-
Thermal sterilization is the most suitable method for delivering safe and shelf-stable food products[1]. Most of the shelf-stable food products on supermarket shelves are processed by this method. In traditional thermal processing, pre-packaged food is exposed to pressurized high-temperature steam or hot water to eliminate spores and bacteria from food to extend its shelf life[2]. However, long processing times due to low heat transfer, especially within solid and semi-solid foods, can lead to severe degradation in the quality and sensory attributes of the processed food products[3,4], which in turn reduces their market appeal.
Microwave-assisted thermal sterilization (MATS) reliant on novel 915 MHz single-mode designed cavities is an advanced in-package food processing technology that was developed at Washington State University, USA; the technique has been accepted by the FDA for thermal sterilization of food and earned a letter of non-objection from the USDA FSIS[4]. MATS not only produces safe foods but also retains the quality attributes of the MATS-processed foods[4−6]. Previous studies have investigated the stability of nutrients such as vitamins A, E, and C in MATS-processed food products[7,8]. The results for MATS showed higher stability of nutrients with a minimal effect compared to conventional thermal processing. Additionally, MATS-processed products better maintained the texture, instrumental colors, and aroma of processed foods[7,9,10]. However, the thermal stability of natural pigments after MATS and their relation to the color of food products, as well as their storage stability without the presence of any additives such as vitamin C or encapsulated vitamin C[8,11], has not been fully investigated.
Regardless of whether a product is processed or fresh, color is a direct indicator of the quality of food and affects the product's appeal to the consumer[12]. Bright natural color as a physical attribute thus adds commercial value to the food product. Food products obtain distinctive colors from either natural pigments or synthetic colorants. Natural color pigments, particularly betalains, beta-carotene, and anthocyanins, are abundant in fruit and vegetables. For example, the intense orange color of carrots comes from beta-carotene pigment and anthocyanins give fruits and vegetables purple, blue, and red colors[12,13]. But these natural pigments are highly sensitive to heat and oxygen[12]. Several studies have shown that the thermal degradation of betacyanins (the red pigment in beetroot), anthocyanins, and β-carotene follows the first-order kinetics model[14−16]. Therefore, theoretically, reducing the processing time of thermal processing should reduce the thermal degradation of such pigments. Compared to conventional thermal retort, the MATS system sharply shortens the processing time with volumetric microwave heating[4,17,18]. Thus, we hypothesize that MATS can create an opportunity to enhance the color and the retention of naturally derived pigments in shelf-stable foods.
Since metal is not transparent to microwaves, high-oxygen and water-barrier polymeric packaging materials are used for processing with MATS[4]. MATS processing conditions involve the exposure of food packages to high temperatures (approximately 124 °C), high moisture (RH = 100%), and high pressure (26−32 psi) environments to meet food safety requirements[1]. Therefore, the packaging materials must be resilient and able to maintain their gas barrier integrity. Among the high barrier polymeric packages, polyethylene terephthalate (PET) metal oxide-based packages are a favorable option for MATS processing since they maintain their superior gas barrier and low-water vapor transmission rate (WVTR) and oxygen transmission rate (OTR) after MATS and during storage[19]. Previous studies highlighted the compatibility of this type of packaging with MATS processing conditions and its capability to enhance the storage stability of MATS-processed food products[3,7,10].
The present study investigated the dielectric heating effect of a 915-MHz single-mode on betalains, anthocyanins, and beta-carotene stability. The reason for selecting these pigments was to represent fat- and water-soluble pigments that are commonly present in food products. We chose red beetroot puree as a model food for betalains, red cabbage puree as a model food for anthocyanins, and carrot puree as a model food for beta-carotene. The purees were then packaged in PET–metal oxide-based pouches and subjected to MATS processing to F0 = 6−11 min. The processed purees were stored under a storage temperature of 37.8 °C for a period of 6 months. Their natural pigments, instrumental color, weight loss, and pH were evaluated after the MATS treatment and monitored during the storage period. Additionally, kinetic data on the degradation of natural pigments and instrumental color during the storage period was developed by using zero, first, and fractional conversion models.
-
The PET–metal oxide-based pouch was selected because its unique multilayer structure provides high protection, which is important for in-package sterilized food products. The pouch had three consecutive PET layers that serve as oxygen and moisture barrier. The three layers of PET were coated with aluminum oxide (AlOx). The first layer served as a barrier layer. The other two layers protect the first layer physically against cracks and pinholes, and they provide additional barrier protection as well. The layers in the structure of this pouch were laminated as follows: AlOx-coated PET (12 μm)//AlOx-coated PET (12 μm)//AlOx-coated PET (12 μm)//Ony (15 μm)//CPP (70 μm) to give a total thickness of 121 ± 0.8 μm. More details about the functionality of this structure in preserving the package integrity, specifically OTR and WVTR after thermal processing, are presented in Parhi et al.[19]. Each pouch (18.5 cm length × 14 cm width) has a maximum filling capacity of 290 g (10.2 oz). The WVTR value measured immediately after processing with MATS was 0.15 ± 0.01 (g m−2 d−1), and the OTR value after MATS processing was below the range of detection that the instrument can measure (< 0.05 cc/m2/d)[19].
Puree preparation
-
Fresh vegetables, specifically beetroot (Mosby Bros Farms, Green Valley, WA, USA), fresh whole carrots (Bolthouse Farms, Bakersfield, CA, USA), and cabbages, were obtained from a local store (Walmart, Pullman, WA) and kept at refrigeration temperatures (4 °C) for 18 h. The vegetables were cleaned by washing them with tap water; the carrot and beetroot were chopped into regular short pieces (approximately 50 mm in size) and the cabbage was cut into four quarters and then sliced into thin pieces. The purees were prepared by mixing each cut vegetable with distilled water. The ratio used for mixing the cut vegetables and water was 1:1. The vegetables were mixed in a kitchen blender that was run at approximately 3,000 rounds/min for 2 min to produce a homogeneous puree. The purees were then de-aerated and an Easy-Pack vacuum sealer was utilized (UltraSource. LLC. Kansas, MO, USA) three times.
The portion of puree put in each polymeric pouch was 220 g. The pouches containing the puree were subjected to a vacuum-sealing utilizing the Easy-Pack vacuum sealer (UltraSource. LLC. Kansas, MO, USA) for 5 s at 0.8 bar. For each type of the puree, 36 pouches were prepared. The pouches were then stored at refrigeration temperature (4 °C) for 12 h before the MATS treatment.
Microwave-assisted thermal sterilization (MATS) treatment and storage conditions
-
A 915-MHz single-mode MATS system was used to process the packaged purees at Washington State University (Pullman, WA, USA). A previous publication by Tang[4] provides a full description of the MATS system. Initial tests were conducted to determine the MATS processing conditions. In these tests, three mobile temperature sensors (Ellab, Centennial, CO, USA) were used. The sensors were installed inside the pouches to record the temperature history of the processed puree's slowest heating point. The results of the initial tests showed that the three purees had microwave heating characteristics different from each other. We thus designed three separate MATS treatments with similar processing conditions to achieve the desired sterilization lethality for the three different purees. The MATS treatments initially started with loading the puree packages into the pre-heating section through a belt conveyor system and holding them for preheating in circulating water set at 61 °C. Next, the pre-heated pouches were moved through the microwave section, where they were subjected to microwave heating inside the microwave cavities that operated on a 915-MHz single-mode. Following the microwave heating, the pouches were advanced through the holding section that included the circulating water set at 124 °C. Finally, the pouches were cooled in the cooling section with circulating water set at room temperature. The processing conditions of the three MATS treatments are summarized in Table 1.
Table 1. Processing conditions of the three MATS treatments for the purees.
MATS processing parameters Unit Product Carrot puree Beetroot puree Cabbage puree Pre-heating temperature °C 61 61 61 Pre-heating time min 20 20 20 Water temperature in microwave heating section °C 124 124 124 Microwave heating time min 3.04 3.40 3.70 Water temperature in the holding section °C 124 124 124 Holding time min 3.12 3.49 3.80 Cooling water temperature °C 20 20 20 Cooling time min 5 5 5 Belt speed inch/min 42.0 37.5 34.5 Microwave power for cavities (both 1st and 2nd/3rd/4th cavities) kW 11.7/3.2/
3.711.7/3.2/
3.711.7/3.2/
3.7The calculated cumulative lethality at the slowest heating position (cold spot) inside the pouches filled with the carrot, cabbage and beetroot purees were 5.90 ± 0.82 min, 11.10 ± 2.42 min, and 6.0 min, respectively. These sterilization lethality values were calculated as follows:
$ {F}_{0}={\int }_{0}^{t}10\dfrac{T-{T}_{Ref}}{z}dt $ (1) where T is the processed food product's temperature at the cold spot, Tref is 121.1 °C, which is reference temperature of the sterilization processing, and z value is 10 °C.
The quality of the purees was evaluated after MATS (i.e., within 8 h) and during storage. To evaluate the storage quality of the purees, an accelerated shelf-life test (ASLT) was conducted. The processed puree pouches were transported into an incubator (Precision Incubator Thermo Fisher, Waltham, MA) controlled at 37.8 °C. The pouches were stored there for 6 months. Three pouches of each puree were drawn monthly and subjected to quality analyses (natural pigments, instrumental color, weight loss, and pH). This ASLT mimics the storage of food products for 3 years at ambient temperature[3].
Measurement of weight loss
-
The starting weight of the pouches was measured before the MATS treatment. The changes in weight of the pouches were determined by a balance (model GK703, Sartorius, Goettingen, Germany) after the MATS treatment and throughout the storage period. Each measurement of weight change was converted and expressed as weight loss percentage (w/w%).
Measurement of instrumental color
-
We utilized a CM-5 spectrophotometer (Konica Minolta, Ramsey, NJ, USA) for instrumental color assessments of the purees. A portion of 20 g from the fresh and the MATS-processed purees were separately transferred into a transparent plastic dish (60 mm × 15 mm). Then, the bottom of the dish was placed on the top of the port of the spectrophotometer, and the color parameters CIELAB were recorded. The color measurements (n = 6) were taken using the reflectance mode with the specular component excluded under the following conditions: the area of measurement was 8 mm, and the angle of observer was 10°, and with the illuminant D65. Total color difference (ΔE) was additionally calculated using Eqn (2 )to assess the magnitude of color changes in purees after the MATS treatment and during storage as follows:
$ \Delta E=\sqrt{({\mathrm{L}}^{*}-{L}_{0}^{*}{)}^{2}+({a}^{*}-{a}_{0}^{*}{)}^{2}+ ({b}^{*}-{b}_{0}^{*}{)}^{2}} $ (2) The values of L0*, a0*, and b0* represent the fresh puree sample's lightness, redness/blueness, and yellowness, while L*, a*, and b* represent the values of the puree sample immediately after MATS.
Based on the sensory perception, the ΔE value of food products can be interpreted as follows[20]:
ΔE < 0.2: no apparent color difference
0.2 < ΔE < 0.5: highly minor color difference
0.5 < ΔE < 2: minor color difference
2 < ΔE < 3: fairly apparent color difference
3 < ΔE < 6: apparent color difference
6 < ΔE < 12: intense color difference
ΔE > 12: different colors
Measurement of natural pigments
Betalains
-
Betalains are normally divided into two subclasses: betacyanins (red pigment) and betaxanthins (yellow pigment). The approach reported by Sonar et al.[12] was used to measure the betalains. A beetroot puree sample of 5 grams was mixed with 25 mL of deionized water and homogenized utilizing a Polytron PT 2500E homogenizer (Bohemia, NY, USA) for 3 min at about 600 revolutions per minute (rpm). The mixture was subjected to filtration by using Whatman No. 1 filter paper to clear it from any impurities. The clear filtered solution was then adjusted to a final volume of 100 mL with deionized water. The absorbance of the diluted solution was measured at three different wavelengths: 536 nm, 485nm, and 650 nm. A total of six absorbance readings were used to calculate the concentration of betacyanins and betaxanthins using the following equation:
$\begin{split}& Betacyanin \; or \; betaxanthin \; concentration\;\left(\dfrac{mg}{L}\right)\\=&\dfrac{A\times MW\times DF\times 1000}{\varepsilon \times l}\end{split} $ (3a) where A is the absorbance of betacyanins (A536−A650) and the absorbance of betaxanthins (A485−A650), MW represents the weight of molecule (550 g mol−1 for betacyanin and 339 g mol−1 for betaxanthin), DF represents the factor of dilution,
$\varepsilon $ $ l $ Anthocyanins
-
The protocol for measuring the concentration of anthocyanins in the fresh and MATS-processed cabbage puree was performed according to the method of Sonar et al.[12] with small modifications. A cabbage puree sample of 5 g was poured into a 50 mL polypropylene tube and mixed with methanol for 3 min at 6,000 rpm utilizing a homogenizer of Polytron PT 2500E (Bohemia, NY, USA). Then, the solution was filtered. The filtration was done through Whatman No. 1 filter paper, and the filtered solution was collected in a 30 mL beaker. Two buffer solutions were used to dilute the collected solution at a ratio of 1:3. These buffer solutions were a potassium chloride buffer, maintaining a pH value of 1, and a sodium acetate buffer, maintaining a pH value of 4.5. For 15 min, the diluted solutions were kept in the dark at ambient temperature. The absorbance was measured at 520 nm and 700 nm, respectively. The total monomeric anthocyanin content (MAC) in the fresh and MATS-processed cabbage purees was calculated using Eqn (3b) as follows:
$ Total\;monomeric\;anthocyanins\;\left(\dfrac{mg}{L}\right)=\dfrac{A\times MW\times DF\times 1000}{\varepsilon \times l} $ (3b) where A is the absorbance of the difference of diluted solutions at pH 1 and pH 4.5 (A520−A700), MW represents cyanidin-3-glucosideas's molecular weight, which is 449.2 g mol−1,
$ \varepsilon $ $ l $ β-carotene
-
Beta carotene in the fresh and MATS-processed carrot purees was extracted using an extraction solvent made of 0.1% BHT, 25% acetone, 50% hexane, and 25% ethanol according to the method described by Sonar et al.[13], with minor alterations. A carrot puree sample of 5 g was mixed with 30 mL of the extraction solvent with the use of the homogenizer of Polytron PT 2500E (Bohemia, NY, USA). The mixture was then vacuum filtered through Whatman No. 1 filter paper and poured into a separation funnel to separate the organic and the aqueous phases in the mixture. The collected organic phase was adjusted with the extraction solution to make an ultimate volume of 50 mL. The diluted solution's absorbance was evaluated at a wavelength of 450 nm. At least six absorbance readings were obtained and used to calculate the beta carotene's concentration in the carrot puree using the following equation:
$ Total\;carotenoids\;\left(\dfrac{\mu g}{g}\right)=\dfrac{A\times Volume\;\left(ml\right)\times 10000}{{E}_{cm}^{1{\text{%}}}\times sample\;weight\,\left(g\right)}$ (4) where A is the absorbance at a wavelength of 450 nm and
$ {E}_{cm}^{1\%} $ Measurement of pH
-
The pH values for the purees were measured using a pH meter. Twelve grams of each puree were sampled and placed in a 25 mL beaker. The pH meter electrode was then inserted into the puree. Once stable, the pH readings were then recorded; pH measurements (n = 6) were performed in duplicate only before and after MATS.
Statistical analysis and kinetic models
-
The test of one-way analysis of variance (ANOVA) was employed on the collected data to find out if there was a significant difference among the means. This test was followed by a Tukey test only when the ANOVA test's results displayed a significant difference among the means. The significant level was 0.05 for both tests. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used to conduct the statistical analysis. For the kinetic data of puree quality attributes, the constants of reaction rate were estimated by modeling the data into zero order (Eqn 5), first order (Eqn 6), and fractional conversion (Eqn 7) models[12]:
$ {C}_{t}={C}_{0}-kt $ (5) $ {C}_{t}={C}_{0}{e}^{-kt} $ (6) $ {C}_{t}={C}_{\infty }+\left({C}_{0}-{C}_{\infty }\right){e}^{-kt} $ (7) where Ct is the concentration of the quality attribute at a given time, C0 is the initial concentration,
$C_\infty $ -
Maintaining food moisture content in storage is important, as moisture loss can affect the quality of food in terms of color and texture as well as the sensory evaluation of the thermally processed food product[8,10,20]. Figure 1 illustrates the weight loss of MATS-processed beetroot, cabbage, and carrot puree samples throughout the storage period at 37.8 °C. MATS treatments did not change the samples' weight significantly (p > 0.05). This might be attributed to the short processing time of the MATS system and the high water vapor barrier properties of the package. However, during storage, the MATS-processed packaged purees experienced a small weight change. By the sixth month at 37.8 °C, the weight loss of the puree samples ranged between 0.43%–0.45%. Results in the present study were comparable to those obtained in previous studies[3,10,21]. These studies demonstrated that the PET–metal oxide-based packages that were tailored with different numbers of metal oxide-coated layers (i.e., one, two, or three layers) could prevent weight loss in thermally processed food products during storage because of their low WVTR permeation properties. This shows that polymeric packages with low WVTR values are essential to prevent weight loss in processed food during an extended storage period.
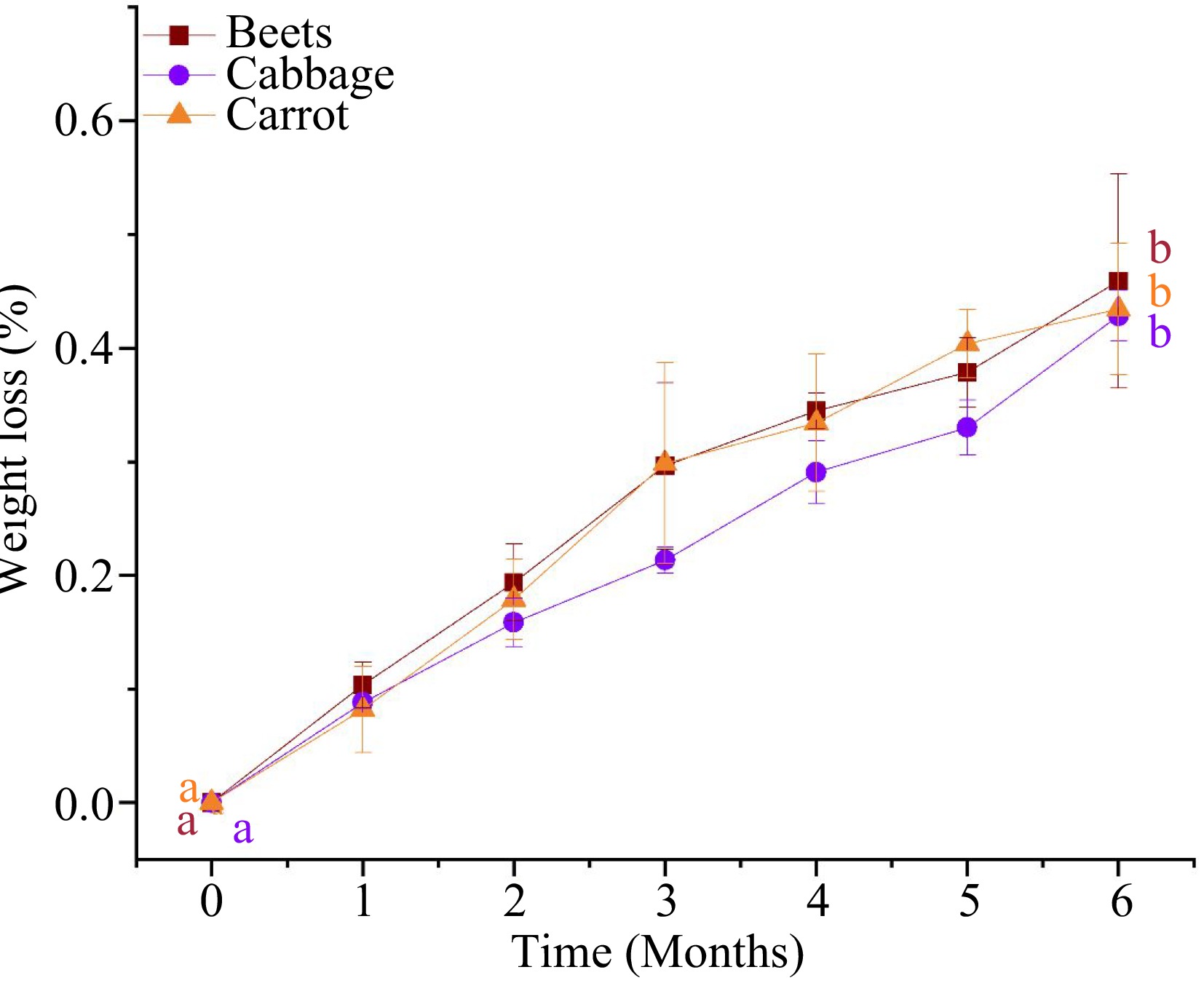
Figure 1.
Weight loss of the MATS- processed beetroot, cabbage, and carrot puree samples during 6 months of storage at 37.8 °C (n = 3). Values with the same superscript letters are not significantly different (p > 0.05).
Instrumental color of purees during MATS processing and storage
Color changes in beetroot puree
-
High temperature and long processing time can alter the color hue of the beetroot-based product as a result of betacyanins degradation[22,23]. Post-MATS, the values of color parameters, redness (a*), lightness (L*), and yellowness (b*) of the beetroot puree increased significantly (p < 0.05) compared to the fresh beetroot puree sample (Table 2). The calculated ΔE value of the MATS-treated beetroot puree was 6.39 ± 1.09, which indicated a strong change in the original color of the beetroot puree before processing[20]. This change of instrumental color parameters was reflected by the change in the visual color, as the deep violet color of fresh beetroot puree became less violet after the MATS treatment (Fig. 2). For the MATS-treated beetroot puree, other studies have reported lower ΔE values, especially for pasteurized beetroot-based product, as shown in Table 3. However, processing time can be a critical factor in the preservation of beetroot-based products with pasteurization or sterilization techniques. However, the high ΔE value of the MATS-processed beetroots purée compared to the reported values can be explained by the sensitivity of natural pigments to high temperatures. Although the MATS system based on 915-MHz is featured with a high penetration depth within the pre-packaged food[4], the localized heating at high temperatures on the surface of the puree may have played a role in the leaching or the degradation of betalains, resulting in a relatively low color retention.
Table 2. Quality attributes of vegetable purees after the MATS treatment, n = 6.
Vegetable puree Quality attribute Before MATS After MATS Beetroot pH 6.30 ± 0.02a 6.03 ± 0.19b L* 12.5 ± 0.65a 14.05 ± 0.53b a* 1.90 ± 0.20a 7.64 ± 0.93b b* 0.22 ± 0.16a 2.36 ± 0.31b ΔE None 6.39 ± 1.09 BC (mg/100 g) 33.7 ± 4.86a 27.0 ± 2.91b BX (mg/100 g) 21.7 ± 2.97a 15.4 ± 1.93b Cabbage pH 5.90 ± 0.02a 5.62 ± 0.01b L* 19.1 ± 0.48a 24.1 ± 1.61b a* 5.32 ± 0.23a 3.13 ± 0.27b b* –6.92 ± 0.17a –4.42 ± 0.22b ΔE None 6.09 ± 1.20 MAC (mg/100 g) 47.45 ± 4.00a 35.09 ± 4.34b Carrot pH 5.57 ± 0.04a 5.35 ± 0.03b L* 40.9 ± 0.52a 36.3 ± 0.55b a* 27.3 ± 0.78a 18.1 ± 0.38b b* 36.0 ± 1.33a 37.0 ± 1.02b ΔE None 10.5 ± 0.97 β-Carotene (μg/g) 55.1 ± 12.1a 57.4 ± 2.07a BC: betacyanin in beetroot puree, BX: betaxanthin in beetroot puree, and MAC: total monomeric anthocyanins in cabbage puree. Values with the same superscript letters are not significantly different (p > 0.05). 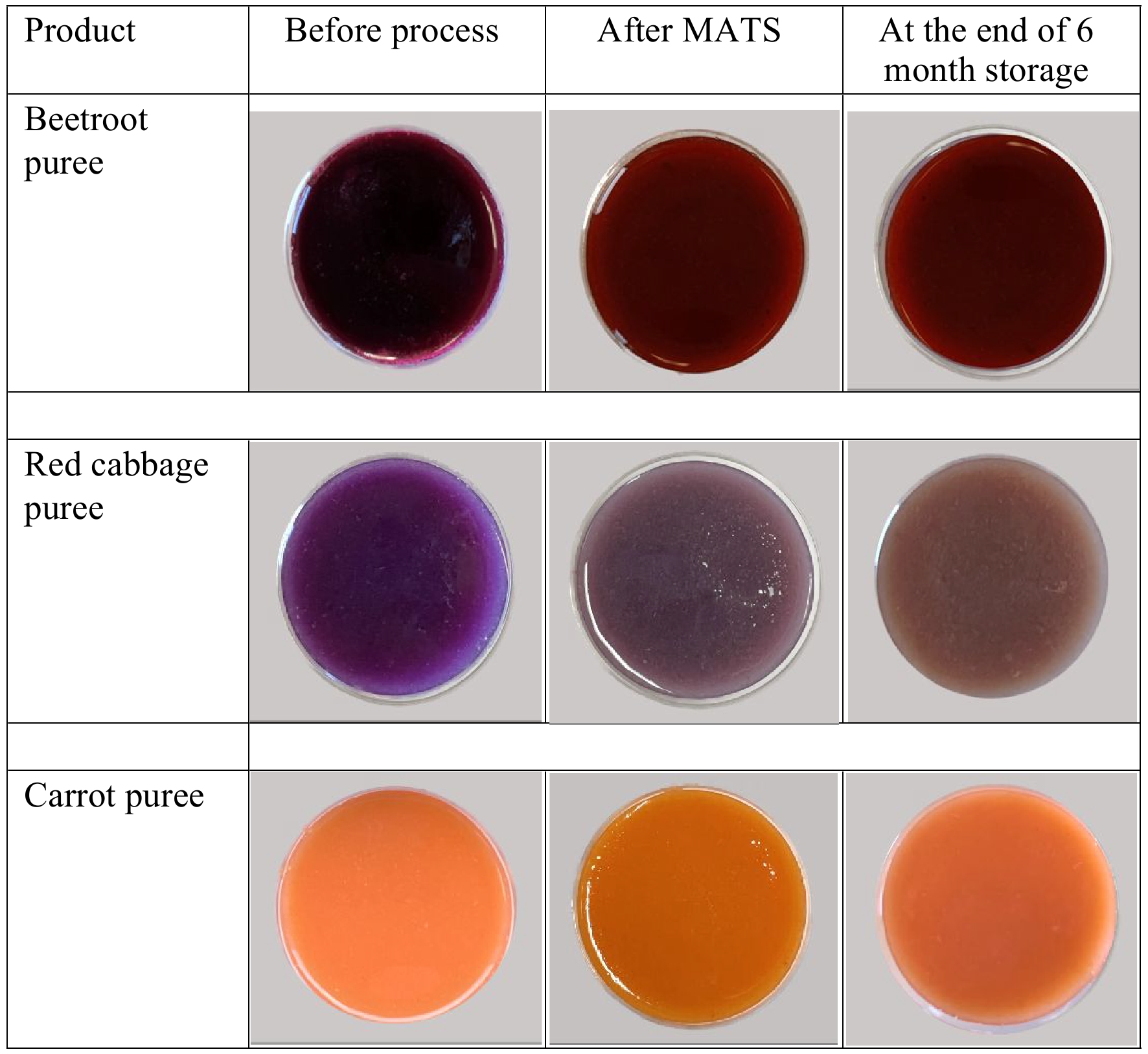
Figure 2.
The changes in visual color in beetroot, red cabbage, and carrot purees after the MATS treatment and at the end of 6 months of storage at 37.8 °C.
Table 3. Color assessment studies of foods processed with high pressure and thermal processes.
Food product Type of processing Processing conditions Total color change (ΔE) Ref. Beetroot juice HPP 600 MPa for 3 min at ambient temperatures 3.49 ± 0.04 [24] Beetroot puree In-package thermal pasteurization 90 °C for 10 min in hot water bath 2.44 ± 0.06 to 2.89 ± 0.12 [12] Beetroot puree PATS 600 MPa at for 5 min at 90 °C 2.49 ± 0.18 [25] Beetroot slices HPP HPP 600 MPa for 3–30 min at ambient temperature 21.80 ± 0.30 to 27.60 ± 0.90 [26] Beetroot puree MATS 124 °C, 6.89 min 6.39 ± 1.09 Present study Cabbage puree In-package thermal pasteurization 90 °C for 10 min in hot water bath 2.81 ± 0.03 to 3.59 ± 0.03 [12] Purple mashed potato PATS 600 MPa at for 5 min at 90 °C 9.99 ± 0.24 [25] Pomegranate juice HTST 110 °C/8.6 s 6.83 ± 1.06 [27] Strawberry puree HPP 600 MPa for 3min 2.12 ± 0.07 [28] Blackberry puree HPP 600 MPa for 3min 2.15 ± 0.03 [28] Cabbage puree MATS 124 °C, 8.8 min 6.09 ± 1.20 Present study Carrot puree In-package thermal pasteurization 90 °C for 10 min in hot water bath 1.02 ± 0.64 to 2.89 ± 0.12 [13] Pumkin puree PATS 600 MPa at for 5 min at 90 °C 3.37 ± 2.59 [25] Carrot juice HPP 600 MPa at for 5 min at 4 °C 5.39 ± 0.01 [29] Carrot puree MATS 124 °C, 8.12 min 10.50 ± 0.97 Present study HPP: high pressure processing, PATS: pressure-assisted thermal sterilization, and HTST: high temperature short time processing. About the changes in color parameters (L*, a*, and b*) during thermal processing, similar trends have been reported in several studies. For example, Singh & Ramaswamy[22] reported an increase in a*, L*, and b* in thermally sterilized red beetroot, attributing this to the degradation of betalains. Similarly, Prieto-Santiago et al.[23] observed an increase in the values of L*, a*, and b* of beetroot puree during a thermal kinetic degradation study at 120 °C in an autoclave. In the present study, the changes in the color parameters indicated the sensitivity of the beetroot puree's color toward the selected MATS processing conditions. Therefore, optimization of MATS processing conditions is needed to maximize the color retention in beetroot puree.
Figure 3 illustrates the changes in L*, a*, and b* of beetroot puree throughout the storage period at 37.8 °C. All color parameters were significantly (p < 0.05) affected by the duration of storage, as L*, a*, and b* increased (p < 0.05) during storage. At the end of the storage, the final values of L*, a*, and b* were 16.7 ± 0.69, 9.25 ± 0.26, and 5.20 ± 0.68, respectively. These values differed significantly (p < 0.05) from their encounters registered after MATS processing. However, visual observation revealed that the color of MATS-processed beet puree at each time point during storage did not differ from that of the puree immediately after MATS (Fig. 2). The ΔE at the end of storage was only 4.23 ± 1.26, indicating an overall perceptible change[20] but also minimal changes in beetroot color after a storage period of 6 months at 37.8 °C. This low value of ΔE can be attributed to the high gas barriers of the metal oxide-PET-based pouches. Sonar et al.[12] observed a significant difference (p < 0.05) in the ΔE values of thermally pasteurized beetroot purees packaged in polymeric packages with varied OTR values were stored at 7 °C for 80 d. They observed that thermally processed beetroot puree packaged in the low OTR (1 cm−3 m−2 d−1) package had the lowest ΔE value at the end of storage compared to the ones packaged in the higher OTR package (81 cm3 m−2 d−1).
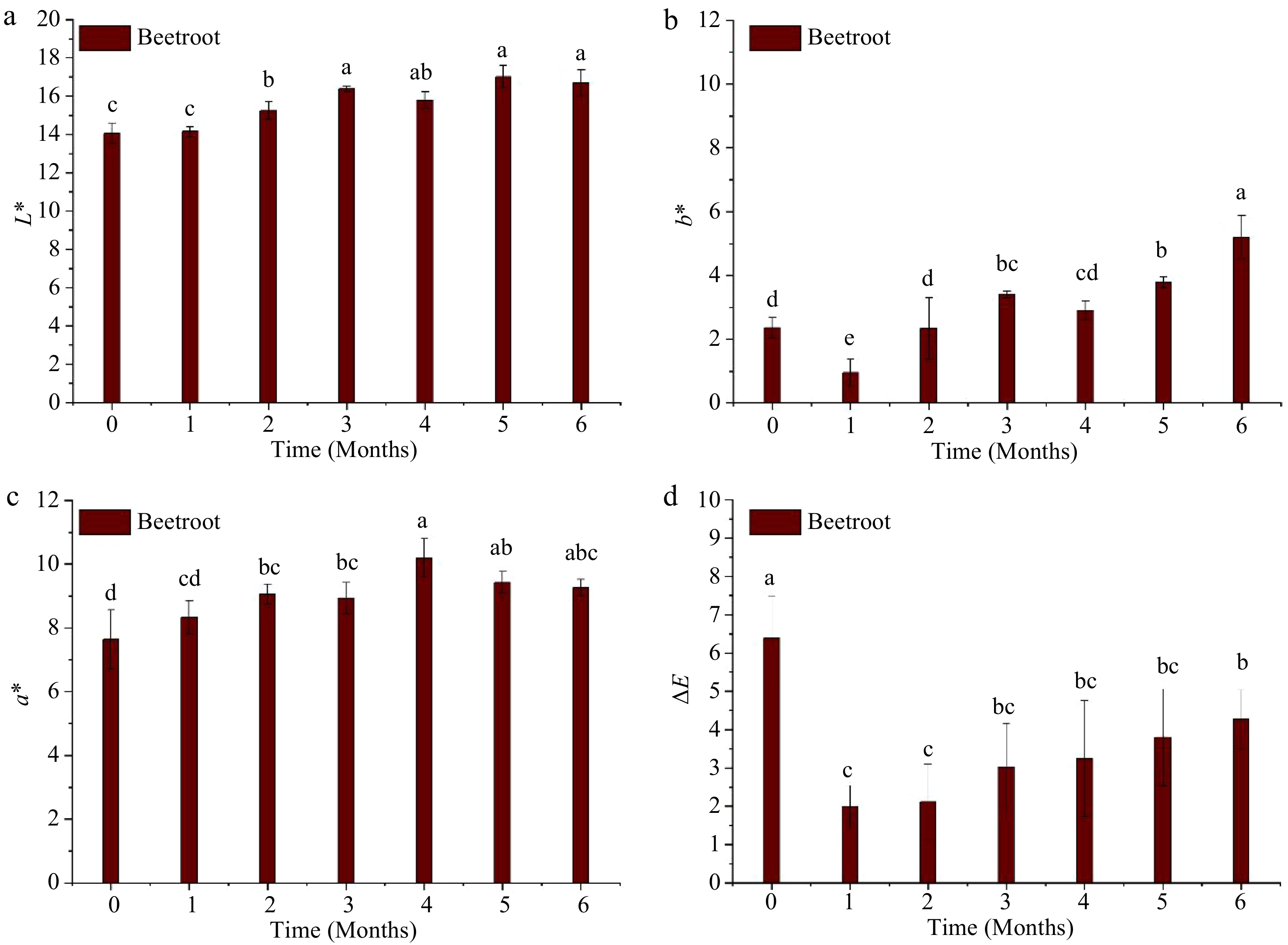
Figure 3.
Instrumental color changes of the MATS-processed beetroot during 6 months of storage at 37.8 °C: (a) lightness, (b) yellowness, (c) redness, and (d) total color difference, values with the same superscript letters are not significantly (p > 0.05) different, n = 6.
Color changes in cabbage puree
-
The values of lightness (L*), blueness (b*), and redness (a*) of the cabbage puree changed (p < 0.05) after the MATS treatment (Table 2). Both L* and b* increased (p < 0.05) after the MATS treatment, but a* decreased (p < 0.05). Sonar et al.[12] observed a comparable trend of instrumental color L*, b*, and a* changes in thermally pasteurized red cabbage packaged in polymeric films with different OTR values (ranging between 1 and 81 cm3 m−2 d−1). Additionally, changes in instrumental color in anthocyanin-rich foods after thermal sterilization processing were reported by Al-Ghamdi et al.[25]. They observed a significant increase in L* in thermally sterilized purple mashed potato, which was attributed to the degradation of anthocyanins. The ΔE of the MATS-processed cabbage puree in the present study was 6.09 ± 1.20. For MATS-cabbage purees, the ΔE value obtained after MATS was comparable to or lower than that reported using different sterilization techniques for anthocyanins rich food products in different studies, as shown in Table 3. For example, Patel et al.[11] reported a ΔE value of 4 for purple mashed potato packaged in a PET-metal oxide-based pouch and processed with MATS. The similar ΔE value of the MATS-processed cabbage puree compared to the ones reported in the literature can be accounted for the high thermal stability of diacylated anthocyanins[12]. Similar to the MATS-processed beetroot puree, optimizing the processing conditions could result in higher color retention for the cabbage puree.
During storage, L* of the cabbage puree exhibited a significant increase (p < 0.05) during the first month (Fig. 4). However, the increase in L* value after the first month was insignificant (p > 0.05) compared to that in the first month. The value of b* increased (p < 0.05) from a negative to a positive value during the first month (i.e., indicated more yellow color) and continued to increase (p < 0.05) throughout the storage period. However, a* increased significantly (p < 0.05) but then decreased toward the end of the storage period. The values of L*, a*, and b* at the end of the storage period were 28.5 ± 0.62, 4.03 ± 0.09, and 7.24 ± 0.20, respectively. These final values differed significantly (p < 0.05) from those obtained on day 0. The significant (p < 0.05) changes in the values of color parameters L*, a*, and b* led to a significant (p < 0.05) increase in the ΔE values throughout the storage period. The ΔE reached 12.3 at the end of storage, indicating the end point of the shelf life of red cabbage puree in accordance with Zhang et al.[20]. Moreover, from visual observation, the increase in ΔE was accompanied by the development of brown layers in different locations in the packaged cabbage puree sample, as shown in Fig. 2. The presence of brown layers could have resulted from enzymatic browning reactions.
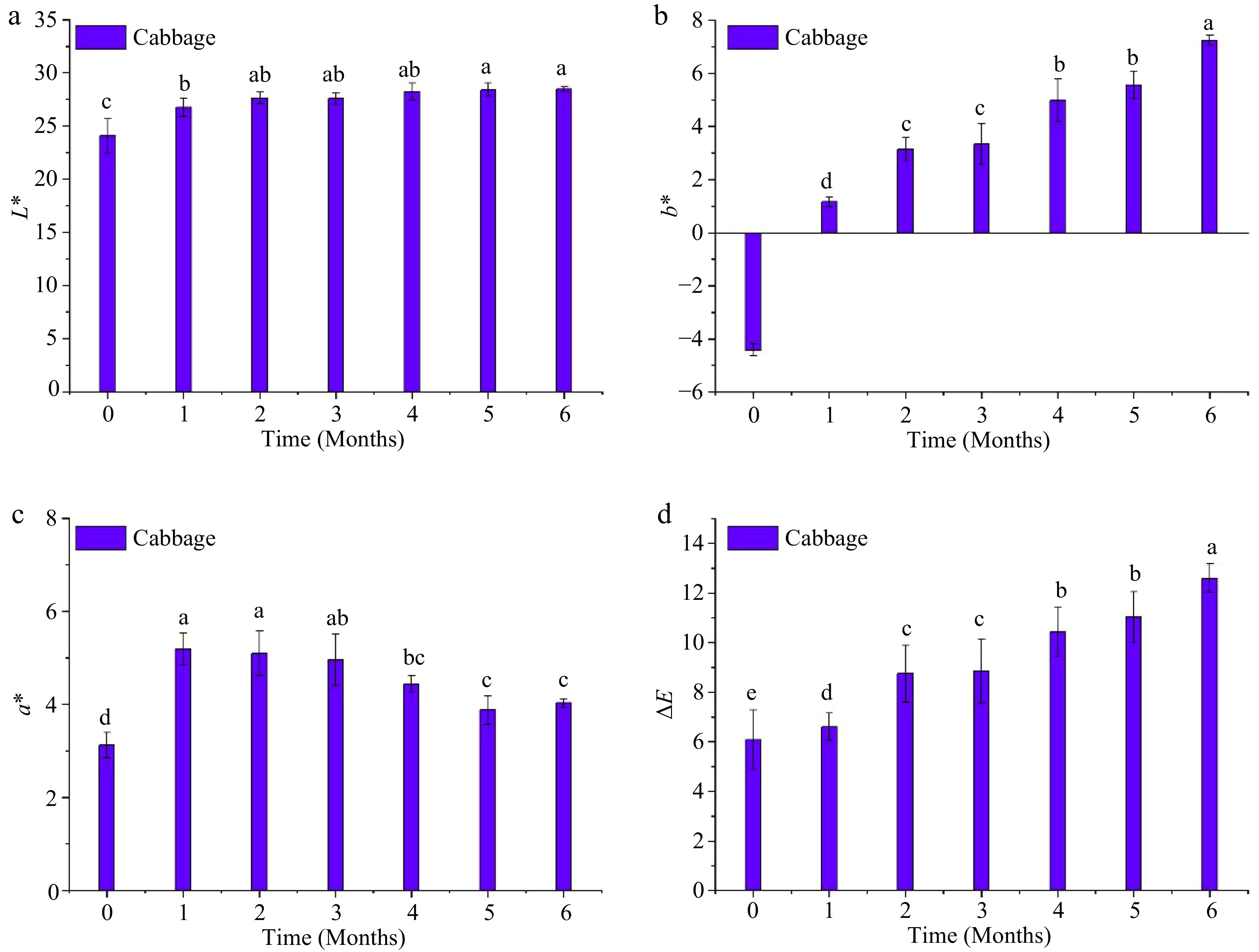
Figure 4.
Instrumental color changes of the MATS-processed cabbage puree during 6 months of storage at 37.8 °C: (a) lightness, (b) redness, (c) blueness, and (d) total color difference. Values with the same superscript letters are not significantly different (p > 0.05), n = 6.
Color changes in carrot puree
-
The color of the carrot puree was affected by the MATS treatment. However, the effect of the MATS treatment was limited to the lightness (L*) and redness (a*) of carrot puree (Table 2). Both L* and redness a* exhibited a significant (p < 0.05) decrease, but yellowness (b*) did not significantly (p > 0.05) change. Zhang et al.[8] observed a decrease in a* along with the changes in other color parameters in sweet potatoes subjected to MATS processing. On the other hand, the canning process and high-pressure-sterilization treatment were reported to significantly impact all color parameters in carrot[30]. Similarly, Chen et al.[31] reported that carrot juice processed with retort at 120 °C for 30 min had a significant decrease in color parameters L*, a*, and b*; with these instrumental color changes, there was a change in the carrot's color from orange to yellow. In this study, the ΔE value of the MATS-processed carrot puree was 10.5 ± 0.97 with a slightly dark orange color, as shown in Fig. 2. In this study, the ΔE value of MATS-processed carrot puree is higher than the values reported in the literature based on pasteurization and sterilization techniques, as shown in Table 3. This difference in color change could relate to the severity of heating methods and processing time used in those studies, as longer exposure to high temperatures could result in significant color changes. In the dielectric heating of a 915-MHz single-mode, the dielectric properties (dielectric constant ε′ and dielectric loss factor ε′′) of the food is essential in determining the efficiency of heating[4].
The carrot's intense orange color may be affected during microwave heating when beta carotene is isomerized; the changes in a* and b* can indicate that[32]. Besides the applied processing conditions, the oxygen permeation of the polymeric packaging can also affect the color of carrot puree during in-package thermal processing. Sonar et al.[13] reported that the value of ΔE was significantly (p < 0.05) higher in thermally pasteurized carrot puree packaged with high OTR film (81 cm−3 m−2 d−1) compared to the one packaged in low and medium OTR films (1−30 cm−3 m−2 d−1). A higher OTR of the package means more oxygen permeation into the packaged foods. During thermal processing that involves high temperatures and pressure, minimizing the oxygen permeation may play a role in color and beta-carotene retention of carrot puree after thermal processing. In this study, the low OTR of the metal oxide PET-based pouch and the short time of the MATS treatment may have minimized the overall changes in both the instrumental and the visual colors of the carrot puree.
Figure 5 shows the changes in L*, a*, and b* of MATS-processed carrot puree during the storage period. Both L* and a* values remained relatively constant during the storage period. However, the value of b* exhibited a decrease (p < 0.05) during the storage period. The final values of L*, a*, and b* at the end of storage were 36.0 ± 0.42, 19.1 ± 1.74, and 30.5 ± 2.83. The ΔE value changed significantly (p < 0.05) during storage; at the end of the storage period, it reached 7.23 ± 2.67. This value was not significantly (p > 0.05) different from the value registered after processing. Ayvaz et al.[33], indicating that the OTR of packaging material impacted the color parameters' stability in pressure-assisted thermally processed carrot, especially a* and b*, during a storage period of three months at 37 °C, and the package with lower OTR maintained the high value of a* at the end of the storage period. Similarly, Sonar et al.[13] reported that thermally pasteurized carrots packaged in a low OTR (1 cm−3 m−2 d−1) package preserved a* and b* during 45−100 d of storage at refrigerator temperatures (4–13 °C). In the present study, the degradation in b* might be caused by the oxidation of beta-carotene due to the high storage temperature.
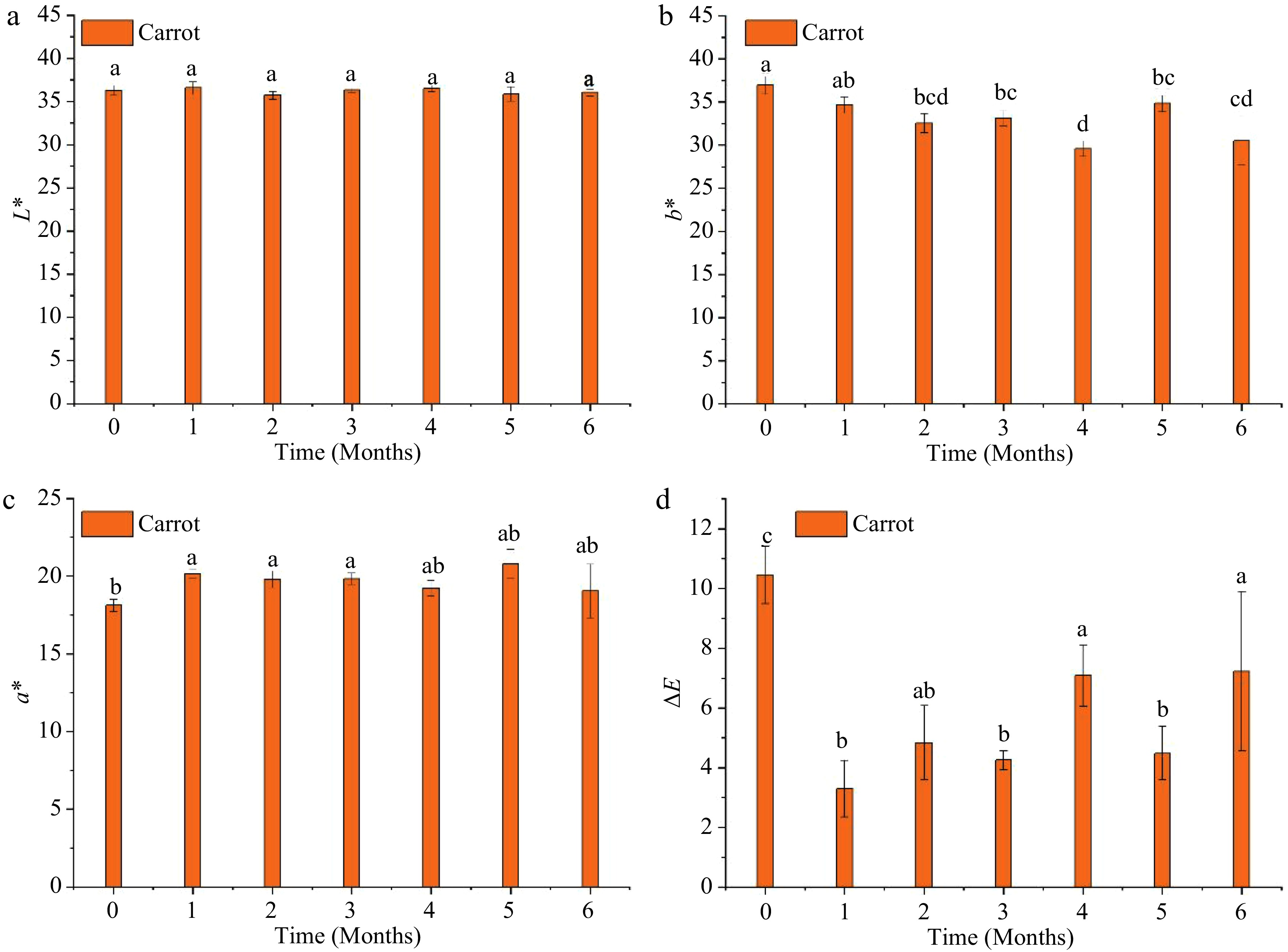
Figure 5.
Instrumental color changes of the MATS-processed carrot puree during 6 months of storage at 37.8 °C: (a) lightness, (b) redness, (c) yellowness, and (d) total color difference. Values with the same superscript letters are not significantly different (p > 0.05), n = 6.
Natural pigments
Betalains
-
The content of betalains in the beetroot puree was affected by the MATS treatment. Betacyanin (red pigment) and betaxanthin (yellow pigment) concentration was reduced by 20% and 29%, respectively (Table 2). Jiratanan & Liu[34] reported that the canning process at 115 °C for 30 min induced degradation in betacyanins and betaxanthins by 62% and 60%, respectively in beetroot. In the same study, the authors reported further degradation in betacyanins and betaxanthins in the beetroot by 81% and 73%, respectively, after a canning process at 125 °C for 30 min. In another study, the canning process of beetroot in a retort at 126 °C for 10 min led to a reduction in betacyanins by 22%[35]. Betanin under a heating treatment can be degraded by several pathways, namely hydrolysis, decarboxylation, and dehydrogenation, which can alter its red color[36]. However, the higher retention of betalains in the present study compared to beetroot that underwent canning processes can be attributed to the shorter processing time in the MATS treatment.
In the MATS-processed beetroot puree, betacyanins had less degradation than betaxanthins. One of the possible reasons could be the regeneration of betacyanins after the MATS treatment, since the quality analysis was conducted within 8 h after the MATS treatment. Von Elbe et al.[35] observed the degradation and retention of betacyanin in beetroot after thermal treatment (126 °C for 10 min). The hydrolysis of a betanin solution during a thermal treatment resulted in the formation of two products, cyclodopa and betalamic acid, and the process of the betanin regeneration involves condensation of these two intermediate products[35]. In addition, according to Huang & Elbe[37], the partial regeneration of betanin after thermal processing depends on the heating treatment's temperature and the concentration of betalamic acid. In the present study, the short processing time in the MATS treatment might result in a minimum degradation of betacyanin and therefore facilitate the regeneration of betacyanin from the betalamic acid and the cyclodopa in the beetroot puree after the MATS treatment.
The concentration of betacyanins and betaxanthins in the MATS-processed beetroot puree samples experienced a significant (p < 0.05) changes during the storage period compared to the value registered after MATS processing (Fig. 6). Despite the higher thermal stability in the MATS treatment, betacyanins showed less stability during storage than betaxanthins. Both pigments demonstrated a semi-linear degradation pattern during storage. At the end of the storage period of 6 months, there was a 73% degradation in betacyanins and 53% in betaxanthins. Sonar et al.[12] observed that the oxygen permeation of the package affected the stability of betalains during a storage period of 80 d at 7 °C. They observed that the beetroot puree in a medium OTR package (30 cm−3 m−2 d−1) had a 27.7% and 25.9% decline in betacyanins and betaxanthins, respectively. In comparison, a high OTR package (81 cm−3 m−2 d−1) showed a higher degradation in betacyanins and betaxanthins by 54.8% and 40.5%, respectively. However, adding natural additives such as vitamin C may help preserve betalains in the MATS-processed beetroot puree during storage. This needs to be investigated in future studies.
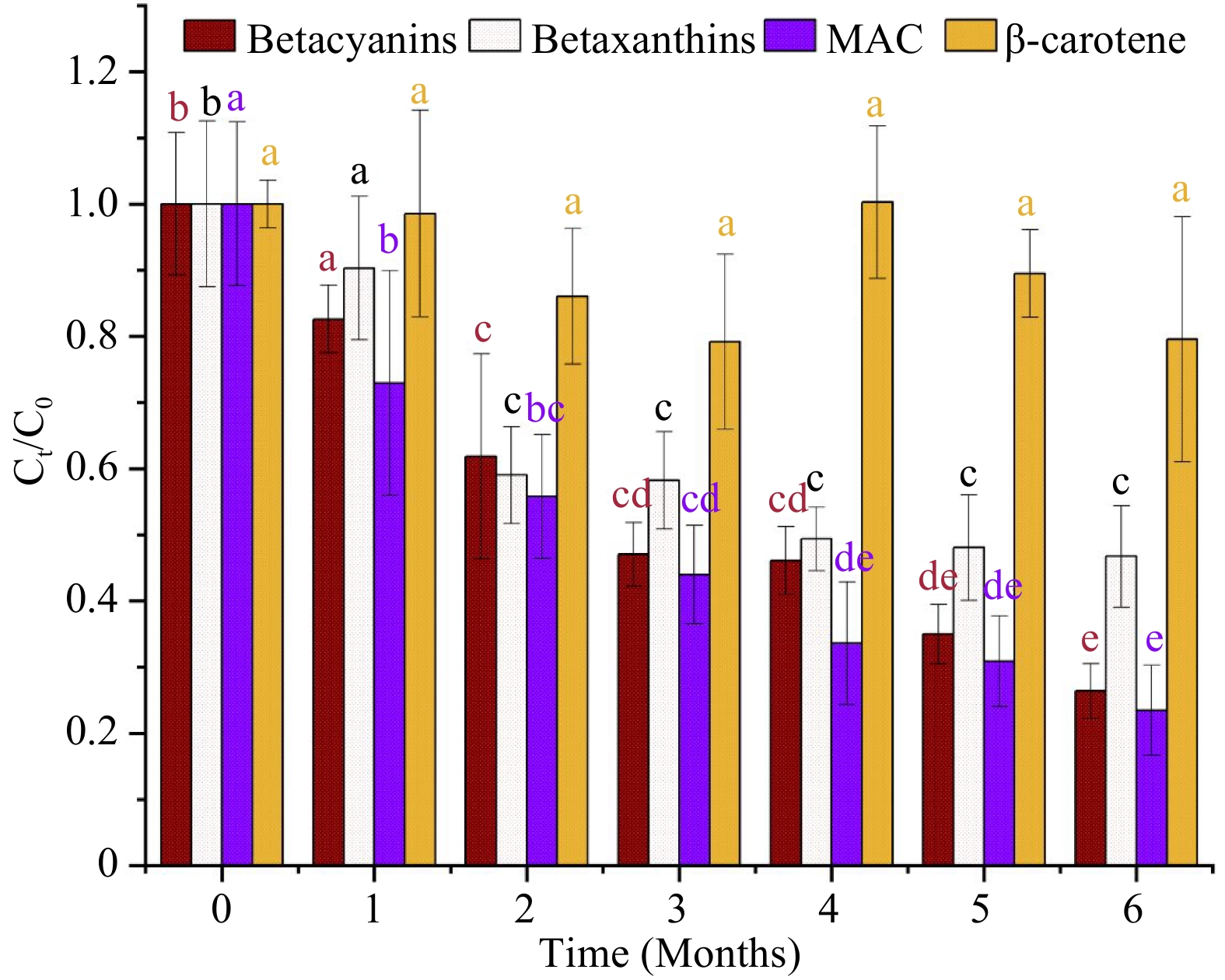
Figure 6.
Concentration of natural pigments (betacyanins, betaxanthins, total monomeric anthocyanins (MAC), and beta-carotene) in MATS-processed beetroot puree samples during 6 months of storage at 37.8 °C (n = 6). Values with the same color and superscript letters are not significantly different (p > 0.05).
Regarding the relations between instrumental color indicators and pigments in this study, we found an inverse relationship between L* and the degradation of betalains in the beetroot puree. The degradation in betacyanins and betaxanthins was negatively correlated with the increase of L* during the storage period by −0.96 and −0.94, respectively. Sonar et al.[12] reported a negative correlation between ΔE and total betalains content in thermally pasteurized beetroot after a storage of 80 d at 7 °C.
Anthocyanins
-
The MATS treatment has a significant (p < 0.05) impact on anthocyanins in the cabbage puree. There was a 26% reduction in the anthocyanin concentration in the MATS-processed cabbage puree compared to the fresh sample (Table 2). This anthocyanin concentration reduction can be ascribed to the exposure to high temperatures during microwave heating and the holding steps in the MATS treatment. But much higher degradation in anthocyanins was reported during conventional thermal processing. For example, degradation in anthocyanins in red cabbage was reported to be 59% and 49% after the cabbage sample was subjected to blanching and steaming processes[38]. Additionally, Al-Ghamdi et al.[29] reported a 57% degradation in anthocyanins in PATS processed puree. Patel et al.[11] observed an increase in anthocyanins in the vitamin-C-fortified purple mashed potato processed with MATS. The degradation in anthocyanins during a heating treatment can be affected by several variables including pH, acylation of the anthocyanins, and the duration of the heating treatment[39].
Figure 6 shows the changes in the concentration of anthocyanins in the cabbage puree during a storage duration of 6 months at 37.8 °C. Similar to the betalains in the beetroot puree, the anthocyanins in the cabbage showed a significant decline with a semi-linear trend during storage. At the end of the storage period, there was approximately 76% degradation in anthocyanins compared to the values obtained immediately after MATS processing (day 0). A similar degradation trend in anthocyanins was observed by Patel et al.[11] in a MATS-processed purple mashed potato fortified with vitamin C and packaged in polymeric pouches with a high barrier during a storage period of 7 months at a storage temperature of 37.8 °C; the authors observed degradation of anthocyanins ranging between 70% and 76% after storage; they attributed the higher anthocyanin degradation to the higher OTR value of the polymeric package. To improve the stability of the anthocyanins in MATS-processed food products, a pre-treatment such as blanching may be effective in preserving such pigment during longer storage periods.
In the present study, there was a negative correlation between the color parameters L*, b*, and ΔE and the degradation of anthocyanins in the cabbage puree samples during the storage period. The increase in L*, a*, and ΔE were negatively correlated by values of −0.96, −0.98, and −0.94, respectively, with the degradation in anthocyanins. Alighourchi & Barzegar[40] also reported a negative correlation between ΔE and the degradation in total anthocyanins in thermally pasteurized pomegranate stored at 37 °C for 210 d.
β-carotene
-
The MATS treatment did not affect the total concentration of beta-carotene in the carrot puree. There was a slight but not statistically significant (p > 0.05) reduction in the total concentration of beta-carotene compared to the fresh carrot puree (Table 2). As expected, MATS had an extremely minor effect on the beta-carotene concentration even without the presence of any additives such as vitamin C. Zhang et al.[8] observed a higher thermal stability of beta-carotene in sweet potato puree fortified with vitamin C subjected to a MATS processing with similar processing conditions. Beta-carotene is a thermally stable substance, and high-temperature processes can enhance its extractability, as observed by Tola & Ramaswamy[41].
Beta-carotene was stable throughout the storage period; at the end of storage, we did not observe a significant difference (p > 0.05) compared to the concentration of beta-carotene after processing (day 0). Zhang et al.[8] observed that the total beta carotene concentration in MATS-processed sweet potato packaged in high-barrier polymeric pouches (OTR ranged between 1−4 cm−3 m−2 d−1) did not experience any significant degradation (p > 0.05) during 6 months of storage at 37.8 °C. Additionally, Sonar et al.[13] observed a high level of stability of the total beta carotene in thermally processed carrot puree stored at temperatures of 13 °C for 45 d. The reason for the higher storage stability of the beta-carotene compared to the water-soluble pigments (betalains and anthocyanins) during storage could be attributed to its fat solubility. Patel et al.[7] reported that fat-soluble vitamins such as A and E exhibited resistance to degradation after MATS processing and after 6 months of storage at 37.8 °C. However, other factors, such as enzymatic activities and changes in pH, may affect the overall stability of water and fat-soluble pigments after MATS and during storage. This needs to be investigated in future studies.
Kinetics of natural pigments and color changes
-
Table 4 shows the estimated parameters of the kinetics models for the degradation of natural pigments (betalains and anthocyanins) and for the overall color changes (ΔE) in the purees during the storage period of 6 months and at a storage temperature of 37.8 °C. Since beta-carotene in the MATS-processed carrot puree exhibited a high level of stability during storage, the changes in the pigments did not fit any kinetics degradation models. For betacyanin degradation, both the first order and fractional conversion models fitted well, with degradation rate of 0.25 month−1, a R2 of 0.986, and a RMSE of 0.029, as shown in Fig. 7a. For betaxanthins, the model of fractional conversion showed the best fit, with a high correlation factor (R2) of 0.937 and a low root mean square error (RMSE) of 0.051, as shown in Fig. 7b. The degradation rate of betaxanthins was 0.52 month−1. In relation to degradation rate values of betalains, betaxanthins had twice the rate of degradation than betacyanin, which suggested the sensitivity of betaxanthins to the high temperature of storage conditions. On the other hand, anthocyanin degradation was also explained well by both the first-order model (with a R2 of 0.989 and a RMSE of 0.026) and the fractional conversion model (with a degradation rate of 0.36, an R2 of 0.998 and a RMSE of 0.011), as shown in Fig. 8. Sonar et al.[12] reported that the degradation of betacyanins and betaxanthins in thermally pasteurized beetroot puree followed the fractional conversion model and the first order model respectively, especially for the beetroot packaged in high and medium OTR pouches (30−81 cm3 m−2 d−1). Buvé et al.[42] reported that the degradation of anthocyanin in thermally pasteurized strawberry juice followed the first-order model. In the present study, the deviation from the first-order model for anthocyanins in the MATS-processed cabbage may be caused by enzymatic reactions during the prolonged storage period.
Table 4. Estimated parameters of the kinetics models for the natural pigments' degradation and overall color changes during the storage of 6 months at 37.8 °C.
Model Natural pigments ΔE of vegetable purees Betacyanins Betaxanthins Anthocyanins Beetroot Cabbage Carrot First order k (month−1) 0.22 ± 0.01 0.14 ± 0.03 0.25 ± 0.01 * * 0.15 ± 0.04 t1/2 (month−1) 3.16 ± 0.20 4.80 ± 0.92 2.82 ± 0.15 * * 4.51 ± 1.23 R2 0.986 0.870 0.989 * * 0.784 RMSE 0.0291 0.070 0.0262 * * 0.740 Fractional conversion kf (month−1) 0.25 ± 0.09 0.52 ± 0.18 0.36 ± 0.03 * * * C∞ 0.06 ± 0.16 0.43 ± 0.06 0.15 ± 0.03 * * * t1/2 (month−1) 2.78 ± 0.98 1.34 ± 0.47 1.93 ± 0.19 * * * R2 0.986 0.937 0.998 * * * RMSE 0.0287 0.0505 0.0112 * * * Zero order k (month−1) 0.11 ± 0.01 0.08 ± 0.02 0.11 ± 0.02 0.46 ± 0.04 1.16 ± 0.07 0.78 ± 0.68 t1/2 (month−1) 4.07 ± 0.45 5.46 ± 1.48 5.46 ± 1.48 3.89 ± 0.61 2.40 ± 0.14 1.55 ± 0.40 R2 0.938 0.771 0.898 0.972 0.957 0.750 RMSE 0.0606 0.0959 0.898 0.138 0.396 0.762 R2 is correlation factor, and RMSE is root mean square error, n = 6, * R2 value was low due to poor fit of the model. 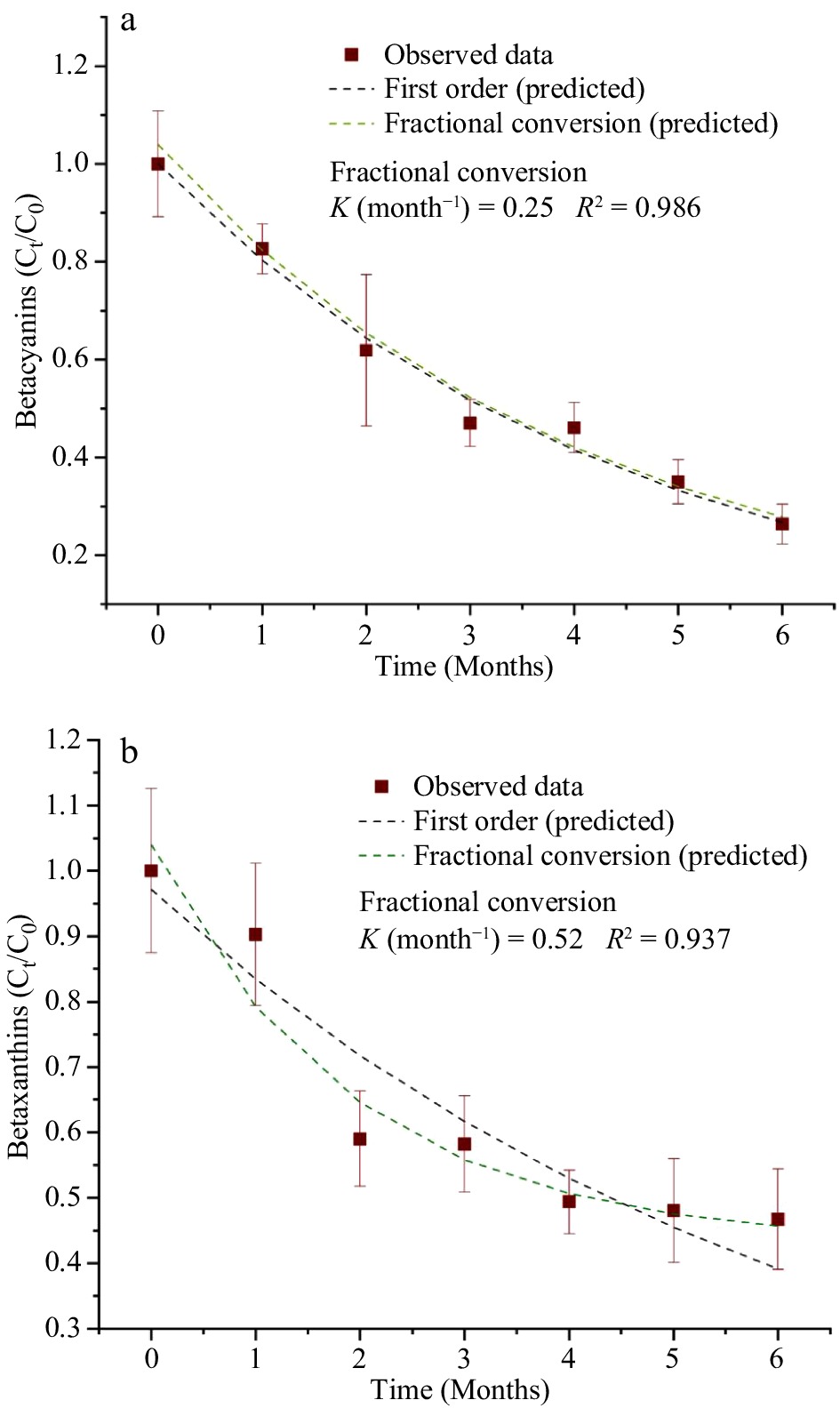
Figure 7.
Concentration of betalains [(a) betacyanins, (b) betaxanthins] during the 6 months of storage at 37.8 °C fitted with the first order and fractional conversion models, n = 6.
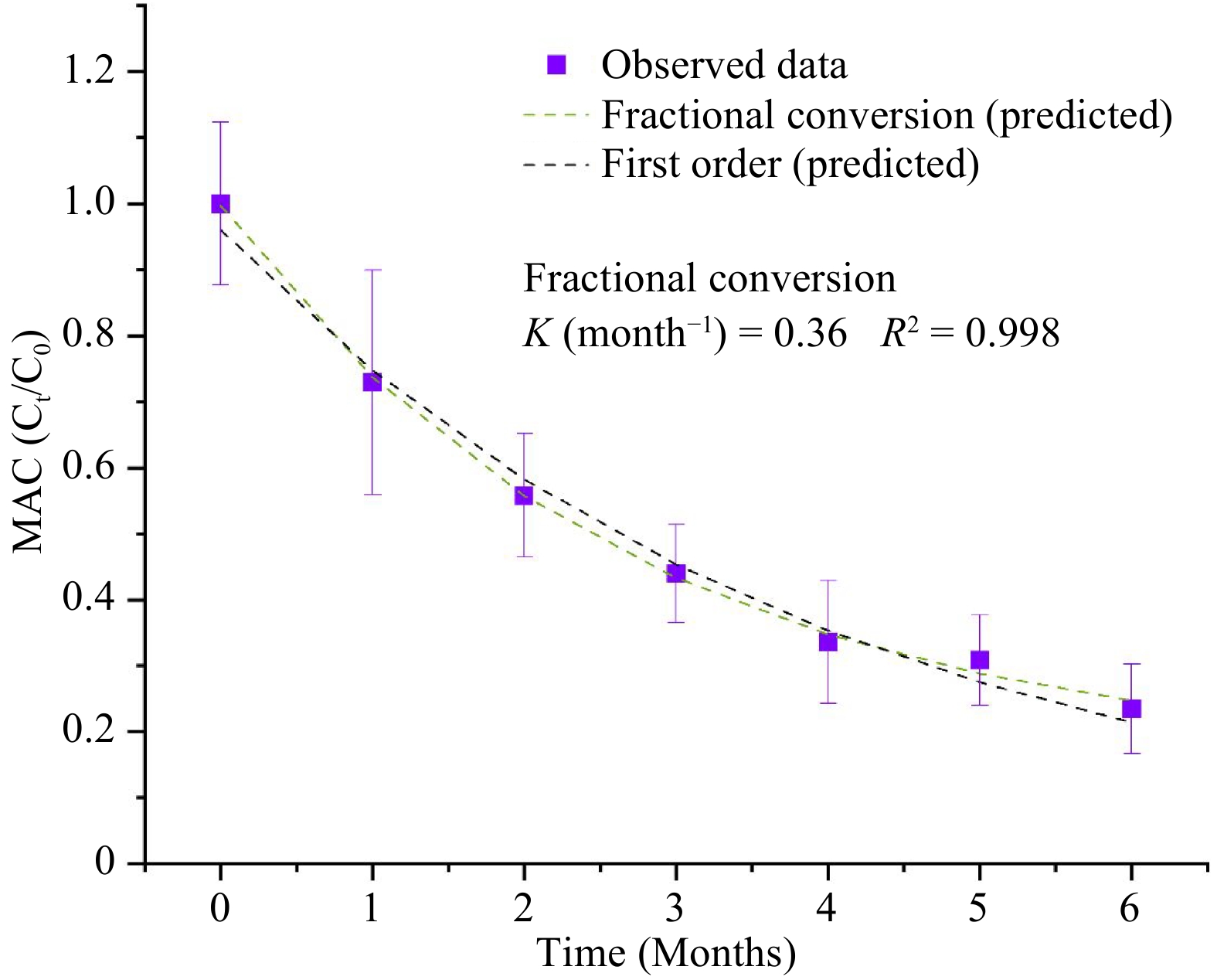
Figure 8.
Concentration of total monomeric anthocyanins (MAC) during the 6 months of storage at 37.8 °C fitted with the first order and fractional conversion models, n = 6.
The changes in ΔE of the beetroot puree and cabbage purees fit the zero-order model well, with a high R2 of 0.972 and 0.957, respectively (Table 4). The ΔE degradation rates of beetroot and cabbage purees were 0.46 and 1.16 month−1, respectively. This shows that the color changes in cabbage puree occurred faster than the beetroot puree during storage, which is consistent with the visual color alteration observed during the storage period. As mentioned earlier, the presence of residual enzymatic activities may affect the stability of the original color of the cabbage puree under the storage conditions. However, carrot puree produced low R2 ranging between 0.74−0.78 with the zero and first-order models. This indicated that the changes in carrot puree's total color during storage was not explained well by the zero, first, and fractional conversion models in the present study.
pH
-
The pH values of all purees significantly (p < 0.05) decreased after the MATS treatment as shown in Table 2. Albahr et al.[26] and Al-Ghamdi et al.[29] also observed a significant (p < 0.05) pH decrease in thermally processed avocado, red beetroot puree, pumpkin, and butternut squash samples. They attributed the decrease in pH to the acidic compounds that are released upon heating from the vegetables and fruit matrix.
-
This study showed that MATS treatments had a smaller effect on the color of carrot, red cabbage, and red beetroot purees. The MATS process also had a smaller effect on the retention of concentration betalains, anthocyanins, and beta-carotene, as the degradation in these pigments was less than 29% due to the short time of the MATS treatment. However, the pH of the purees declined significantly (p < 0.05) after the MATS treatment presumably as a result of the processing temperature. The three-layer PET-coated metal oxide-based pouches showed effectiveness in preserving the weight of the puree samples as well as minimizing the change in the colors and the natural pigments of the samples during the storage period of 6 months at 37.8 °C. Beta-carotene in the carrot puree was stable during the storage period, with no significant (p > 0.05) reduction. Betaxanthins (the yellow pigment) in beetroot puree and beta-carotene in carrot puree showed a higher stability than both betacyanins (the red pigment) in the beetroot puree and anthocyanins in the cabbage puree at the end of the storage period. The first and fractional conversion models explained the degradation in betacyanins in the MATS-processed beetroot and anthocyanins in the MATS-processed cabbage purees. However, a high-performance liquid chromatography (HPLC) study is necessary to investigate the degradation mechanism in betalains and anthocyanins after the MATS treatment and during storage. Overall, based on the total color difference, beetroot puree is the most suitable puree to be processed with MATS compared to other evaluated purees in this study. However, improving the beetroot puree formulation may help to preserve its quality attributes during longer storage periods.
-
The authors confirm contribution to the paper as follows: conceptualization: Albahr Z, Sablani SS; investigation, methodology: Albahr Z, Promsorn J, Tang Z; data handling, draft manuscript preparation, formal analysis: Albahr Z; data curation: Promsorn J; resources: Albahr Z, Tang J; manuscript reviewing and editing: Tang Z, Tang J, Ganjyal G, Sablani SS; supervision: Tang J, Sablani SS; funding, project administration: Sablani SS. All authors reviewed the results and approved the final version of the manuscript.
-
The data of the findings is available from the corresponding author upon reasonable request.
This research was partially supported by the (USDA) National Institute of Food and Agriculture Research grants numbers 2016-67017-24597 and 2016-68003-24840, and Hatch project #1016366.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Albahr Z, Promsorn J, Tang Z, Ganjyal GM, Tang J, et al. 2024. Storage and thermal stability of selected vegetable purees processed with microwave-assisted thermal sterilization. Food Innovation and Advances 3(2): 99−110 doi: 10.48130/fia-0024-0010
Storage and thermal stability of selected vegetable purees processed with microwave-assisted thermal sterilization
- Received: 20 December 2023
- Revised: 30 April 2024
- Accepted: 01 May 2024
- Published online: 20 May 2024
Abstract: The impact of microwave-assisted thermal sterilization (MATS) on three natural pigments and their storage stability in vegetable purees was investigated. We selected carrot puree for beta carotene, red cabbage puree for anthocyanins, and red beetroot puree for betalains. The purees were packaged in multilayer flexible pouches of AlOx-coated PET (12 μm)//AlOx-coated PET (12 μm)//AlOx-coated PET (12 μm)//ONy (15 μm)//CPP (70 μm), then processed with the MATS system to Fo = 6 to 11 min. After MATS treatment, the pouches were stored for 6 months at a storage temperature of 37.8 °C. The MATS treatment had a significant impact (p < 0.05) on the instrumental colors of three purees, with the total color difference (ΔE) ranging between 6.0 and 10.5. Similarly, the concentration of betalains experienced degradation by 20%−29% after the MATS treatment, while beta-carotene concentration showed a high retention. In addition, the pH of the purees declined considerably (p < 0.05) after the MATS treatment. Over the 6 months of storage at 37.8 °C, the PET-metal oxide pouches maintained the moisture content in all the purees, as the weight loss was only 0.43%−0.45%. The pigments in the MATS-processed purees had different levels of stability; ΔE values varied between 4.23 and 12.3. Beta-carotene was the most stable pigment, followed by betalains and anthocyanins. The degradation of both betalains and anthocyanins during storage was explained by first and fractional conversion models. MATS processing and packages with high gas barriers can therefore be used to preserve selected vegetable purees rich in natural pigments.
-
Key words:
- MATS /
- Short processing time /
- Natural pigments /
- Thermal stability /
- Storage stability /
- Vegetable purees












