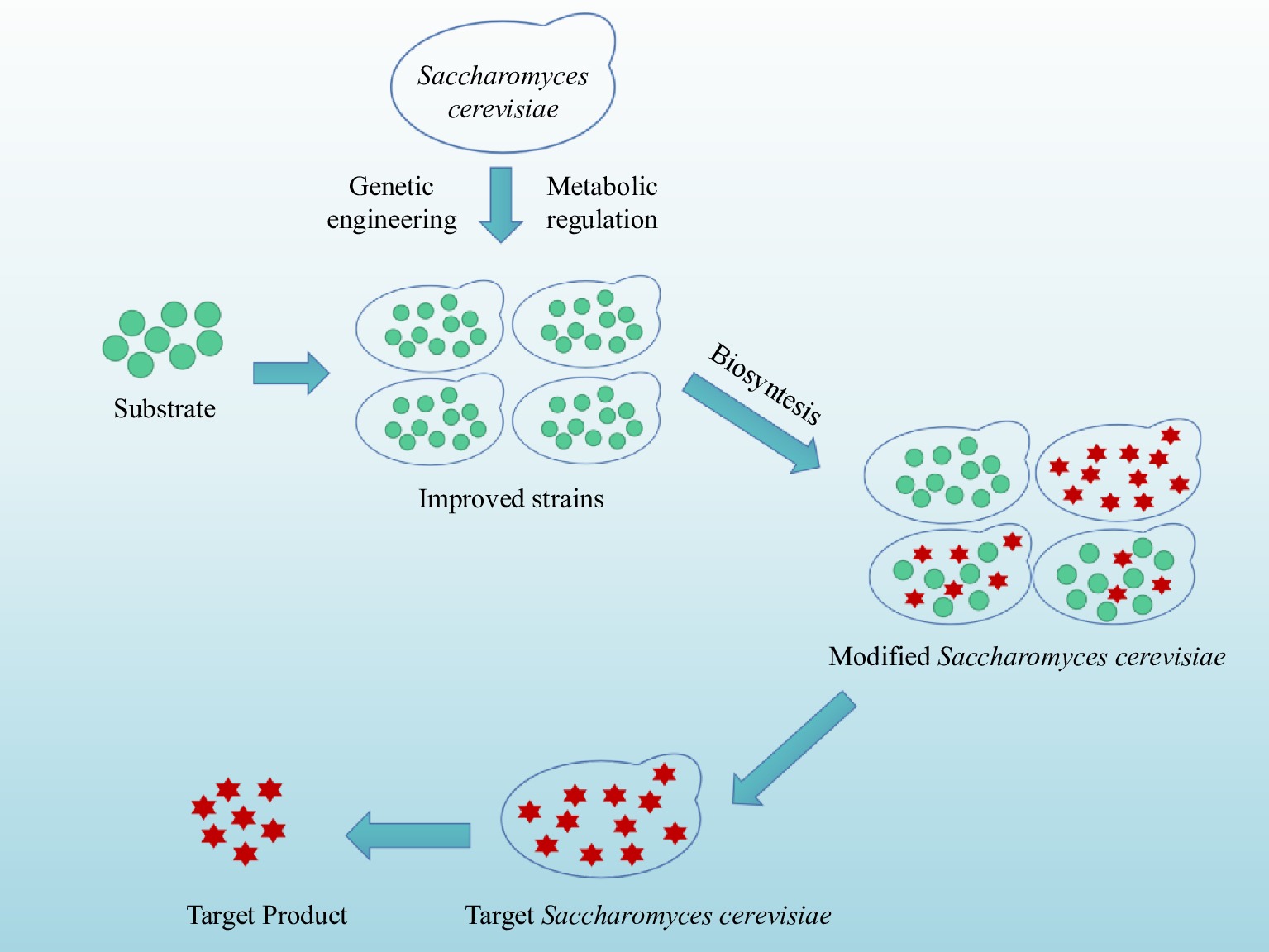
-
Saccharomyces cerevisiae, a single-celled eukaryote that was artificially cultivated nearly 10,000 years ago, is one of the microorganisms most closely related to humans[1]. Saccharomyces cerevisiae belongs to the Saceharomycetaceae and is a single-celled fungus with an oval or spherical cell shape and a cell size of 2.5−10 μm × 4.5−21 μm (Fig. 1a). The communities of Saccharomyces cerevisiae on the solid medium are milky white and convex, the surfaces are moist and shiny, and the edges are neat (Fig. 1b). Saccharomyces cerevisiae has three types of reproduction, including budding reproduction, spore reproduction and conjugative reproduction of which budding reproduction is the main one (Fig. 1c). Saccharomyces cerevisiae is a facultative anaerobic fungus that can use glucose, maltose, fructose, sucrose, galactose, and raffinose for fermentation, but cannot use lactose and cellobiose[2]. It converts sugars into CO2, energy, and biomass under aerobic conditions, while in the absence of oxygen, it converts sugars into ethanol, CO2 and glycerin through alcohol fermentation[3−6].
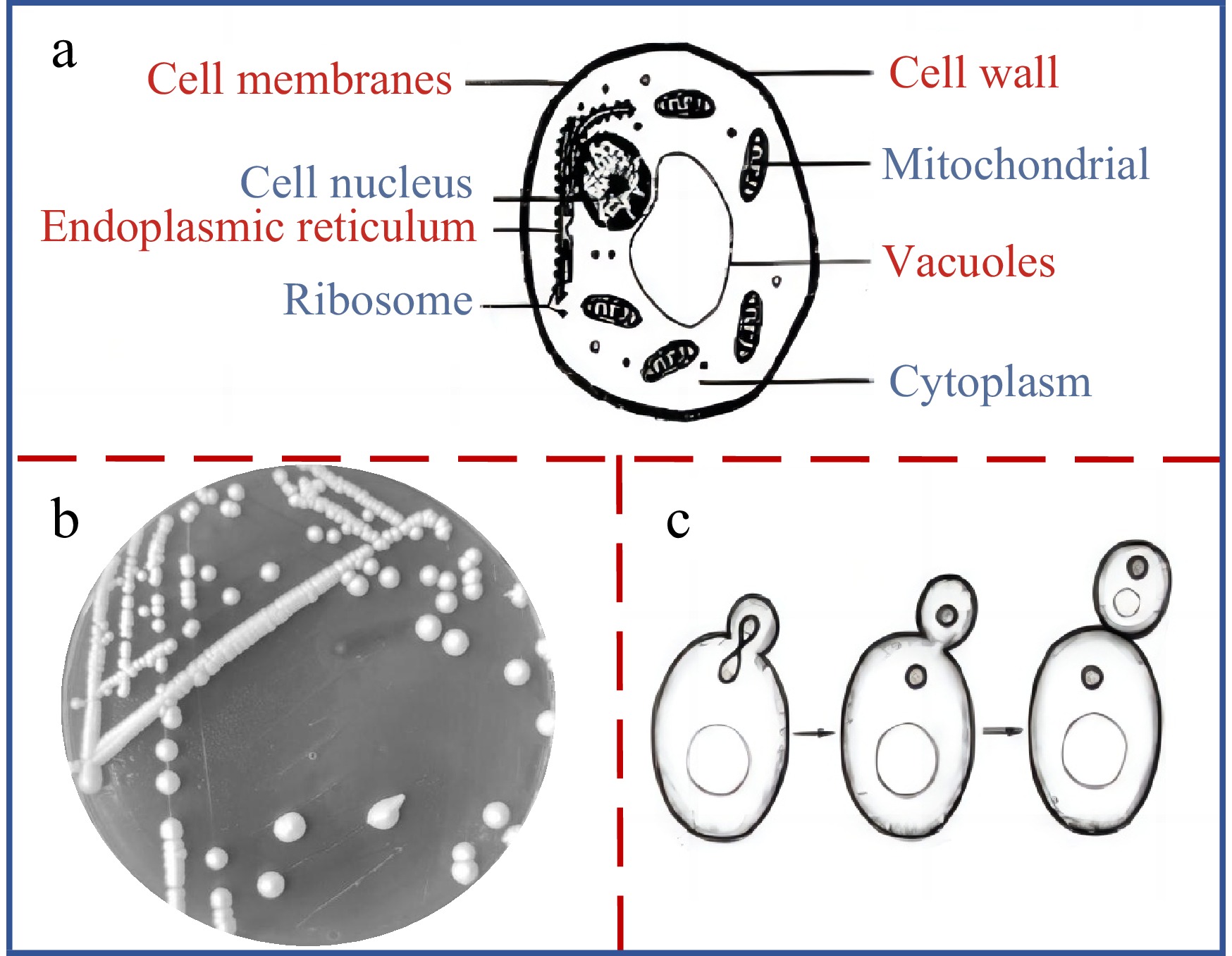
Figure 1.
The cell morphology, colony morphology and reproduction mode of Saccharomyces cerevisiae. (a) Cell morphology of Saccharomyces cerevisiae. (b) colony morphology of Saccharomyces cerevisiae. (c) reproduction mode of Saccharomyces cerevisiae.
Humans have used Saccharomyces cerevisiae for thousands of years. Today, Saccharomyces cerevisiae has been widely used in fields such as fermented food, synthetic biology, environmental protection, trace element supplements, and life sciences (Fig. 2)[3,7−11]. Saccharomyces cerevisiae has a unique aroma, taste, and good palatability, rich in protein, nucleic acid, vitamins, and various enzymes, has strong acid resistance during the fermentation process, and is not easy to be contaminated. Therefore, it has a wide range of applications in the field of fermentation. In the diet, Saccharomyces cerevisiae is often used in brewing and baking, such as the production of wine, liquor, beer, and alcoholic beverages, the production of bread and steamed bread, etc[3,4,12,13]. Saccharomyces cerevisiae is responsible for the fermentation of converting sugars in raw materials into alcohol and other compounds. The flavor substances produced by Saccharomyces cerevisiae mainly include alcohols, aldehydes, ketones, volatile acids, advanced alcohols, esters, fatty acids, etc[12]. Controlling the fermentation parameters of Saccharomyces cerevisiae precisely is the key to producing high-quality fermented food.
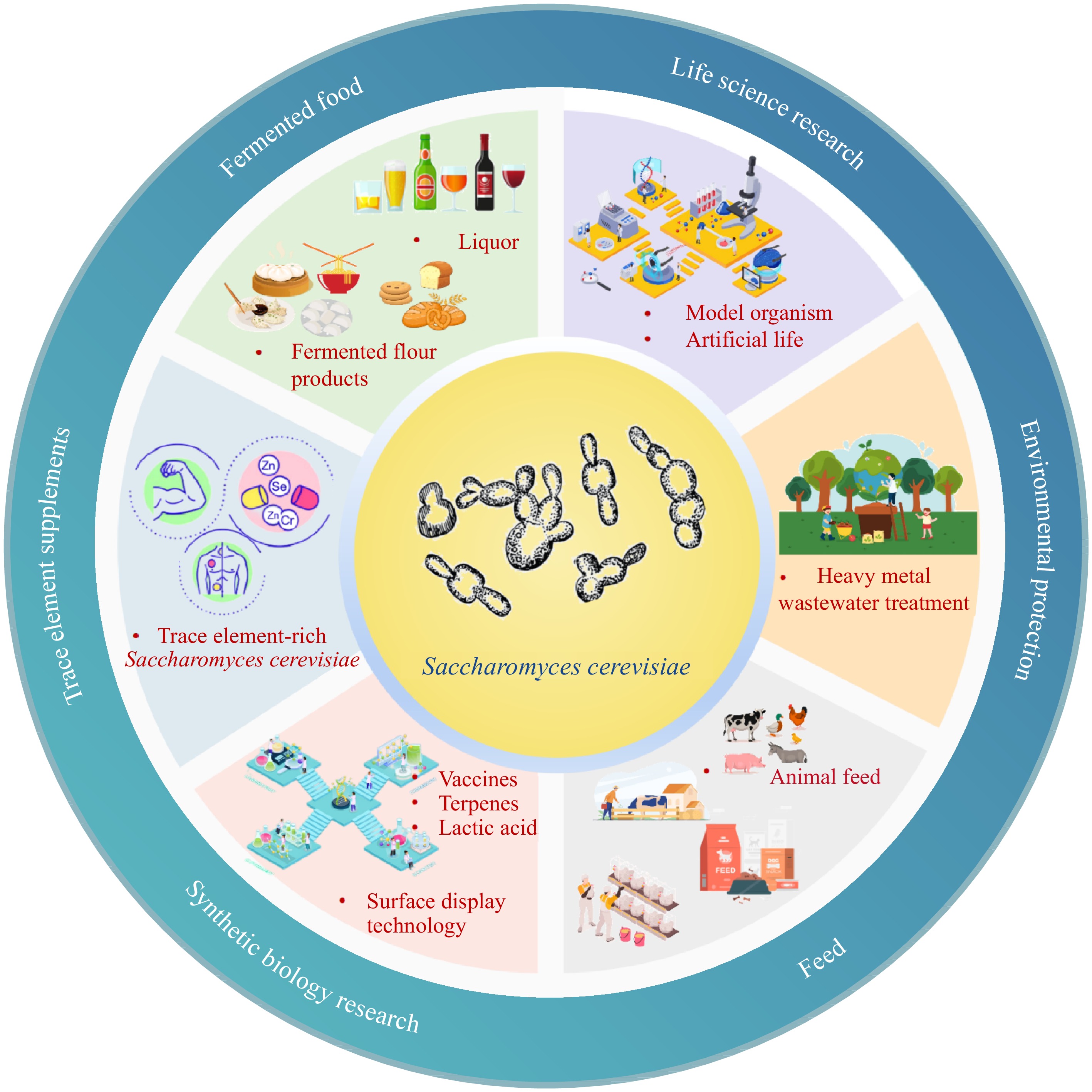
Figure 2.
Application of Saccharomyces cerevisiae in the fields of fermented food, trace element supplements, synthetic biology research, feed, environmental protection and life science research.
In industry, the products produced using Saccharomyces cerevisiae can be divided into two categories: one type of product is produced by Saccharomyces cerevisiae through anaerobic fermentation, which is Saccharomyces cerevisiae's metabolites, such as alcohol, various alcohols, and glycerol[12,14]. The developed yeast strains can survive and ferment at higher alcohol concentrations, thereby reducing possible disruptions in the fermentation process and improving the continuity and efficiency of the production of alcoholic beverages (such as whiskey, vodka, and other spirits)[15−17]. Production of second-generation ethanol from lignocellulose will require the development of robust strains of Saccharsaccharus cerevisiae, which is capable of growing and producing ethanol from at least glucose and xylose, and exhibiting heat resistance and tolerancing to inhibitors such as phenols, furans, and weak acids[16]. The other type of product is produced by Saccharomyces cerevisiae through aerobic fermentation, which is the cell or cell component of Saccharomyces cerevisiae, such as Saccharomyces cerevisiae is often added to feed as a cellular protein. Saccharomyces cerevisiae is large in size and rich in nutrition, with protein content up to 60%[9].
With the development of molecular biology, the research of Saccharomyces cerevisiae has become more and more in-depth. In 1996, Saccharomyces cerevisiae S288C strain completed genome sequencing and Saccharomyces cerevisiae became the first eukaryotic organism to sequence the whole genome[18]. In recent years, with the continuous research, a series of assembly tools suitable for Saccharomyces cerevisiae pathway have been developed, making Saccharomyces cerevisiae an ideal chassis organism for synthetic biology research, such as the biosynthesis of artemisinin[19] and opioids[20]; the production of hepatitis B vaccines and human papillomavirus vaccines[21].
Saccharomyces cerevisiae is a model organism for eukaryotic research and it has the advantages of simple gene manipulation, clear regulation mechanism of gene expression, mature high-density fermentation technology, and is also a recognized biologically safe strain. It has been used in the production of various biological drugs, such as hepatitis B surface antigen, hirudin, insulin, glucagon, urate oxidase, macrophage colony stimulating factor, etc[22,23], and has also been successfully modified for the production of artemisinic acid[24], salvianone[25], resveratrol[26], ginsenoside[27] and other natural products. However, the expression system of Saccharomyces cerevisiae has its shortcomings, mainly because the post-translational processing of eukaryotic gene products is different from that of higher eukaryotes, and the expressed recombinant protein often undergoes hyper glycosylation, which makes product separation difficult. In addition, Saccharomyces cerevisiae plays a key role in the long process of human research on its genome[28] For example, Saccharomyces cerevisiae is used to realize the functional identification of new human genes; and is also used to study the mechanisms of human cell cycle, cell morphology, cell decay, and other actions[29].
This article reviews the application and latest research progress of Saccharomyces cerevisiae in the fields of the food industry, human health, life science fields, environmental protection and animal husbandry. Specifically, it includes fermented foods, trace element supplements, synthetic biology applications, feed, and environmental protection. The advantages and disadvantages of Saccharomyces cerevisiae application in these fields are analyzed, and the direction for future research of Saccharomyces cerevisiae is put forward.
-
Saccharomyces cerevisiae has been the cornerstone of fermented food production for centuries and is the most widely used microorganism in the production of traditionally fermented foods, typically in fermented foods such as fermented alcoholic beverages and baked goods. In modern times, advances in biotechnology and synthetic biology have allowed the development of specialized yeast strains that further enhance the properties of fermented foods.
For alcoholic drinks production
-
Saccharomyces cerevisiae is an important component in the production of alcoholic beverages (wine, beer, Chinese rice wine, etc.) and significantly affects the quality, flavor, and aroma of the final product. During the winemaking process, Saccharomyces cerevisiae converts sugar into alcohol to give life to alcoholic beverages, and at the same time produces a series of flavor substances that give alcoholic beverages a special flavor profile, namely, the soul of the alcohol.
Saccharomyces cerevisiae is responsible for the fermentation process that converts the sugars in the raw material into alcohol and other compounds (Fig. 3)[3−6]. Various styles of alcoholic beverages can be produced using different matrices, vinification processes, and conditions, as shown in Table 1. Flavor substances produced by Saccharomyces cerevisiae mainly include alcohols, aldehydes, ketones, volatile acids, higher alcohols, esters, and fatty acids, etc.[12], and their metabolic pathways are summarized in Fig. 3. They are metabolites of Saccharomyces cerevisiae during alcoholic fermentation and play an essential role in the aroma and taste of alcoholic beverages such as fermented and distilled wines. Table 2 summarizes the flavor substances and characteristics from the metabolites of Saccharomyces cerevisiae. Moderate amounts of these substances are thought to enhance the organoleptic qualities of alcoholic beverages, however, excessive amounts can lead to flavor imbalances[4,15,30]. Therefore, it is crucial to manage the metabolites by precisely controlling the fermentation process and post-processing of winemaking to produce high-quality alcoholic beverages.
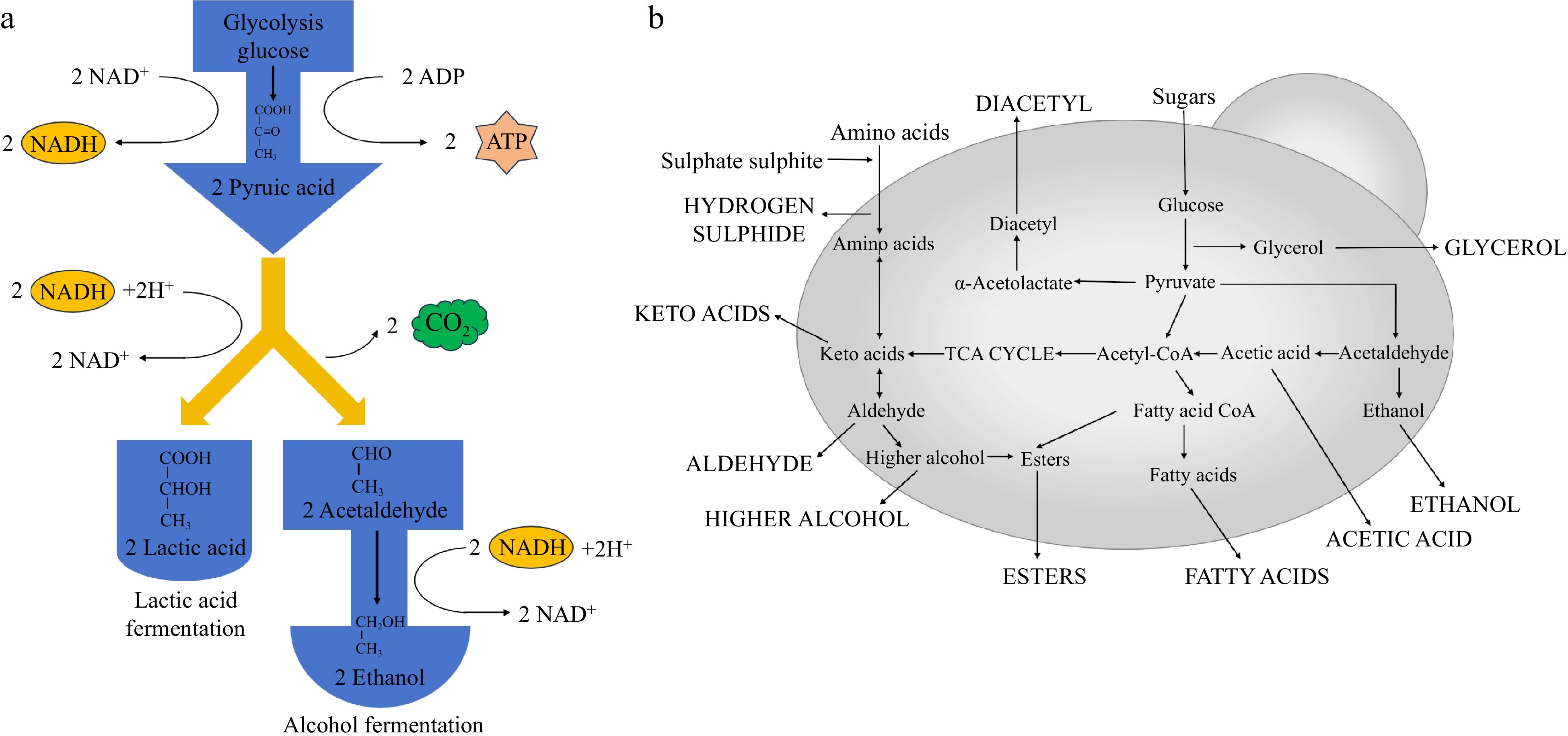
Figure 3.
(a) Ethanol fermentation by Saccharomyces cerevisiae, and (b) summary of Saccharomyces cerevisiae metabolic pathways yielding fermentation products[107].
Table 1. .Saccharomyces cerevisiae used in the production of alcoholic beverages, media and comments.
Beverage Media Comments Ref. Wine Grape must Modern, large-scale wineries use specially selected starter cultures of Saccharomyces cerevisiae strains available in dried form (e.g., active dry yeast) from specialist yeast supply companies. [107] Beer (ale) Barley malt wort Lager yeasts are likely a natural hybrid (Saccharomyces cerevisiae & Saccharomyces eubayanus).Relatively few strains employed in lager fermentations. Lager strainsutilise maltotriose more efficiently than ale strains, and they ferment atcooler temperatures. Ale yeasts are polyploid strains. Numerous strainsemployed in ale brewing. Ale yeasts ferment at warmer temperaturescompared with lager yeasts. [107] Chinese rice wine (Huangjiu) Glutinous rice (Oryza sativa var. glutinosa) Saccharomyces cerevisiae play a key role in the yeast flora of Chinese rice wine, and are responsible for converting the main carbohydrates into alcohol and other organic compounds, as well as influencing the flavor, aroma and texture of Chinese rice wine. [108] Whisky Grains include barley, corn, rye, and wheat. Scotch whisky producers currently use selected distilling strains of Saccharomyces cerevisiae in three main formats, cream yeast, pressed (cake) and driedyeast. Malt whisky distilleries traditionally use pressed yeast, but largergrain distillers have now adopted cream yeast. Dried yeasts are not asprevalent as pressed and cream formats in whisky fermentations. [15,107] Rum Sugar cane molasses Saccharomyces cerevisiae strains in rum fermentations are developed as startercultures and provide faster fermentation with more higher alcohols andfatty acids, but less esters resulting in lighter style rums. [107] Brandy, Gin,Vodka, etc. fruit juices, grains or molasses, wheat or rye For brandies, cognac, etc. the base wine is produced by pure startercultures of Saccharomyces cerevisiae. For gin, vodka, ete. selected distilling strains of Saccharomyces cerevisiae will be used. [107] Table 2. Fermentation metabolites of Saccharomyces cerevisiae in alcoholic beverages.
Compound class Example Flavour Comments Ref. Higher alcohols n-propanol; Isobutanol;
Iso-amyl alcohol
(3-methylbutan-1-ol); Phenylethanol;Alcoholic; Pharmacy;
Fusel, alcoholic, fruity, banana; Roses, perfumeIn moderate amounts, higher alcohols can enhance the flavor complexity and mouthfeel richness of alcoholic beverages, improving the organoleptic qualities of the drink. However, the presence of excessive amounts of higher alcohols may lead to an imbalance in the flavor of alcoholic beverages, producing an irritating or unpleasant sensation. [15] Esters Ethyl acetate;
Ethyl butyrate;
Ethyl caproate;
Ethyl caprylate;
Ethyl hexanoate;
Ethyl lactate;
Ethyl octanoate;
Iso-amyl acetateSolvent, acetone; Pineapple, banana, mango; Apple, aniseed; Apple; Pineapple, unripe banana; Butter/cream; Sour apple, apricot, fruity; Banana, fruity Esters increase the aromatic complexity of alcoholic beverages, improve the mouthfeel, and give them a rounder, fuller body. A moderate amount of esters gives alcoholic beverages a pleasant aroma of fruits, flowers, vanilla, etc. An excessive amount of esters can bring overly strong fruity or chemical solvent flavors. [109] Carbonyl compounds Acetaldehyde; Benzaldehyde; Green apple; Almond Aldehydes are usually present in trace amounts in alcoholic beverages and give them a floral and fruity flavor. Excessive amounts of aldehydes can give alcoholic beverages an irritating and unpleasant odor, and the winemaking process needs to be carefully controlled. [109] Organic acids Succinic acid,
Citric acid,
AceticacidSourness Acidity regulation of alcoholic beverages, the right amount of organic acid will increase the complexity of the drink and the richness of the mouthfeel, too much organic acid will produce an unpleasant sensation and affect the balance of the taste. [109] Polyols Glycerol A colorless, odorless, and non-toxic compound that has a sweet taste Glycerin has no direct effect on the aroma of alcoholic beverages, but its effect on texture and mouthfeel can indirectly influence the perception of flavor by moderating the harshness of alcohol and making other flavor compounds more pronounced. [15] Vicinaldiketones Diacetyl,
Pentane-2,3-dioneButter, butterscotch Diacetyl is a compound produced in the brewing of beer and some wines that gives liquors a buttery texture and aroma; Excess diacetyl is undesirable in most beers, resulting in an imbalance of flavors and a rancid butter or 'butterscotch' taste. [109] Sulphur compounds Hydrogen sulphide, Dimethyl sulphide, Sulphur dioxide, Thiols Rotten eggs The role of sulfides in alcoholic beverages is complex and varied, and they have a significant impact on the flavor of alcoholic beverages, both positively and negatively. For example in white wines, thiols contribute to the pleasant aroma of tropical fruits and blackcurrants. In excess, however, it can produce unpleasant rotten egg or rotting odors. [15] Phenolic
compounds4-Vinylguiacol Clove-like Some yeasts, including wild yeasts, that are POF+ (phenolic off-flavour) can produce undesirable phenolic flavoursand aromas. However, the clove-like compound, 4-vinylguiacol, is desirable in certain beer styles and can beproduced by hefe ale yeast strains of Saccharomyces cerevisiae. [15] In recent years, alcohol beverage production has been devoted to improving and controlling the fermentation characteristics of Saccharomyces cerevisiae by utilizing advanced technologies and bioengineering methods. To improve the fermentation efficiency of yeast, add its new functional properties and expand the range of applications, and improve the flavor characteristics of alcoholic beverages. Firstly, Saccharomyces cerevisiae was modified by genetic engineering techniques to improve its ethanol yield, fermentation efficiency and enhance resistance. First-generation ethanol production required yeast strains capable of producing ethanol directly from starch without the need to isolate the saccharification process and able to withstand stresses such as high ethanol concentrations and high temperatures during fermentation. By enhancing the expression of genes related to sugar metabolism in Saccharomyces cerevisiae, yeast can be made more efficient at converting sugar to ethanol[31−33]. These improved yeasts are especially valuable in the production of alcoholic beverages such as beer and wine. Yeast strains developed could survive and ferment at higher alcohol concentrations, thus reducing possible interruptions in the fermentation process and improving the continuity and efficiency of the production of alcoholic beverages (e.g., spirits such as whisky, vodka, etc.)[15−17]. Second-generation ethanol production from lignocellulosic will require the development of robust strains of Saccharomyces cerevisiae that are capable of growing and producing ethanol from at least glucose and xylose, and that exhibit heat tolerance and tolerance to inhibitors such as phenolic compounds, furans, and weak acids[16]. This technology not only improves the efficiency of resource utilization, but also provides new ways to produce environmentally friendly alcoholic beverages. However, overcoming major limitations such as incomplete substrate catabolism, low titer of heterologous protein expression, heat resistance, ethanol tolerance, and barriers caused by inhibitor/toxic byproduct accumulation remains a challenge. It is necessary to both improve existing industrial strains and develop new phenotypes utilizing the rich biodiversity available.
Secondly, customizing the flavor of alcoholic beverages by metabolically engineering yeast. Metabolic engineering involves adjusting the metabolic pathways of yeast to optimize or introduce new metabolites. On the one hand, the production of specific flavor compounds (e.g., esters, and higher alcohols) is enhanced by augmenting or introducing new metabolic pathways. Modifying Saccharomyces cerevisiae by metabolic engineering to increase the production of certain ester compounds gives beer or wine a richer fruity flavor, e.g., modified yeasts with low production of higher alcohols, engineered yeasts with high production of aromatic thiols[34−36]. On the other hand, controlling and reducing the content of undesirable flavor substances and harmful compounds in alcoholic beverages, e.g., acid-producing/acid-reducing yeasts, low-producing hydrogen sulfide yeasts, yeast engineered for low urea production[34,35,37]. Also increasing yeast tolerance to environmental stresses such as high alcohol concentration, low pH and temperature fluctuations[38].
Furthermore, multiple fermentation techniques are essential for improving yeast fermentation efficiency and regulating flavors. Mixed yeast inoculated fermentation brewing process brings unique flavor and quality to alcoholic beverages. It usually consists of a commercial, or laboratory Saccharomyces cerevisiae strain and a non-Saccharomyces cerevisiae strain, with mixed promoters used in a co-inoculation or sequential fashion, often in varying proportions of cell numbers. Parapouli et al. compared most studies using co-inoculation and sequential inoculation modes for mixed fermenters of Saccharomyces cerevisiae and non-Saccharomyces cerevisiae to obtain the results that increased the production of volatile compounds, esters, and terpenes, reduction of volatile acidity, total acidity and pH[6,39]. Also, the use of Saccharomyces cerevisiae in combination with other microorganisms, such as lactic acid bacteria, can create unique flavor combinations. This multi-fermentation technique has been adopted by several craft beer producers to develop new beers with complex mouthfeel and unique flavor layers[40].
In addition, the integrated use of Saccharomyces cerevisiae with novel physical processing techniques to enhance the overall quality of alcoholic beverages. For example, Saccharomyces cerevisiae combined with technologies like ultrasound and pulsed electric field treatments to improve and accelerate the fermentation and maturation process of alcoholic products[41].
However, a current challenge is that overgrowth of yeast may lead to uncontrolled fermentation processes, affecting flavor and quality. New sensing technologies and real-time monitoring systems are applied to enable precise control of the yeast fermentation process to alleviate this difficulty, e.g. through Raman spectroscopy and machine learning to assess yeast activity[42,43]. Moreover, the waste and by-products generated by yeast during the production process need to be properly disposed of to avoid negative impacts on the environment, and the development of the utilization of yeast by-products is imminent.
Overall, the use of Saccharomyces cerevisiae in the production of alcoholic beverages continues to contribute to technological innovation and enhancement of beverage quality. Future developments will focus on enhancing flavor diversity, increasing production efficiency and sustainability, and using advanced technologies to improve the fermentation properties of yeast.
For the production of fermented flour products
-
Saccharomyces cerevisiae also known as baker's yeast, is an essential microorganism in the production of fermented flour products such as steamed bread and bread. Flour products fermented with Saccharomyces cerevisiae exhibit excellent qualities in terms of texture, flavor, and nutrition (Fig. 4). During dough making, Saccharomyces cerevisiae plays a key role in the expansion, elasticity, and extensibility of the dough. Saccharomyces cerevisiae uses sugars (such as glucose, fructose, maltose, sucrose, etc.) in the dough to produce carbon dioxide and ethanol through glycolysis. Among them, carbon dioxide is captured by the gluten lattice network in the dough, causing the dough to expand and produce the typical bread pore structure, and the rate of carbon dioxide production determines the rate of dough fermentation[13]. Alcohol evaporates as the temperature rises during the baking process, contributing to the porous structure and characteristic flavor of the bread[44]. In addition, the metabolites such as glycerin produced by Saccharomyces cerevisiae in the early stage of fermentation can effectively improve the properties of the dough[45].
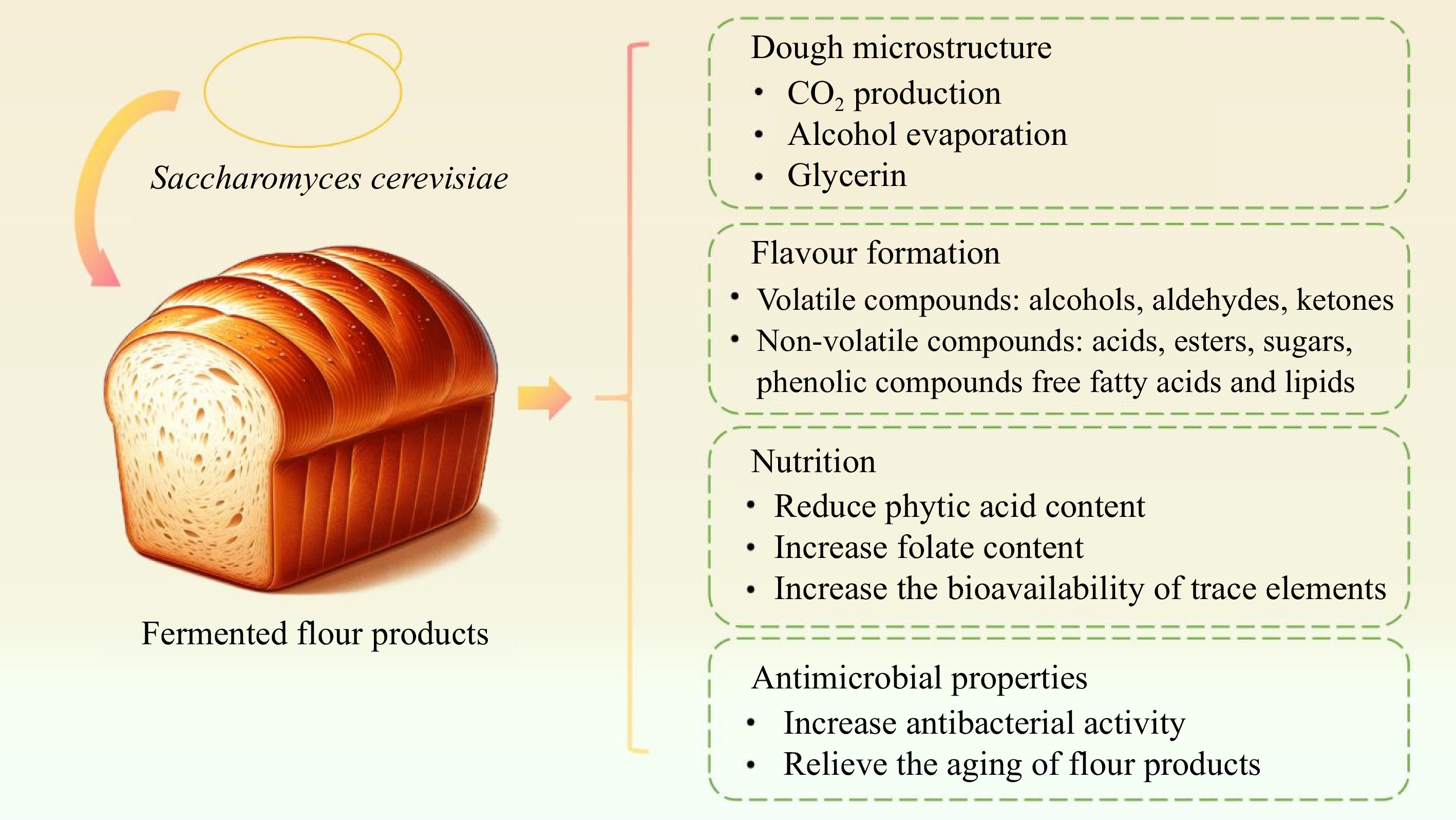
Figure 4.
Contribution of Saccharomyces cerevisiae to fermented flour products characteristics from several aspects.
During fermentation, Saccharomyces cerevisiae also produces other metabolic by-products in the baking of flour products, such as esters, aldehydes, ketones, and organic acids, which together form the unique flavor profile of pasta products[14]. Saccharomyces cerevisiae produces a variety of esters and other aroma compounds during fermentation, which contribute most to the aroma of flour products. Of these, the most important are alcohols and aldehydes such as 2,3-butanedione and 3-hydroxy-2-butanone and esters[46]. Some non-volatile compounds act as precursors for later reactions to form new flavor compounds. Sugars remaining in the fermentation react in the Maillard reaction and have a significant impact on the aroma[47]. Organic acids like lactic acid and acetic acid are produced by Saccharomyces cerevisiae to increase the acidity of the flour product, which in turn affects its flavor profile. The types and amounts of volatile flavor substances produced by different Saccharomyces cerevisiae strains fermentations are different, and their range of applicability varies. Therefore, in the fermentation process of flour products, the selection of strains is particularly important. Traditional Saccharomyces cerevisiae yeasts typically produce mild alcoholic and ester aromas that are suitable for most standard flour products. Further, specialized strains of Saccharomyces cerevisiae have been selected and developed based on consumer demand to produce specific flavor profiles, such as enhanced fruity or more complex ester aromas, for special flour products or craft breads. Compared to these commercial yeast strains, wild yeasts produce more complex and diverse flavors that are suitable for longer-fermented flour products, such as traditional sourdough breads made by co-fermentation of Saccharomyces cerevisiae with Lactobacillus with a distinctive flavor profile[48]. Therefore, the selection of strains is particularly significant in the fermentation process of flour products.
More interestingly, existing studies have shown that Saccharomyces cerevisiae can increase the nutrient content of flour products. A previous study found that during the fermentation process, the folate content in the dough increased seven-fold, which well compensated for the loss of folate caused by baking[49]. In addition, Saccharomyces cerevisiae can also reduce the content of phytic acid through the action of phytase, thereby increasing the bioavailability of trace elements such as magnesium and phosphorus. Katina et al.[49] also found that fermenting rye bran with Saccharomyces cerevisiae can increase the content of free ferulic acid.
The fermentation of Saccharomyces cerevisiae in the dough also improves the preservative capacity of the pastry and prolongs its shelf life. Studies have found that the aging speed of bread is significantly slowed down after ethanol treatment. During the fermentation process, Saccharomyces cerevisiae produces a large amount of ethanol, lipase, protease, and other enzymes, which can effectively alleviate the aging of flour products. Heitmann et al.[13] found that bread fermented with different yeast strains have significant differences in hardness during storage, which indicated the selection of strains is particularly important again. The organic acids produced by yeast fermentation can lower the pH of bread and increase its antimicrobial properties, thus improving its freshness.
Although Saccharomyces cerevisiae has been involved in the bakery industry for a long time, its performance is somewhat limited due to various industrial constraints and requirements. First, the fermentation of Saccharomyces cerevisiae is a time-consuming process that may not be suitable for all commercial operations seeking rapid production of baked goods. Second, on an industrial scale, Saccharomyces cerevisiae is exposed to a variety of multiple and fluctuating environmental stresses, which ultimately reduces product yields and negatively impacts the quality of baked goods[50]. What's more, the fermentation process of Saccharomyces cerevisiae is sensitive to environmental factors such as temperature and humidity, which need to be carefully controlled to ensure consistent results. If not handled properly, the shelf life of fermented flour products may be limited by continued yeast activity or microbial spoilage. To overcome these limitations, many studies have utilized advanced biotechnology to modify Saccharomyces cerevisiae strains to increase flavor induction, tolerance to various industrial stresses, and enhance fermentation capabilities. (I) Strain improvement for flavor induction: pathway engineering using recombinant DNA technology to introduce four genes[51], overexpression of ILV2, ILV3, ILV5, and BAT2 genes involved in valine metabolism[52], and in silico stoichiometric modeling[53] were used to obtain mutant strains with high production of vanillin to enhance the production of vanillin, a natural flavor reagent; Metabolic engineering of Saccharomyces cerevisiae by heterologous overexpression of a gene encoding phenylalanine for the production of trans-cinnamic acid derivatives (cinnamaldehyde, cinnamyl alcohol, and hydrocinnamyl alcohol) for the production of high-value aromatic compounds[54]. (II) Strain improvement for stress tolerance: producing self-cloning baker's yeast strains that harbor the TDH3p-PDE2 gene heterozygously and homozygously by self-cloning procedure[55], deletion of NTH1 in combination of MAL62 gene over-expression[56], alteration of the POG1 gene by breeding method[57] to increase freezing tolerance during dough fermentation. (III) Strain improvement for fermentation efficiency: disruption of MIG1 and or TUP1 and/or SSN6 genes[58], over-expressing the GSY2 and SNF1 gene and deleting NTH1 gene[58,59] to improve maltose metabolism and leavening ability of yeast during dough fermentation.
In addition, precise control of Saccharomyces cerevisiae fermentation parameters are required in the baking industry, where bioreactors and computer control systems can be used to automate the fermentation process, control the texture and flavor of pasta products, and improve production efficiency and product consistency.
-
Trace elements are essential nutrients for life maintenance and development, and they play important functions in the living body. When the intake of trace elements in the diet is unbalanced or insufficient, various diseases will occur in the human body. Happily, trace elements can be supplemented through nutrient intake. Saccharomyces cerevisiae is a good carrier of trace elements and is often used as a carrier for enrichment[7].
Saccharomyces cerevisiae is a simple single-celled eukaryotic organism with simple cultivation requirements and fast reproduction speed. Its eukaryotic structure also endows it with good stress tolerance. At present, it has developed widely produced selenium-enriched Saccharomyces cerevisiae, and Saccharomyces cerevisiae rich in other elements such as chromium-enriched Saccharomyces cerevisiae[60], zinc-enriched Saccharomyces cerevisiae[61] and germanium-enriched Saccharomyces cerevisiae[62]. Selenium-enriched Saccharomyces cerevisiae is discussed below as an example. In addition, the fermentation of Saccharomyces cerevisiae can synthesize rich nutrients and active substances, including amino acids, proteins, polysaccharides, esters, and B vitamins[63] . There are two ways of enrichment of trace elements in Saccharomyces cerevisiae: (1) biosorption process, which is fast and has nothing to do with metabolism. It is affected by pH value, microorganism type and cultivation time, ion type and concentration, other competing ions, and enrichment time. The defect is that Saccharomyces cerevisiae cannot convert inorganic zinc into organic form, its biological function is poor, and its absorption and utilization rate is low; (2) the active transportation process is slow and consumes energy, which is related to the metabolic process and is mainly affected by the activity of microorganisms, culture temperature, nutritional conditions, the influence of metabolic inhibitors and other factors[64].
For selenium supplement
-
Selenium-enriched Saccharomyces cerevisiae is the earliest edible fungus developed for selenium enrichment. Selenium in Saccharomyces cerevisiae is organic selenium[65]. Compared with inorganic selenium, selenium-enriched Saccharomyces cerevisiae is safe and non-toxic, nutritious, and selenoprotein stable. Saccharomyces cerevisiae has a high selenium-rich capacity, a short fermentation cycle, and mature technology. Selenium in Saccharomyces cerevisiae directly scavenges oxygen free radicals in a biologically combined form, inhibits lipid peroxidation, stimulates immune response, promotes immune protein synthesis, and stimulates lymphocyte proliferation[66]. The effect is significantly better than inorganic selenium compounds. The selenomethionine in Saccharomyces cerevisiae is not only biologically active, but also easier to be absorbed, with less loss from urine and feces, and stays longer in the body.
The metabolism of selenium in Saccharomyces cerevisiae is shown in Fig. 5. Saccharomyces cerevisiae converts inorganic selenium into organic selenium through fermentation and cultivation, which effectively reduces the toxicity of selenium and contributes to the efficient use of selenium in the body. According to reports, the body's absorption and utilization of Saccharomyces cerevisiae selenium is 20 times that of inorganic selenium[65]. Inorganic selenium and Saccharomyces cerevisiae selenium are absorbed differently in the small intestine. Inorganic selenium can only pass through the small intestine through passive diffusion, while yeast selenium can be actively transported through the intestinal wall, so yeast selenium can maintain a high retention rate in the body[67]. The influencing factors during the selenium-enriched culture process mainly include: nutrient composition and ratio of the medium, pH value, temperature, dissolved oxygen, fermentation time, selenium source, selenium addition method, and selenium addition time. Extending selenium-enriched yeast to bread, steamed bread, biscuit production, and the brewing industry (fermented condiments and fermented wine) can increase product value and enrich the types of selenium foods. Studies have shown that bread made with selenium-enriched yeast as a starter is rich in selenomethionine, which is significantly better than selenium-enriched bread with direct sodium selenite addition in terms of quality and nutrition[68]. Selenium-enriched Saccharomyces cerevisiae was inoculated into grape juice to successfully prepare selenium-enriched wine. It can be seen that the selenium-enriched yeast used as a starter to participate in the food industry has shown good biological acceptability, effectively increasing the selenium content of food and increasing the added value of the product.
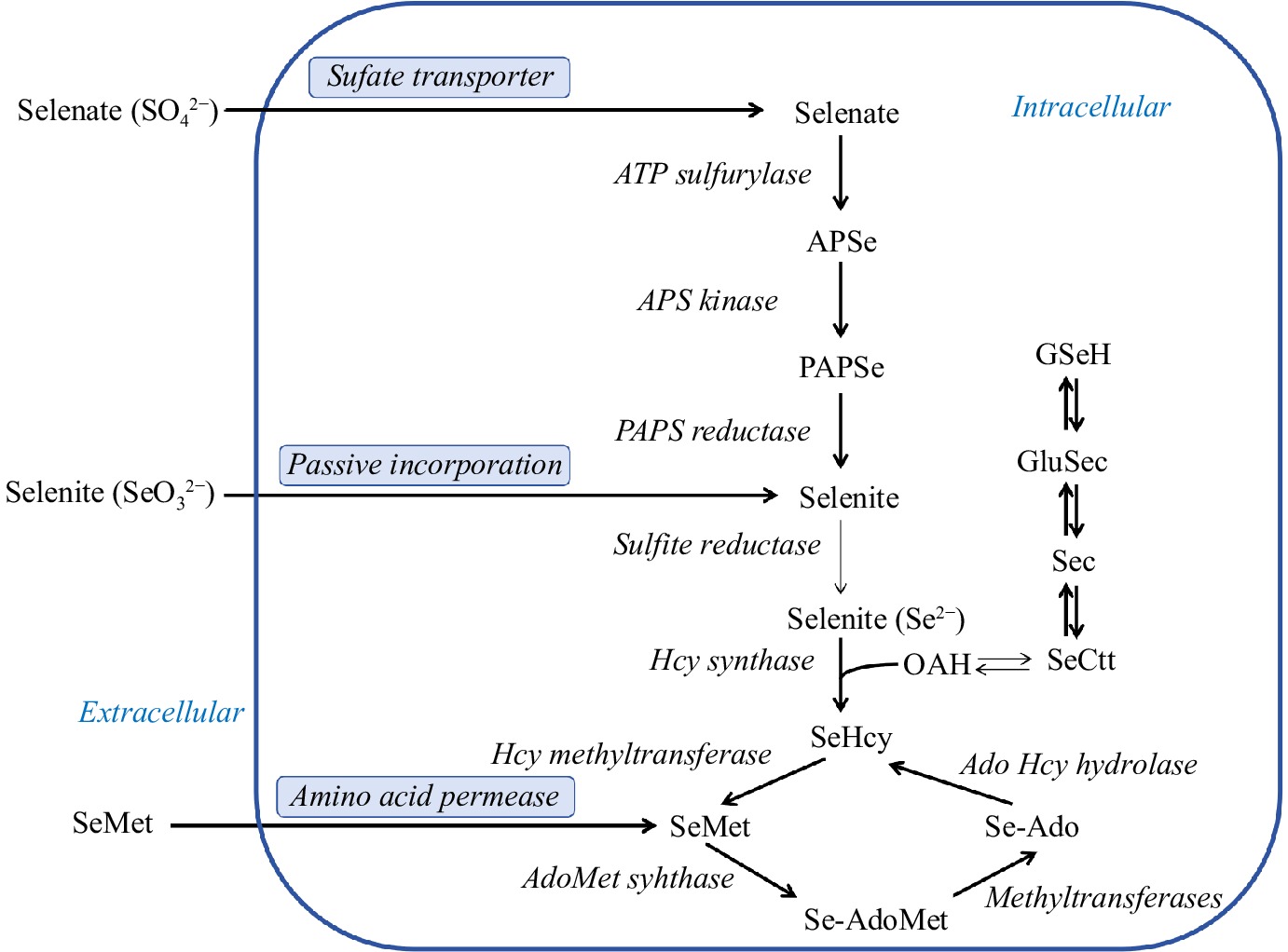
Figure 5.
Selenium metabolism in Saccharomyces cerevisiae. APS, adenine sulfate; APSe, adenine selenate; GluSec, γ-glutamine selenocysteine; GSeH, selenoglutathione; OAH, O-acetylhomoserine; PAPS, 3′-phosphoadenosine sulfate; PAPSe, 3′-phosphoadenosine selenate; SeCtt, selenocyst thionine.
Adding yeast selenium in the preparation of animal feed can enrich the nutritional value of feed. Selenium supplementation can promote growth and increase the immunity of animals, thereby improving the quality of breeding and the quality of meat sources. Pigs fed with selenium-enriched feed have slower water loss after slaughter and prolonged fresh-keeping time, which has a significant advantage in similar meat products. After adding a certain amount of yeast selenium to dairy cow feed, selenomethionine is circulated and metabolized in the mammary glands of dairy cows, and high-quality selenium-enriched milk can be obtained, and the offspring will also have better health[69]. The partial replacement of fish meal with selenium-enriched Saccharomyces cerevisiae in the feed can promote fish and shrimp weight gain and increase protein nutrition; broiler chickens fed with selenium-enriched Saccharomyces cerevisiae have a significant increase in antioxidant capacity during the freezing process.
-
Synthetic biology (Fig. 6) is based on the knowledge and technology of genetics, molecular biology, and metabolic engineering, applying standardized modular engineering principles to the design of biological systems, and transforming existing natural systems to make the biological system have unprecedented new functions or rebuild a new artificial biological system[8,70]. Saccharomyces cerevisiae was the first eukaryotic microorganism to perform whole-genome sequencing. The gene expression regulation mechanism of Saccharomyces cerevisiae is clear, the gene operation is simple, and the high-density fermentation technology is mature. It can be genetically modified by recombination technology and is suitable for the expression of foreign genes and genetic engineering transformation. In recent years, a series of assembly tools suitable for the Saccharomyces cerevisiae pathway having been developed, and Saccharomyces cerevisiae is generally recognized as a safe (GRAS) strain, making Saccharomyces cerevisiae an ideal chassis organism for synthetic biology research[8]. Saccharomyces cerevisiae is often used to produce lactic acid, terpenes, steroids, vaccines, and so on[71−74].
Vaccines
-
Saccharomyces cerevisiae has two vector systems (free and integrated) through which heterologous proteins are expressed: (1) The episomal vector system uses a shuttle vector to simplify the transformation of Saccharomyces cerevisiae. The target gene is inserted into the plasmid vector and then cloned into E. coli and transformed into a Saccharomyces cerevisiae host. The episomal plasmid provides a high copy number of the expression cassette, but it is unstable. (2) The integrated vector system integrates heterologous genes into Saccharomyces cerevisiae chromosome, and it shows high stability[22,75]. Compared with other eukaryotic expression systems, the Saccharomyces cerevisiae host provides high-level expression of target genes and is used in various biopharmaceutical industries, such as hepatitis B surface antigen, hirudin, insulin, glucagon, urate oxidase, macrophage colony-stimulating factor[22,23].
Saccharomyces cerevisiae is one of the commonly used protein expression systems for vaccine production[76]. With the rapid development of Saccharomyces cerevisiae-related biotechnology and the excellent characteristics of Saccharomyces cerevisiae itself, the Saccharomyces cerevisiae protein expression system-often used for the production of heterogeneous seedling proteins[77]. The Saccharomyces cerevisiae protein expression system has the following advantages: (1) high production efficiency, low production cost, and large-scale production; (2) the protein expression level is very high and can be post-translationally modified; (3) it does not produce endotoxins and is not affected by viruses epidemic pollution; (4) the glucan and mannan contained in the cell wall of Saccharomyces cerevisiae can enhance the body’s immunity and act as a natural immune adjuvant[78]. The first commercial recombinant vaccine-hepatitis B vaccine is produced by Saccharomyces cerevisiae through genetic engineering technology[79]. Cervical cancer is a disease with high morbidity and mortality in women and is common in young women. Human papillomavirus vaccine is a vaccine against cervical cancer, and the Saccharomyces cerevisiae platform currently provides most of the Human papillomavirus vaccine[80]. What is more, in the early 1980s, ZymoGenetics used Saccharomyces cerevisiae as a host for the industrial production of recombinant human insulin in the early 1980s and co-marketed NOVOLIN L with NOVO NORDISK INC in 1987. Currently, the Saccharomyces cerevisiae platform provides half of the world's insulin.
Terpenes
-
Nowadays, more and more compounds (like terpenoids, flavonoids) have been found to have important medicinal and economic values[81]. However, due to the scarcity of plant resources, the low content of active substances, and the difficulty of chemical synthesis, the wide application of these compounds is limited. With the development of synthetic biology, the biosynthetic pathways of these compounds have gradually been elucidated. The biosynthetic cell factory obtains target products through the targeted transformation of host cells and targeted biosynthetic pathways, which can reduce production costs, shorten production cycles, improve production capacity and have very broad application prospects.
Although terpenoids have different structures and functions, they all start from acetyl-CoA and synthesize common precursors IPP and DMAPP through the MVA pathway in Saccharomyces cerevisiae. Then, IPP and DMAPP combine to form GPP, FPP, and GGPP, and further monoterpenes, sesquiterpenes, and diterpenes are generated by cyclization or rearrangement under the action of terpene synthases[82].
Carotenoids are the general term for a class of important natural pigments and are tetraterpenoids. In 1994, Yamano at al.[83] first expressed crtE, crtB, crtY, crtI, and other carotenoid pathway genes from bacteria in Saccharomyces cerevisiae, and successfully constructed a strain of Saccharomyces cerevisiae that produces lycopene and carotene. For the first time, the heterogeneous expression of multi-gene pathways in eukaryotic systems has been realized. Subsequently, other terpenoids such as artemisinic acid, purpurene, rosinol and other metabolic pathways were gradually successfully constructed and expressed in Saccharomyces cerevisiae[84,85].
The antimalarial drug artemisinin is currently the most successful example of the metabolic conversion of terpenoids in Saccharomyces cerevisiae. By combining metabolic engineering, pathway engineering, and fermentation engineering methods, artemisinin precursors such as artemisinin and artemisinic acid are synthesized in Saccharomyces cerevisiae, and these biosynthetic precursors are further converted into artemisinin[19]. Naturally, the industrial production of artemisinin has been successfully realized. At present, the product has been commercialized by Sanofi. Paclitaxel is a diterpene alkaloid compound with anticancer activity, which has aroused great interest of researchers[86]. Natural paclitaxel is extracted from the bark of yew, with low yield and high price. Therefore, the construction of high-yielding paclitaxel engineering strains has great application value. At present, by truncating the TASY sequence, the yield of taxene in Saccharomyces cerevisiae has reached about 130 mg/L[87], which provides a basis for further development of the biosynthesis of paclitaxel by Saccharomyces cerevisiae, which is conducive to the realization of industrialization.
Lycopene, also known as ψ-carotene, is a tetraterpene carotenoid. Lycopene is a high-quality nutritious food additive approved by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) Food and Agriculture Expert Committee (Vita., 2007). However, the production of heterologously synthesized lycopene by Saccharomyces cerevisiae is much lower than that of Escherichia coli[88]. Therefore, there is still great potential for the establishment of a Saccharomyces cerevisiae-cell factory that efficiently synthesizes lycopene. At present, the heterogeneous synthesis of lycopene in Saccharomyces cerevisiae mainly has the following problems: (1) The lycopene synthesis pathway is a multi-enzyme combination pathway, and the effective coordination of multiple enzymes is the key to increasing the flux of the heterogeneous pathway; (2) Saccharomyces cerevisiae Acetyl-Coenzyme a synthesis pathway and the MVA synthesis pathway are highly regulated, increasing the supply of terpenoid synthesis precursors, and balancing the flux of endogenous precursor synthesis pathways and heterologous precursor synthesis pathways are the key to increasing yield; (3) Lycopene Vegetarian is a lipophilic substance. The accumulation of high levels of lycopene will produce metabolic stress and affect the normal physiological functions of cells. Therefore, improving the tolerance of cells to lycopene is the key to breaking the yield limit. Therefore, in the process of transforming Saccharomyces cerevisiae to synthesize lycopene, it is necessary to continuously optimize and adapt the chassis cells and heterogeneous pathways at the same time, so that the two are fully compatible, to realize the efficient production of lycopene.
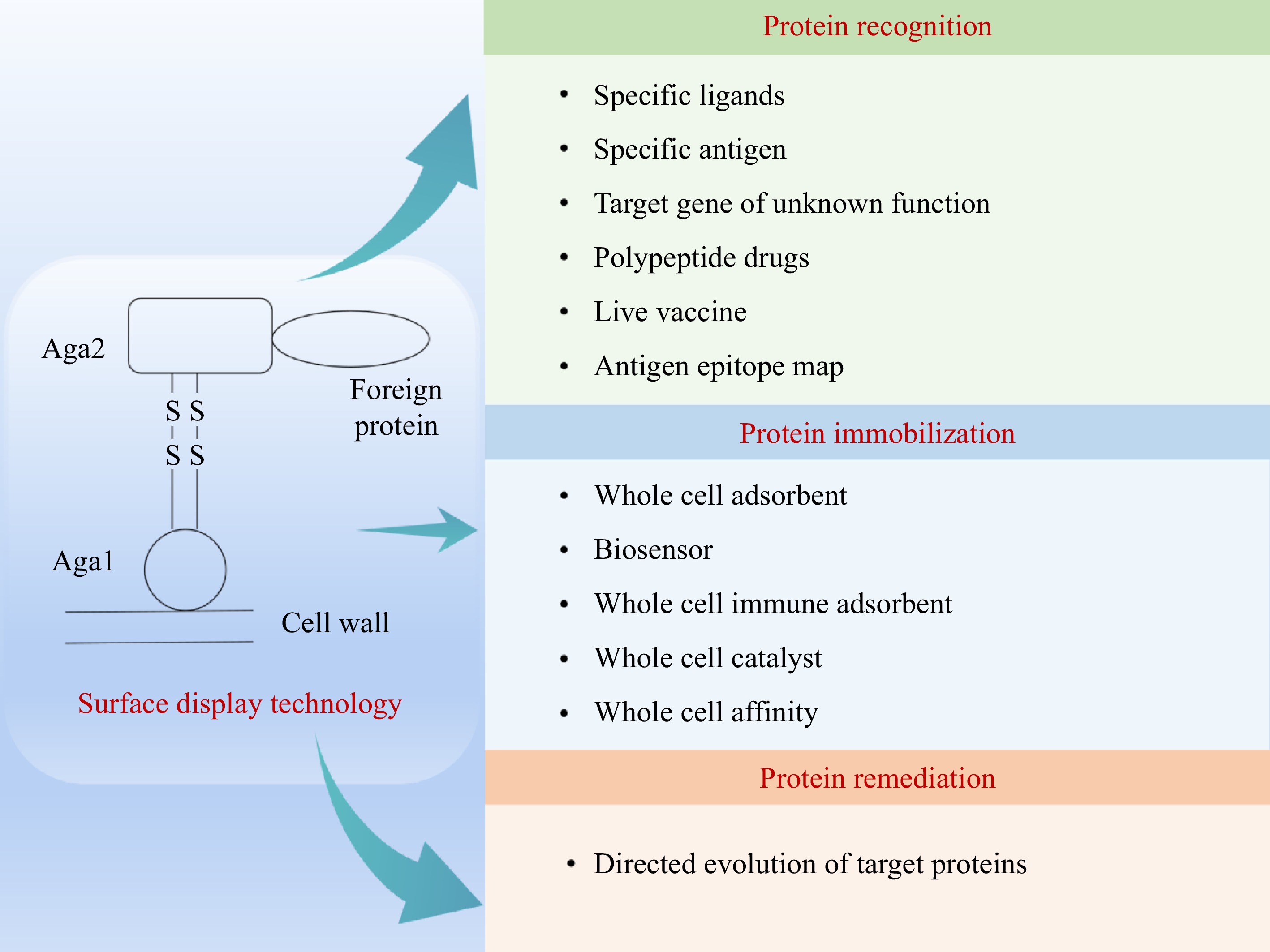
Saccharomyces cerevisiae surface display technology
-
In recent years, the surface display technology of Saccharomyces cerevisiae has attracted increased attention. It is to fuse the target protein with the cell wall protein of Saccharomyces cerevisiae, and then connect the cell wall protein to the cell wall through covalent and non-covalent bonds. β-1,3-D-glycans and mannans of Saccharomyces cerevisiae cell walls are an immunostimulant, which imbue yeast cells with the properties of natural adjuvants[89]. These characteristics have been proven to have high value in the use of Saccharomyces cerevisiae as a vaccine carrier. Secondly, Saccharomyces cerevisiae has post-translational mechanisms (such as disulfide isomerization, glycosylation), which can fold and secrete large and complex glycosylated proteins containing multiple disulfide bonds. It has obvious advantages over bacteriophages and bacteria when expressing complex proteins derived from eukaryotes. Thirdly, Saccharomyces cerevisiae is easy to cultivate, has a short growth cycle, and can be applied to industrial production on a large scale[90,91]. Therefore, it has a wide range of applications in antibody library screening, enzyme engineering, biosorption, vaccine research and development (Fig. 7). At present, exogenous proteins expressed by Saccharomyces cerevisiae surface display technology include fluorescent proteins, various enzyme molecules, and drug proteins[74,91−93].
Feed
-
Cell protein is also called microbial cell protein. Cell protein is widely used in feed and food processing industries because of its rich nutrition, wide range of raw materials, high output rate, and industrial production. It is a feed protein with high nutritional value. Saccharomyces cerevisiae is often used as cell protein to add to feed. Saccharomyces cerevisiae is large in size and rich in nutrients, with a protein content of up to 60%[9]. A wide range of amino acids, rich in essential amino acids such as lysine, leucine, methionine, threonine, etc., are rich in vitamin types and content. Among them, B vitamins are extremely rich, and are beneficial microorganisms in the intestinal tract[94]. In addition, its abundant enzymes can cooperate with the intestine to participate in the metabolism in the animal body, break down nutrients, improve the animal's gastrointestinal digestibility, and increase the utilization rate of feed. The combination of mannan and dextran contained in the cell wall of Saccharomyces cerevisiae can promote the growth of immune organs, improve immune function, and disease resistance[95]. Related experiments have also found that mannan can reduce Salmonella and Escherichia coli in animal feces. Saccharomyces cerevisiae has the following functions in feed: 1) Regulate the balance of animal intestinal flora. Because there are many kinds of microorganisms in the intestinal tract of livestock and poultry, they used to restrict each other and cooperate with each other to establish a normal intestinal balance flora, which is of great significance to resist pathogenic microorganisms; 2) inhibit and kill harmful bacteria. Saccharomyces cerevisiae can produce antibacterial peptides and antibiotics, such as subtilisin, nisin, etc.; 3) promote animal growth. Fermented feed contains acids, peptides, enzymes, metabolites and other beneficial factors, which have a variety of expression effects, which are conducive to the growth and development of livestock and poultry and the digestion and absorption of nutrients; 4) improve the immune function of animals. Saccharomyces cerevisiae can stimulate the immune cells of the original host to exert specific immune functions, improve the resistance of livestock and poultry, and prevent the occurrence of diseases; 5) reduce feed costs[9,95,96]. Compared with the raw materials before fermentation, all indicators are significantly improved. Under the premise of reducing costs, the quality of feed has not declined. However, the only disadvantage of Saccharomyces cerevisiae is that it cannot use polysaccharides as a carbon source, but it can greatly improve the quality of feed through the multiple pressures of compound carbon source cultivation, with the help of molds, bacillus, and lactic acid bacteria that combine hydrolyzed sugars.
Compared with traditional animal and plant protein feed, Saccharomyces cerevisiae cell protein feed has many advantages. The specific manifestations are as follows: First, the protein content of Saccharomyces cerevisiae is relatively high. Second, Saccharomyces cerevisiae grows and reproduces faster than animals and plants, and its production rate is much higher than that of traditional animal and plant protein feeds. Third, Saccharomyces cerevisiae is easy to be mutagenized and ideal high-yielding mutant strains can be obtained, which is convenient to quickly and greatly improve the production efficiency of Saccharomyces cerevisiae cell protein. Fourth, the production of Saccharomyces cerevisiae protein feed is not affected by region, climate, and season compared with traditional animal and plant protein feed production, and it is easy to promote and utilize. Fifth, the production of Saccharomyces cerevisiae protein requires a wide range of sources of raw materials, and various industrial and agricultural wastes, such as industrial sewage, beer waste, waste beet meal, pomace, straw, waste after petroleum processing, which can be used for production raw materials and single-cell protein feed, which can not only reduce environmental pollution caused by pollutants, but also create new value by turning waste into treasure[97−99].
-
Traditional heavy metal wastewater treatment methods include chemical methods, ion exchange methods, adsorption methods, electrolysis methods, and membrane separation methods. However, these methods are cumbersome to operate and have secondary pollution, especially when using heavy metal ions, the effect is not ideal. When the concentration is low, it is often difficult to put into practical application due to the high operating cost and raw material costs. Happily, the application of Saccharomyces cerevisiae to treat heavy metal wastewater is simple, low cost, and has no secondary pollution. Saccharomyces cerevisiae is a good metal-enriched and immobilized microorganism[10,100]. On the one hand, it has safety characteristics, on the other hand, it is easy to cultivate and grow. Saccharomyces cerevisiae cells can accumulate a variety of heavy metals, maintain their ability to accumulate heavy metals under extreme environmental conditions, and can also be used to prepare multi-purpose bioremediation Saccharomyces cerevisiae[101,102].
Nowadays, with the strengthening of environmental protection, the research interest in biofuel ethanol continues to increase[11]. Biofuel ethanol is a promising and one of the most popular alternatives to fossil fuels, with potential for energy and environmental security. Second-generation biofuel ethanol is a typical representative of advanced biofuels and one of the most promising alternatives to fossil fuels[103]. The second-generation biofuel ethanol has the advantages of rapid production and no competition for land, showing economic and environmental advantages, and is the direction of large-scale and sustainable development of fuel ethanol[104,105]. Saccharomyces cerevisiae can effectively ferment glucose to produce ethanol. However, it is difficult for natural Saccharomyces cerevisiae to utilize xylose[2]. The recombinant Saccharomyces cerevisiae obtained through genome engineering has realized the common utilization of glucose and xylose. However, there are still many technical difficulties in the industrial application of ethanol, such as 1) how to improve the level of efficient simultaneous co-fermentation of xylose/glucose; 2) how to increase the tolerance of upstream processes such as pretreatment to inhibitors; 3) how to improve residual oligomerization sugar in lignocellulose raw materials conversion efficiency. Recombinant Saccharomyces cerevisiae can ferment simple sugars (glucose, xylose) to produce ethanol and its utilization of oligosaccharides (such as low xylose, and cellooligosaccharides) is often neglected[11,104,106]. These are also the main challenges faced by Saccharomyces cerevisiae, using lignocellulosic biomass as a raw material to produce bio-based products.
-
In this article, the application and recent progress of Saccharomyces cerevisiae in the fields of food fermentation trace element supplements enrichment, synthetic biology, and environmental protection are summarized. Saccharomyces cerevisiae has an important impact on the quality, flavor, and aroma of fermentation products. Precise control of fermentation parameters and increasing tolerance of Saccharomyces cerevisiae to various industrial stresses are the key to improving fermentation quality. Future research will focus on using advanced technologies to improve Saccharomyces cerevisiae fermentation characteristics, enhancing flavor diversity, automating fermentation processes with bioreactors and computer control systems to improve production efficiency, sustainability, and product consistency. At the same time, Saccharomyces cerevisiae is also an ideal chassis organism for synthetic biology research, can reduce production costs, shorten production cycle, improve production capacity, and have a very broad application prospect. However, in the process of transforming Saccharomyces cerevisiae to synthesize target substances, it is necessary to continuously optimize and adapt chassis cells and heterogeneous pathways at the same time, so that the two are completely compatible, to achieve efficient production of target substances, which will provide a basis for further development of Saccharomyces cerevisiae biological target substances to achieve industrialization. In addition, with increased environmental protection, bio-fuel ethanol is a promising and one of the most popular alternatives to fossil fuels, with the potential for energy and environmental security. Nevertheless, it is difficult for natural Saccharomyces cerevisiae to utilize xylose, thus using lignocellulosic biomass as raw material to produce bio-fuel products is the main challenge for Saccharomyces cerevisiae, which is also a potential research difficulty and hot spot.
-
The authors confirm contribution to the paper as follows: writing - original draft: Que Z; writing - review & editing: Wang S; resources: Wei M; visualization: Ma T; validation: Wang X; data curation: Wang S, Wei M; form analysis: Que Z; project administration: Sun X; supervision & funding acquisition: Sun X, Wang X, Fang T. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This study was supported by the National key research and development program (2023YFD2100304), and the Innovation Capacity Support Plan of Shaanxi Province (2023-YBNY-176, 2023KXJ-171).
-
The authors declare that they have no conflict of interest.
-
Authors contributed equally: Zhiluo Que, Shengnan Wang
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Que Z, Wang S, Wei M, Fang Y, Ma T, et al. 2024. The powerful function of Saccharomyces cerevisiae in food science and other fields: a critical review. Food Innovation and Advances 3(2): 167−180 doi: 10.48130/fia-0024-0016
The powerful function of Saccharomyces cerevisiae in food science and other fields: a critical review
- Received: 07 April 2024
- Revised: 13 May 2024
- Accepted: 23 May 2024
- Published online: 20 June 2024
Abstract: Saccharomyces cerevisiae is the earliest domesticated fungus, researched deeply and widely used fungus. When used in food fermentation, Saccharomyces cerevisiae has an important influence on the quality, flavor, and aroma of products. Future developments will focus on enhancing flavor diversity, increasing production efficiency, sustainability, and product consistency, as well as improving the fermentation characteristics by using advanced technologies. Saccharomyces cerevisiae is an ideal substrate for synthetic biology research, usually used in the production of lactic acid, terpenes, steroids, vaccines, etc., which helps to reduce production cost, shorten the production cycle, improve production capacity, and has a very broad application prospect. In addition, in the field of environmental protection, biofuel ethanol is one of the promising and popular fuels with potential for energy and environmental security. However, there are major challenges for Saccharomyces cerevisiae that use lignocellulosic biomass as feedstock to produce biofuel ethanol.