-
Amaranth, a green leafy vegetable with high edible value, is rich in flavonoids, carotenoids, betalains, and other secondary metabolites[1−4]. Aromatic amino acids are precursors of secondary metabolites such as flavonoids, carotenoids, and alkaloids[5].
Flavonoid compounds are important secondary metabolites produced by plants[6]. They are widely found in seeds, flowers, leaves, and fruits of plants, and most of them accumulate in the vacuoles of plant cells[7,8]. Flavonoids have a variety of biological functions. They can not only affect the transport of plant hormones[9,10], but also regulate the level of reactive oxygen species (ROS) in plants[11−13]. Phenylalanine ammonia-lyase (PAL) is the first enzyme in the phenylpropane metabolic pathway and a key enzyme in the flavonoid metabolic pathway[14,15]. Chalcone synthase (CHS) is the first key enzyme in the flavonoid synthesis pathway, which leads the phenylpropane metabolic pathway to the flavonoid synthesis pathway[16−18]. Chalcone isomerase (CHI) is an important rate-limiting enzyme in the synthesis pathway of flavonoid compounds, which catalyzes the chalcone cyclization[19,20]. Flavanone 3-carboxylase (F3H) catalyzes the synthesis of flavonols and substrates for anthocyanin synthesis[21−23].
Carotenoids are a group of yellow, red, or orange-red polyene substances. There are a wide variety of carotenoids, about 850 kinds[24], and they are widely found in the chloroplast and chromoplast membranes in plants. Carotenoids could participate in photosynthesis in plant cells and reduce photooxidation in precursor cells[24,25]. Carotenoids participated in the synthesis of plant hormones[26,27]. In the metabolic pathway of carotenoids, the transcription level of related enzymes affects the synthesis of carotenoids, geranylgeranyl diphosphate (GGPP) is a direct precursor in carotenoid biosynthesis pathway[28,29]. Two GGPPs are condensed into colorless phytoene under the catalysis of phytoene synthase (PSY)[30], which is the rate-limiting enzyme in the carotenoid synthesis pathway[31−33]. Phytoene is converted to red phytoene by the co-catalysis of phytoene dehydrogenase (PDS) and carotene dehydrogenase (ZDS)[34−36]. The content of phytoene was changed in maize by the PDS gene mutation[37], and the content of carotenoids was changed in Arabidopsis because of the ZDS gene mutation[38].
Plant tissue culture is an important part of biotechnology and has become one of the most effective methods for the production of secondary metabolites[39,40]. During plant tissue culture, callus growth, and accumulation of secondary metabolites are affected by many factors, such as plant growth regulators, exogenous additives, light conditions, and so on[41−43]. Amino acids, a sort of exogenous additive, are important factors affecting callus biomass, and different amino acids have different effects on the accumulation of secondary metabolites[44,45]. The addition of amino acids could increase the accumulation of alkaloids in the suspension callus of motherwort (Leonurus heterophyllus Sweet)[46]. Low concentrations of L-proline increased biomass and alkaloid accumulation in callus of Hydrocotyle bonariensis, while high concentrations inhibited it[47].
Our previous study showed that flavonoids could be produced using amaranth callus[48]. However, the effects of aromatic amino acids on the callus growth, the accumulation of secondary metabolites, and the expression of related genes in amaranth are still unclear. Therefore, based on the previous research, an amaranth callus was used as the material to analyze the effects of aromatic amino acids on the growth of amaranth callus and the synthesis of secondary metabolites, suitable conditions were screened out for the production of flavonoids and carotenoids from amaranth callus, and the effect of exogenous amino acids on the gene expression of flavonoids and carotenoids were determined. The results provide a scientific basis and method for callus culture and flavonoid production by the callus of amaranth.
-
The callus was induced by 'Suxian No.1' as material, which were provided by Suzhou Academy of Agricultural Sciences (Suzhou, China).
Experimental method
Treatment of amaranth callus with aromatic amino acids
-
Amaranth callus was inoculated into a basic medium (MS + 0.5 mg/L 2,4-D + 6.0 mg/L 6-BA + 30 g/L sucrose + 7 g/L agar)[48], supplemented with different concentrations of tyrosine (0, 2.0, 4.0, 6.0, and 8.0 mg/L), phenylalanine (0, 1.0, 2.0, 3.0, and 4.0 mg/L), and tryptophan (0, 0.5, 1.0, 1.5, and 2.0 mg/L). Each concentration was inoculated into 30 bottles, and three small pieces of amaranth callus (each piece was about 0.2 g fresh weight) were in each bottle. Three biological repeats were performed for each treatment. After 35 d of treatment, the growth of amaranth callus was observed, and the fresh weight and dry weight of callus were counted and sampled.
Proliferation coefficient = Callus biomass after proliferation (g/bottle) / Initial callus biomass (g/bottle)
Determination of flavonoid and carotenoid content in amaranth callus
-
The flavonoid content in Amaranthus tricolor was determined according to the flavonoid extraction and determination protocol (Comin Biotechnology Co., Ltd., Suzhou, China). 10 mL 60% (v/v) ethanol solution was added into a conical flask with 20 mg dried powder of amaranth callus. Flavonoid extraction was performed with shaking at 60 °C for 2 h, followed by centrifugation at 10,000 rpm at 25 °C for 10 min. The supernatant, containing flavonoid, was detected at a wavelength of 510 nm in a ultraviolet-visible spectrum spectrophotometer (UV-900, Shanghai Yuan Analysis Instrument Co., Ltd, Shanghai, China). For quantitation, rutin was used as an internal standard for calibration.
Standard curve: y = 5.02x + 0.0007,R2 = 0.9996
Total flavonoid content (mg·g−1 DW) = (△A−0.0007) / 5.02 / (w/v)
Carotenoid content was determined by ultraviolet-visible spectrum spectrophotometer. 2 ml of extraction solution of acetone : petroleum ether (1:1, v/v) was added into a tube with 20 mg dried powder of amaranth callus. And then the mixture was placed on a 200 rpm shaker to extract carotenoid for 8 h under dark conditions. Subsequently, the supernatant was collected by centrifuging at 10,000 rpm for 5 min at room temperature. Finally, the absorption peak of carotenoid at 445 nm was determined by a ultraviolet-visible spectrum spectrophotometer (UV-900, Shanghai Yuan Analysis Instrument Co., Ltd., Shanghai, China).
Effects of aromatic amino acids on the expression of genes involved in carotenoid and flavonoid metabolic pathways in amaranth
-
Total RNA was extracted was from all samples using a MolPure Plant Plus RNA Kit (Yeasen, China) according to the manufacturer's instructions. First-strand cDNA was then synthesized from 1 mg of total RNA using Recombinant M-MLV reverse transcriptase (TransGen Biotech, Beijing, China). Quantitative real time-PCR (qRT-PCR) was performed in optical 96-well plates using the Roche LightCycler 480II instrument (Roche, Sweden). The reactions were carried out in a 20 μL volume containing 10 μL of Hieff qPCR SYBR Green PCR Master Mix (Yeasen Biotechnology, China), 0.5 μL of gene-specific primers, 2 μL of diluted cDNA, and 7.0 μL of ddH2O. The PCR conditions were as follows: 30 s at 95 °C, 40 cycles of 10 s at 95 °C and 12 s at 59 °C, followed by 12 s at 72 °C. Three biological repeats were performed for each material. EF1α[49] was used as the reference gene. The 2−ΔΔCᴛ method was used for quantitative analyses of gene expression. The primers used for qRT-PCR are listed in Supplemental Table S1.
Data analysis
-
Data are presented as mean ± standard error and were submitted to analysis of variance (ANOVA). Values of p < 0.05 were significant in comparisons between the treatments and controls. All statistical analyses were performed using SPSS 26 (IBM Corp., Armonk, NY, USA). GraphPad Prism 8.1 (GraphPad Software Inc., La Jolla, CA, USA) was used for the bar chart drawing.
-
After treatment with different concentrations of aromatic amino acids for 35 d, the amaranth callus growth was normal without browning (Fig. 1). On the medium with tyrosine, the callus color was green and yellow, but on the medium with phenylalanine, the callus color was white. The best medium was with tryptophan, where the callus color was yellow.

Figure 1.
Effect of amaranth callus growth treated with aromatic amino acids. A1-A5 represents the amaranth callus with 0, 2, 4, 6, 8 mg/L tyrosine for 35 d; B1-B5 represents the amaranth callus with 0, 1, 2, 3, 4 mg/L phenylalanine for 35 d; C1-C5 represents the amaranth callus with 0, 0.5, 1.0, 1.5, 2.0 mg/L tryptophan for 35 d.
Phenylalanine, tyrosine, and tryptophan all had significant promoting effects on the proliferation and dry matter accumulation of amaranth callus, besides 2.0 mg/L tryptophan (Fig. 2). With the increase in tyrosine concentration, the proliferation coefficient of amaranth callus increased first and then decreased (Fig. 2a). When the tyrosine concentration was 4.0 mg/L, the proliferation coefficient and dry weight of callus reached the maximum, reaching 15.6 and 0.43, respectively, which were significantly higher than those of other concentrations and control groups.
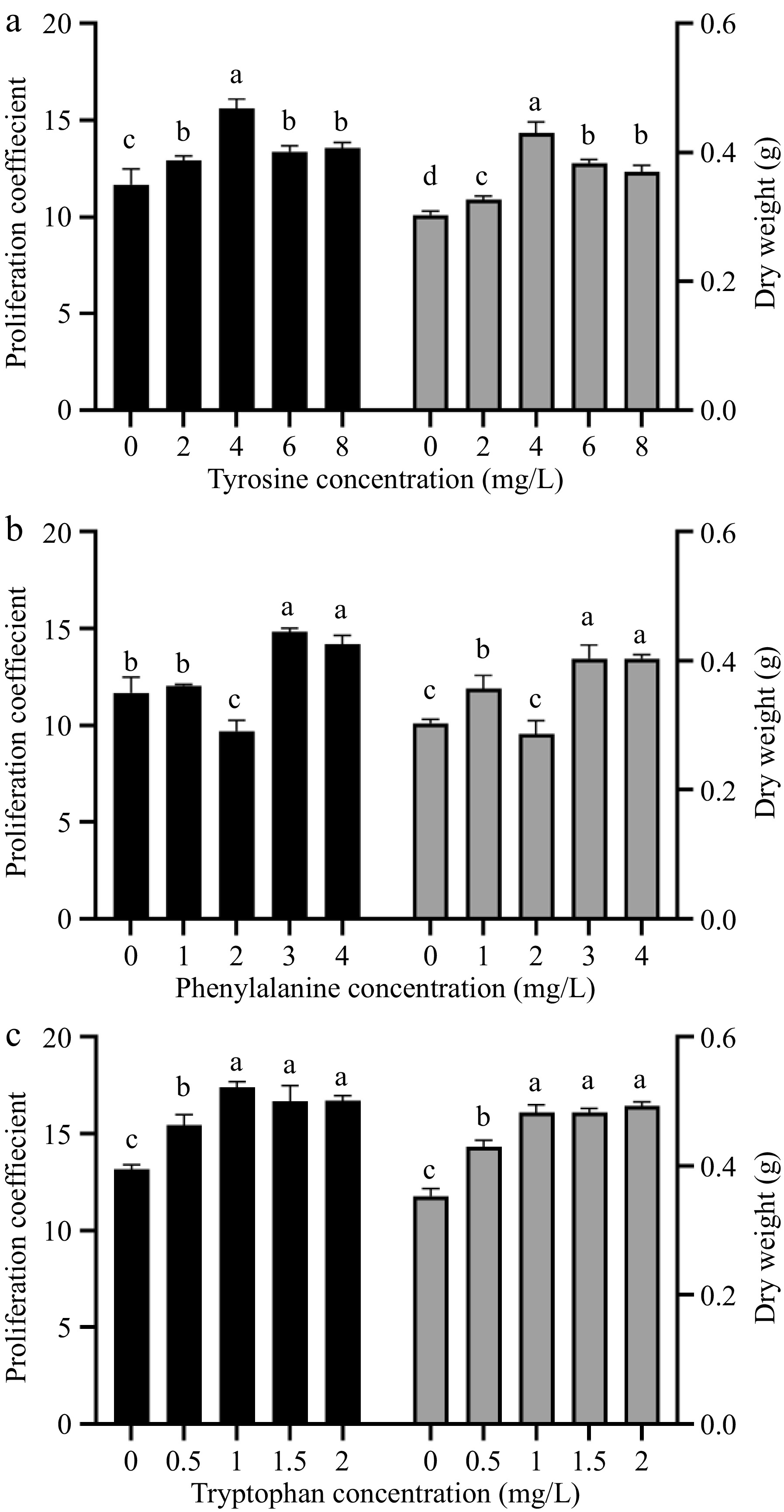
Figure 2.
The effect of different concentrations of aromatic amino acids on amaranth callus proliferation and dry weight. Treatment of (a) tyrosine, (b) phenylalanine, and (c) tryptophan.
With the increase in phenylalanine concentration, the callus proliferation coefficient and dry weight decreased first and then increased (Fig. 2b). When the concentration of phenylalanine was 2.0 mg/L, the callus proliferation coefficient and dry weight decreased to 9.7 and 0.29, respectively, and the callus proliferation coefficient was significantly lower than that of the control group, but the dry weight was not significant. When the concentration of phenylalanine was 3.0 mg/L, the callus proliferation coefficient and dry weight reached 14.82 and 0.4, respectively, which were significantly higher than those of the control group.
With the increase of tryptophan concentration, the proliferation coefficient of amaranth callus showed a trend of first increasing and then decreasing (Fig. 2c). The proliferation coefficient increased to the highest at the concentration of 1 mg/L of tryptophan, reaching 17.39, which was significantly different from the control group. The dry weight showed an upward trend, and the highest at the concentration of 2 mg/L of tryptophan, reaching 0.4, which was significantly different from the control group.
Effect of different concentrations of aromatic amino acids on flavonoid content in amaranth callus
-
The effect of different concentrations of aromatic amino acids on the flavonoid content in amaranth callus is shown in Fig. 3. With the increase in tyrosine concentration, the content of flavonoids in the callus reached the highest level (4.77 mg/g) when the tyrosine concentration was 2.0 mg/L, which was significantly different from that of the control (Fig. 3a).
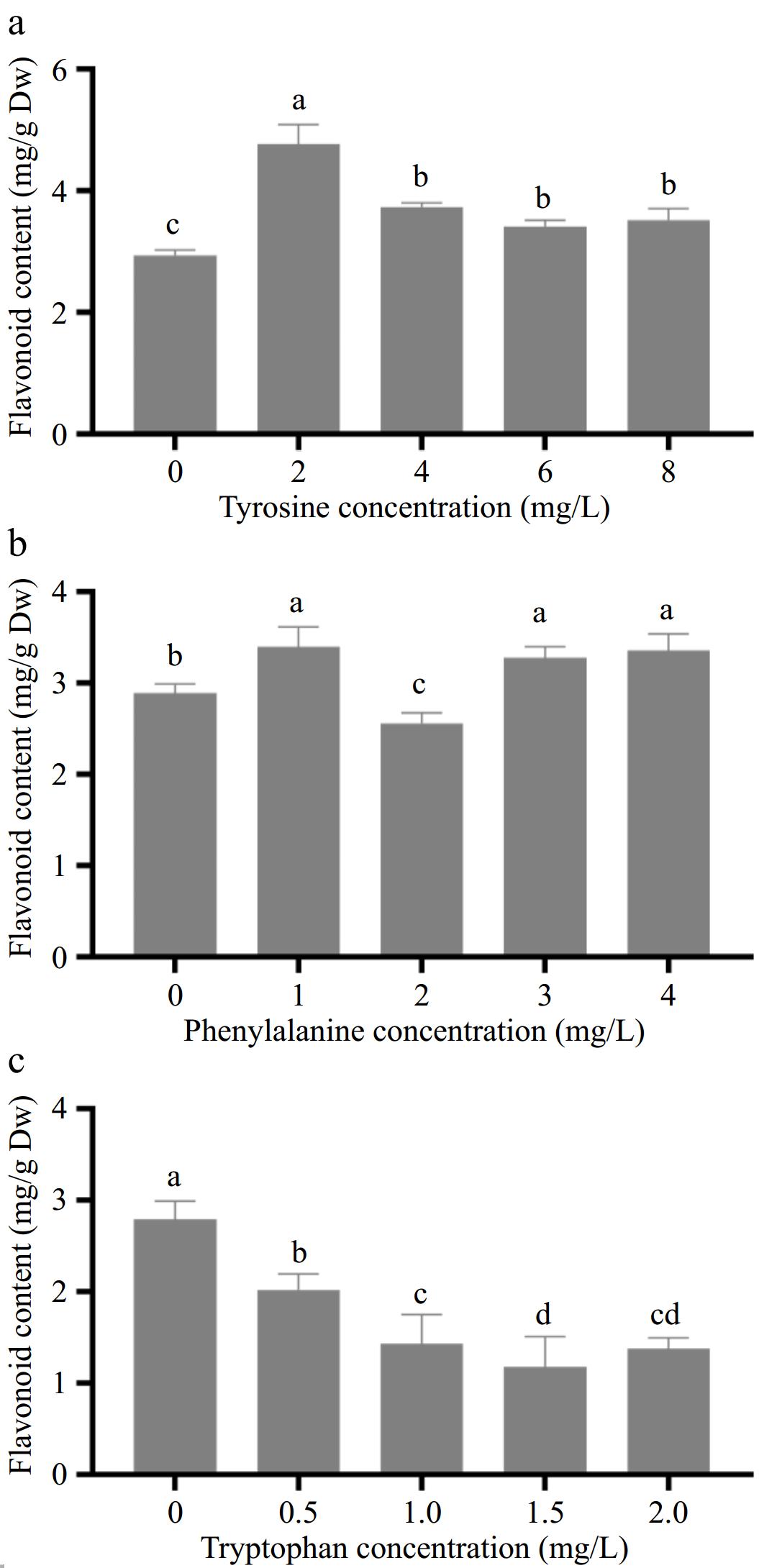
Figure 3.
Effects of different concentrations of aromatic amino acids on flavonoid content in amaranth callus. (a) Tyrosine, (b) phenylalanine and, (c) tryptophan.
With the increase in phenylalanine concentration, the content of flavonoids in the callus showed an upward trend (Fig. 3b). When the concentration of phenylalanine was 2.0 mg/L, the content of flavonoids decreased to 2.63 mg/g, which was lower than that of the control group (2.94 mg/g). When the concentration of phenylalanine was 1.0 mg/L, the content of flavonoids in the callus were the highest. It reached 3.42 mg/g, which was significantly different from the control group.
With the increase in tryptophan concentration, the content of flavonoids in the callus decreased gradually, and the lowest was 1.18 mg/g when the tyrosine concentration was 1.5 mg/L, which was significantly different from the control group (Fig. 3c).
Effects of different concentrations of aromatic amino acids on carotenoid content in amaranth callus
-
The effect of different concentrations of aromatic amino acids on carotenoid content in amaranth callus is shown in Fig. 4. With the increase in tyrosine concentration, the content of carotenoids in the callus decreased, and the minimum was 99.2 μg/g when the tyrosine concentration was 4.0 mg/L (Fig. 4a). With the increase of phenylalanine concentration, the content of carotenoids in callus decreased. When the concentration of phenylalanine was 2.0 mg/L, the content of carotenoids decreased to the lowest, reaching 64.6 μg/g (Fig. 4b).
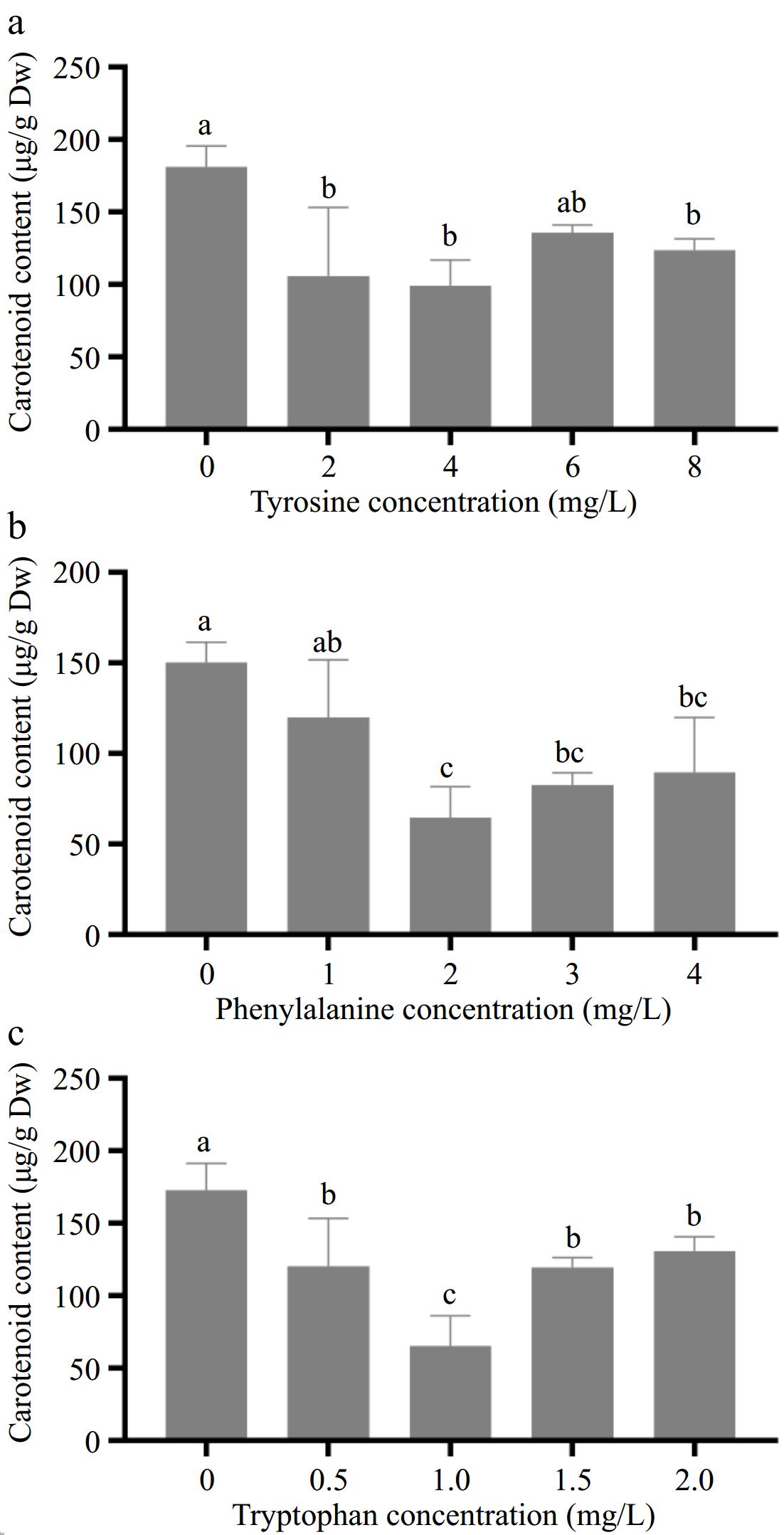
Figure 4.
Effects of different concentrations of aromatic amino acids on carotenoid content in amaranth callus.
With the increase in tryptophan concentration, the content of flavonoids in the callus decreased first and then increased. When the concentration of tryptophan was 1 mg/L, the content of flavonoids in callus was the lowest (63.6 μg/g), and when the concentration of tryptophan was 2.0 mg/L, the content of carotenoids was the highest (140.6 μg/g), which was lower than that of the control group (166 μg/g) (Fig. 4c).
Effect of aromatic amino acids on the related genes expression of flavonoid biosynthesis in amaranth callus
-
The relative expression levels of flavonoid synthesis-related genes in amaranth callus treated with different concentrations of phenylalanine are shown in Fig. 5. The results showed that the gene expression of PAL, F3H, and CHS increased to the highest at the level of 1.0 mg/L phenylalanine, which was significantly different from the control.
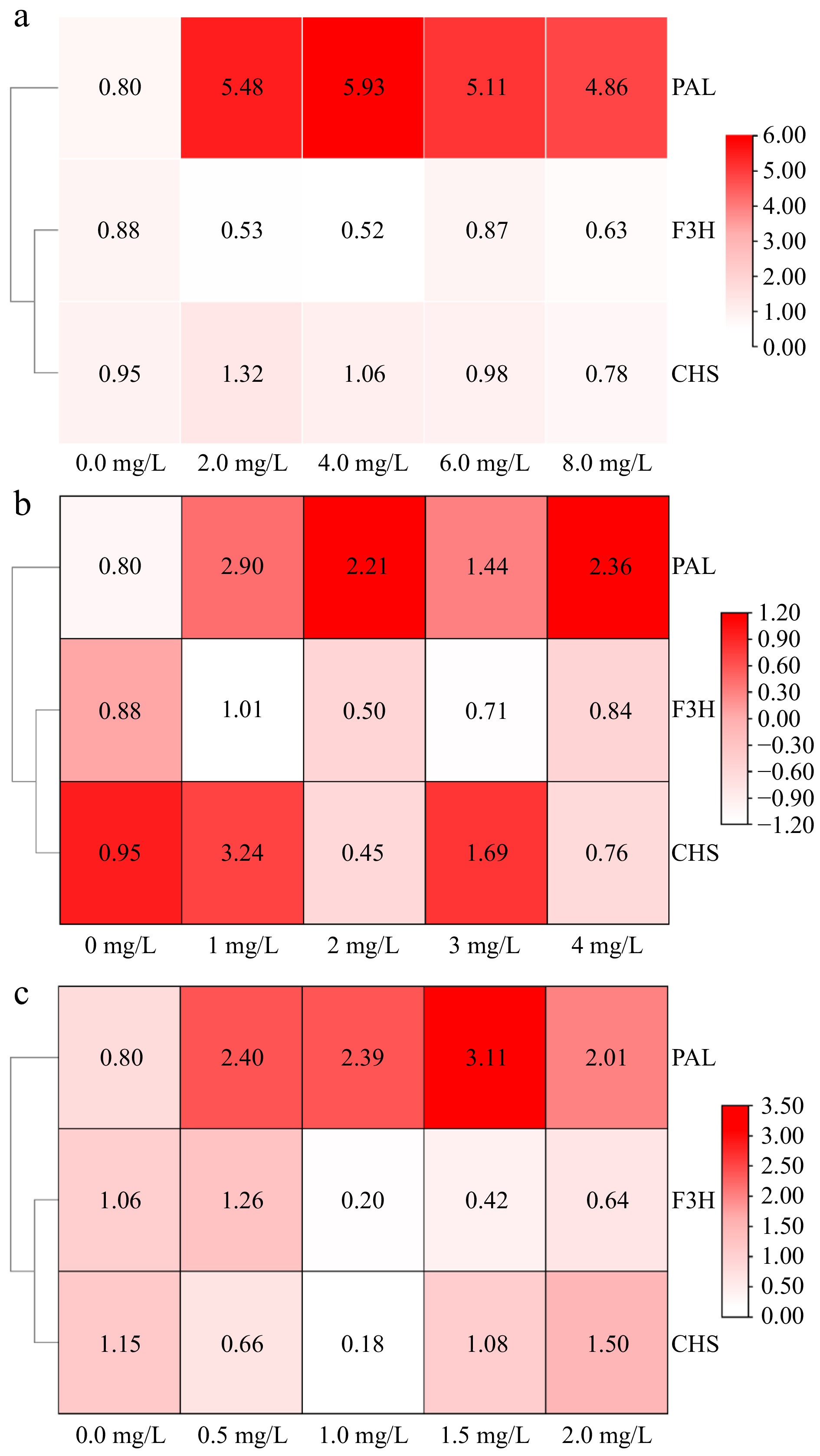
Figure 5.
The effect of aromatic amino acids on the expression of flavonoids metabolism related genes in amaranth callus. (a) Tyrosine, (b) phenylalanine, and (c) tryptophan.
The relative expression levels of genes involved in flavonoid synthesis in amaranth callus treated with different concentrations of tyrosine are shown in Fig. 5. The addition of tyrosine promoted PAL gene expression, reaching a significant difference compared with the control. However, there was no significant difference between different concentrations of tyrosine. The relative expression of the F3H and CHS genes were the highest when the concentration of tyrosine was 6.0 and 2.0 mg/L, respectively.
The relative expression levels of genes involved in flavonoid synthesis in amaranth callus treated with different concentrations of tryptophan are shown in Fig. 5. The relative expression of PAL, F3H, and CHS genes were the highest when the concentration of tyrosine was 1.5, 0.5, and 2.0 mg/L, respectively. And they all reached a significant difference compared with the control.
Correlation analysis between flavonoid content and genes related to flavonoid synthesis
-
SPSS 26 software was used to analyze the correlation between flavonoid content and flavonoid biosynthesis-related genes in amaranth callus treated with three aromatic amino acids (Table 1). The results show that the content of flavonoids was positively correlated with PAL under tyrosine treatment, but not under phenylalanine and tryptophan treatments. There was a significant positive correlation between F3H and flavonoid content under phenylalanine and tryptophan treatments, besides tryptophan treatment. There was a significant positive correlation between flavonoid content and CHS only under tyrosine treatment.
Table 1. Correlation analysis of flavonoid content and flavonoid-related genes.
Aromatic
amino acidsPAL F3H CHS Tyrosine Pearson correlation 0.615* −0.659** 0.694** Significance (two-tailed) 0.015 0.007 0.004 Phenylalanine Pearson correlation −0.183 0.669** 0.258 Significance (two-tailed) 0.513 0.006 0.353 Tryptophan Pearson correlation −0.858 0.753 0.076 Significance (two-tailed) 0.063 0.142 0.90 * indicates significant correlation at the p < 0.05 level, ** indicates extremely significant correlation at the p < 0.01 level. Effect of aromatic amino acids on the expression of carotenoid biosynthesis-related genes in amaranth callus
-
Tyrosine had different effects on the expression of carotenoid synthesis genes in amaranth callus (Fig. 6a). Low concentrations of tyrosine (0−4 mg/L) could promote gene expression, whereas high concentrations inhibit PSY gene expression. When the tyrosine concentration was 4.0 mg/L, the relative expression reached the highest level. Tyrosine had no effect on the PDS gene expression However, it could inhibit the expression of the ZDS gene.
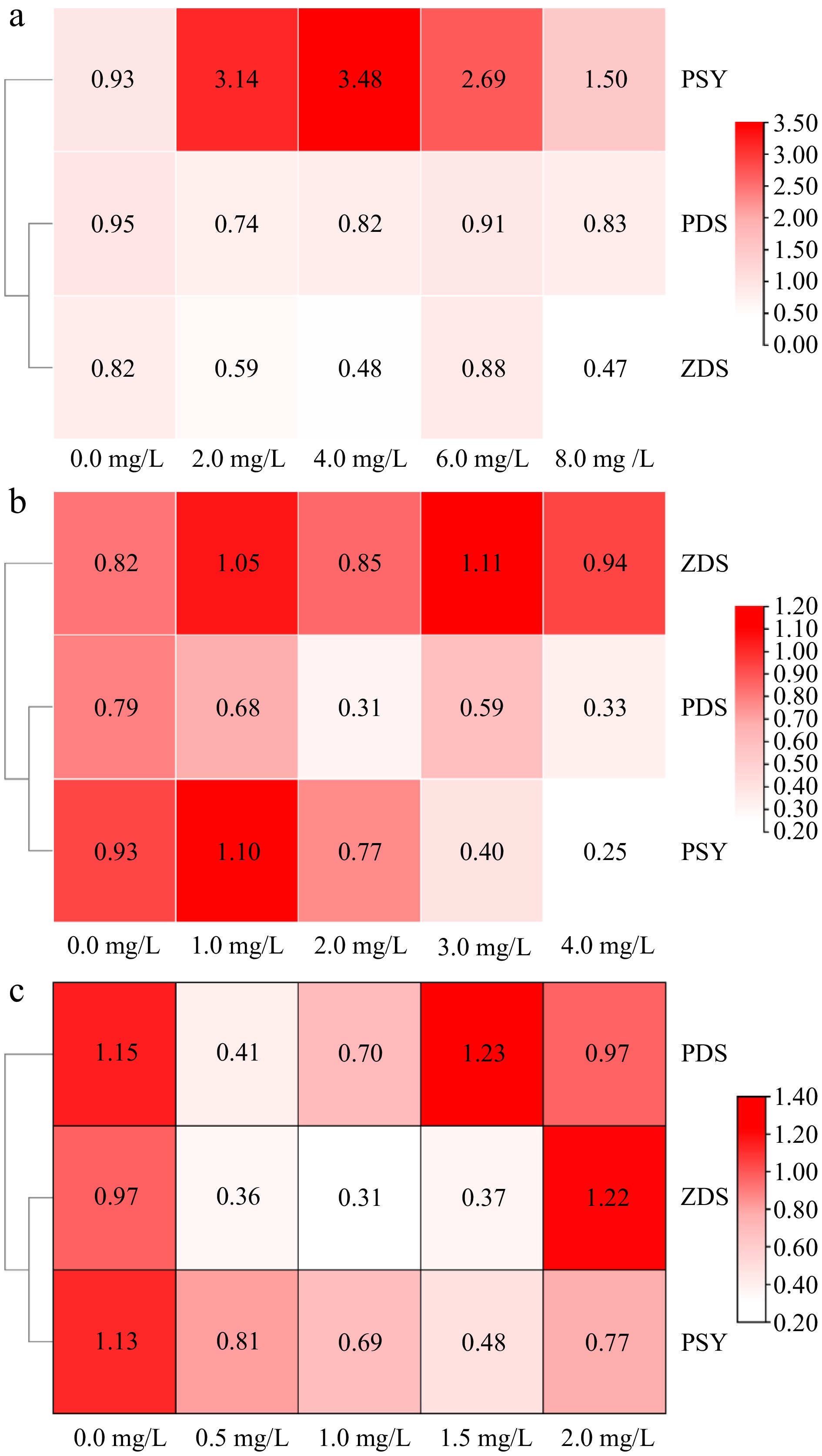
Figure 6.
The effect of aromatic amino acids on the expression of carotenoid metabolism related genes in amaranth callus. (a) Tyrosine, (b) phenylalanine, and (c) tryptophan.
The relative expression levels of carotenoid synthesis-related genes in amaranth callus treated with different concentrations of phenylalanine are shown in Fig. 6b. Phenylalanine could inhibit the expression of PDS and PSY, and there was no significant difference in the expression of the ZDS gene between the phenylalanine treatment and control. Under tryptophan treatment, the carotenoid synthesis gene (PDS, PSY, and ZDS) expression was inhibited in amaranth callus. There were significant differences from the control (Fig. 6c).
Correlation analysis between carotenoid content and genes related to carotenoid synthesis
-
SPSS 26 software was used to analyze the correlation between carotenoid content and carotenoid biosynthesis related genes in amaranth callus treated with three aromatic amino acids (Table 2). The results show that the content of carotenoid was positively correlated with PDS under phenylalanine treatment, but not under tyrosine and tryptophan treatments. There was a significant positive correlation between PSY and carotenoid content under tryptophan treatments. There was a significant positive correlation between flavonoid content and ZDS under tyrosine and tryptophan treatment.
Table 2. Correlation analysis of carotenoids content and carotenoids-related genes.
Aromatic
amino acidsPDS ZDS PSY Tyrosine Pearson correlation −0.348 −0.356 0.638* Significance (two-tailed) 0.204 0.192 0.011 Phenylalanine Pearson correlation 0.694** −0.025 0.442 Significance (two-tailed) 0.004 0.93 0.099 Tryptophan Pearson correlation 0.342 0.591* 0.57* Significance (two-tailed) 0.212 0.02 0.026 * indicates p < 0.05, ** indicates p < 0.01. -
Amino acids as a source of organic nitrogen can promote plant growth and development, and play key roles in plant cells and tissues[45,50], and are important regulators in vitro[51]. Exogenous supplementation of amino acids and casein hydrolysate could enhance callogenesis from plumular explants of coconut (Cocos nucifera L.)[52]. The present results showed that phenylalanine, tyrosine, and tryptophan all had significant promoting effects on the proliferation and dry matter accumulation of amaranth callus. When the concentration of tryptophan was 1 and 2.0 mg/L, the effect of callus proliferation and dry matter accumulation was the best, and the effect was better than that of phenylalanine and tyrosine. It was hypothesized that tryptophan is an important precursor substance for auxin biosynthesis in plants and its structure is similar to IAA, and auxin is beneficial for callus induction and proliferation. So the addition of tryptophan 1.0–2.0 mg/L in the culture medium was most conducive to callus proliferation and dry matter accumulation.
Aromatic amino acids are precursors of secondary metabolites such as flavonoids, carotenoids, and alkaloids[5]. Flavonoid compounds are important secondary metabolites produced by plants[6]. Flavonoids have a variety of biological functions. They can not only affect the transport of plant hormones[9,10], but also regulate the level of reactive oxygen species (ROS) in plants[11−13]. Phenylalanine ammonia-lyase (PAL) is the first enzyme in the phenylpropane metabolic pathway and a key enzyme in the flavonoid metabolic pathway[14,15]. PAL and C4H can convert phenylalanine to p-coumaric acid. Meanwhile, tyrosine can be directly converted to p-coumaric acid[53]. Subsequently, p-coumaric acid is converted to flavonoids under the action of a series of enzymes, including 4CL, CHS[19,20], CHI, and F3H[21−23]. The present results show that tyrosine was beneficial to increase the content of flavonoids in amaranth callus, and it was superior to phenylalanine. The two precursor amino acids are converted to flavonoids by different pathways. Through correlation analysis, there was a significant positive correlation between the PAL gene, CHS gene, F3H gene, and flavonoid content under tyrosine treatment, indicating that tyrosine affected the synthesis of flavonoids by regulating the expression of the three key genes. Under phenylalanine treatment, there was a significant positive correlation between F3H and flavonoid content, indicating that phenylalanine regulates the expression of F3H.
Carotenoids are a sort of yellow, red, or orange-red polyene substance[24], which could participate in photosynthesis[24,25] and the synthesis of plant hormones[26,27]. Two geranylgeranyl diphosphates (GGPPs) are condensed into colorless phytoene under the catalysis of phytoene synthase (PSY)[30], which is the rate-limiting enzyme in the carotenoid synthesis pathway[31−33]. Phytoene is converted to red phytoene by the co-catalysis of phytoene dehydrogenase (PDS) and carotene dehydrogenase (ZDS)[34−36]. The present results showed that aromatic amino acids inhibited the carotenoids biosynthesis. Perhaps the degradation and transport of carotenoids affected their concentrations. However, the mechanism is not still clear.
-
Aromatic amino acids, especially tyrosine, could promote the growth of amaranth callus and flavonoid synthesis, and regulate related gene expression. In contrast, aromatic amino acids inhibited carotenoids synthesis in amaranth callus.
-
The authors confirm contribution to the paper as follows: writing – original draft, writing – review & editing: Liu S, Xuan Y; validation: Xuan Y; conceptualization: Liu S, Lai Z; methodology: Xuan Y, Feng W. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
This work was supported by the Natural Science Foundation of Fujian Province (2023J01449), Innovation Foundation of Fujian Agriculture and Forestry University (KFb22024XA), Rural Revitalization Social Service Team of Fujian Agriculture and Forestry University (11899170125).
-
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
-
accompanies this paper at (https://www.maxapress.com/article/doi/10.48130/tp-0024-0034)
-
Received 18 April 2024; Accepted 26 July 2024; Published online 20 September 2024
-
Aromatic amino acids could promote the growth of amaranth callus.
Optimum concentrations of tyrosine and phenylalanine were beneficial for flavonoid content in the callus.
- Supplemental Table S1 The primer pairs for the qRT-PCR analysis.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xuan Y, Feng W, Lai Z, Liu S. 2024. Effects of aromatic amino acids on callus growth and accumulation of secondary metabolites in amaranth. Tropical Plants 3: e032 doi: 10.48130/tp-0024-0034
Effects of aromatic amino acids on callus growth and accumulation of secondary metabolites in amaranth
- Received: 18 April 2024
- Revised: 16 July 2024
- Accepted: 26 July 2024
- Published online: 20 September 2024
Abstract: Amaranth, a green leafy vegetable with high edible value, is rich in flavonoids and carotenoids. Aromatic amino acids are favored to secondary metabolites biosynthesis as precursors. Our previous study showed that flavonoids could be produced using amaranth callus. However, the effects of aromatic amino acids on the callus growth, the accumulation of secondary metabolites, and the expression of related genes in amaranth are still unclear. In this study, the results showed that aromatic amino acids could promote the growth of amaranth callus. Meanwhile, tyrosine and phenylalanine within fitting concentrations were beneficial to flavonoid accumulation and expression regulation of the related gene. In contrast, aromatic amino acids reduced carotenoid accumulation. The results provide a scientific basis and method for callus culture and flavonoid production by the callus of amaranth.
-
Key words:
- Amaranthus tricolor /
- Callus /
- Aromatic amino acids /
- Flavonoids /
- Carotenoids /
- Gene expression