-
Throughout human history, medicinal plants have been the main source of secondary metabolites utilized in traditional medicine[1]. Medicinal plants offer a sustainable and eco-friendly approach to healthcare[2]. They can be cultivated in diverse climates, including urban areas, and often require fewer resources compared to synthetic drug production. As the global demand for sustainable solutions increases, medicinal plants can provide a viable alternative to conventional pharmaceuticals. Genetic engineering and plant tissue culture have been promising technologies for improving the formation of secondary metabolites in plants for the last decade[3−5]. The secondary metabolites are not vital for the basic functions of growth and development but often contribute to ecological interactions and defense mechanisms. Photoperiod, Light intensity, water availability, temperature, soil composition, and biotic interactions are key environmental variables shaping secondary metabolite synthesis[6,7]. For instance, studies have shown that increased light intensity can regulate the secondary metabolism of plants, such as terpenoids, alkaloids, and flavonoids[8].
Similarly, temperature fluctuations can modulate secondary metabolite synthesis, with certain compounds being more abundantly under specific temperature regimes[9]. Water availability also plays a critical role; drought stress, for example, can induce the synthesize of secondary metabolites involved in osmotic regulation and antioxidant defense[10]. Furthermore, soil composition affects nutrient availability, influencing secondary metabolite biosynthesis[11]. Biotic factors such as herbivores or pathogen attacks can trigger the production of defense-related secondary metabolites as part of a plant's response to stress[12].
Understanding the intricate interplay between environmental cues and secondary metabolite production is essential for optimizing the cultivation conditions of medicinal plants and the sustainable production of bioactive compounds for pharmaceutical and agricultural applications. Additionally, elucidating the molecular mechanisms underlying these responses can facilitate the development of strategies to manipulate secondary metabolite biosynthesis for desired traits in crops and other organisms.
-
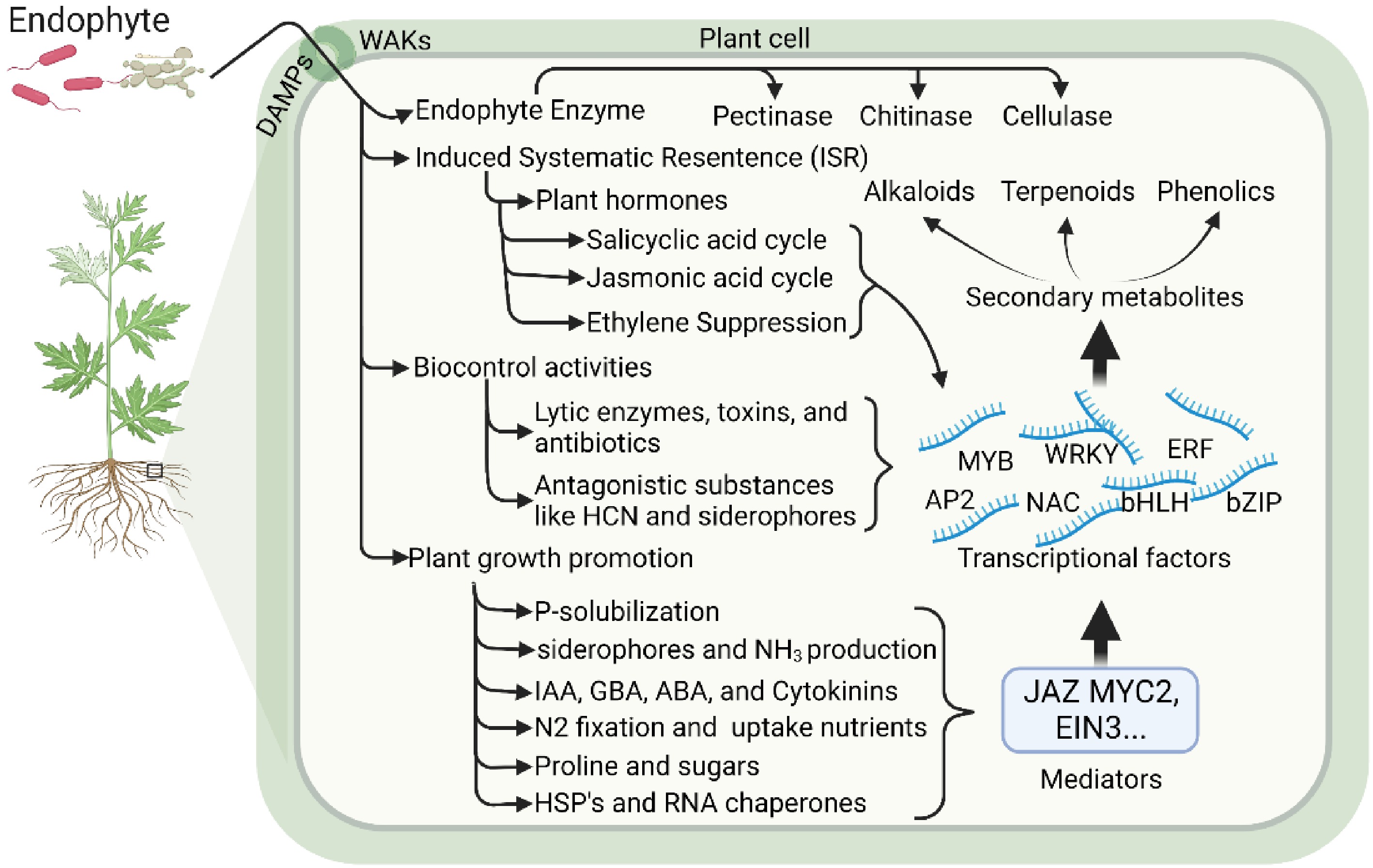
An endophyte is a kind of endosymbiont that dwells inside a plant for at least part of its life cycle without producing visible illness. Endophytes are abundant within the tissues of live plants and play a vital role in these micro-ecosystems. The quality and quantity of crude pharmaceuticals derived from medicinal plants can be significantly impacted by the unique interaction that some co-existing endophytes and their host plants have established over time. This association can also substantially impact the synthesis of metabolic products in plants (Fig. 1)[13]. Nowadays, endophytes are often understood to be microorganisms (mostly bacteria and fungi) that can invade the internal tissues of healthy plants without producing indications of disease. However, when the host plant experiences senescence, it could become pathogenic. Various organisms called endophytes include fungi[14], bacteria[15], and archaea[16]. In addition, they live a large part of their life cycle within the living tissues of plants. Medicinal plants, as shown in Table 1, showed that stimulating factors like endophytes induced the production of metabolites, and it could be a better and more stainable way to use endophytes as green fertilizer for the significant increase of secondary metabolites in plants. Here, some common endophytes in medicinal plants are reviewed and the advantages, disadvantages, and applications are covered.
Table 1. Endophytes increase the accumulation of secondary metabolites in medicinal plants.
Metabolites Endophytes Accumulation change Plants Ref. Ginsenoside Bacillus altitudinis KX230132.1 Increased Panax ginseng [17] Ginsenoside Rg3 Burkholderia sp. GE 17-7 Increased Panax ginseng [18] Camptothecin Kytococcus schroeteri Increased Ephedra foliata [19] Berberine Microbacterium and Burkholderia Increased Coptis teeta [20] Guignarderemophilanes A-E Guignardia mangiferae Increased Gelsemium elegans [21] Grignard dene A Guignardia mangiferae Increased Gelsemium elegans [21] Grignard lactone A Naphthomycins A, D, E, L, K, O-Q Streptomyces sp. Increased Maytenus hookeri [22] Cedarmycin B Daunorubicin Paenibacillus polymyxa Increased Ephedra foliata [19] Hookerolide Streptomyces sp. Increased Maytenus hookeri [22] Benzoic acid phthalic acid Bacillus atrophaeus and Bacillus mojavensis Increased Glycyrrhiza uralensis [23] 5,7-Dimethoxy-4-phenylcoumarin Streptomyces aureofaciens Increased Zingiber officinale [24] Bis (2-ethylhexyl) phthalate Bacillus subtilis Increased Thymus vulgaris [25] 1,3-dimethyl-, p-xylene dibutyl phthalate Tetracosane 1- –Heptacosano Nocardiopsis sp. Increased Zingiber officinale [26] Sesquiterpenoids Pseudomonas fluorescens Increased Atractylodes macrocephala [27] Essential oil Pseudomonas fluorescens Increased Atractylodes lancea [28] Ligustrazine Bacillus subtilis Increased Ligusticum chuanxiong [29] Morphine Marmoricola sp. and Acinetobacter sp. Increased Papaver somniferum L. [30] Forskolin Fusarium redolens, Phialemoniopsis cornearis, and Macrophomina pseudophaseolina Increased Coleus forskohlii [31] Endophytic fungi
-
Endophytic fungi are diverse, polyphyletic groups of microorganisms that can live in various healthy tissues of living plants, both above and below ground, without causing any symptoms. These tissues could consist of roots, leaves, and stems. Many different types of plants contain endophytic fungi[32]. It is believed that about one million different species of endophytic fungi may be found in nature. Endophytic fungus, not their host plants, are required to produce bioactive compounds that are specific to them. These substances are unique to endophytic fungus.
Furthermore, these substances can promote the synthesis of numerous novels and well-known physiologically active secondary metabolites. Humans may be able to use and employ these metabolites as important sources of medicinal medicines. Alkaloids, diterpenes, flavonoids, and iso-flavonoids are only a few of the bioactive substances that endophytic fungi produce to increase the resistance of their host plant against biotic and abiotic stresses. Endophytic fungi would be the producers of these substances. Some endophytic fungi can potentially encourage the accumulation of secondary metabolites that plants initially created. These secondary metabolites may include essential therapeutic components or medicines. According to the analyzed references, these metabolites may have been created by the host plants or endophytic fungi. Huperzia serrata, found in the tropical zone, may yield compounds containing Huperzine-A that are thought to be activated by the endophytic fungus Acremonium and Shiraia species[33].
It is well known that endophytic fungal colonization is not a chance phenomenon. Because of chemotaxis, a process that the host plants use to produce specific chemicals. Simultaneously, some secondary metabolites, including saponin and essential oils from medicinal plants, are produced through protracted co-evolution as a defense mechanism against the pathogens, most likely including endophytic fungi. The secondary metabolites consequently became barriers that stopped endophytic fungus from effectively colonizing the plant. To get beyond the defense mechanisms of the remaining host plants, endophytic fungi must develop the proper detoxifying enzymes, such as cellulases, lactases, xylanases, and proteases, to break down these secondary chemicals. It makes this obstacle surmountable for the endophytic fungus[34]. Endophytic fungi go into a dormant, or quiescent, stage when they have established themselves within the tissues of a host plant. This state can persist for the whole of the host plant's life (neutralism), or it can persist for a considerable amount of time (mutualism or antagonism) until either the host's ontogenetic state changes in a way that benefits the fungi or the environmental conditions become more favorable for endophytic fungi. It was shown that when cultured in vitro under axenic conditions, an endophytic fungus known as Taxomyces andreanae could produce taxol. A Taxus brevifolia tree's bark was used to isolate the fungus. Some endophytic fungi can create many phytochemicals, secondary metabolites originating in plants. One such toxin is podophyllotoxin, camptothecin and structural analogs, hypericin and emodin, deoxypodophyllotoxin, and azadirachtin. Many endophytic fungi that had colonized various host plants, such as Metarhizium anisopliae, and Tubercularia sp. strain TF5 were also found to produce taxol. Our research shows that certain endophytic fungi are the production of secondary metabolites in host plants. This enhancement affects both the quantity and quality of medicinal compounds. This insight explains why traditional Chinese medicine often emphasizes using specific medicinal plants from particular regions or habitats where desired chemical compounds are likely to be abundant. The key advantage lies in leveraging the abilities of endophytic fungi to promote the accumulation of secondary metabolites originally produced by plants. By harnessing this capability, we can enhance the synthesis and accumulation of bioactive compounds in medicinal plants, leading to higher-quality pharmaceuticals. Achieving this involves introducing specific endophytic fungi to the plants. This strategy, once the interaction between endophytic fungi and their host plants is well-understood, holds great promise for revolutionizing natural medicine production, offering a highly effective approach to enhancing medicinal qualities[35].
Endophytic bacteria
-
Endophyte species of archaea and bacteria make up prokaryotic endophytes, and it is currently thought that prokaryotic endophytes may be classified into anywhere from two to 21 different phyla[36,37]. Surprisingly, most prokaryotic endophytes are located inside one of four distinct phyla of bacteria: proteobacteria, bacteroidetes, firmicutes, and actinobacteria. By lowering ethylene concentration, bacterial endophytes help plants become more resilient to abiotic stress and alleviate it. When there is stress and a spike in ethylene concentration, it is crucial. Under stressful conditions, ethylene reduction in plant organs has been linked to the bacterial enzyme ACC (1-aminocyclopropane-1-carboxylate) deaminase. ACC deaminase converts ACC, which the plant generates and is an ethylene precursor, into 2-oxobutanoate and ammonia, inhibiting the ethylene signaling pathway[38].
Additionally, cleaved chemicals provide nutrients to endophytic bacteria, and ACC deaminase promotes bacterial colonization of plants to maximize bacterial development. Further investigation is necessary to ascertain the limitations of ACC deaminase in plant-endophyte relationships. This is due to the lack of inconsistent studies about its ability to reduce the levels of ethylene and aid in accumulating secondary metabolites in medicinal plants under abiotic stress. Recent studies have found that bacterial endophytes benefit secondary metabolites. Numerous articles provided evidence for this[39]. For example, Mishra et al. reported that the two most potent bacterial endophytes, Bacillus amyloliquefaciens (MPE20) and Pseudomonas fluorescens (MPE115), were able to individually and in combination modulate the withanolide biosynthetic pathway and tolerance against Alternaria alternata in Withania somnifera. It's interesting to note that plants treated with the microbial consortium under A. alternata stress had higher expression levels of genes involved in the withanolide biosynthesis pathway (3-hydroxy-3-methylglutaryl co-enzyme A reductase, 1-deoxy-D-xylulose-5-phosphate reductase, farnesyl di-phosphate synthase, squalene synthase, cytochrome p450, sterol desaturase, sterol-7 reductase, and sterol glycosyl transferases)[40].
According to the findings of some studies, the creation of secondary metabolites that have pharmacological action may be boosted by pathogenic bacteria or other naturally occurring elicitors[41]. Plant-growth-promoting rhizobacteria (PGPR) are bacteria that colonize the rhizospheres of plants. These bacteria promote plant development via various processes under normal settings and in situations detrimental to plant growth. Through a process that botanists call induced systemic resistance, PGPR can encourage the manufacture of secondary metabolites in plants. The PGPR proteins are efficient elicitors of the main enzymes engaged in secondary metabolites' biosynthesis pathways. These pathways are associated with the defensive responses of plants against pathogenic pathogens[42].
Pathogens
-
Plants do not possess innate immune systems like animals, yet plants can resist many diseases via secondary metabolites (Fig. 2). Some plant secondary metabolites (PSMs), known as phytoalexins, show antimicrobial actions. These PSMs serve as a line of defense for plants against various diseases[43]. During the process of plants defending themselves against pathogens, the need for large concentrations of PSMs causes their production to be triggered fast. In lupin (Lupinus angustifolius), secondary metabolites such as phenolics exhibit variations in their amounts as a defense mechanism against fungal infection caused by Colletotrichum lupini. Effector-triggered immunity and basal immunity are the two processes responsible for activating a plant's innate immune system in response to a pathogen assault. Plants develop innate immune systems as a defense mechanism against the invasion of pathogens. In the system of basal immunity, microbe-associated molecular patterns, also known as MAMPs, are recognized by pattern recognition receptors (PRR) located on the cell surface.
Conversely, the impact is brought about by effectors that stimulate immunity, which include low molecular weight natural products, phytotoxins, and microbial proteins or peptides[44]. Because they interpret these effectors as an infection signal, plants respond by turning on several metabolic pathways. The activation of these metabolic pathways results in the production of a variety of secondary metabolites. When a pathogen attacks, PSM concentrations rise to protect the plant; however, once the threat has passed, they diminish[45]. Consequently, raising the secondary metabolite content may strengthen the plant's resistance to diseases and infections. Furthermore, employing specific endophytes as green fertilizers may encourage the production of secondary metabolites and lessen the likelihood of plant illnesses.
Herbivores
-
Plants create PSMs, or secondary metabolites, to defend themselves against herbivores. These PSMs regulate the signaling pathways involved in plant defense and support defensive functions. Herbivore injuries set off a complex series of processes that ultimately lead to the synthesis and accumulation of PSMs. PSMs consist of amines, glucosinolates, quinones, phenolics, peptides, polyacetylenes, terpenes, and cyanogenic glucosides (Fig. 2)[12,41]. PSMs do not participate in the fundamental processes of a plant's existence, but they are essential to its ability to adapt to its environment and defend itself against herbivores. PSMs are generated through a series of metabolites and intermediates involved in plants' defensive mechanisms. Herbivores may interfere with the production of defense chemicals at multiple levels, including the last stages of the biosynthesis process. Therefore, integrating them directly into regulatory feedback loops could help plants monitor and control their defense mechanisms more effectively[46].
While some insects serve as pollinators, others consume just plant matter. We may classify herbivores into polyphagous, oligophagous, and monophagous. Polyphagous herbivores eat a wide variety of plant species, whereas oligophagous herbivores have a preference for just a few plant types. Oligophagous herbivores have a narrower range of plant preferences than monophagous herbivores. The PSMs in their food plants presented a challenge for the herbivorous insects, and they still do. Many systems have been developed to allow them to tolerate or detoxify PSMs. Generally, generalists have highly active enzymes that either deactivate harmful PSMs (via the CYP pathway) or swiftly remove them (through the ABC transporter pathway). Another tactic is to consume not just one plant but also samples from different species (with low PSM concentrations), diluting the impact of any poisonous substance consumed. Herbivores often have a rapid digestive process, which allows them to absorb nutrients more rapidly than any poisons, which are then rapidly expelled via the feces. Some herbivores get the assistance they need for detoxification from symbiotic gut bacteria. These microorganisms may often break down or deactivate harmful substances. If there are enough of them, they may cause significant damage to the plants that serve as their hosts. This is something that may be seen in regions where there are a lot of Senecio jacobaea plants (which produce PAs). Senecio populations have a high risk of experiencing severe declines if the PA-specialized moth Tyria jacobaeae is found in the same region. However, even under these dire circumstances, Tyria cannot eradicate its host plants[47].
Herbivores can adjust to secondary metabolites by sequestering or detoxifying toxic substances and by upregulating or downregulating genes related to sensory information processing. PSMs regulate the interactions of herbivores, pollinators, natural enemies, and hosts in a multitrophic environment. Monophagous insects who enjoy their deadly host plants exhibit certain specializations. These insects are known to exist. These specialists frequently actively sequester the toxic PSMs generated by the host plant in addition to being able to withstand them. As a result, these experts can stockpile significant quantities of poisonous PSMs and employ them as part of their defensive mechanisms against enemies. These researchers have examined a variety of hazardous alkaloids, including quinolizidines, pyrrolizidines, aconitine, and cyanogenic glucosides, as well as poisonous cardiac glycosides, aristolochic acids, and cyanogenic glucosides[48]. These professionals often display colors that serve as warnings. That is to say. They broadcast their potential toxicity to any possible predator under their aposematic nature.
Since we cannot observe how these specialists circumvent the inherent toxicity of PSMs, we typically do not know how they manage it. In the case of certain insects that can sequester cardiac glycosides, it is feasible to show that point mutations have changed the binding site of their molecular target, the Na, K-ATPase, to prevent cardiac glycosides from attaching to it. Cardiac glycosides would be unable to sequester them as a result. As a result, heart glycoside concentrations that would be fatal to every other species, polyphagous or oligophagous, cannot be tolerated by monarch butterflies. In most other instances, we do not have clear information about how an act of insensitivity was carried out. It has been observed that herbivorous insects that eat by piercing or sucking plants cause ethylene release and JA buildup in the plants they consume[49].
-
Eliciting the production of phytochemicals in plants by applying chemical or physical stimuli is called abiotic elicitation. Experiments have been conducted using these elicitors alone and in combinations, in hydroponics and sprays, during various phases of plant development and even after harvesting[50,51] (Fig. 3).
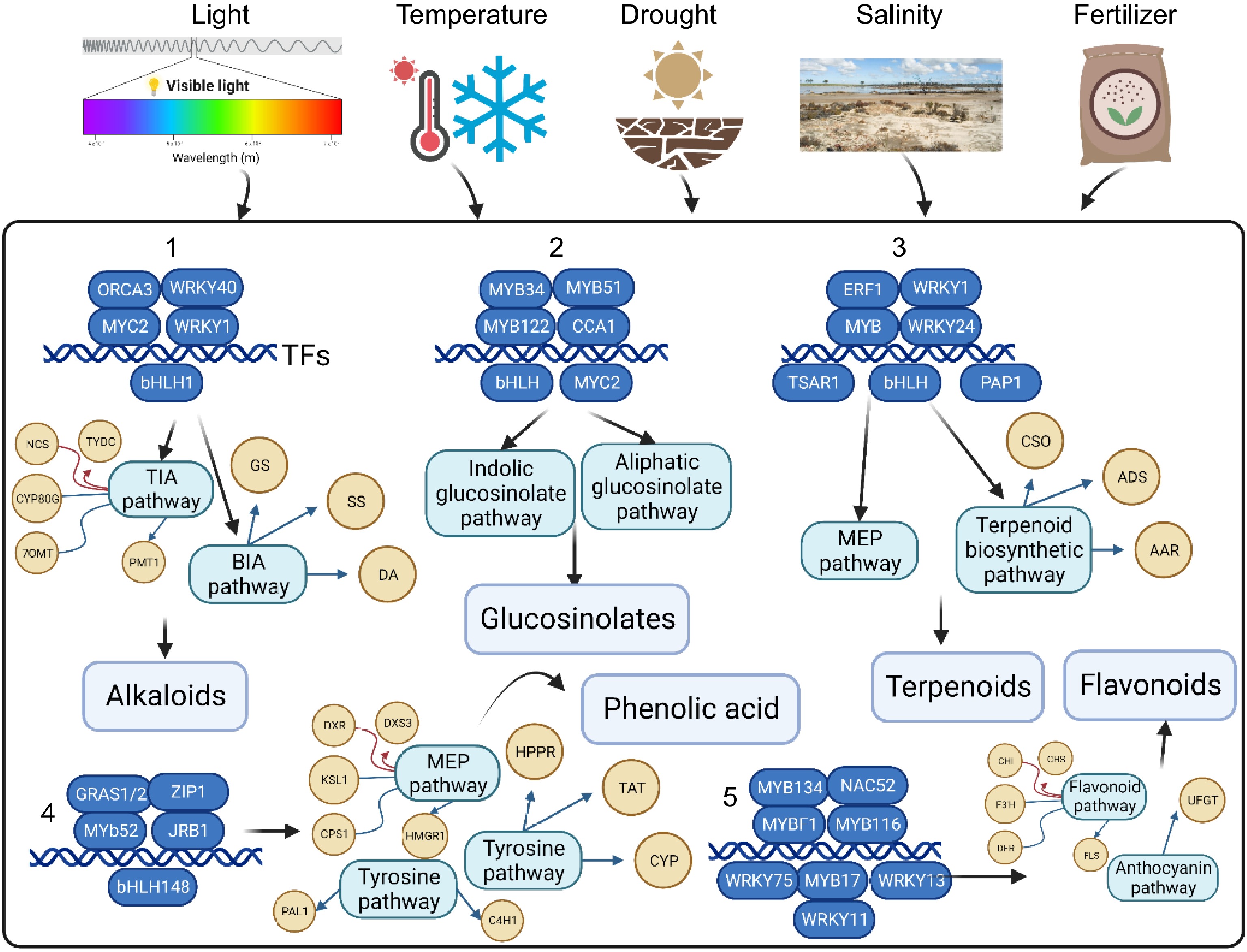
Figure 3.
Plant synthetic responses to the abiotic factors 1-5; majore group of secondary metabolites biosynthesis pathways.
Light
-
The physical aspect of light influences many different plant species' metabolite synthesis. Light exposure in Zingiber officinale callus culture can stimulate the production of secondary metabolites, including zingiberene and gingerol. VIB The production of secondary metabolites is also enhanced by ultraviolet (UV) radiation. An increase in UV-B exposure causes plants in the field to produce more essential oils and phenolic content, whereas a decrease in toxic beta-asarone is observed. In addition to being essential for photosynthesis and plant growth, light controls the quantity and caliber of secondary metabolites (PSMs) plants produce. Sunlight exposure promotes coumarin accumulation in M. glomerata. Plants with longer light periods have much higher levels of coumarin in their leaves and stems, while shorter light periods result in much lower levels of coumarin[52]. The length of the photoperiod had a considerable impact on the amount of coumarin found in the stems and leaves. Therefore, the amount of accumulated PSMs is significantly influenced by both the light intensity and the photoperiod.
Based on the varied expression patterns of terpene synthase genes seen throughout plant development and in response to both biotic and abiotic environmental stimuli, terpenoid metabolites have been linked to some ecological and physiological activities[53]. These links have been made possible by the observation that these genes are expressed differently. For instance, the ent-copalyl diphosphate (CPS1) and kaurene synthase (KS1) genes are activated in rice leaves when the plant is treated with an elicitor or ultraviolet light. Gibberellin is produced in plants via the action of these genes that control the process. Quantitative and qualitative investigations demonstrate that light exposure may impact the concentration of bioactive triterpenes in grasses like Centella asiatica, which has high levels of secondary metabolites. They had the greatest acetic acid concentration but the lowest asiaticoside concentration when grown under 70% shade[54]. The solid seasonal variations in the transcript levels of mRNA from 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXRs), the first committed enzyme in the 2-methyl-D-erythritol 4-phosphate (MEP) terpenoid biosynthetic pathway, isoprene synthase (ISPS), have been reported by Mayrhofer et al. These changes depended on the developmental stage and were strongly correlated with temperature and light levels. The process of terpenoid manufacture and the expression of genes encoding terpene synthase are generally significantly influenced by light intensity[55].
Temperature
-
An essential environmental component that affects enzyme activity and metabolic pathways, influencing the synthesis of secondary metabolites, is temperature[56,57]. Research has demonstrated that temperature variations can impact the diversity, concentration, and makeup of secondary metabolites produced by marine species, microorganisms, and plants[58,59]. A rise in temperature speeds up the aging process of Panax quinquefolius's leaves and the buildup of secondary metabolites in the roots. It should be highlighted that thermal stress dramatically slows down plant development and causes senescence, even though it has also been shown to promote or decrease the synthesis of secondary metabolites in plants. When the temperature of the plants of the Panax quinquefolius genus is raised, the amount of root ginsenosides they contain increases. Temperate plants produce certain cryoprotectant molecules during the overwintering season. These molecules include soluble sugars (trehalose, stachyose, saccharose, and raffinose), sugar alcohols (sorbitol, ribitol, and inositol), low molecular weight nitrogenous substances (proline and glycine betaine, protective antifreeze proteins, and others). Plants exhibit enhanced resistance to low temperatures due to lignification and suberin deposition in their cell walls[17].
The term 'cold stress' refers to temperatures below 20 °C, which have a deleterious effect on the growth and development of plants and drastically reduce their production (Table 2). It hinders plants from expressing their full genetic potential, which directly inhibits metabolic responses and indirectly inhibits water intake and cellular dehydration. This prevents plants from reaching their full genetic potential. The levels of chlorophyll a and total chlorophyll are reduced due to cold stress, whereas the quantity of apoplastic and total soluble protein in the leaf is increased[60]. According to the findings of the research, cold stress has a substantial influence on the variance of the number of PSMs present.
Table 2. List of metabolites that are affected by temperature variation in different medicinal plants.
Metabolites Stress Affects Plants Ref. Flavonol, quercetin, kaempferol, and isorhamnetin Cold stress Increased Brassica oleracea L., var. sabellica [61] Terpenoids Cold stress Increased Polygonum minus [62] Carotenoid Cold stress Increased Sugarcane [62] Alkaloids Cold stress Increased Arabidopsis [17] Total phenolic Cold stress Decreased Eleutherococcus senticosus
(Rupr. & Maxim.) Maxim.[63] Flavanol Cold stress Decreased Polygonum minus Huds. [62] Artemisinin Cold stress Increased Artemisia sp. [64] Autrescine Heat stress Increased Oryza sativa L. [65] Tryptophan, tyrosine, and phenylalanine Heat stress Decreased Ricinus communis [66] Flavonoids Heat stress Decreased Vigna radiata (L.) R. Wilczek [67] Hypericin, pseudohypericin, and hyperforin Heat stress Increased St. John's wort [17] Alkaloid ricine Heat stress Increased Caster bean [66] Drought
-
Drought is one of the most important environmental stresses that can alter plants' physiological and biochemical features and increase the concentration of secondary metabolites in plant tissues. Drought is an abiotic physical elicitor[68−70]. Drought is classified as a physical elicitor and is an example of an abiotic physical elicitor. There is a wide range of variability in the ability of plant species to withstand drought. The circumstances that lead to drought are characterized by low levels of available water and are coupled with elevated temperatures and intense levels of solar radiation[71−73]. In Glechoma longituba, a lack of water results in a reduction in the total flavonoid content, and a water treatment of field capacity between 80 and 85% effective is ideal for achieving the highest possible total flavonoid content[74]. According to research published in 2015 by Gupta and colleagues, drought stresses significantly raise the amount of rebaudioside A in the suspension culture of Stevia rebaudiana more so than stevioside[75].
Plant growth and photosynthesis are altered due to drought, which also changes the biochemical features of the plant[73]. Lack of water increases the amount of the secondary metabolite artemisinin in Artemisia, whereas it increases the amount of betulinic acid, quercetin, and rutin in Hypericum brasiliense. The concentration of secondary metabolites like hypericin and pseudohypericin, as well as the photosynthetic rate of the leaves, are both lowered by water stress. Conversely, in situations with insufficient water, the concentration of the major secondary metabolite hyperforin increases[73]. When exposed to water stress, herbs like sweet basil (Ocimum basilicum) and American basil (Ocimum americanum) lose vegetative growth, total carbohydrates, essential oil, proline, nitrogen, phosphorus, potassium, and protein content. Stress from water leads to an increase in the percentage of essential oils and a decrease in nitrogen, phosphate, potassium, and protein. For the production of herbs and essential oils, the field's water capacity must be at least 75% to maximize the potential for both species[76]. These results demonstrate that drought stress could fluctuate the biosynthesis of PSMs.
Salinity
-
Salt in the environment encourages the development of many secondary metabolites in plants, such as phenols, terpenes, and alkaloids. Some plant species have a higher anthocyanin concentration in response to salt stress, whereas salt-sensitive species have a lower anthocyanin content. A correlation exists between an increase in salinity and an increase in the polyphenol content of certain plant tissues. In Chamomilla (Matricaria chamomilla), the essential oil content, number of branches per plant, number of flowers per plant, peduncle length, and other characteristics are all negatively affected by salinity and dryness[77]. Salt stress causes a rise in the content of the alkaloids reserpine and vincristine, which are both classified as plant stress metabolites (PSMs) in Rauvolfia tetraphylla and C. roseus, respectively. The alkaloid ricinine present in Ricinus communis was higher in the plant's shoots but lower in its roots. The enhancement of phenols in Mentha pulegium[78] and Nigella sativa[79] has been noticed under salt stress. Isabgol (Plantago ovata) was subjected to salt stress, which resulted in a rise in the plant's proline, flavonoids, and saponins content[80]. Plants' accumulation of various ions may also change the concentration of primary and secondary metabolites. This can happen in both cases. According to the findings of these investigations, salinity is a factor that positively influences the accumulation of PSMs.
Plant growth regulators
-
Because of their ability to stimulate the gene expression of various photosynthetic pathways, several plant growth regulators have also been used for elicitation[81,82]. Salicylic acid and jasmonic acid are the most often used plant growth regulators as elicitors. These two plant growth regulators are important signals that influence gene production. Abscisic acid is a stress hormone in plants due to its rapid buildup in response to stress. In addition to producing systemic acquired resistance in plants in response to different pathogens, SA is also known to produce secondary metabolites in plants[83]. The JA signaling pathway is necessary for synthesizing several plant secondary metabolites, including terpenoids, flavonoids, alkaloids, and phenylpropanoids. It is acknowledged that this route is a crucial signal for this operation. The cyclopentanone class of compounds known as jasmonates, which includes JA and methyl jasmonates (MeJA), affects a range of plant responses and functions as an excellent elicitor to encourage the synthesis of secondary metabolites in vitro cultures. Jasmonate a and jasmonate b are the two categories into which jasmonates can be divided. They make up a significant subclass of elicitors for a wide variety of metabolic pathways, which is most often seen as the induction of secondary metabolite production in response to environmental challenges experienced by plants. The phytohormones jasmonic acid (JA) and salicylic acid (SA) are responsible for inducing plant defenses, and the linked pathways interact convolutedly at the transcript and protein levels. Following the application of JA and SA, it was shown that both chewing insects (Heliothis virescens) and sucking insects (Myzus persicae) had detrimental impacts on their ability to survive[84].
In plant cell cultures, proteins, carboxylates, and enzymes may all function as biotic elicitors and set off defensive processes. Protein elicitors have been employed to understand how ion channels in plant cell membranes relate to the signal transmission brought on by outside stimuli. In plant cell cultures, glycoproteins trigger the creation of phytoalexins. Plant defense mechanisms include proteins that attach to carbohydrates and function as lectins or agglutinins, protecting plants from various animals that prey on them. It is associated with secondary metabolite production, which plays a part in plant defense mechanisms. Oligogalacturonides (OGAs), molecules that function as elicitors, are produced from the pectic polysaccharides in plant cell walls. Biosynthesis of phytoalexins is prompted in the cotyledons of Glycine max plants by OGAs, whereas Nicotiana tabacum plants' defensive mechanisms are activated. Chitin, a component of fungal cell walls, performs the function of a powerful elicitor signal in some plant-based systems[85].
Different chemicals, such as minerals, heavy metals, fertilizers, pollutants, gaseous toxins (such as elevated CO2 and ozone), insecticides, and so on, may all contribute to developing chemical stress (Table 3). In Cassia angustifolia, the administration of micronutrients causes an increase in primary metabolites, which may generate a corresponding increase in secondary metabolites. The amounts of chlorophyll, protein, and phenol are not the only things affected by FeSO4, ZnSO4, Miczink, and CuSO4. The amount of flavonoid in the St. John's Wort plant (Hypericum perforatum) may be altered by adding nitrogen and phosphorus fertilizers. Nitrogen and phosphorous are essential nutrients that play a significant role in the development and maturation of the plant[86].
Table 3. Elevated CO2 affected the accumulation of secondary metabolites.
Metabolites Chemicals Affects Plants Ref. Total phenolics Elevated CO2 Increased Populus tremula L. [87] Tannins Elevated CO2 Increased Zingiber officinale Roscoe [88−90] Total and individual phenolics and antioxidant Elevated CO2 Decreased Oryza sativa L. [91] Terpene Elevated CO2 Increased Phaseolus lunatus L. and Gossypium hirsutum L. [92,93] Morphine, codeine, papaverine, and noscapine Elevated CO2 Increased Papaver setigerum L. [17] Furthermore, plants use nitrogen as fertilizer as signals to control the expression of specific genes, including those in Arabidopsis and other plant species. In response to the varying amounts of nitrogen available in their surroundings, plants have evolved a variety of responses. Scientists use various methods, including genetics and bioinformatics, to identify the regulatory pathways that plants use in response to different nitrogen concentrations. Plants respond to nitrogen supply by changing gene expression and metabolic, physiological, and developmental changes. Phosphorus influences not just the development of plants but also the secondary metabolites they produce. Phosphorus increases the leaf biomass, total phenolic concentrations, and rosmarinic acid (RA) concentrations in Salvia officinalis (garden sage), but does not influence the quality or quantity of the essential oils produced by the plant. Therefore, phosphorus is crucial in the growth and manufacture of secondary metabolites in plants[17].
Apart from these food factors, plants also require specific atmospheric gases, such as nitrogen, oxygen, and carbon dioxide, to complete their biological activities and create secondary plant metabolites. A rise in CO2 concentration raises the concentration of secondary plant products in Taxus bacatta, H. perforatum, and Echinacea purpure, either directly or indirectly[94]. Plant metabolism is altered by heavy metals, which also impact the synthesis of carbohydrates, proteins, non-protein thiols, pigments used in photosynthetic processes, and sugars. Metals can alter secondary metabolism in certain ways, which could impact the synthesis of physiologically active molecules. The formation of secondary metabolites may be affected by various metal ions, including Ag+, Eu3+, Cd2+, La3+, and oxalate[95]. According to these studies on the chemical impacts on secondary metabolites, the chemicals that plants require for growth and development also influence PSM synthesis, which implies that plant concentrations of PSMs may vary in response to these chemicals. PSMs are necessary for plants' growth and development, so naturally, these substances would have this impact.
Soil fertility
-
Mineral elements, especially mineral nitrogen, affect primary metabolism and the production of secondary metabolites. This has a major impact on the quality of the raw materials that plants generate and their growth and development. The amount of flavonol in the seedling tissues of Arabidopsis and tomato plants was found to have a highly significant inverse connection with the availability of nitrogen and phosphate. Furthermore, it was discovered that in both species, the concentrations of quercetin, kaempferol, and isorhamnetin rose in response to either phosphate or nitrogen stress. Labisia pumila Benth's capacity to produce total phenolics and flavonoids was noticeably influenced by the nitrogen levels present in the environment. In addition, the increase in fertilizing was associated with a modest rise in antioxidant activities[96].
Furthermore, different genes encoding flavonoid biosynthesis enzymes may be affected differently by N-deficiency stress. For example, mRNA levels for chalcone synthase (CHS) and dihydroflavonol-4-reductase (DFR) increased in response to N stress, whereas mRNA levels for a chalcone isomerase homologous band (CHI) decreased. Insufficient amounts of specific nutrients may impact the concentration of alkaloids in the soil. However, the content of alkaloids was greatly enhanced by 9%–17% by nitrogenous fertilizers; the largest increase was observed upon application of NH4NO3. Under conditions of acute potassium deficit, the alkaloid contents in the seeds of sweet cultivars of lupins increased drastically by 205%. When magnesium and nitrogen were applied to seeds, the number of alkaloids present was reduced. When the rate of fertilization was raised in Datura innoxia, the number of total alkaloids that were produced also rose. The metabolic route that produces amino acids, nucleic acids, lipids, and enzymes, all of which immediately impact cell division, has nitrogen as one of its essential components. When Pseudotsuga menziesii was cultivated with a high concentration of ammonium nitrate fertilizer, the monoterpenoid concentrations showed an ascending pattern. When 200 milligrams of N fertilizer were applied to Thuja plicata during the active growth season, the amount of monoterpenoid increased. This was in contrast to the 400 mg high fertilizing amount. However, the higher amount of fertilizer applied resulted in a higher monoterpenoid content[97].
-
Plant secondary metabolites represent natural compounds plants synthesize, pivotal for their adaptation and survival mechanisms. These compounds, categorized by their chemical structures and biosynthetic pathways, intrigue humans due to their potential in pharmaceuticals, nutraceuticals, and various industrial applications. Both biotic and abiotic factors influence the synthesis of these metabolites in medicinal plants. Biotic factors encompass interactions with organisms like herbivores, pathogens, and symbionts. Herbivores can trigger secondary metabolite production as a defense mechanism, elevating their concentration. Pathogens also stimulate their production, often leading to compounds with antimicrobial properties. On the other hand, abiotic factors, including temperature, light, soil nutrients, and water availability, can significantly impact secondary metabolite biosynthesis. Understanding these factors is crucial for harnessing the full potential of plant secondary metabolites.
-
Plant tissue culture techniques, such as cell suspension cultures, organ cultures, and hairy root cultures can produce secondary metabolites in a controlled environment. By optimizing culture conditions, such as nutrient composition, hormone levels, and elicitation strategies, researchers can stimulate the production of secondary metabolites, offering the advantage of year-round output independent of seasonal variations. Applying stress factors, such as UV radiation, temperature changes, or exposure to specific chemicals can trigger the plant's defense mechanisms and stimulate secondary metabolite production through a process known as elicitation. Understanding the signaling pathways involved in stress responses enable the development of innovative approaches to induce secondary metabolite production in plants.
Metabolic engineering involves manipulating the metabolic pathways of plants to redirect the flow of precursors toward the synthesis of desired secondary metabolites. This can be achieved through gene editing, pathway engineering, or modulation of enzyme activities, enabling the production of specific secondary metabolites or the creation of novel compounds with improved therapeutic properties. Biotechnological methods, such as using plant cell cultures, bioreactors, and genetic transformation can scale up secondary metabolite production. These approaches provide controlled and efficient systems for the large-scale production of bioactive compounds and can be combined with other techniques, like metabolic engineering, to enhance productivity further.
Employing precision agriculture techniques coupled with remote sensing technologies can enable real-time monitoring of plant health and stress levels, facilitating timely interventions to mitigate adverse effects on biosynthesis. Additionally, harnessing microbial biofertilizers and elicitors can modulate plant metabolism, enhancing the synthesis of bioactive compounds. Furthermore, integrating traditional knowledge with modern biotechnological approaches holds promise for the sustainable cultivation of medicinal plants. Utilizing indigenous cultivation practices that promote plant resilience to environmental stressors and genetic enhancement through marker-assisted breeding and genetic engineering can further augment the yield and quality of medicinal crops. Continued research, advancements in biotechnology, and the integration of multidisciplinary approaches will contribute to unlocking the full potential of these valuable bioactive compounds.
-
The authors confirm contribution to the paper as follows: conceptualization: Alami MM, Yang G, Wang X; methodology: Alami MM, Guo S; resources: Mei Z; management: Mei Z, Yang G, Wang X; funding acquisition: Yang G, Wang X; writing – original draft preparation: Alami MM; writing – review & editing: Guo S, Mei Z, Yang G, Wang X. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
We thank the Chinese Scholarship Council (CSC) for providing scholarships for our Ph.D. studies. This study is supported by the enterprise entrusted project: Multiomics analysis and evaluation of core germplasm resources of Tinospora sagittata. and National Natural Science Foundation of China (81872948).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Alami MM, Guo S, Mei Z, Yang G, Wang X. 2024. Environmental factors on secondary metabolism in medicinal plants: exploring accelerating factors. Medicinal Plant Biology 3: e016 doi: 10.48130/mpb-0024-0016
Environmental factors on secondary metabolism in medicinal plants: exploring accelerating factors
- Received: 30 November 2023
- Revised: 23 May 2024
- Accepted: 11 June 2024
- Published online: 26 August 2024
Abstract: Medicinal plants are vital in synthesizing crucial substrates, fortifying stress resilience, and serving clinical and industrial domains. The optimization of pharmacological potential necessitates a nuanced understanding of the factors governing the synthesis of secondary metabolites sourced from plants. Cultivation success hinges upon many factors dictating the production of these vital compounds. Biotic factors, encompassing pathogens and herbivores, alongside abiotic factors such as light exposure, altitude, temperature variations, irrigation patterns, soil fertility, drought susceptibility, and salinity levels, collectively orchestrate medicinal plants' growth, development, and metabolic pathways. This comprehensive review delves into the intricate interplay of factors influencing the formation of secondary metabolites, exploring the roles of endophytes, pathogens, light availability, temperature fluctuations, drought stress, pollution impacts, and plant growth regulators. Grasping the dynamics of these factors is imperative for devising strategic interventions to enhance secondary metabolite production, thereby ensuring the sustainable and efficient cultivation of medicinal plants.
-
Key words:
- Secondary metabolism /
- Biotic factor /
- Abiotic factor /
- Medicinal plants /
- Plant growth regulator