-
Oral hygiene is an extremely important aspect of human life. The first oral care products have been around for thousands of years. Ancient Egyptians used dental cream with ashes from oxen hooves and egg shells. Persians added burnt shells of snails, herbs, and honey. At the same time in China, people formulated their toothpaste with a flavor using ginseng, and herbal mints[1].
The mouth is the first section of the digestive system (Fig. 1). There, food is mechanically broken down and mixed with saliva, which has an enzyme - salivary amylase. The most exposed to weakening agents is tooth enamel. Sugars and acids in food, as well as improper oral care, can lead to demineralization – which leads to tooth decay, or too much mineralization, with the symptom being the build-up of dental calculus. The main purpose of good oral hygiene is to provide a reduction in bacteria, proper pH, get rid of food debris, and dental plaque and strengthen teeth. Fourty five percent of the world's people have oral disease problems, and the number has been rising steadily for the past 30 years. The greatest increase in incidence is observed in low- and middle-income countries. An interesting fact is that oral health seems to be taken for granted, however, this aspect is neglected on a global level. The best treatment is prevention and prophylaxis[2]. Therefore, the development of cosmetic chemistry should also include oral hygiene products.
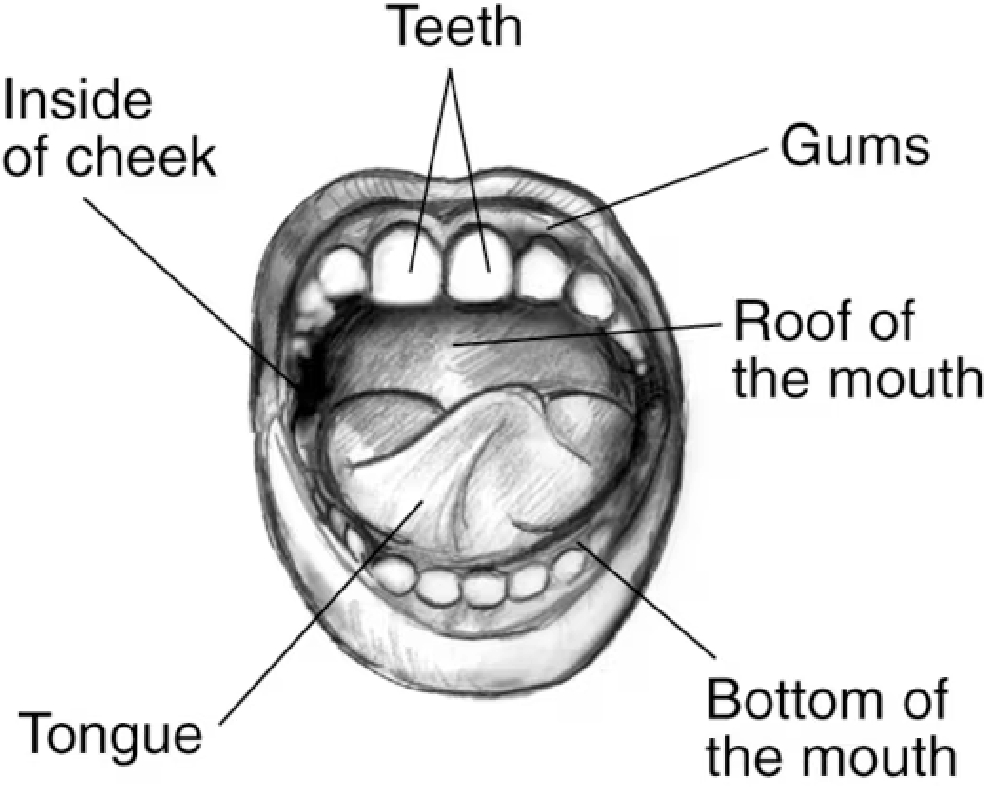
Figure 1.
Drawing showing labels pointing to teeth, gums, roof of the mouth, bottom of the mouth, tongue, and inside of the cheek[3].
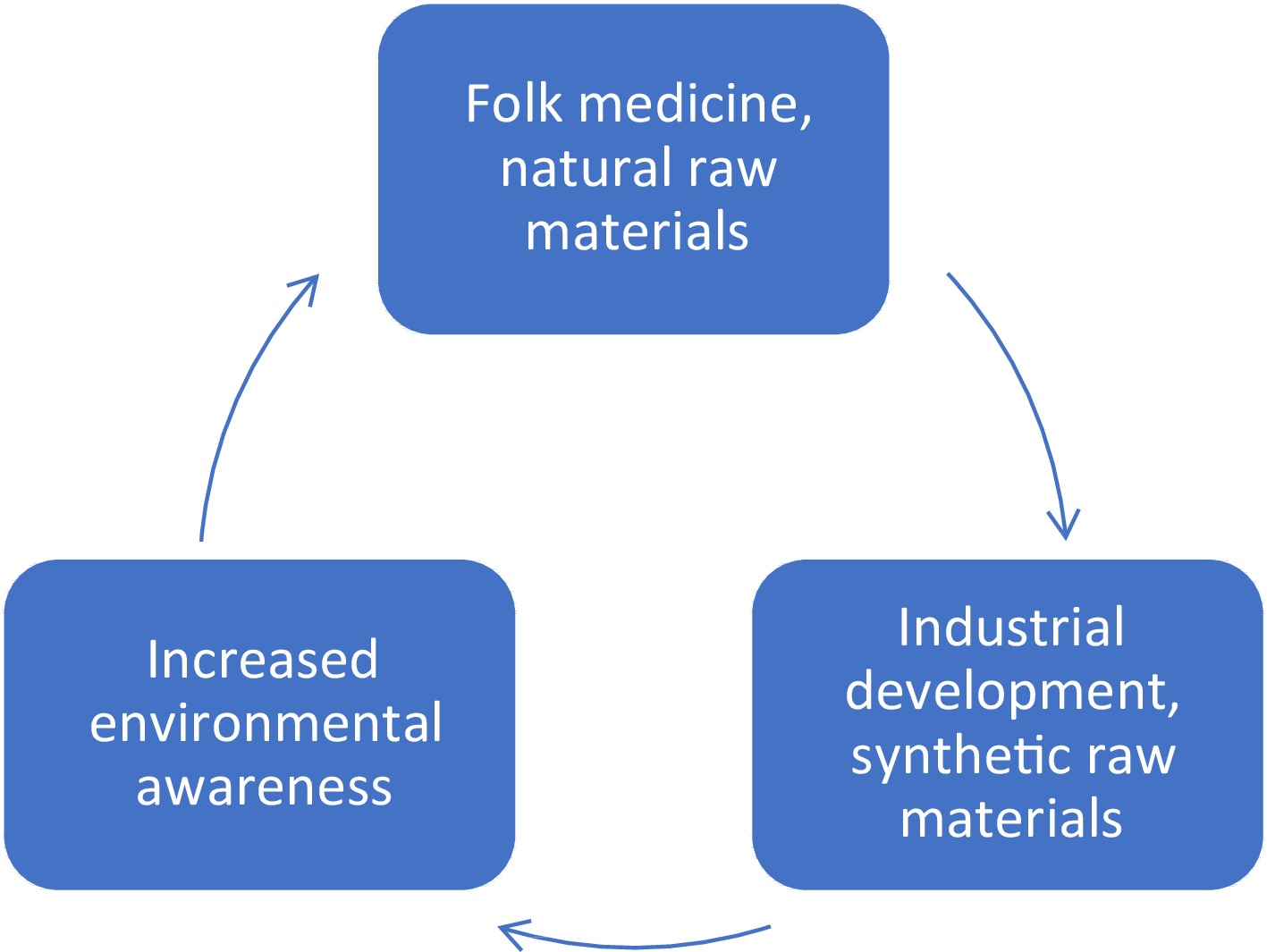
Natural cosmetics continue to grow in popularity. In 2019, the global market for natural cosmetics was worth US
${\$} $ ${\$} $ Folk medicine is known for its wide range of proven healing methods. It mainly uses plants, which are a rich source of biologically active substances. Plant products, i.e. extracts, essential oils, decoctions, or tinctures, show antimicrobial, anti-inflammatory, analgesic effects. Raw materials extracted from plants can be active substances and carriers for other ingredients[5,6].
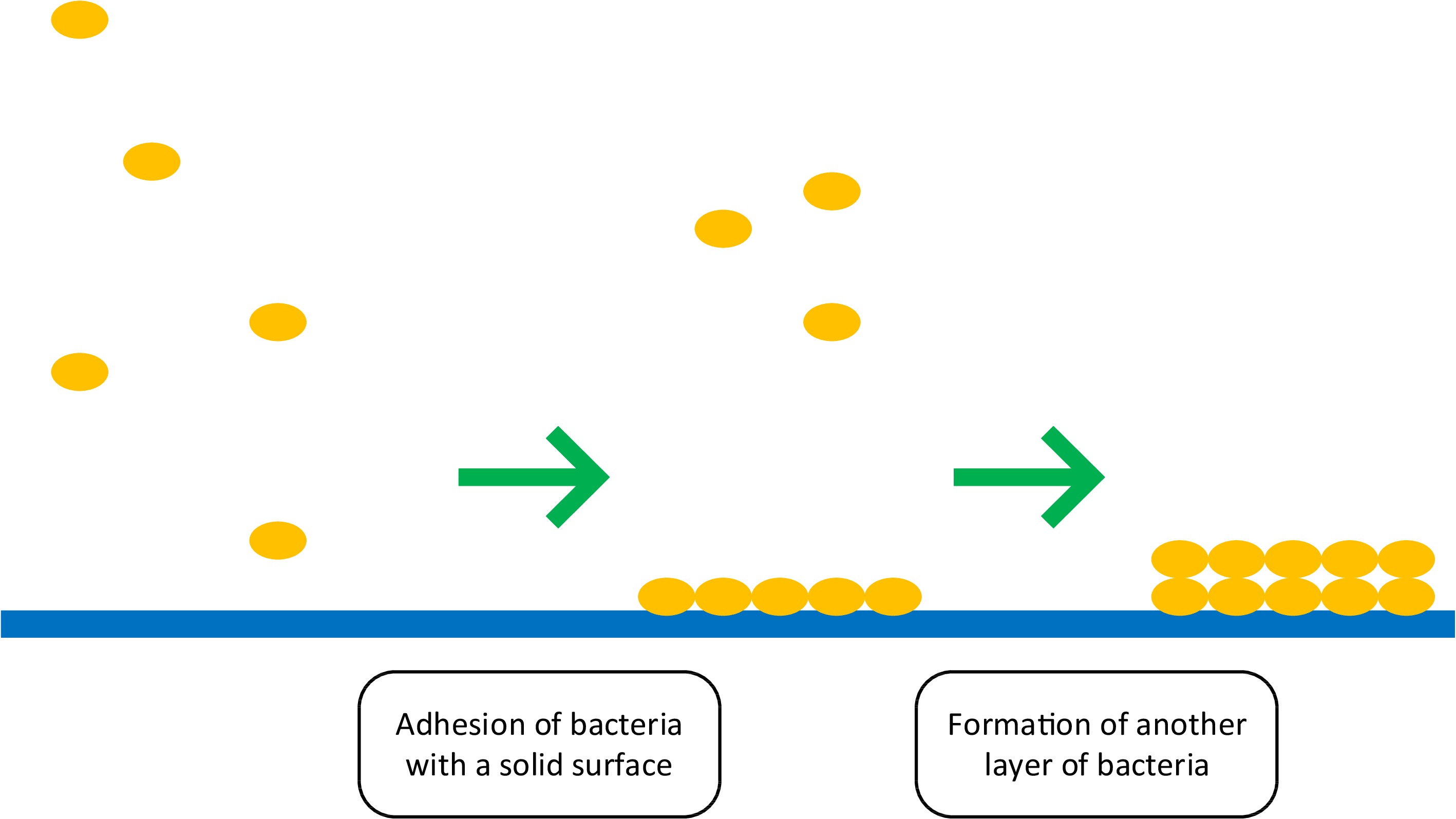
Most oral diseases result from the formation of a bacterial biofilm. A biofilm is a complex community of microorganisms. It consists of cells and extracellular substances (polysaccharides, proteins, lipids, extracellular DNA). Biofilm formation begins with adhesion, whereby bacteria attach to a solid surface. The next step is the polyfusion of bacterial cells and the secretion of extracellular polymeric substances. This creates another layer of biofilm (Fig. 3)[7].
Biofilm control methods are divided into physical, chemical, and biological. Phytochemicals contained in various plant parts biologically act on biofilm. They are inhibitors of the Quorum Sensing (QS) system, an intercellular communication mechanism that controls cell density-dependent gene expression. Active compounds from the plant material have an inhibitory effect on virulence factors and regulatory genes, as well as blocking pyocyanin biosynthesis and reducing the regulation of QS genes. This prevents the biofilm from forming properly[7].
Within the European Union, a cosmetic product also with herbal ingredients must meet the requirements of Regulation (EC) No. 1223/2009 of the European Parliament and the Council of November 30, 2009, concerning cosmetic products. In the act, one can find several definitions and rules that must be met for the product to enter the EU market. There are also annexes to the Regulation, which, among other things, mention prohibited substances, substances that can be used under appropriate concentration limits, colorants, preservatives. In terms of the plant world, the Regulation's annexes contain information on which plants may not be used in cosmetic products[8].
-
Gum diseases represent the most common problem that patients go to visit a dentist with, which maybe associated or not with dental plaque. However, the most frequent periodontal disease is gingivitis, which is associated with growing plaque[9].
Plaque is a soft layer covering the teeth. A significant part of dental plaque is made up of bacteria, i.e. grains, chopsticks, and filamentous bacteria. In addition, the plaque is also built by an organic substance called a matrix, in which microbes are also present. Growing plaque promotes the formation of tartar between teeth and in the gum gap[10].
It is recognized that plaque is a biofilm formed by more than 700 species of bacteria.
In the absence of lesions, these species live in symbiosis with our oral cavity. However, nutrition can alter the ecosystem that prevails in the oral cavity. Disruption of the ecology upsets the balance between microorganisms and host[11].
An effective way to fight gingivitis may be to inhibit bacterial growth. That kind of action can be taken by designing preparations containing specific plant extracts. Al-Mujamamii & Al Waheb investigated the effect of Rhamus prinoides extract on Streptococcus mutans biofilm. R. prinoides is a plant rich in flavonoids, saponins, alkaloids, terpenoids, and tannins, which have antibacterial effects[12]. R. prinoides, belonging to the Rhamnaceae family[13]. The leaves of R. prinoides were extracted using a Soxhlet apparatus and 70% methanol, then purified with diethyl ether to obtain a solution with pH = 8. A mouthwash was prepared and used by the patients twice daily for 3 weeks. The antibacterial effect was evaluated by counting streptococci before and after treatment with the rinse and placebo. The results are shown in Table 1. The study presented showed that R. prinoides extract reduces the activity of S. mutans bacteria[12].
Table 1. Average number of S. mutans at the beginning and end of treatment for the trial with R. prinoides extract and for the control trial[12].
Average no. of S. mutans at the beginning of therapy (CFU/mL) Average no. of S. mutans after therapy (CFU/mL) Research sample 14.23 × 10−5 ± 7.67 0.45 × 10−5 ± 0.28 Control sample 13.79 × 10−5 ± 11.11 30.22 × 10−5 ± 33.57 Mouthwashes can also be used to prevent gum disease. In this way, propolis extract was used in the research[14]. Propolis is a resinous material collected by bees[15]. Due to its therapeutic properties and the chemical compounds, it contains, it is used in medicine and dentistry[16]. It is a component rich in flavonoids, phenols, and aromatic compounds, due to it having an antibacterial effect. In the present study, 60 children aged 12 to 14 years old participated, who had no dental caries and had orthodontic braces for more than a month. Children were separated into two groups, where group A consisted of 40 children who were given mouthwashes with a product containing 5% propolis extract. Group B (the control group) consisted of 20 children who were given distilled water instead of mouthwash. The product was applied twice daily after meals, in 30-day cycles for 3 months. The study compared the average plaque index score. The results are presented inTable 2. Analyzing the data presented in it, it may be seen that the use of both rinse variants reduces the amount of plaque, but the preparation with 5% propolis extract has better results[14].
Table 2. Results of the mean values of the plaque index in both groups.
Time of therapy Average plaque index Group A Group B Before mouth wash 1.493 ± 0.017 1.483 ± 0.027 First day after mouth wash 1.493 ± 0.017 1.483 ± 0.027 After 1 month 1.173 ± 0.045 1.293 ± 0.018 After 2 months 0.853 ± 0.055 1.065 ± 0.049 After 3 months 0.683 ± 0.050 0.795 ± 0.022 In India, an herbal mouthwash was developed from a hydroalcoholic extract of Pongamia pinnata to combat the bacteria that cause gingivitis, i.e. S. mutans, Porphyromonas gingivalis, Staphylococcus and Lactobacillus[17]. P. pinnata is a plant found throughout India, which is a rich source of flavonoids and their derivatives. The seeds, seed oil, flowers, and stems yield caranjin, pongapin, pongaglabron, canugin, desmethoxycanugin, and pinnatine[18]. Dried powdered leaves (132 g) of P. pinnata were placed in the thimble of a Soxhlet apparatus and double the extraction process was carried out using 800 mL of a mixture of 70% ethanol and water in a 1:1 ratio, for 48 h. The resulting material was then evaporated at 70 °C for 8 h and dried. In addition to the leaf extract of P. pinnata, the essential oil of Mentha × piperita was also obtained. For this purpose, a steam distillation process was carried out in a Clevenger apparatus. The extract obtained contained: alkaloids, carbohydrates, glycosides, saponins, phytosterols, resins, terpenoids, phenols, tannins, and flavonoids. Three formulations of herbal mouthwash differing in the content of P. pinnata leaf extract (250, 500, 1,000 mg) were prepared. The other ingredients used are: peppermint oil (0.1 mL), saccharin (0.1 mg), PEG-40 (4 g), glycerol (8.5 mL), salt solution (2 mL), Lemon Yellow Color (1−2 drops) and purified water (up to 100 mL). The resulting formulations were compared with a commercially available formulation with chlorhexidine. It turned out that formulation 2 had similar parameters to the commercial liquid. However, the cited study did not test for plaque inhibitory activity. This provides an opportunity for further in vitro and in vivo studies of P. pinnata leaf extract in other formulations and oral hygiene products.
Inhibition of growth of pathogenic bacteria (P. gingivalis, A. actinomycetemscomitans, and S. viridans) by coffee bean extract was studied by Sari et al.[19]. The antibacterial properties of coffee result from the presence of compounds such as flavonoids, caffeine, trigonelline, or chlorogenic acid[19]. Coffee comes from the Rubiaceae family[20]. It is characterized by a neutral, weak flavor with a pronounced bitterness[21]. The chemical composition of robusta coffee depends on the growing region, but we can distinguish chemical compounds, i.e.: diterpenes (e.g. cafestol, 16-O-methylcafestol), sterols (e.g. β-sistosterol, 24-methylencycloartanol), tocopherols (γ-tocopherol)[22,23]. Robusta coffee extract was prepared by macerating coffee beans in 96% ethanol in a ratio of 1:5 for 72 h. The macerate was then filtered and the solvent evaporated. Aqueous solutions have been prepared from the extract obtained at the following concentrations from 50% to 6.25%. The resulting extracts were applied to paper discs. The bacterial colony was prepared on an agar medium. The discs with the extracts were placed in the bacterial colonies and the diameter of the growth inhibition was measured. The results of the studies are presented in Table 3. The study showed that Robusta coffee extract has an antibacterial effect from 12.5%[19]. Such results allow testing of the extract in specific oral hygiene products.
Table 3. Results of the mean periopathogenic bacterial growth inhibition zone with standard deviation after using Robusta coffee extract at different concentrations.
Concentration
of Robusta
coffee extractMean growth inhibition zone [mm] +
standard deviationP. gingivalis A.
actinomycetemcomitansS. viridans 0% (control test) 0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 6.25% 0.00 ± 0.00 0.00 ± 0.00 0.00 ± 0.00 12.50% 13.14 ± 0.24 8.40 ± 0.22 12.15 ± 0.25 25.00% 16.59 ± 0.17 12.20 ± 0.10 14.95 ± 0.10 50.00% 19.18 ± 0.18 16.15 ± 0.12 18.15 ± 0.19 Inflamed gums require antimicrobial action in the periodontal pockets. For this purpose, researchers set out to test the efficacy of Sarang Semut (Myrmecodia pendens) extract as an ingredient in an irrigation and mouthwash solution[24]. It grows in Sumatera, Papua New Guinea, the Philippines, Cambodia and Malaysia[25]. The composition of M. pendens includes glycoside, flavonoids, tocopherols, polyphenols, and tannins[26]. In the present study, male rats (Rattus norvegicus) aged 4−6 months were used. The plant was powdered and then macerated in 96% ethanol for 5 d. A 1% Carboxymethylcellulose Sodium (NaCMC) solution was used as a control preparation. Rats were divided into four groups (five individuals in each group). Observation followed within 3 h of administration of the preparation. The study showed minimal toxicity of M. pendens extract at a dose of 0.1 g/kg body weight to the kidneys and livers of the test rats. It was concluded that there is a need to test a lower dose of the extract ultimately in mouthwash preparations and the results obtained may be a reference for further studies[24].
Other scientists have investigated the activity of water hyacinth (Eichhornia crassipes) leaf extract against bacteria found in the plaque of patients suffering from gingivitis[27]. The water hyacinth is a perennial floating plant. It competes with domestic aquatic plant species by completely covering the surface of water reservoirs[28]. The phytochemical composition of water hyacinth includes numerous secondary metabolites: polyphenols, flavonoids, fatty acids, alkaloids, sterols. The leaves contain phosphatidylethanolamine, phosphatidylcholine, and phosphatidylglycerol and are rich in lecithin, asparagine, and glutamine[29]. Water hyacinth leaf extract was used in the study, which was obtained by maceration in 70% ethanol, filtration on a Büchner funnel, and evaporation on a rotary evaporator using a water bath. Antimicrobial activity was checked by counting the number of microbial colonies on Mueller Hinton Agar (MHA). Microorganisms were extracted from the plaque of patients aged 20−30 years with gingivitis. The extracts tested had concentrations ranging from 100% to 0.78%. The study showed that water hyacinth extract inhibited the growth of plaque bacteria already at a concentration of 3.125%[27].
-
Dental caries are a chemical localized decay of the tooth surface that is caused by the metabolic activity of microorganisms in the biofilm. These microorganisms transform sugars supplied with food, converting them into acid. The product of microbial metabolism demineralizes the tooth enamel. The carious lesions themselves develop in areas where biofilm can accumulate and mature[30]. S. mutans has long been considered the caries-causing bacterium because it produces lactic acid and also increases at low pH values. In addition to S. mutans, there are other species of acid-producing microorganisms. These include bacteria from the types Veillonella, Scardovia, Propionibacterium, as well as other Streptococci that grow at acidic pH[31].
One of the most effective ways to prevent tooth decay is through good oral hygiene, by brushing and flossing. Unfortunately, mechanical removal of biofilm, is not enough. There is a need to include additional substances in hygiene to counteract biofilm formation. One such substance is chlorhexidine dihydrochloride, but regular use leads to unwanted effects. For this reason, the essential oil of the leaves of Stachytarpheta cayennensis was used as an alternative medicine in studies described by Oliveira et al.[32]. S. cayennensis is a plant that can be found in tropical climates. In Brazil, its leaves are used in folk medicine as an anti-inflammatory, analgesic, antipyretic, constipation, and liver and kidney disorders. This study investigated the antibacterial properties of essential oil against decay bacteria. Fresh leaves of S. cayennensis were a source of essential oil, which was obtained from the plant material by hydrodistillation in a Clevenger apparatus (400 g leaves and 500 mL distilled water). The activity of the essential oil from S. cayennensis against decay bacteria was tested by microdilution in broth. The bacterial strains used were Streptococcus mutans, Streptococcus mitis, Streptococcus salivarius, Streptococcus sanguinis, Streptococcus sobrinus, Enterococcus faecalis, and Lactobacillus casei, which were cultured on agar medium. The essential oils were dissolved in Dimethyl Sulfoxide (DMSO). The positive control was chlorhexidine dihydrochloride placed in broth. The study showed the activity of S. cayennensis leaf essential oil against the bacteria S. mutans, S. mitis, S. salivarus, and S. sobrinus[32]. The results of this study show the potential for the use of S. cayennensis essential oil in oral care products and encourage further research.
Another publication examined the effects of Mentha spicata and Eucalyptus globulus essential oils on S. mutans biofilm[33]. M. spicata is otherwise known as spearmint. Cultivation concentrated on essential oil production is located in the USA[34]. The predominant component of the oil is carvone (40%−76%). In addition, limonene, 1,8-cyneol are found in the oil. E. globulus is a tree that reaches up to 60 m in height. The main component of the oil is 1,8-cineole, which can be up to 84%[35]. Evaluation of the inhibitory activity of the oils was determined by agar well diffusion and calorimetric microdilution methods. In contrast, activity against biofilms was determined on pieces of bovine enamel using Buffered Minimal Methanol (BMM) medium under anaerobic conditions with daily exposure to sucrose to mimic oral conditions. Essential oils were applied at a concentration of 0.4% in saline solution with 1% polysorbate-20. The study showed a significant reduction in biofilm after 72 h. This was assessed by turbidity of the suspension and microbial counts. The studies discussed here demonstrated the efficacy of both essential oils[33]. This provides an opportunity for further research into the oils and testing their antimicrobial activity in oral hygiene preparations.
Butterfly pea (Clitoria ternatea Linn.) extract has also shown activity against Streptococcus mutans. Butterfly pea flower is used in Ayurvedic medicine[36]. C. ternatea contains antifungal compounds, i.e. taraxerol, taraxerone, p-Hydroxycinnamic acid, β-sitosterol, delphinidin, kaempferol, clitorin[37]. In a study conducted in Indonesia, an ethanolic (70% ethanol) extract of C. ternatea was used. In vitro tests were carried out using the diffusion method, using 12 test groups of 4-fold replicates. A 0.2% chlorhexidine solution (positive) and distilled water (negative) were used as control groups. Aqueous solutions of the extract were then prepared at concentrations ranging from 10% to 90%. S. mutans bacteria were cultured on Brain Heart Infusion Broth (BHIB) medium (at 37 °C under anaerobic conditions for 24 h) and then bacteria were cultured in a petri dish containing MHA. Samples were divided into control (positive and negative) and test groups. Inhibition of bacterial growth was shown at concentrations of 50% and above, but the best result was obtained for the pure extract. This is an expected result, as the pure extract contains the highest concentration of active substances[36]. The study found that an extract from C. ternatea could be an ingredient in caries prevention products.
In Indonesia explored the antibacterial properties of Piper crocatum Ruiz & Pav. and Mentha × piperita extracts as toothpaste ingredients. The anti-caries ingredient in the pastes is fluoride, but it cannot be used in pastes for children under 4 years of age. In addition, the use of fluoride in excess can lead to osteoporosis and damage to the nervous system. The study described here aimed to find an alternative to fluoride[38], P. crocatum Ruiz & Pav. This is one of the more popular plants used in herbal therapies in Indonesia[39]. P. crocatum is rich in tannins, saponins, alkaloids, and flavonoids[40]. Mentha × piperita is a triple hybrid of M. aquatica and M. spicata. It has reddish-purple stems. It is used most commonly in gastrointestinal disorders and as a taste and odor enhancer[34]. In the present study, extracts of Piper crocatum Ruiz & Pav. and Mentha × piperita were prepared. The extracts were prepared by maceration in 70% ethanol for 24 h, filtration, and evaporation using a vacuum evaporator. The resulting extracts were used in five toothpaste formulations. A zone of inhibition of S. mutans bacterial growth was observed in the study. The microorganisms used in the study were cultured on an agar medium. Five test groups, a negative control (toothpaste without plant extracts), and a positive control (herbal toothpaste) were then prepared. The test materials were incubated for 24 h at 37 °C. Studies have shown the effectiveness of a paste containing two extracts in its composition in concentrations: 10% of both extracts, 15% of P. crocatum extract and 5% of Mentha × piperita extract, 5% of P. crocatum extract and 15% of Mentha × piperita extract[38].
Another study under discussion was conducted in Brazil. An article by Camilo et al. investigated the antimicrobial properties of extract of Stryphnodendron adstringens[41]. The stem bark is commonly used medicinally for its anti-inflammatory and antimicrobial properties[42]. S. adstringens is a rich source of alkaloids, terpenes, flavonoids, steroids, and tannins[43]. The researchers prepared an extract from the dried leaves of S. adstringens by maceration in 95% ethanol (process time: 7 d), followed by concentration at 50 °C using a rotary evaporator[41,44]. The Kirby-Bauer plate diffusion method was used to determine the value of the minimum inhibitory concentration. The following bacterial cultures were used in the study: Enterococcus faecalis, Lactobacillus casei, Streptococcus mitis, S. mutans, S. salivarius, S. sanguinis, S. sobrinus. DMSO solutions at concentrations ranging from 1 to 10% were used as the negative control in the study, and chlorhexidine digluconate solutions at concentrations ranging from 0.015 to 5.9 mg/mL were used as the positive control. S. adstringens extract was tested at dilutions from 50 to 500 mg/mL. Bacterial cultures were developed on broth medium in plates. The previously mentioned solutions were added to the prepared colonies. Each sample was incubated at approximately 35 °C for 24 h. After incubation, 0.01% resazurin (30 μL) was added to the samples so that microbial growth could be observed immediately. Blue coloring indicated no growth and red coloring indicated microbial growth. The results showed that the extract from S. adstringens has activity against decay bacteria[41].
The efficacy of three plant extracts of S. mutans compared to a 0.2% Chlorhexidine solution is described in an article by Mehdipour et al.[45]. The first plant discussed in the study is Carum copticum. Ajwain (C. copticum) is a plant of the Apiaceae family. The seeds contain carbohydrates, fats, proteins, fiber, tannins, glycosides, saponins, and minerals[46]. The second plant was Phlomis bruguieri. It is a plant belonging to the Lamiaceae family. It exhibits antioxidant and antimicrobial activities. It is a rich source of flavonoids and glycosides[47]. The last plant used in the study by scientists was Marrubium parviflorum. It is used in folk medicine as an antipyretic[48]. The phytochemical composition of M. parviflorum may include diterpenes, caffeic acid derivatives, as well as sterols and flavonoids[49]. Methanolic extracts were prepared from the plants discussed by maceration. The agar well diffusion method using DMSO was used to assess antimicrobial activity. Bacteria were cultured on BHI agar medium. The wells in the agar used for testing were filled with extracts at concentrations ranging from 0.390 to 200 mg/mL and bacterial material. The positive control was 0.2% chlorhexidine solution and the negative control was DMSO. After incubation, the diameter of the inhibition zones was measured. The biofilm inhibition properties were then tested using the O'Toole's procedure. The standard medium for the S. mutans biofilm test was brain-heart infusion broth with 2% sucrose (BHIS). Bacterial suspension and extracts were placed in a microtiter plate at concentrations ranging from 0.39 to 200 mg/mL. The positive control was the wells filled only with the bacterial suspension and the negative control was the BHIS medium. The study discussed here showed antibacterial activity against S. mutans and antibiofilm activity against S. mutans. The effect on biofilm was lower than 0.2% chlorhexidine. There is a need for further studies on extracts from C. copticum, Phlomis bruguieri and M. parviflorum to see the in vivo effects[45].
An article by Bance et al. describes research on the aqueous extract of Prosopis africana[50]. P. africana is a common tree that grows in arid and semi-arid areas of the world[51]. In traditional medicine, it is used to treat the oral cavity[50]. It is a source of flavonoids, steroids, alkaloids, and fatty alcohols[52]. The bacterial strains used in the study were S. mutans, Staphylococcus aureus, Pseudomonas aeruginosa, Chromobacterium violaceum. Each was stored in BHI liquid medium (S. mutans, S. aureus) and Lauria-Bertani (P. aeruginosa, C. violaceum) with 50% glycerol at −80 °C until use. The collected plant material was dried and then powdered. Extraction was carried out using powder and distilled water (time of process: 30 min). Then the extract was cooled, filtered through nylon cloth and concentrated in an oven. The whole mixture was lyophilized and sealed in airtight white vials. Anti-inflammatory activity was evaluated by lipoxygenase inhibitory activity. Oxidative properties were evaluated by the radical cation decolorization method and antibiofilm activity was tested using crystal violet and solution absorbance studies. The study showed an inhibitory effect of P. africana extract on the growth of bacterial biofilm, which justifies the use of this plant in folk medicine for the treatment of dental diseases, including dental caries[50]. This of course creates opportunities for further investigation of P. africana in in vivo tests in cosmetic preparations.
-
Oral candidosis is the most common fungal diseases of the oral cavity. It is caused by the fungus Candida spp. in particular Candida albicans[53]. C. albicans belongs to the oral microflora and approximately 30%−50% of the population is a carrier of this organism, with the frequency of being a carrier increasing with age[54]. Candidiasis is commonly referred to as 'thrush'. It involves infections of the tongue and other areas of the mucosa. The characteristic feature is fungal overgrowth and invasion of tissue surfaces. Candidiasis was already known at the time of Hippocrates, who wrote about it in his book 'Of the epidemics'[55]. The disease itself is due to lowered immunity, use of antibiotics, steroids[53,56]. Contributing to the search for alternative treatments has been the overuse of fluconazal, to which the Candida species have begun to become immune[57].
The antifungal properties of glycolic plant extracts were investigated in an article by Meccatti et al. Extracts from Rosmarinus officinalis, Punica granatum, Rosa centifolia, and Curcuma longa were used in the study[57]. Rosemary (Rosmarinus officinalis) is an aromatic plant belonging to the Lamiaceae family. In folk medicine, it has been used as an oral remedy to relieve renal colic, painful menstruation, and muscle spasms. It is characterized by antifungal, antiviral, antibacterial, and anti-inflammatory properties, among others. R. officinalis is a rich source of flavonoids, polyphenols, and terpenes[58]. The pomegranate (Punica granatum) is a small tree. Each part of the pomegranate has different pharmacological properties and is the source of a wide range of active ingredients such as ellagitannins, gallotannins, ellagic acid derivatives, catechins, and many others[59]. Rosa centifolia L. is a perennial plant, belonging to the Rosaceae family. It is a hybrid of varieties such as Rosa gallica L., Rosa moschata Herrm., Rosa canina L., and Rosa damascene Mill. In traditional medicine it has found use in the treatment of arthritis, coughs, asthma, bronchitis, wounds, and ulcers[60]. Turmeric (Curcuma longa) is a medicinal herb in the Zingiberaceae family. It is used as a spice but has also found use in folk medicine due to its medicinal properties. It is characterized by its antibacterial, anti-inflammatory action. These effects are attributed to a compound present in turmeric – Curcuminoids[61]. In the study in question, the researchers examined, using High-Performance Liquid Chromatography (HPLC), which active ingredients were present (Table 4). Mixtures of extracts were used: R. centifolia + C. longa and R. officinalis + P. granatum against C. albicans, C. dubliniensis, C. tropicalis, and C. krusei. It was shown that C. albicans biofilm significantly decreased after application of the extracts[57].
Table 4. Active compounds detected by HPLC from plant extracts (Rosmarinus officinalis, Punica granatum, Rosa centifolia, and Curcuma longa).
Plant Rosmarinus officinalis L. Punica granatum L. Rosa centifolia L. Curcuma longa L. Active ingredients Gallotannin, chlorogenic acid,
p-coumaric acidGallotannin, quercetin or kaempferol Gallic acid, gallium, p-coumaric acid, derivative of quercetin Curcumin In India, research has been conducted on the antifungal activity of grapefruit extract. This study evaluated the effect of the volatile oil extract of grapefruit leaves against Candida species[62]. Citrus paradisi (grapefruit) belongs to the Rutaceae family[63]. Grapefruit is a natural cross between sweet orange and pomelo[35]. The active compounds present in the essential oils of C. paradisi are terpenes, sesquiterpenes, aldehydes, alcohols, esters, and sterols[64]. The oil was obtained from the leaves of grapefruit trees by hydrodistillation in an aqueous-glycerol solvent in a Clevenger apparatus. The antifungal properties of the raw material were tested on Candida albicans, Candida krusei, Candida tropicalis, and Candida parapsilosis strains. The zone of inhibition of microbial growth was determined by a dilution technique in broth, using oils at concentrations of: 100% and 50% to 3.75%. A cytotoxicity test was then performed on human gingival fibroblasts. The tests carried out showed the efficacy of grapefruit oil on Candida fungi in the following order: Candida parapsilosis > Candida krusei > Candida tropicalis > Candida albicans. The results obtained for Candida albicans and Candida krusei showed greater activity of the extract than the commercially available Amphoreitin B[62].
In an article by Proškovcová et al. describes research on the antifungal and antibiofilm effects of five essential oils (Salvia officinalis, Thymus vulgaris, R. officinalis, Origanum vulgare, and Hyssopus officinalis)[65]. Sage (Salvia officinalis) is a perennial shrub in the Labiatae/Lamiaceae family[66]. Salvia essential oil is used for inflammation and infections of the mucous membranes of the throat and mouth. S. officinalis is a rich source of metabolites with healing properties, e.g. α- and β-thujone, 1,8-cineole, camphor, carnosic acid, oleanoic and ursolic acids or phenolic compounds[67]. Thyme (Thymus vulgaris) is a spicy herb in the Lamiaceae family native to southern Europe. Thyme has been used for centuries as a flavoring, spice, and in herbal medicine[68]. The essential oil from Thymus vulgaris has a broad spectrum of antimicrobial activity. The main constituents of the oil such as p-cymene and thymol show strong antifungal activity[69]. Rosemary essential oil is obtained from rosemary. It is usually obtained by steam distillation of fresh leaves. Its composition depends on the chemotype of the raw material. There are three chemotypes: camphor, 1,8-cineol, and verbena. The oil can contain eucalyptol, camphor, α-pinene, borneol, and verbenone in its composition[35]. Oregano (Origanum vulgare) is another member of the Lamiaceae family. It has been used in folk medicine to treat respiratory disorders, digestive disorders, and as an ointment for wounds. The essential oil is extracted from the stems, leaves, and flowers. It is a rich source of phenolic compounds, flavonoids, tannins, and polysaccharides[70]. Hyssop (Hyssopus officinalis) is also a representative of the Lamiaceae family. In herbal medicine, it was used to treat coughs and stomach diseases. The essential oil extracted from H. officinalis has a variable composition depending on its growing regions. The oil may include cis-pinocamphene, elemol, β-pinene, and 1,8-cyneol. It is characterized by antiviral, antibacterial and antifungal activity. Previous studies have shown its efficacy against Candida sp.[71]. In the study in question, Slovak researchers determined the antifungal properties of each of these essential oils in the concentration range of 200−0.4 mg/mL on C. albicans cells. The study used 13 C. albicans isolates from clinical patients with suspected candidiasis. In addition, each oil was evaluated for biofilm inhibition efficacy. A crystal violet test was used. The effect of essential oils on inhibiting biofilm growth was determined for the following concentration ranges: 25−0.05 mg/mL for Origanum vulgare, H. officinalis and T. vulgaris; 200−0.4 mg/mL for S. officinalis and 100−0.2 mg/mL for R. officinalis. Essential oils at the indicated concentrations and 100 μL SG were added to the wells of the yeast microtiter plate. Results were obtained after 48 h of incubation. Studies have shown that each of the essential oils in question has antifungal and antibiofilm activity. Oil of oregano has proven to be the best alternative for the treatment and complementary therapy of mycoses. Thyme oil, on the other hand, showed potential in the prevention and treatment of mycoses. The whole study encourages further research[65].
In an article by Alves-Silva et al. also decided to investigate the anti-biofilm properties of an essential oil. They chose lavender oil to test the potential against strains of dermatophytes and C. albicans[72]. Lavender (Lavandula multifida L.) is a perennial plant belonging to the Lamiacea family. Lavender is used to obtain essential oil. Its composition varies depending on the part of the plant and the place of cultivation. The oil may contain the following compounds: carvanol, linalool, 1-octen-3-ol, carvacrol, anethole, bisabolene[73]. The lavender oil used in the study was obtained by hydrodistillation. The antibiofilm activity of lavender essential oil was tested on yeasts and filamentous fungi. The dermatophytes used were Microsporum gypseum, Trichophyton mentagrophytes var. Interdigitale, T. rubrum, Epidermophytom floccosum, M. canis, T. mentagrophytes; and yeast: C. albicans. Biofilm mass, extracellular matrix and viability were examined quantitatively using crystal violet, safranin and XTT assays. In contrast, morphological changes were confirmed by optical and scanning microscopy. The results proved that lavender oil showed a strong inhibitory effect on the biofilm formation of both dermatophytes and C. albicans. In addition, the oil showed the ability to eliminate mature biofilm[72].
Essential oils were also chosen for testing in an article by Shah et al. Scientists selected four essential oils to test their properties against Candida yeast[74]. One of the oils was lemongrass (Cymbopogon citratus) essential oil. C. citratus belongs to the Poaceae family[75]. The main constituent of C. citratus essential oil is citral, with up to 85%[35]. The second essential oil tested was cinnamon bark oil (Cinnamomum zeylanicum). Cinnamon is uses in Ayurvedic medicine, it is considered a remedy for respiratory and digestive diseases and is also used for gynecological ailments[76]. The main chemical compound of cinnamon bark oil is cinnamaldehyde[77]. The third oil was the oregano oil already discussed. The last of the essential oils was oil from Trachyspermum ammi (ajwain oil). It belongs to the Apiaceae family. It is characterized by its stimulant, windmilling, antispasmodic, and tonic properties. The main ingredient of ajwain oil is thymol, which has antibacterial properties and is also known for its healingprop-erties (e.g. for alleviating the symptoms of cholera)[35,78]. Each essential oil was prepared by hydrodistillation in a Clevenger apparatus. Microbiological material for the study was collected from 50 subjects with early childhood caries, oral candida, and removable dentures and orthodontic appliances. The study material was divided into eight groups: (1) ajwain essential oil; (2) cinnamon bark oil; (3) lemon grass oil; (4) oregano oil; (5) essential oil blends; (6) probiotics; (7) clotrimazole; (8) chlorhexidine. The efficacy of the aforementioned agents was tested against C. albicans using the agar well diffusion method (positive control: clotrimazole and chlorhexidine; negative control: DMSO), followed by testing the minimum inhibitory concentration (visual method with Alamar Blue dye). Studies have shown that selected essential oils can be used as antimicrobial agents against Candida[74].
Sudanese researchers decided to test clove extract for antimicrobial activity and develop a suitable medicinal preparation to treat oral candidiasis[79]. Clove (Syzygium aromaticum) is a dried flower bud belonging to the Myrtaceae family. Cloves exhibit strong antimicrobial and antioxidant activity. Cloves are a source of phenolic compounds, flavonoids, and tannins[80]. The plant material was dried and extracted with 97% ethanol (process time: 24 h). The total was then separated from the solvent, filtered, and concentrated at 65 °C. To determine the antifungal potential on the yeast C. albicans of the clove extract, the agar well diffusion method was used. Pharmaceutical formulations were developed to determine the minimum concentration of the extract that would be effective against C. albicans. Formulations containing concentrations ranging from 25% to 100% of clove extract were tested. The positive control was 5 mg of amphocerin B. The gel formulation with the extract tested consisted of glycerol (15 mL), carboxymethylcellulose (5 g), sodium benzoate, vanillin (25 mL), sucrose syrup, polysorbate 20, and distilled water. To evaluate the antifungal efficacy of the resulting formulation, it was compared with an oral gel with miconazole. The diameters of the zones of inhibition were then measured. The study showed the efficacy of the developed gel, where the minimum concentration of clove extract was 25%[79].
-
Oral hygiene is a key part of taking care of your health. Over the years, the problem of oral diseases has become increasingly prominent. The growing industry and synthetic applications have made most pathogens resistant to commonly used preparations. This fact and the growing trend toward eco products have forced manufacturers of oral preparations to look for alternatives by drawing knowledge from folk medicine and hitherto unexplored substances of plant origin. This review presents the latest reports on the effectiveness of extracts, macerates, and essential oils used alone or in hygiene preparations in the fight against oral diseases.
Gingival disease is primarily influenced by the growing bacterial biofilm that constitutes plaque. It has been proven that a good solution is to find substances that inhibit the pattern of biofilm. For this purpose, a good solution is to use rinses that will contain in their composition plant extracts that inhibit the development of biofilm and plaque.
In the case of caries, results have been presented showing that appropriate essential oils inhibit the growth of S. mutans bacteria. In addition to antimicrobial properties, oils can impart flavor and aroma in formulations. In addition to OEs, plant extracts play a large role in the fight against S. mutans as in gingival diseases.
In the treatment of what is commonly known as thrush, extracts and essential oils that show properties against the fungal growth of Candida sp. play an important role. As above, finding suitable plants offers great opportunities for the development of future formulations and a variety of market preparations.
Active substances extracted from plants may also find other uses in addition to their inhibitory effect on microorganisms that cause oral diseases. Biocidal compounds contained in plants are also being studied for their toxic effects on organisms, i.e. insects. A paper by Xiao et al. gives an example of using a paste of Chinese herbs to test toxicity on imported red ants[81]. The findings are promising and show an interesting direction for today's researchers. At a time when environmental sustainability and ecology are of extreme importance, it is necessary to look for new solutions that work universally in different aspects.
In summary, oral diseases are a problem affecting more and more people. It is the task of scientists as well as industry to draw knowledge from traditional therapeutic methods and seek new solutions using what the plant world gives us. This contributes to increasing sustainability and meeting consumer expectations.
This work was supported by Science Club: Substances of plant origin - research perspectives, potentials applications in the prevention and treatment of various diseases in University of Engineering and Health of Warsaw.
-
The authors confirm contribution to the paper as follows: writing and figure preparation: Świątek IM; review and editing: Adamska-Szewczyk A. Both authors reviewed, read, and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Świątek IM, Adamska-Szewczyk A. 2024. An overview of the potential use of plants in oral care products. Medicinal Plant Biology 3: e015 doi: 10.48130/mpb-0024-0015
An overview of the potential use of plants in oral care products
- Received: 30 December 2023
- Revised: 09 May 2024
- Accepted: 05 June 2024
- Published online: 06 August 2024
Abstract: Problems such as periodontal disease, tooth decay, and oral candidiasis are common conditions that affect people of all ages and geographical zones. They are often associated with poor oral hygiene. Pathogenic microorganisms, their metabolic activity, and inflammation are considered to be the basis of their formation. The search for active substances, components of oral care products, and hygiene products expands this possibility to include research on plant substances with antibacterial, antifungal, and anti-inflammatory properties. Plant extracts such as Rhamus prinoides, Pongamia pinnata, Myrmecodia pendens, Eichhornia crassipes, or the well-known propolis or coffee can effectively reduce the formation of dental plaque and protect against periodontitis. The effect of reducing tooth decay has been demonstrated in relation to extracts from plants such as: Stachytarpheta cayennensis, Mentha spicata, Piper crocatum, Mentha × piperita, Eucalyptus globulus, Clitoria ternatea, Stryphnodendron adstringens, Carum copticum, Phlomis bruguieri, Marrubium parviflorum and Prosopis africana. Rosmarinus officinalis, Punica granatum, Rosa centifolia, Curcuma longa, numerous essential oils (sage, mint, lavender, thyme, hyssop, oregano, lemongrass and others) and other known aromatic plants (including cloves, cinnamon, or Citrus paradisi) had anti-yeast properties. This study aimed to present an up-to-date review of the literature in relation to the latest research and possible potential sources of biologically active plant ingredients for use in preparations, both in prophylaxis and oral hygiene.
-
Key words:
- Without overviews /
- Potential /
- Oral products /
- Plants /
- Essential oils