrose transport activity, respectively. The CDS sequence of SlSWEET10a was cloned into pDR195 vector with XhoI and BamHI sites, which was further transformed into mutant yeast strains. The transformants were selected on solid synthetic deficient (SD)/-uracil medium containing 2% (w/v) maltose or glucose for further analysis. Hexose transport activity was monitored on an SD/-uracil medium containing 2% (w/v) maltose or glucose. Sucrose transport activity was monitored on SD/-uracil medium containing 2% (w/v) glucose or sucrose. Growth of yeast cells was photographed after 3−4 d at 30 °C.
Agrobacterium-mediated tomato plant transformation
-
The CDS sequences of SlSWEET10a and SlSWEET14 without stop codons were cloned into the pCAMBIA3301-LUC vector carrying the CaMV35S promoter with SacI and XmaI sites. The recombinant plasmids were transformed into A. tumefaciens strain GV3101. The transformation was performed as described previously[29]. The positive plants were selected by PPT (glufosinate-ammonium, an herbicide) resistance and PCR analysis in the T1 generation. SlSWEET10a and SlSWEET14 lines in the T2 generation were used for functional study.
Virus-induced gene silencing (VIGS)
-
VIGS was performed as described previously with slight modification[6]. The 300 bp specific fragment of SlSWEET10a was cloned into a pTRV2 vector with a KpnI site to produce the pTRV2-SlSWEET10a construct. TRV1, TRV2 and pTRV2-SlSWEET10a were transformed into A. tumefaciens strain GV3101. Transformants were grown overnight at 28 °C in Luria-Bertani (LB) medium (50 mg/L Kanamycin and 50 mg/L Rifampicin, 10 mM MES, 20 μM acetosyringone). The cells were collected and suspended to OD600 of 1.0 in the infiltration buffer (10 mM MgCl2, 10 mM MES, 200 μM acetosyringone). Three hours later at room temperature, a mixture of equivalent cultures was infiltrated into cotyledons of about 1-week-old tomato plants with a 1 mL syringe. The uniformly sized plants were used for infiltration, and the experiment was repeated three times. Two weeks after infiltration, the effect of virus-induced gene silencing in young leaves was determined by qRT-PCR.
Soluble sugars, starch, and enzyme activity measurement
-
Fruits (0.5 g) and mature leaves (0.2 g) were used for sugar and starch content analysis. The fruit-soluble solid content was measured by a hand-held sugar measurement instrument (PAL-fu, ATAGO, Japan) as described previously[30]. Sucrose, glucose, and fructose were extracted and analyzed as described previously[31]. Starch was extracted as described previously[32]. Fruits (0.5 g) were used to extract enzymes. The frozen samples were ground in a mortar with a small amount of quartz sand and 5 mL HEPES buffer (50 mM HEPS-NaOH, pH 7.5, 1 mM EDTA, 10 mM MgCl2, 2.5 mM DTT, 10 mM VC, and 5% insoluble PVPP). The extracted enzyme solution was used for invertase activity, SS as well as SPS activity analysis as described previously, respectively[25,33].
Construction and screening of cDNA yeast libraries of tomato fruits
-
The experiments were performed by Shanghai OE Biotech. Co., Ltd (Shanghai, China). A split-ubiquitin-based membrane yeast two-hybrid system (DUALsystems biotech, Schlieren, Switzerland) was used to screen for proteins that may interact with SlSWEET14. The CDS sequence of SlSWEET14 without ATG codon and stop codon were cloned into the pBT3-STE bait vector using the SfiI site. To construct the cDNA yeast libraries of tomato fruits (Micro-Tom), the fruits at different stages (including fruits about 28 d after anthesis, 35 d after anthesis, 38 d after anthesis, 42 d after anthesis, 45 d after anthesis, 50 d after anthesis) were sampled and pooled, and then the total RNA was extracted from various tissues using a Trizol reagent (Tiangen, Beijing, China) according to the manufacturer's instructions. cDNA was synthesized using a Dual systems Biotech Easy Clone cDNA synthesis kit, and then transformed into yeast strain NMY51 according to the user manual (DUALsystems biotech). The co-transformation efficiency of yeast transformants was confirmed by culturing on synthetic dropout (SD) medium without leucine (Leu) and tryptophan (Trp). The yeast transformants were further screened on SD medium lacking histidine (His), leucine (Leu), tryptophan (Trp), adenine (Ade) and supplemented with 20 mM 3-amino-1,2,3-triazole (3-AT) and the presence of β-galactosidase. The plasmids of positive clones were extracted using a Yeast Plasmid Mini Kit (Omega, USA) and transformed into E. coli for sequencing.
Statistical analysis
-
The data are presented as the mean ± SD (Standard Deviation). Significance tests were carried out in SPSS software (version 17.0) based on Student's t-tests at p < 0.01 or p < 0.05.
-
In our previous study, SlSWEET14 played an important role in sugar accumulation during tomato fruit development. When silence SlSWEET14, it caused an increase of hexose and sucrose concentration in tomato fruits[17]. To further explore the possible action mechanism that SlSWEET14 is involved in regulating the sugar homeostasis of tomato fruits, a cDNA yeast library of tomato fruits (MT) was created and screened for potential SlSWEET14 interactors using a split-ubiquitin membrane yeast two-hybrid (mY2H) system. Thirty four potential interaction proteins were obtained (Supplemental Table S1), in which a clade III member (SlSWEET10a, Solyc03g097580) was identified, as previously reported that oligomerization of SWEET might be involved in regulating sugar transport[34]. Subsequently, interaction of SlSWEET10a and SlSWEET14 was tested by mY2H, BiFc, fluorescence co-localization and LUC complementation (Fig. 1a−d, Supplemental Table S2 & Supplemental Fig. S1). As shown in these results, SlSWEET10a interacted with SlSWET14 to form hetero-oligomers at the margin of N. benthamiana epidermal cells. Similarly, when co-infiltrated with SlSWEET10a-nLUC and SlSWEET14-cLUC constructs, strong luminescence signals were detected. In addition, SlSWEET14 and SlSWET10a could also form homo-oligomers with themselves, respectively.
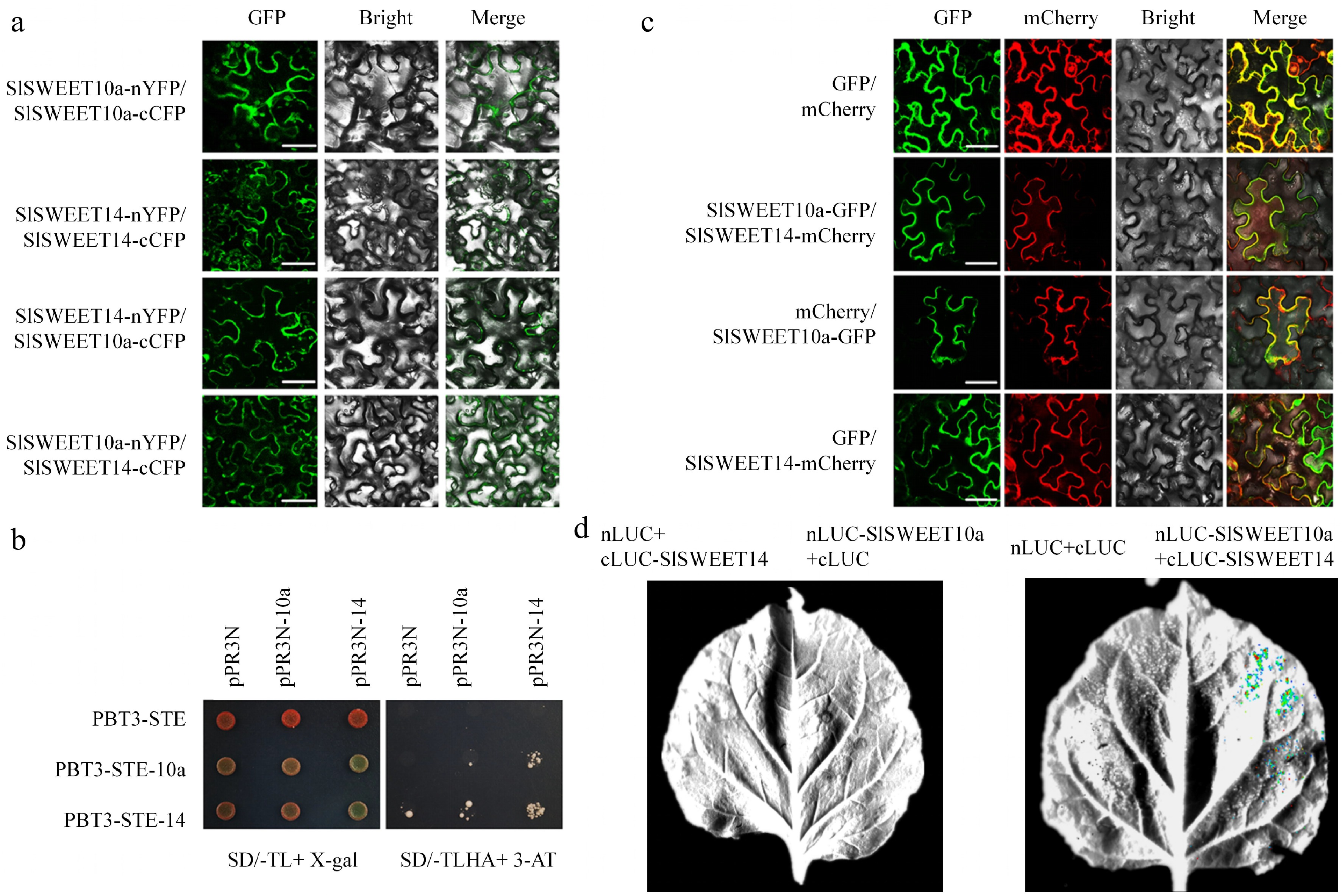
Figure 1.
SlSWEET10a interacts with SlSWEET14. (a) Interaction of SlSWEET10a and SlSWEET14 was verified by bimolecular fluorescence complementation (BiFC). Leaves of N. benthamiana were co-infiltrated with fusion proteins, and the green fluorescence signals were detected 2 d later with a confocal microscope. (b) Interaction of SlSWEET10a and SlSWEET14 was revealed by split-ubiquitin yeast two-hybrid. Histidine (His) and adenine (Ade) reporter genes were used for testing the interaction; X-gal (100 μg/mL) staining assay (left panel) and 3-amino-1,2,4-triazole (10 mM) (right panel) were also used. pPR3N with pBT3-STE, pPR3N-SlSWEET10a with pBT3-STE, pPR3N-SlSWEET14 with pBT3-STE, pPR3N with pBT3-SlSWEET10a, and pPR3N with pBT3-SlSWEET14 were used as negative controls. The experiment was performed at least three times. SD-TL, SD/-Trp/-Leu; SD-TLHA, SD/-Trp/-Leu/-His/-Ade. (c) SlSWEET10a and SlSWEET14 were co-localised in N. benthamiana leaves. The fusion proteins were co-expressed in N. benthamiana leaves; the combinations of SlSWEET10a-GFP with empty vector carrying mCherry reporter gene, and of SlSWEET14-mCherry with empty vector carrying GFP reporter gene were used as negative controls. (d) Firefly luciferase complementation imaging assays of SlSWEET10a and SlSWEET14 in N. benthamiana leaves.
Subcellular localization analysis of SlSWEET10a
-
Previous results showed that SlSWEET14 was mainly localized on the plasma membrane. To further determine the subcellular localization of SlSWEET10a, pCAM35S::SlSWEET10a-GFP was co-expressed with a plasma membrane marker (pCAM35S::AtPIP2A-mCherry) (Fig. 2). The mCherry-labelled red fluorescence and GFP-labelled green fluorescence were overlapped on the plasma membrane. The results demonstrated that SlSWEET10a was also a plasma membrane-localized protein.
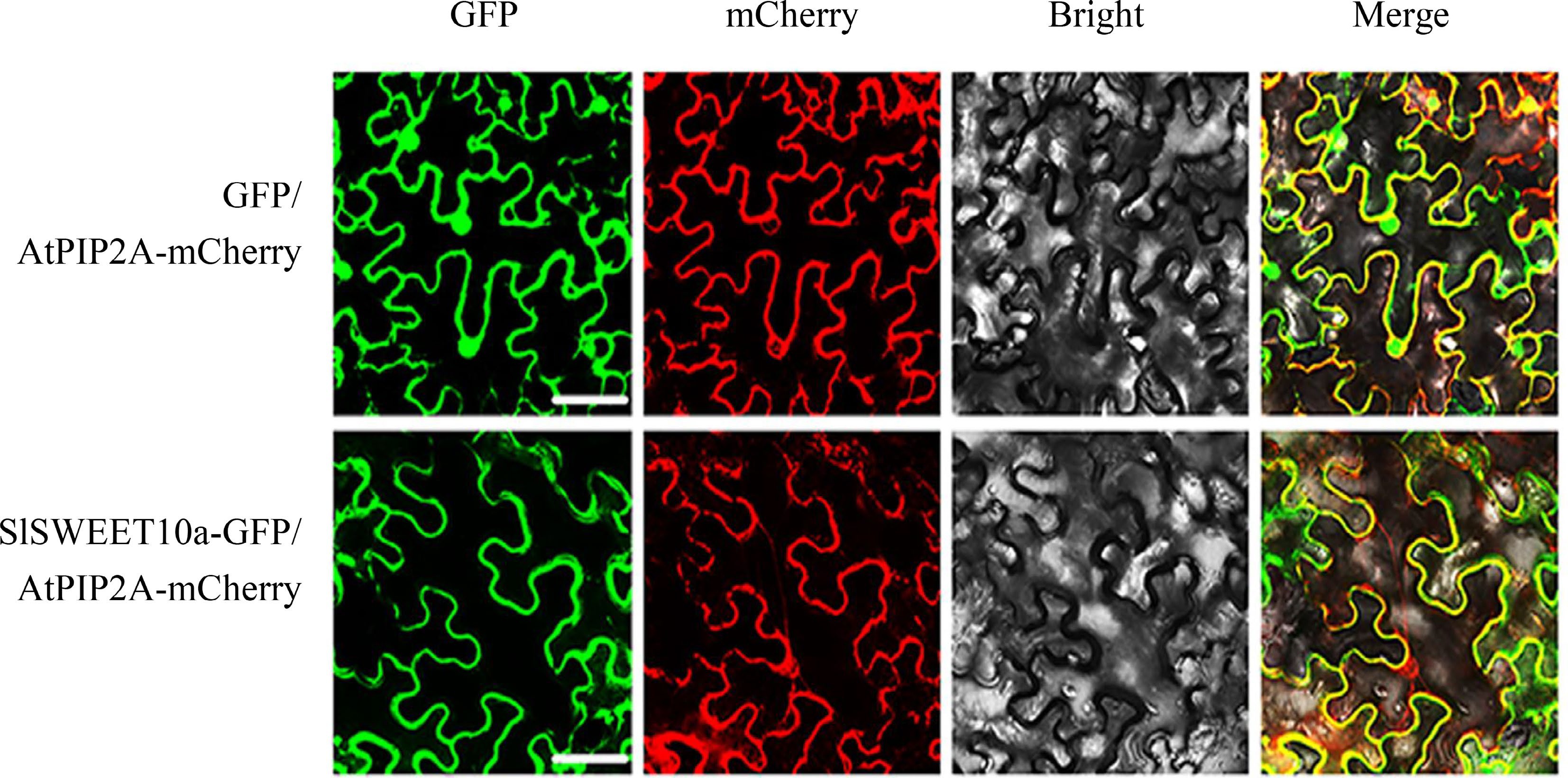
Figure 2.
The subcellular localization of SlSWEET10a. An mCherry-labelled plasma membrane protein, AtPIP2A, was used as positive control. Scale bars correspond to 25 μm. These experiments were performed at least three times, representative results are shown.
Expression assay of SlSWEET10a
-
To further investigate the possible roles of SlSWEET10a and the relationship with SlSWEET14, the expression pattern of SlSWEET10a from different tissues during different developments of MT plants was examined (Supplemental Table S3 & Supplemental Fig. S2). RT-qPCR analysis showed that SlSWEET10a was highly expressed in mature green (MG) fruits and seeds, especially in the former. In addition, we also performed GUS activities analysis of the SlSWEET10a promoter (Fig. 3). The promoter activity of SlSWEET10a were observed in pollen grains of stamens in flowers, fruits (especially in vascular bundles of pericarps and placenta in MG fruits), seeds, and stem. These results showed that SlSWEET10a could also play important roles in sugar allocation of sink tissue and the development of seeds and stem, similar to SlSWEET14[17].
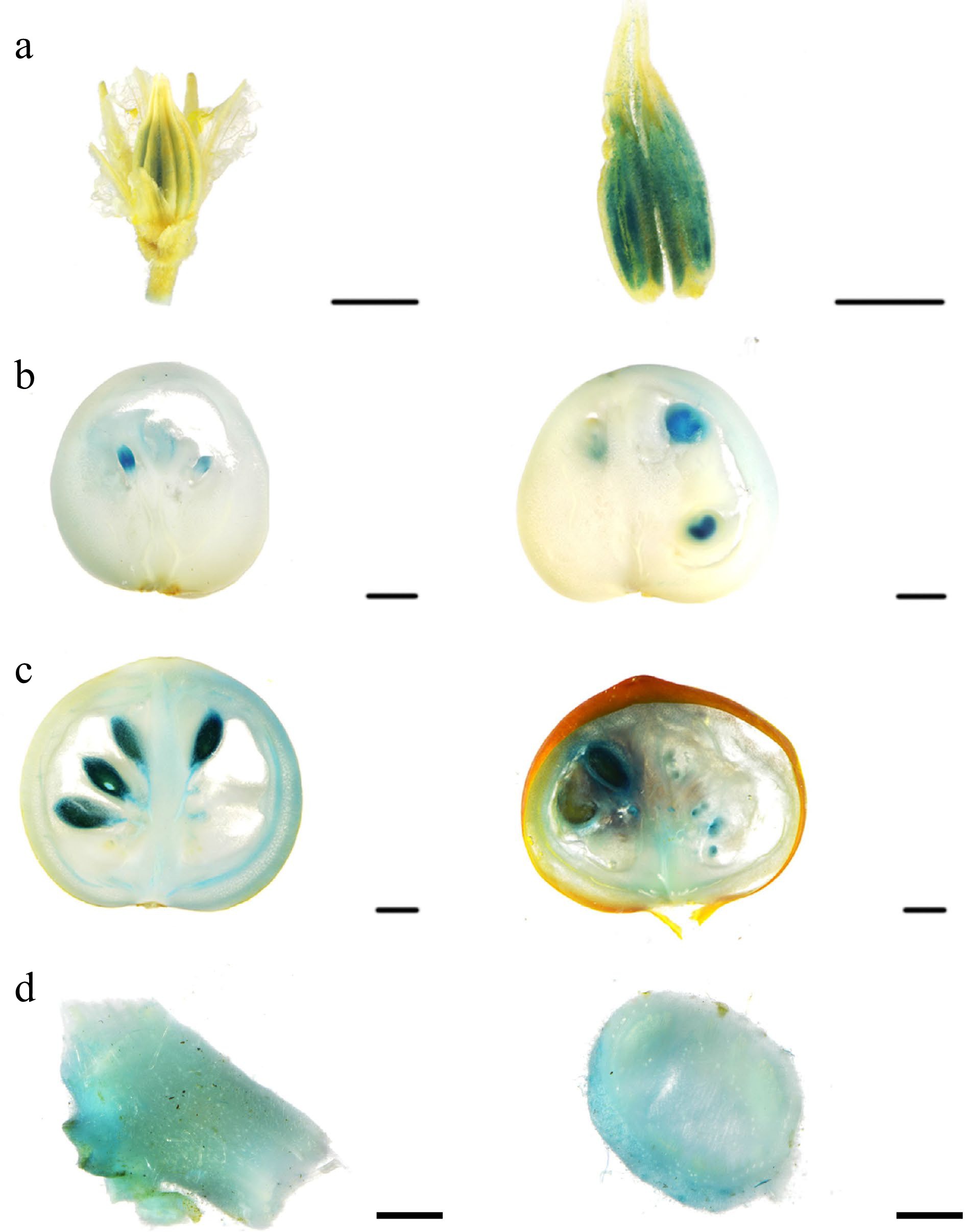
Figure 3.
Spatiotemporal expression analysis of SlSWEET10a. (a) Histochemical analysis of pSlSWEET10a: GUS activity in flower. (b) Fruits of expanding period, (c) fruits of mature green stage and red ripe stage, and (d) stem. Scales bars = 2,000 μm.
Functional characterization of SlSWEET10a in yeast
-
SlSWEET14 was found to transport hexose and sucrose in our previous study. As an interactor of SlSWEET14, the transport activity of SlSWEET10a was analyzed. The cDNA of SlSWEET10a was heterologously expressed in the hexose uptake-deficient yeast (Saccharomyces cerevisiae) strain EBY.VW4000 and the sucrose uptake mechanism-deficient yeast (Saccharomyces cerevisiae) strain SUSY7/ura3 (Fig. 4). AtSWEET1a (a hexose transporter) and AtSUC2 (a sucrose transporter) were used as positive controls. AtSUC2 and SlSWEET10a fused SUSY7/ura3 strains grew better on the sucrose-containing media compared with the empty vector (Fig. 4). Fructose and glucose transport activity was also analyzed. AtSWEET1a fused EBY.VW4000 strain could restore growth on both glucose-containing and fructose-containing media but not the yeast cells transformed with SlSWEET10a (Supplemental Fig. S3). Collectively, these results indicated that SlSWEET10a could only transport sucrose.
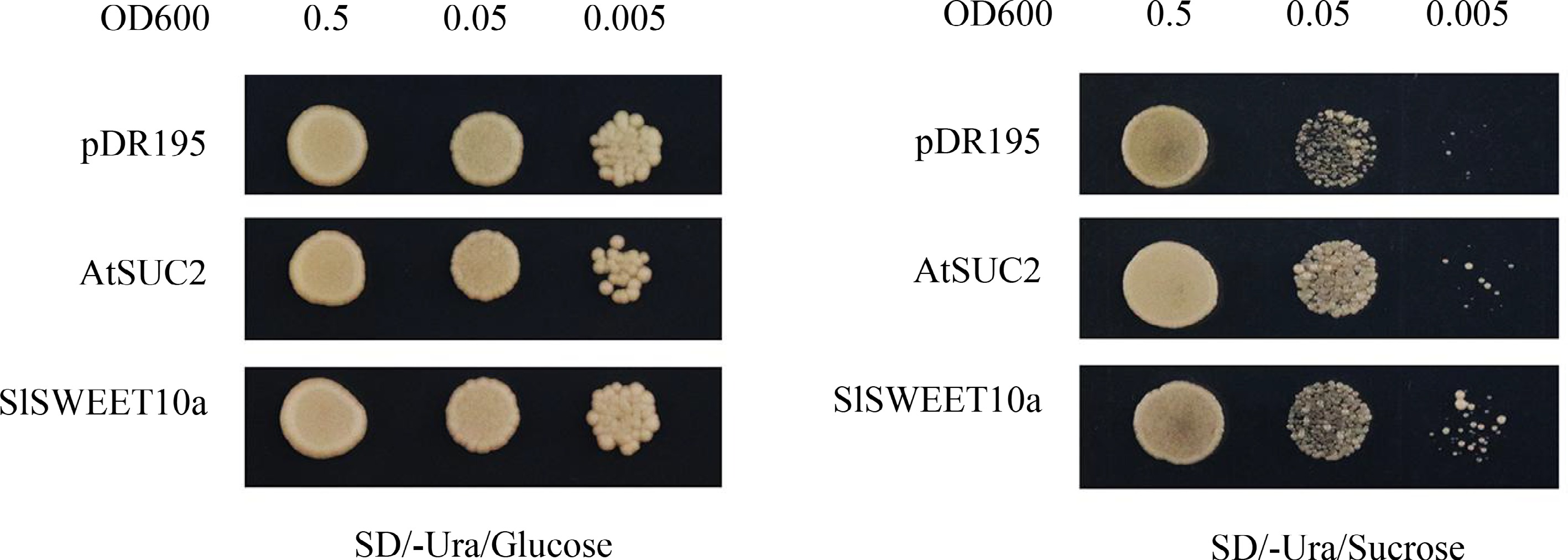
Figure 4.
Sucrose transport activity of SlSWEET10a in yeast. Growth status of SUSY7/ura3 expressing SlSWEET10a. Yeast cells were cultured on SD/-Ura medium supplemented with 2% glucose or 2% sucrose. Yeast cells expressing AtSUC2 and pDR195 empty vector were used as a positive and negative control, respectively. Yeast cells of SUSY7/ura3 were grown at 30 °C for 4 d.
Expression of SlSWEET10a caused sugar accumulation alter in tomato fruits and growth status in tomato plants
-
To further characterize the function of SlSWEET10a in tomato fruit sugar allocation and possible relation with SlSWEET14, we obtained SlSWEET10a overexpression MT plants (OE10a) under the constitutive 35S promoter and used VIGS (virus-induced gene silencing) to transiently silence the expression of SlSWEET10a in MT plants (TRV-10a). The expression level of SlSWEET10a was up-regulated in the OE10a lines either MG or RR fruits (Fig. 5a). Control (CK, only injected with infiltration buffers) and non-silenced (TRV) plants were compared with TRV-10a plants. In TRV-10a plants, the expression level of SlSWEET10a was down-regulated by 10%−90% compared with control lines. In addition, in MG and RR fruits of TRV-10a lines, the expression level of SlSWEET10a was also down-regulated (Fig. 5a, Supplemental Fig. S4a). We also measured the soluble sugar (fructose, glucose, and sucrose) and starch concentrations in OE10a and TRV-10a lines (Fig. 5b). The sucrose concentration of leaves in OE10a lines was significantly decreased 22%, compared with control lines. In the MG fruits, the soluble sugar concentration was decreased in OE10a lines, especially the glucose and sucrose concentrations significantly reduced by 24% and 56%, compared to control lines, respectively. On the contrary, the fructose and glucose concentrations in TRV-10a lines was increased by 35% and 50% compared with non-silenced lines, respectively. The sucrose concentration was also slightly increased relative to that of CK. The starch concentration of OE10a lines was markedly increased by 41% relative to those of WT, but the starch concentration of TRV-10a lines was significantly reduced by 26% compared with non-silenced lines.
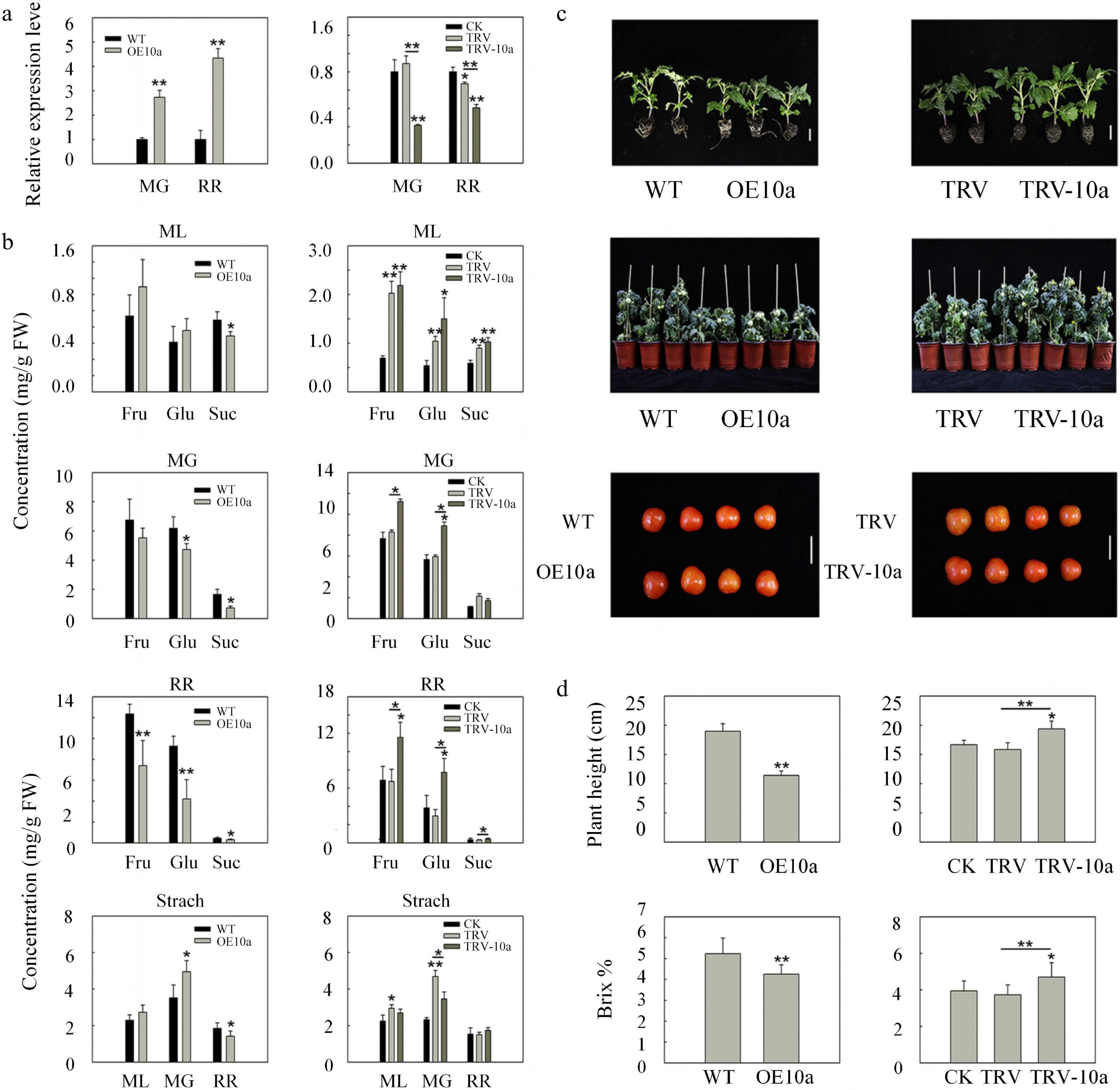
Figure 5.
Sugar concentrations and morphology phenotype as well as phenotypic parameters in SlSWEET10a overexpression lines and silencing lines. (a) The relative expression levels of SlSWEET10a in wild type (WT), SlSWEET10a overexpression (OE10a), control (CK, only injected with infiltration buffers), non-silenced (TRV), and silenced SlSWEET10a (TRV-10a) fruits at the mature green stage (MG) and red ripe stage (RR), respectively. The expression level of WT and CK was set to 1, respectively. The ACTIN was used as internal control. Data are mean values ± SD of at least three biological replicates. (b) Fructose (Fru), glucose (Glu), sucrose (Suc), and starch concentrations in mature leaves (ML), mature green fruits (MG), and red ripe fruits (RR) in different lines. FW, fresh weight. Data represent the mean values ± SD of at least five biological replicates. (c) Plant architecture of the SlSWEET10a (OE10a) lines and SlSWEET10a silencing (TRV-10a) lines about 30 and 80 d after sowing the seeds, respectively, and fruits at the stage of red ripen fruits. Scale bars = 2 cm. (d) Plant height (n ≥ 15) about 90 d after sowing the seeds and soluble solid content (indicated by brix %) of fruits (n ≥ 20) at the red ripe stage in OE10a lines and TRV-10a lines. Data represent mean values ± SD. * p < 0.05, ** p < 0.01 according to Student's t test.
During the RR stage, OE10a fruits obtained lower sugar concentrations compared with control lines. The fructose, glucose, and sucrose concentrations were reduced by 40%, 55%, and 30%, respectively. Conversely, the soluble sugar concentrations were increased in TRV-10a RR fruits compared with the non-silenced lines. The fructose and sucrose concentrations were increased by 72% and 39%, respectively, compared to the non-silenced lines. The glucose concentration in TRV-10a RR fruits was almost 2-fold that in the non-silenced lines. However, only OE10a lines showed decreased starch concentration about 23% compared with control lines. Taken together, the results suggested that SlSWEET10a might function as a sucrose exporter. During the MG stage, silencing SlSWEET10a inhibited the export of sucrose which resulted in sugar accumulation in MG fruits. While over-expressing SlSWEET10a promotes the sucrose export, which caused a decrease in the sugar content.
In addition, OE10a lines displayed an obvious dwarf phenotype, However, TRV-10a lines showed a higher plant height (Fig. 5c). The height of OE10a lines was reduced by 40% relative to that of control lines, while the height of TRV-10a lines was increased by 22% compared with non-silenced lines (Fig. 5d). The soluble solid content (Brix %) was decreased by 19% in OE10a lines, compared with control lines (Fig. 5d). The soluble solid content was significantly increased by 26% in TRV-10a lines, compared with non-silenced lines (Fig. 5d). There was no obvious difference between OE10a lines and TRV-10a lines in the single fruit weight and fruit diameter (Fig. 5c, Supplemental Fig. S4b−d). These results indicate that the change in the transcript levels of SlSWEET10a could affect plant growth and sugar accumulation (hexose and sucrose) in tomato fruits, which is similar to SlSWEET14.
SlSWEET10a regulated sugar accumulation in tomato fruit via sugar metabolism pathway
-
Enzyme activities and genes expression level which are associated with sugar metabolism were further analyzed (Supplemental Table S4 & Fig. 6). In OE10a lines, only HK4 changed, compared with control lines. For the cell wall invertase encoding genes, the expression levels of LIN5 and LIN6 were sharply up-regulated and the expressed level of LIN9 was down-regulated in OE10a lines, compared to control lines. In addition, VI (encoding vacuolar invertase) was also down-regulated in OE10a lines. Among the eight genes encoding cytoplasmic invertase (CIN), the transcript levels of CIN5-8 were decreased in OE10a lines. In the OE10a lines, the transcript levels of SPSA2 and SPSB (encoding sucrose phosphate synthase) and SS1 (encoding sucrose synthase) were down-regulated, however, the expression level of SS3 was up-regulated. In addition, HT3 showed high expression, compared with control lines. While the expression of SUT2 was decreased. Overexpression of SlSWEET10a also affected the expression patterns of SWEETs genes. Among all the SWEET members (divided into four phylogenetic clades), clades I, II, and IV mainly transport hexose, while clade III prefers sucrose. Correspondingly, clade I members (SlSWEET1a, SlSWEET1e, and SlSWEET2a) and clade II members (SlSWEET5a) and clade III members (SlSWEET10b, SlSWEET10c, SlSWEET11b, SlSWEET12c, SlSWEET12d, and SlSWEET15) were highly expressed in OE10a lines. However, the expression levels of SlSWEET1c, SlSWEET14, and SlSWEET17, belonging to clades I, III, and IV, respectively, were decreased.
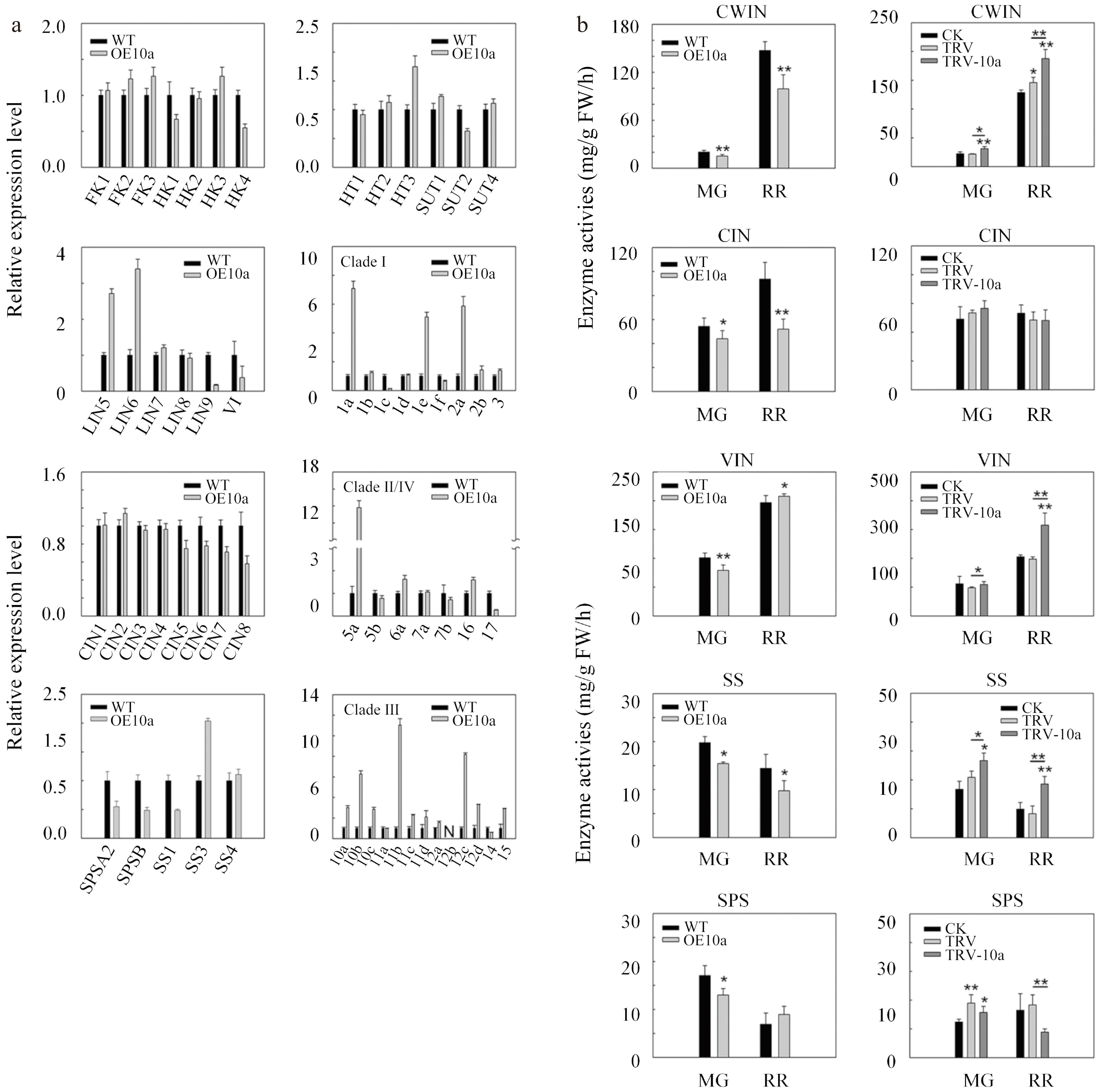
Figure 6.
Relative expression levels of genes associated with sucrose metabolism in mature green fruits and assays of enzymes in fruits for SlSWEET10a. (a) Transcript levels of sucrose metabolism genes in OE10a fruits at the mature green stage. FK, fructokinase; HK, hexokinase; SPS, sucrose phosphate synthase; SS, sucrose synthase; LIN, cell wall invertase; VI, vacuolar invertase; CIN, cytoplasmic invertase; HT, hexose transporter; SUT, sucrose transporter; 1a-17, Sugar will eventually be exported transporters (SWEETs). The expression data of WT was set to 1. The ACTIN gene was used as internal control. (b) Enzyme activity assay in mature green fruits (MG) and red ripe fruits (RR) in OE10a and TRV-10a lines. CWIN, cell wall invertase; CIN, cytoplasmic invertase; VIN, vacuolar invertase; SS, sucrose synthase; SPS, sucrose phosphate synthase.
To further identify key enzymes involved in sugar metabolism in the study, the enzyme activities of cell wall invertase (CWIN), cytoplasmic invertase (CIN), vacuolar invertase (VIN), sucrose synthase (SS), and sucrose phosphate synthase (SPS) were measured in the MG fruits and the RR fruits (Fig. 6b). CWIN activity was significantly reduced in OE10a fruits (including MG and RR), by 25% and 33%, respectively, relative to those of control lines. On the contrary, CWIN activity in TRV-10a MG fruits and RR fruits significantly increased, by 44% and 29%, respectively, compared with those in non-silenced lines. The activity of CIN was only significantly changed in OE10a fruits, with a reduction of 19% and 45% respectively, compared to those in control lines. The activity of VIN was significantly elevated in OE10a RR fruits, by 6% compared with control lines. However, in OE10a MG fruits, it sharply decreased, by 22% compared with control lines. The activities of SS showed consistently changed patterns with the CWIN activities, with a reduction of 22% and 32% in OE10a fruits and an increase of 28% and 124% in TRV-10a fruits. However, the SPS activities were significantly reduced in OE10a MG fruits and TRV-10a RR fruits, by 24% relative to those of control lines and 52% relative to those of non-silenced lines. These results indicate that SlSWEET10a is also involved in the regulation of the sucrose metabolism pathway, in which CWIN and SS may be two key players.
-
It has been reported that SWEET transporters are divided into four clades: clades I, II, and IV predominantly transport hexose, whereas clade III are sucrose transporter and localized on the plasma membrane[14,35]. Phylogenetic analysis shows that SlSWEET14 and SlSWEET10a are classified into clade III[6]. Consistently, SlSWEET14 and SlSWEET10a are both plasma membrane-localized sucrose transporter. SlSWEETs differed in sugar transport activity. For clade I, SlSWEET1a can transport both glucose and fructose[6]. SlSWEET5b which belongs to clade II, can transport hexose[36]. SlSWEET7a also belongs to clade II, and functions as a hexose and sucrose transporter[17]. When it comes to clade III, SlSWEET11b, SlSWEET12c and SlSWEET14 can transport glucose, fructose and sucrose[17,18,37]. SlSWEET15, another member of clade III only has sucrose transport activity[19]. In our study, SlSWEET10a also belongs to clade III, which has the same sugar transport activity as SlSWEET15. It can only transport sucrose (Fig. 4; Supplemental Fig. S3).
Sucrose is the major form of photosynthetic product and is transported long distance from leaves to various sink organs, the process is necessary to enable the growth and development of flowers, fruits, and seeds[6,38]. The efficient unloading of sucrose transported from leaves into fruit is an essential determinant of fruit quality. It is worth noting that SlSWEET14 and SlSWEET10a are both expressed in vascular bundle of fruit, implicating the role of SlSWEET14 and SlSWEET10a in sucrose unloading of fruit. Considerable studies have suggested that SWEETs play an irreplaceable role in sucrose unloading in sinks, it may accordingly be inferred that SWEETs must be tightly regulated by the plant to ensure the proper distribution of sucrose to sink organs. The regulation of sucrose flux may be mediated by SWEET family proteins at both transcriptional and post-translational levels. It has been reported that transcription factors modulate SWEET gene expression to further affect sugar allocation[15,39]. Therefore, SlSWEET14 and SlSWEET10a may also be co-regulated by some transcription factors to mediate sucrose distribution in fruit, which remain to be investigated in the future.
SWEETs can form homo- or hetero-oligomers by oligomerization to produce a functional pore to transport sugar[27]. Recently, it was reported that SWEET11 interacted with FT homolog StSP6A to block the leakage of sucrose to the apoplast, which indicates sucrose allocation in sink organs may be attributed to oligomerization-dependent regulation[40]. Many membrane transporters can form oligomeric states to play roles in membrane trafficking, function, turnover, and regulation[41]. The structure, function, and stability of membrane-localized transporters are closely related to oligomerization[42]. Crystal structure and biochemical characterization revealed OsSWEET2b and AtSWEET1 form homo-oligomers to function[43]. Mutational studies indicated that oligomerization regulates sugar transport. Mutation of CST1 (a Clade I of SWEET family member) impaired glucose transport activity of CST1 at least in part by preventing its oligomerization in maize[44]. Similarly, the mutated form of OsSWEET11 interacted with normal OsSWEET11 inhibited OsSWEET11 mediated sucrose transport by forming a trimer[45]. Therefore, homo-dimerization of SWEETs maybe necessary for its normal transport activities[34]. Recent report showed that CsSWEET1a and CsSWEET17 may function synergistically in tea plants by forming homo-/heterodimers to transport sugars[46]. It was also observed that SlSWEET10a and SlSWEET14 could form homo-/heterodimers in split-ubiquitin -based yeast two-hybrid system and split GFP system. SlSWEET10a was screened as an interactor for SlSWEET14, more important is that its expression pattern, subcellular localization, and substrate specificity are all consistent with those of SlSWEET14. These results suggest that it may be possible that SlSWEET14 and SlSWEET10a exist as hetero-oligomers to function synergistically in tomato.
At present, it is unclear how the heterodimers of SWEET affect the sugar transport activity in plants, but previous studies showed that the hetero-oligomerization of SUT1 and SUT2 inhibited sucrose transport[47]. Based on the above results, a possible event is that the established hetero-oligomerization between SWEETs might cease the transport activity of SWEETs. To investigate the potential affection of the interaction between SlSWEET14 and SlSWEET10a on sucrose transport, we obtained transgenic plants of SlSWEET10a. In the OE10a lines, the sucrose concentration was both decreased in MG fruit and RR fruit. When the expression of SlSWEET10a was suppressed, the sucrose concentration was increased in RR fruit, as well as slightly increased in MG fruit. Consistent results were also observed in MG fruit of SlSWEET14 suppression lines[17]. Accordingly, SlSWEET14 and SlSWEET10a may function synergistically to inhibit sucrose unloading in MG fruit by forming heterodimers. However, we could not exclude the possibility that SlSWEET14 and SlSWEET10a exist as homo-oligomers to regulate the sucrose unloading in MG fruit.
SlSWEET10a regulates hexose accumulation in tomato fruit and affects tomato plant height
-
In addition to sucrose transport, sugar accumulation in fruits also depend on sucrose metabolism. In most fruits, hexose (fructose and glucose) accounts for the major proportion of soluble sugars and increased hexose can contribute to improving fruit taste[48,49], which means that sucrose unloaded in fruits must be degraded via sucrose metabolism. Sucrose metabolism is a process of the degradation and resynthesis in the cytosol, vacuole, and apoplast, mainly regulated by invertase (CWIN, CIN, VIN), sucrose synthase (SS), and sucrose phosphate synthase (SPS)[15,25]. Sucrose-cleaving enzymes reduce sucrose concentration to form sucrose concentration gradient, which is helpful for sucrose phloem unloading to continue[50]. Unloaded sucrose can be taken up into fruit parenchyma cells (PCs) by sucrose transporter (SUTs) or hexose transporters (HTs) following a degradation by CWIN[4]. Therefore, sucrose metabolism is necessary for sugar unloading in fruit.
Our expression analysis showed that over-expression of SlSWEET10a led to the differential expression of many hexose transporter genes and sucrose transporter genes, including HT, SUT, especially SWEETs which are responsible for hexose and sucrose transport. In addition, the expression levels of sucrose metabolic enzyme-related genes were also affected in OE10a fruit. A similar study was also reported in our previous research on SlSWEET14[17]. These findings suggest that genetic manipulation of SlSWEET10a or SlSWEET14 influences the pathway of sucrose metabolism. In our study, the alteration of sucrose concentration in OE10a or TRV-10a lines was accompanied by the activity changes of sucrose metabolic enzymes. Among these sucrose metabolic enzymes, CWIN and SS are two key enzymes catalyzing sucrose degradation and play an important role in sugar unloading[51]. It is well accepted that CWIN hydrolyzes sucrose into fructose and glucose in cell wall space, and SS degrades sucrose into UDP-glucose and fructose in cytoplasm. Furthermore, it has been showed that the increase of CWIN and SS activity results in hexose accumulation in fruits[23,52]. As expected, CWIN activity and SS activity were most obviously changed among five investigated sucrose metabolic enzymes in either MG fruit or RR fruit along with the altered expression of SlSWEET10a, demonstrating the coupling between sucrose degradation and transport. These results also indicate the alteration of hexose concentration in transgenic fruit for SlSWEET10a can majorly result from the elevated CWIN activity and SS activity.
Evidence showed that SWEETs also play an important role in plant growth and development. SlSWEET15 was highly expressed in development fruits, especially in vascular tissues and seed coats, elimination of the SlSWEET15 function by CRISPR/Cas9 gene editing leads to reduced size and weight of fruits and defects in seed development[19]. In the present study, SlSWEET10a and SlSWEET14 were mainly expressed in mature green fruit, also accumulated in vascular tissues and seed coats; however, there were no obvious defects in the seed and fruit development in the SlSWEET10a and SlSWEET14 suppression lines. It is a possibility that SWEETs play different roles in different development stages of fruits. SlSWEET10a and SlSWEET14 are mainly responsible for sugar accumulation in tomato fruits.
OsSWEET14, which is homologous to SlSWEET10a and SlSWEET14 has been found that overexpression reduced rice height but function knockout increased plant height[53,54]. OsSWEET11 is also homologous to SlSWEET10a and SlSWEET14, and overexpression of OsSWEET11 exhibited a severe dwarf phenotype[45]. In our previous report, the suppression of SlSWEET14 could also improve plant height. Consistent results were also observed in the research on SlSWEET10a. It is possible that SlSWEET10a and SlSWEET14 regulate plant height due to the altered efficiency of sugar transportation in the stem. We observed that SlSWEET10a and SlSWEET14 were both expressed in the stem tissue (Fig. 3), whereas, no obvious difference was shown in the investigated soluble sugar of stem tissues (data not shown). In addition to sugar, plant hormones are also important regulation factors for plant growth and development[55]. Furthermore, SWEETs are not only involved in sugar transport but also in regulating plant hormone response and transport[56]. Notably, in the present study, SlSWEET14 and SlSWEET10a participate in the regulation of CWIN activity. It has been shown that CWIN does not only function in sucrose unloading, but is also involved in sugar signaling to modulate downstream auxin signaling[22]. Suppression of gene coding cell wall invertase lead to alterations in hormone metabolism[57]. Accordingly, SlSWEET14 and SlSWEET10a could regulate plant height via a direct or indirect plant hormones pathway, which requires further study.
-
In the study, a new SlWEET member in tomato was identified, SlSWEET10a, which is an interaction factor of SlSWEET14 and a plasma membrane-localised sucrose transporter. Overexpressing SlSWEET10a led to sucrose and hexose reduction in MG fruits, as well as the alteration of CWIN and SS activities. While silencing SlSWEET10a displayed opposite results. These results indicate that SlSWEET10a can modulate sugar accumulation in tomato fruits, especially during the MG stage. Based on the above results, a regulatory relationship of sugar metabolism and accumulation in tomato fruits mediated by SWEET10a, CWIN, and SS were shown, which further clarified the key role of SWEET in sugar allocation especially in sucrose metabolism in fruit crops.
-
The authors confirm contribution to the paper as follows: conceptualization, funding acquisition: Liu X, Jiang J; investigation, methodology, data curation, writing: Zhang X, Sun J; writing–review & editing, supervision: Jiang J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was supported by the National Key Research and Development Program of China (2022YFF1003022) and the Science and Technology Program of Liaoning Province (2022020768-JH1/102-02).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Xinsheng Zhang, Jiaqi Sun
- Supplemental Table S1 Putative protein interactors of SlSWEET14.
- Supplemental Table S2 Primers used for generating vector construction and identification.
- Supplemental Table S3 Primers used for qRT-PCR analysis of SWEETs.
- Supplemental Table S4 Primers used for qRT-PCR analysis of sucrose metabolism genes and sucrose transporter genes.
- Supplemental Fig. S1 Negative controls for interaction of SlSWEET14 and SlSWEET10a in bimolecular fluorescence complementation assays.
- Supplemental Fig. S2 Expression analysis of SlSWEET10a.
- Supplemental Fig. S3 Glucose and fructose transport activity of SlSWEET10a in yeast.
- Supplemental Fig. S4 Relative expression levels of SlSWEET10a in VIGS leaves and phenotypic characteristics in SlSWEET10a overexpression lines and silencing lines.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang X, Sun J, Liu X, Jiang J. 2024. SlSWEET10a negatively regulates sucrose transport in tomato fruit. Vegetable Research 4: e018 doi: 10.48130/vegres-0024-0018
SlSWEET10a negatively regulates sucrose transport in tomato fruit
- Received: 26 February 2024
- Revised: 16 April 2024
- Accepted: 14 May 2024
- Published online: 12 June 2024
Abstract: SWEETs (Sugars Will Eventually be Exported Transporters) family plays important roles in many physiological processes in plants, but how SWEETs are involved in regulating sucrose metabolism in tomato fruit remains unclear. In this study, SlSWEET10a was screened from a cDNA yeast library of tomato fruits (Micro-Tom) with SlSWEET14 as a bait protein. Based on yeast functional complementation, sub-cellular localization, and GUS activity assay, SlSWEET10a protein was a plasma-localized sucrose transporter and accumulated in mature green fruits (especially in vascular bundles and seeds). The interaction between SlSWEET14 and SlSWEET10a was confirmed using yeast two-hybrid, bimolecular fluorescence complementation, and fluorescence co-localization assays. Overexpression of SlSWEET10a led to decreased hexose and sucrose concentration, lower activities of CWIN and SS in mature green fruit, and dwarfing plant height. In contrast, virus-induced gene silencing of SlSWEET10a expression produced opposite results. Taken together, these results suggest that SlSWEET10a is essential for modulating tomato fruit sugar allocation and plant height development, which is of importance in enriching the cognition of SWEETs function in tomato.













