-
As world population growth, urbanization, and industrialization shrink arable land, food production capacity is rapidly increasing the demand. Additionally, climate change's major threats to sustaining agriculture, including varying temperatures, droughts, and floods, affect agricultural practices[1]. Most importantly, exportation has a high fiscal assessment. However, sugarcane fields provide an alternative to traditional fossil fuels as an energy source. In addition, these plants are often very tolerant of changing climatic conditions, developing a sustainable option in specific regions[2]. These impacts have had a long-term effect on the planet and climate change manifests itself more and more every day[3]. To achieve this balance, agro-farming systems are important by using ecological resources for food production to minimize the negative impact of the manufacturing process on the natural environment[4−6]. Therefore, this information clearly shows that soil resources play an important role in agriculture and very careful maintenance of essential resources are needed to ensure the extended duration and sustainable agricultural systems[6−9].
Soil microbial communities perform a vital function in influencing ecosystem functioning nutrients driving plant health and soil structure. The symbiosis of AM fungal species with plant roots is crucial in these processes. Some research has determined that mycorrhization can substantially change the composition and function of association with soil microbial communities[10−12]. AMF fungi significantly shift in the soil microbiome, enhancing nutrient availability and plant growth. Similarly, ectomycorrhizal fungi (EMF) also promote the entophytic related bacteria and suppressed pathogenic microbes in forest ecosystems[10].
AM fungi are often associated with soil microbes and are incorporated into plant roots to change morphology and physiology[10]. Among other physiological changes, root quality and quantity of exudation are altered, and thus the microbial composition rhizosphere modifications. Several research studies have revealed that AM fungi and its inter relationship with the plant roots, which provides nutrients and signals to the other part of plants[6,11]. Once mycorrhizae are available, the number and imperativeness of these nitrogen fixers improve. Mycorrhizae too help the plant to withstand disease from other parasites and indeed microbes. This may be because the plant, being superiorly fed, is more advantageous and has superior resistance to the trespasser.
Only limited studies have been carried out on the interaction of root-associated fungi with other microorganisms in rhizospheric soils. AM fungi attached roots had low vulnerability to some cultivar’s pathogens. Therefore, in recent years, researchers have expanded their knowledge of the control of AM fungi in the rhizosphere of plants harboring soil-borne pathogens. Much research has reported that the colonization of roots by AM fungi develop immunity resistance against plant pathogens[12−15]. However, some studies revealed that AM fungi make the roots of the host plant susceptible to root pathogens which increase the chances of infection rather than prevent infection[16,17]. It has also been reported[12] that certain rhizospheric microorganisms trigger the association of roots with AM fungi. However, it is still unclear which of the rhizospheric organisms help in the successful colonization of AM fungi into the plant host. It is believed that rhizospheric microbes bring about a measurable shift in the absorptivity of root cells by damaging root tissue, altering root metabolism, utilizing certain root exudates, or secreting toxins.
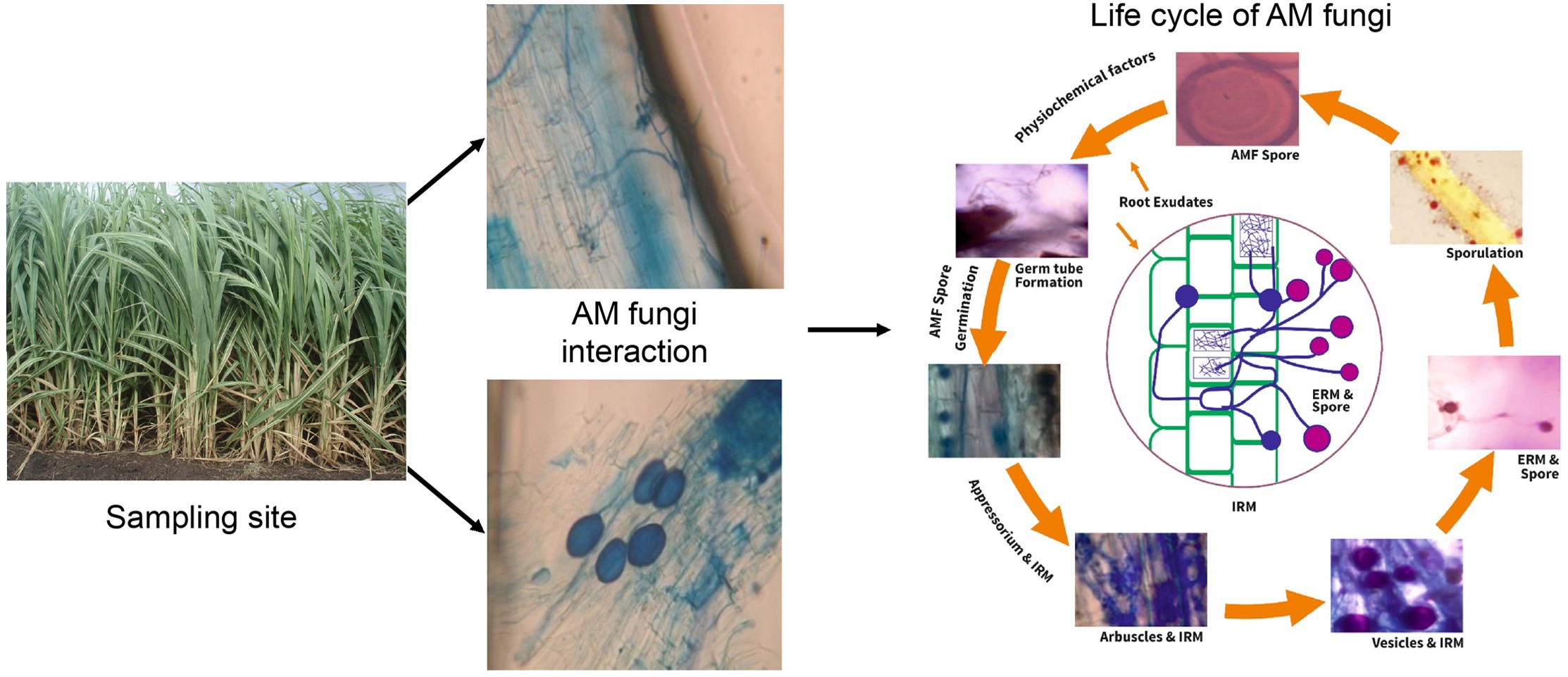
The present investigation deals with the fungi isolated from the rhizosphere of AM fungi mediated and uninfected (AM fungi free) sugarcane plants, monthly and seasonal fluctuations and abundance of individual organisms, comparative monthly and seasonal fluctuations in AM fungi populations in soil, the percentage of AM fungi interaction in roots, and occurrence of total rhizosphere fungi of AM fungi infected and uninfected plants of Saccharum officinarum.
-
In Bihar (India), sugarcane crops are grown mainly in the western region namely Pusa, Samastipur District, the districts are known as main burgeoning areas. Sugarcane is also cultivated in other parts of the region. Soil samples were collected in different months from January 2019 to December 2020 at 10 different taluks and locations. Temperature 30−40 °C, environmental and soil variations (clay and red soil). These samples were collected at the end of each month in different locations and stored in polyethylene bags at 4 °C for further analysis.
AM fungi spores isolation, identification, and inoculum preparation
-
AM fungal species were subjected and isolated from sugarcane field soil samples. Initially, the place for the collection of rhizospheric soil samples from various places of the sugarcane field were selected, then the collected samples were maintained at 4 °C. AM fungi single spores were collected by using slightly modified wet sieving and decanting methods[18,19]. Surface sterilization of spores was achieved by applying 2% (w/v) chloramine T and streptomycin sulfate (200 μg ml−1) for 20 min and then cleaning several times using sterile distilled water. Single AM fungal spore cultures were raised by following the standard funnel technique[20] with Zea mays as a host plant. Three months of soil samples were collected, and spores were harvested. The samples were first subjected to a morphogenetic and micrometric study where individual AM fungal spore morphology, size, shape, color, surface, and structure of hyphae using Melzer’s reagent were determined. The present AM fungal genera morphological identification study results support previous findings where Glomus spp. was found in the sugarcane soil samples (Table 1)[21−24].
Table 1. AM fungi infection in roots and spores' population spores in rhizosphere in soil for different sugarcane fields.
S. No. Sugarcane
cultivated fieldHyphal type Arbuscles Vesicles AMF infection
levelAMF spore of
10 gm soilAMF spp. Broad Thin 1 Field 1 − +++ ++ +++ 56% 12 Rf, Ga 2 Field 2 − +++ ++ +++ 71% 13 Rf, Ga, Gi, Gc 3 Field 3 − + + + 34% 8 Rf, At 4 Field 4 − − − − − 9 Rf, Gal, Gac 5 Field 5 ++ − − + 48% 11 Rf, Ri, Gc, Gg 6 Field 6 − ++ + ++ 52% 12 Rf, Ga, AL 7 Field 7 ++ − + +++ 39% 14 Rf, G, Sc 8 Field 9 − − − − − 10 Rf, Ri, Gm 9 Field 9 − ++ ++ +++ 53% 12 Rf, Ga, Fm 10 Field 10 − ++ + ++ 56% 10 Rf, Ga, Fm +, Poor; ++, Moderate; +++, Abundant; −, Absent. Rf, R. fasciculatus; Ga, G. aggregatum; Ri, R. intraradices; Gm, F. mosseae; Gc, G. constrictum; Gac, G. macrocarpum; Gal, Gigaspora albida; At, Acaulospora tuberculate; AL, A. laevis; Sc, Sclerocystis spp. Assessment of AM fungi root colonisation percentage in sugarcane root systems
-
Fresh samples containing 2−3 g of roots were used to assess the colonization percentage by staining. Fixed roots were cleaned with tap water, applied in 10% KOH, acidified with 1N HCL, and stained with 0.05% trypan blue. Quantification of root colonization of AM fungus was conducted by using the gridline cross-section technique[25] and 100 root sections of each sample were observed under a light microscope[26,27]. AM fungi colonization in plant root systems such as vesicles, arbuscules, and hyphae at fixed points were observed and the percentage of AM fungal colonization in the sugarcane root systems were calculated.
Soil preparation and treatment
-
Experimental pots (60 cm × 50 cm × 50 cm) were filled with 50 kg of sieved and sterilized soil. The soil was crushed through a 4 mm sieve. The soil used was phosphorus deficient (Olsen P. 6.87 μg−1 soil) with a pH of 8.4 (1:2, Soil : water suspension). The inoculum of Rhizophagus fasciculatus was applied through a layering technique[28]. Roots of non mycorrhizal plants served as the control.
Procurement of sugarcane seeds
-
Seeds of sugarcane were obtained from the Sugarcane Research Institute, Pusa, Samastipur, Bihar, India.
Seed treatment and planting
-
Surface sterilized seeds (0.1% aqueous HgCl2 for 30 min) of the B.O.109 variety of sugarcane were used. Two eye seeds were used in each pot. The seedlings were thinned out into one in each pot after 25 d of sowing. The potted plants receiving the above treatments were left on greenhouse benches with the temperature ranging between 25 to 35 °C in a randomized complete block design to minimize any positional effects with six replications per treatment. After 30 d, all plants were given 60 ml of half-strength Hoagland's nutrient solution without phosphate once a week. Water was added as needed to maintain the growth medium at 60% of its limit water holding capacity.
Isolation of fungi from rhizosphere
-
To isolate fungi from the rhizosphere of AM fungi, plants were dug up with an intact root system using a specially designed sharp and long spade. Roots of both plants were carefully collected at intervals of 30 d. Excess soil adhering to the roots was removed by gentle shaking, and root tips (about 2.3 cm long) were cut with sterilized scissors and transferred to 100 ml of sterilized water (in a 250 ml Erlenmeyer flask). The flask was then subjected to vigorous shaking to obtain a homogeneous suspension of rhizosphere soil. From this suspension, a 1:10,000 dilution was prepared. About 20 ml of sterile Martin Rose Bengal agar medium (10.0 g glucose, 5.0 g peptone, 1.0 g KH2PO4, 0.5 g MgSO4 7H2O, 0.020 g rose bengal, 0.03 g agar-agar and 1,000 ml distilled water) was added. The Petri plates were rotated in different directions so that the suspension was completely mixed with the culture medium, and the inoculum was uniformly distributed on the plate. Five replicates were used for each set. The sealed petri plates were incubated at 28 ± 2 °C for 1 week.
Qualitative analysis of rhizosphere fungi
-
After the necessary incubation period, individual fungal colonies developing on agar plates were aseptically transferred to sterilized potato-dextrose agar slants. The pure culture of each isolate was obtained by successive sub-culturing. The morphological characters of different isolates were studied microscopically (450×) and identified by comparing the characters of known species mentioned in the relevant literature. Some of the cultures whose identities were doubtful were sent to C.A.B. Mycological Institute, Kew (UK), for identification and confirmation. The various fungal species isolated from rhizospheres of AM fungi treated and untreated plants gave the qualitative number of fungi associated with the rhizospheres of aforesaid plants.
Quantitative analysis of rhizosphere fungi
-
Quantitative analysis of rhizosphere fungi was performed by calculating the percentage frequency and abundance of different isolated fungi in different months. The formulas used to determine percentage frequency and percentage abundance were similar to those followed by Prasad & Bilgrami[29].
$ \begin{split} & \rm{P}ercentage\; frequency= \\ &\rm{\dfrac{No.\ of\; observations\; in\; which\; species\; appeared}{Total\; no.\; of\; observations}}\times 100 \end{split} $ $ \begin{split} & \rm{P}ercentage\; abundance= \\ &\rm{\dfrac{Total\; no.\; of\; colonies\; of\; a\; species\; in\; all\; observations}{Total\; no.\; of\; colonies}}\times 100 \end{split} $ Statistical analysis
-
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) to evaluate the colonization and spore density of AM fungi in root tissues. Obtained results were analyzed using one-way variance, and significant differences were expressed at the significance level (p < 0.05) by Duncan's multiple range test.
-
In this experiment, an assessment of AM fungal species association into the root part of sugarcane crop plants, as well as fungal spore density in soil samples of different fields is presented in Table 1. The results of the occurrence of species, fields out of a total of 10 fields were found to have the ability to host mycorrhizal root infection because of this inquiry. Based on the results the length of the roots that were analyzed, the level of mycorrhizal fungal association of root tissues varied from 34−71. Field 2 plants had root length infection, whereas Field 3 had the least amount of root colonization (Table 1). The values for the infection spectrum of examined fields were determined to be 56%, 71%, 34%, 48%, 52%, 39%, 53%, and 56% accordingly for Fields 1−10 respectively (except Field 4 and Field 8 as they didn't yield any occurance). The fungal infection that caused AM fungi was made up of hyphae, vesicles, and arbuscules of the fungus. There was a significant amount of variation in the infection rate amongst the different field plants. The gathered soil samples to determine the AM fungi density were variations of sorts and exhibited a broad assortment of soils. In this soil range, the soil had a high population of mycorrhizal spores, and Glomus sp. were most abundant.
The AM fungal concentration in the soil varied from 8−14 in 10 g−1 soil. Field 7 plants were found to have the highest spore density, whereas Field 3 had the lowest. A wide range of spores, the major species of which belonged to the genus Glomus, were extracted from the soil and the root washings. However, a zygospore of Acaulospora and Gigaspora as well as sporocarps of Sclerocystis were also found, although very rare.
The determination of the various sugarcane plants, soil samples taken from the rhizospheres of all the sugarcane fields were treated to isolate the various fungal propagules. Following are some of the fungi that have been identified based on the characteristics of their spores: six species of Glomus, namely Rhizophagus fasciculatus, Glomus aggregatum, Rhizophagus intraradices, Funneliformis mosseae, and Glomus constrictum and Glomus macrocarpum; two species of Gigaspora, namely Claroideoglomus claroideum and G. gigantea and two species of Acaulospora namely Acaulospora tuberculate and A. laevis and one species of Sclerocystis spp. The results showed that AM fungal species were predominant, and mostly belong to these species of the current soil samples at different sugarcane fields followed by R. fasciculatum (10 sugarcane fields), G. aggregatum (five fields), R. intraradices (three fields), F. mosseae (two fields), G. constrictum (two fields), and G. macrocarpum (one field). Additionally, the species of Gigaspora (Gigaspora albida, and G. gigantea) were observed in the sampling soil from one field. The presence of spores and mycorrhizal root colonization was not found to have any definitive relationship with one another. As a result of the fact that the proliferation of an endomycorrhiza relies on its contact with plant roots, the quantity of its spores in soils is likely to change, as was shown in the current experiment. AM fungus has one of the major roles in symbiotic relationships with plant roots and enhances the growth development in humid soil nature, particularly in waterless zones. Soil conditions have a significant effect on the extent to which mycorrhizal fungal populations are active, as measured by the number of spores produced and the extent to which roots are infected[28].
Qualitative features of rhizosphere fungi
-
Many varieties of soil fungal species were isolated from AM fungal-treated plants when compared to non-treated plants that showed a huge number of isolates. Statistical analysis revealed significantly higher root colonization compared to non-inoculated plots (control). Altogether, 14 fungi belonging to nine different genera (Tables 2 & 3) were isolated from AM fungi-infected plants. Out of the total isolates, two species belonged to Phycomycetes, one Ascomycete species, and the remaining 11 species to Deuteromycetes. The rhizosphere soil of Control (AM fungi-free) sugarcane plants (Tables 2 & 3) however, yielded 22 fungi belonging to 16 different genera. Among the isolated organisms, three species belonged to Phycomycetes, two species to Ascomycetes, and the remaining 17 species to Deuteromycetes. In all, 10 fungi viz., M. recemosus, Rhizopus oryzae, Chaetomium globosum, Aspergillus candidus, A. flavus, A. niger, A. terreus, Cladosporium herbarum, Pestalotia glandicola, and Macrophomina phaeolina were found to be common in both the rhizosphere of AM fungi inoculated and uninoculated sugarcane plants. Besides the aforementioned fungi, the rhizosphere of uninoculated plants also yielded 12 more fungi viz. Rhizopus varians, Neocosmospora vasifecta, Alternaria alternata, Curvularia lunata, Helminthosporium halodes, C. sacchari, F. moniliformae, F. solani, F. semitectum, Rhizoctonia solani, Myrothecium roridum, and Verticillium albo-atrum. The rhizosphere of AM fungi inoculated plants, however, yielded only four fungi that were not found in the rhizospheric soil of uninoculated plants viz., Aspergillus sydowi, P. chrysogenum, P. lilacinum, and T. harzianum.
Table 2. Presence (+) and absence (−) of genera of arbuscular mycorrhizal fungi in infected and uninfected (AM fungi free) plants of Saccharum officinarum L.
Fungi AM fungi infected Control
(AM fungi free)Phycomycetes Mucor racemosus Fres + + Rhizopus oryzae Went & Gerling + + Rhizopus varians Povah − + Ascomycetes Chaetomium globosum Kunze & Shorr + + Neocosmospora vasinfecta Smith − + Deute romyetes Alternaria alternata (Fr), Keisler − + Aspergillus candidus Link. + + Aspergillus flavus Link. + + Aspergillus niger Van Tiegh. + + Aspergillus sydowi (Bainer & Sartory) + − Aspergillus terreus Thom + + Cladosporium herbarum (Pors). Link + + Cephalosporium sacchari Butler − + Curvularia lunata (Wakker) Boedijn − + Fusarium moniliforme Sheldon − + Fusarium solani (Mart.) Sace. − + Fusarium semifectum Berk & Rev. − + Helminthosporium halodes Drechs. − + Macrophomina phasealina (Tassi) Goid + + Mycothecium roridum Tode exfr − + Penicillium chrysogenum Thon + − Penicillium lilacinum Thon + − Pestalotia glandicola (Cast) Stey + + Rhizoctonia solani Kuhn. − + Trichoderma harzianum Rafai + − Verticillium albo-atrum Reink & Berthold. − + Total number of fungi 14 12 Table 3. Monthly fluctuations in the percentage frequency of rhizosphere mycoflora of AM fungi infected and uninfected plants of Saccharum officinarum L.
Fungi Jan Feb. March April May June July Aug. Sep. Oct. Nov. Dec. AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU F F F F F F F F F F F F F F F F F F F F F F F F Mucor racemosus 60 65 58 64 57 60 − − − − − − 30 − 35 30 38 35 45 48 54 55 58 56 Rhizopus oryzae 85 88 70 85 65 84 60 72 35 70 30 65 70 40 74 35 79 75 80 80 84 86 86 86 Rhizopus varians − 64 − 80 − 68 − − − − − − − 66 − 58 − 60 − 60 − 66 − 72 Chaetomium globosum − − 34 52 − 40 − − − − − − − − − − 26 45 32 42 34 50 36 − Neocosmospora vainfecta − − − 17 − 10 − − − − − − − 15 − − − 14 − 10 − − − 18 Alternaria alternata − 28 − 35 − 32 − − − − − − − − − 35 − 30 − 28 − − − − Aspergillus candidus 66 70 − 62 − − 36 40 − − − − − − 60 65 62 70 52 60 56 66 60 76 Aspergillus flavus 74 82 − − − − 52 65 60 62 − − 70 80 71 75 − − 75 80 76 85 82 82 Aspergillus niger 80 86 78 86 70 78 65 65 52 67 35 65 40 45 50 64 64 70 75 72 76 75 80 86 Aspergillus sydowi 52 − − − − − − − 34 − − − − − 52 − 60 − 56 − − − 54 − Aspergillus terreus 70 85 − − − − − 70 55 − 50 62 56 64 68 68 64 70 75 78 76 72 66 80 Cladosporium herbarum − − − − 29 35 − − − − − − 40 50 48 48 − 45 32 38 34 35 − − Cephalasporium sacchari − − − 40 − 33 − − − − − − − 50 − 48 − 42 − − − 34 − 30 Curvularia lunata − 50 − 45 − − − − − − − − − 43 − 45 − 50 − 52 − 55 − − Fusarium moniliforme − − − 40 − 36 − − − − − − − 45 − 50 − 45 − 30 − 32 − 28 Fusarium solani − 45 − 50 − 44 − 36 − − − − − 26 − 30 − 35 − 55 − 52 − 48 Fusarium hemitectum − 42 − 50 − 45 − 36 − − − − − 20 − 46 − 48 − 56 − 55 − 54 Helminthosporium halodes − − − 35 − 32 − − − − − − − 40 − − − 36 − 45 − − − − Macrophomina phaseolina − − − − 35 40 − 35 − − − − − − 22 30 40 35 36 45 − 46 − 50 Myrothecium roridum − − − 40 − 35 − − − − − − − 25 − 30 − 22 − 35 − 20 − 30 Penicillum chrysogenum 42 − 52 − − − 30 − − − − − 32 − 28 − 33 − 47 − 52 − 60 − Penicillum lilacinum 50 − 60 − − − − − − − − − − − 64 − 70 − 55 − 54 − 60 − Pestalotia glandicola − 55 52 52 46 − − − − − − − 54 55 − − − − 64 65 58 62 60 55 Rhizoctonia solani − 30 − 35 − − − − − − − − − − − 32 − 35 − 40 − 25 − − Trichoderma harzianum − − 78 − − − − − 56 − 60 − 68 − 64 − 76 − 70 − 76 − 80 − Verticillium albo-atrum − 30 − 25 − 35 − − − − − 26 − 30 − 45 − 35 − 22 − 30 − 50 AI, AMF infected ; AU, AMF Uninfected; F, Percentage frequency; −, Percentage frequency NIL. It is noteworthy that C. sacchari, and F. moniliforme, which cause sugarcane wilt in this region, were not obtained from the rhizosphere soil of AM fungal inoculated plants. Although these two fungi were isolated from rhizosphere soil, they were not inoculated into plants.
Quantitative features of rhizosphere fungi
-
The data presented in Tables 4 & 5 show that the percentage frequency of AM fungi and the percentage of various rhizospheric fungi were higher in non-inoculated sugarcane plants and different months. Generally, the percentage of different fungi is highest in August, September, October and November. The percentage of AM fungal frequency and abundance of these fungi decreased during April, May, and June. The rhizosphere of AM fungi was compared with the fungi extracted from the soil, and the level of frequency and abundance of AM fungi were found to be different. The percentage frequency and percentage abundance of different species of Mucor, Rhizopus, Aspergillus, Penicillium, and Trichoderma were significantly higher in the rhizosphere soil of AM fungi and non-inoculated plants.
Table 4. Monthly fluctuations in the percentage abundance of rhizosphere mycoflora of AM fungi infected and AM fungi free plants of Saccharum officinarum L.
Fungi Jan. Feb. March April May June July Aug. Sep. Oct. Nov. Dec. AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU AI AU F F F F F F F F F F F F F F F F F F F F F F F F Mucor racemosus 7.70 7.85 7.60 8.75 7.56 7.70 − − − − − − 5.45 − 6.15 6.45 6.80 6.15 6.95 7.80 7.35 7.90 7.62 8.50 Rhizopus oryzae 14.42 14.92 13.72 14.45 13.10 14.40 13.05 13.76 8.25 13.70 8.80 13.10 10.70 11.25 14.20 14.85 12.43 14.25 14.43 15.25 14.40 14.65 14.62 14.70 Rhizopus varians − 12.25 − 14.75 − 12.82 − − − − − − − 12.05 − 11.96 − 11.95 − 12.95 − 11.95 − 12.08 Chaetomium globosum − − 6.15 8.10 − 6.80 − − − − − − − − − − 4.45 7.15 4.78 7.25 4.95 7.70 5.05 − Neocosmospora vainfecta − − − 1.95 − 1.88 − − − − − − − 1.95 − − − 1.75 − 1.70 − − − 2.02 Alternaria alternata − 5.45 − 5.90 − 5.85 − − − − − − − − − 5.70 − 5.68 − 5.55 − − − − Aspergillus candidus 8.72 13.10 − 12.85 − − 3.40 10.25 − − − − − − 8.60 13.10 9.35 14.20 8.95 12.45 − 11.85 10.96 14.25 Aspergillus flavus 12.76 14.25 − − − − 12.80 12.95 12.65 12.75 − − 13.12 13.10 13.10 13.95 − − 13.45 14.10 13.48 14.95 14.56 14.56 Aspergillus niger 13.60 15.45 13.20 15.45 12.10 14.85 11.45 11.40 5.12 14.05 8.95 13.85 9.85 9.50 10.26 13.75 11.40 14.15 12.75 14.20 12.80 14.60 13.65 15.45 Aspergillus sydowi 9.05 − − − − − − − 8.70 − − − − − 8.60 − 9.35 − 8.95 − − − 10.96 − Aspergillus terreus 11.76 14.78 − − − − − 13.10 12.32 − 11.70 12.80 12.35 14.05 13.15 13.65 11.48 13.10 12.75 13.75 13.76 13.80 13.10 14.45 Cladosporium herbarum − − − − 5.25 5.45 − − − − − − 6.48 7.55 7.48 7.45 − 7.35 5.45 6.65 5.52 5.65 − − Cephalasporium sacchari − − − 6.05 − 5.45 − − − − − − − 7.52 − 7.48 − 6.46 − − − 5.52 − 5.52 Curvularia lunata − 6.70 − 6.40 − − − − − − − − − 6.30 − 6.40 − 6.75 − 6.75 − 6.85 − − Fusarium moniliforme − − − 5.10 − 4.95 − − − − − − − 6.15 − 8.16 − 7.15 − 4.60 − 4.75 − 4.35 Fusarium solani − 6.15 − 7.06 − 6.05 − 4.95 − − − − − 4.32 − 4.60 − 5.09 − 5.75 − 5.65 − 5.45 Fusarium semitectum − 5.90 − 7.15 − 6.78 − 5.16 − − − − − 5.05 − 6.25 − 6.35 − 6.85 − 6.80 − 6.75 Penicillum chrysogenum 5.80 − 10.20 − − − 5.15 − − − − − 5.20 − 4.90 − 5.15 − 6.35 − 7.96 − 10.56 − Helminthosporium halodes − − − 6.10 − 5.35 − − − − − − − 6.15 − − − 6.15 − 6.60 − − − − Macrophomina phaseolina − − − − 8.95 9.15 − 8.25 − − − − − − 3.30 4.10 3.15 5.15 3.10 6.10 − 7.06 − 8.32 Myrothecium roridum − − − 4.75 − 3.65 − − − − − − − 3.15 − 3.64 − 3.10 − 3.46 − 2.60 − 3.95 Penicillum lilacinum 9.15 − 11.10 − − − − − − − − − − − 11.15 − 12.30 − 10.50 − 12.10 − 10.70 − Pestalotia glandicola − 10.15 10.05 10.10 9.86 − − − − − − − 10.20 10.25 − − − − 11.35 11.40 10.60 11.15 10.85 10.15 Rhizoctonia solani − 3.42 − 4.15 − − − − − − − − − − − 4.08 − 4.26 − 4.96 − 2.15 − − Trichoderma harzianum − − 13.20 − − − − − 12.30 − 12.40 − 12.70 − 10.35 − 13.10 − 12.76 − 13.10 − 13.40 − Verticillium albo-atrum − 2.01 − 2.05 − 3.09 − − − − − 2.15 − 2.96 − 4.98 − 3.26 − 1.95 − 2.35 − − AI, AMF infected ; AU, AMF Uninfected. F, Percentage frequency; −, Percentage frequency NIL. Table 5. Monthly fluctuations in the percentage abundance of rhizosphere mycoflora of AM fungi infected and AM fungi free plants of Saccharum officinarum L.
Fungi Summer Monsoon Winter Annual March AMF
infectedJune AMF free July AMF
infectedOctober AMF free November AMF
infectedFebruary AMF free March AMF
infectedFebruary AMF free F A F A F A F A F A F A F A F A Mucor racemosus 14 1.89 15 1.92 37 6.33 28 5.1 57 7.56 60 8.25 36 5.26 34 5.09 Rhizopus oryzae 47 10.80 73 13.74 76 12.94 57 13.90 81 14.29 86 14.68 68 12.67 72 14.10 Rhizopus varians − − 17 3.20 − − 61 12.22 − − 65 12.74 − − 49 9.39 Chaetomium globosum − − 10 1.70 14 2.30 22 3.60 26 4.03 26 4.12 13 2.11 19 3.08 Neocosmospora vainfecta − − 3 0.47 − − 10 1.35 − − 9 0.99 − − 7 0.16 Alternaria alternata − − 8 1.46 − − 23 4.23 − − 16 2.83 − − 13 2.38 Aspergillus candidus 9 0.85 10 2.56 43 6.72 49 9.93 45 4.92 68 13.01 33 4.16 42 8.50 Aspergillus flavus 28 6 32 6.47 54 12.43 59 13.63 50 9.65 62 10.75 47 9.36 51 10.29 Aspergillus niger 55 9.40 68 13.53 57 11.06 62 12.90 79 13.31 83 15.23 67 11.26 71 13.88 Aspergillus sydowi 8 2.17 − − 42 6.72 − − 56 5.00 − − 26 4.63 − − Aspergillus terreus 22 6.00 50 6.47 66 12.43 70 13.63 53 9.65 59 10.75 48 9.36 53 10.29 Cladosporium herbarum 7 1.31 9 1.36 30 4.85 42 7.25 9 1.38 9 1.41 15 2.51 21 3.34 Cephalasporium sacchari − − 8 1.36 − − 35 5.36 − − 27 4.21 − − 23 3.64 Curvularia lunata − − − − − − 47 6.55 − − 38 4.98 − − 28 3.86 Fusarium moniliforme − − 9 1.23 − − 43 6.51 − − 25 3.55 − − 26 3.76 Fusarium solani − − 20 2.75 − − 37 4.94 − − 49 6.07 − − 35 4.58 Fusarium semitectum − − 20 2.98 − − 46 6.12 − − 50 6.65 − − 38 6.25 Helminthosporium halodes − − 8 1.34 − − 30 4.73 − − 9 1.52 − − 16 2.53 Macrophomina phaseolina 9 2.23 19 4.35 25 2.38 28 3.83 − − 24 3.84 11 1.54 23 4.01 Myrothecium roridum − − 9 0.91 − − 28 3.33 − − 23 2.82 − − 20 3.36 Penicillum chrysogenum 8 1.28 − − 35 5.40 − − 52 8.63 − − 31 5.11 − − Penicillum lilacinum − − − − 47 8.48 − − 57 10.28 − − 35 6.26 − − Pestalotia glandicola 11 2.46 − − 30 5.38 30 5.41 43 7.87 59 10.38 28 5.24 30 5.27 Rhizoctonia solani − − − − − − 27 3.32 − − 27 2.43 − − 16 1.92 Trichoderma harzianum 29 6.17 − − 70 12.22 − − 59 9.92 − − 52 9.44 − − Verticillium albo-atrum − − 15 1.31 − − 33 3.28 − − 34 3.09 − − 27 2.56 F, percentage frequency; A, percentage abundance; −, percentage frequency NIL. Whereas the proportion of frequency and abundance of M. phaseolina in the rhizosphere soil of AM fungi is less. Additionally, the remaining species were moderate in the soil samples. It was generally observed that the frequency of representation of saprophytic fungi like Rhizopus, Aspergillus, Penicillium, and Trichoderma was high, while root rot-causing fungi like Fusarium, Cephalosporium, and Rhizoctonia were almost nil in AM fungi inoculated plants. The percentage frequency and abundance of fungi run parallel to each other during different months throughout the year.
A marked variation in the percentage frequency and abundance (Table 5) of different fungi occurs during different seasons in both AM fungi inoculated and uninoculated in the rhizospheric soil of sugarcane plants. A minimum number of fungi and minimum percentage of frequency of different fungi was recorded during the summer. C. globosum, and P. lilacinum were found to be absent during the summer in the rhizosphere soil of AM fungi-infected plants and C. lunata and P. glandicola were found to be absent during the summer season of uninoculated plants. Winter proved to be the most favorable season for most of the fungi. The occurrence and distribution of several fungi were shown to be higher in the rhizosphere soil of inoculated and non-inoculated plants than AM fungi during this season. In general, the late monsoon and early winter period was found to be the best for growth and development of these fungi. Among the various organisms isolated from the rhizosphere soil, R. oryzae and A. niger proved to be most dominant and tolerant of environmental conditions as they were found throughout the year in the rhizosphere soil of both AM fungi inoculated and uninoculated sugarcane plants. A. sydowi, C. globosum and P. chrysogenum in AM fungi inoculated and Neocosmospora vasinfects, A. alternata, A. candidus, C. lunata, T. harzianum, F. semifectum, M. phaseolina, R. solani, M. roridum and V. albo-atrum in uninoculated plants, however, were found to be sensitive to environmental changes as they appeared only for a few months.
Rhizosphere fungi in relation to AM fungi
-
The recovered number of fungal populations in the rhizosphere soil of AM fungi inoculated and uninoculated sugarcane plants fluctuated during different months (Tables 3−5). The sampling results indicated that total fungal frequency was highest in the rhizosphere soil of AM fungi inoculated and non-inoculated plants during the different seasons of winter (November–February), monsoon (July–October), and summer (March–June). The AM fungal population of spores in the rhizosphere soil and the concentration of mycorrhizal attachment in the roots of sugarcane plants also fluctuated in different months. The maximum population of AM fungal spores and severity of root infection was observed in July and the minimum population of AM fungal spores occurred in February, while the maximum fungal population (i.e., total frequency and abundance of fungi) occurred in October and the minimum frequency, and an abundance of fungi occurred in May. The fluctuations in spore population and root infection were caused by environmental factors and seasonal variation. The maximum concentration of AM fungi spores and root colonization were recorded from April to July and the minimum concentration from November to March. In winter, an inverse relationship was observed between rhizosphere fungi and the AM fungi. The concentration of different rhizosphere fungi was higher in winter but the number of AM fungi and their attachment in roots were lower. Control soils contain opportunistic fungi that are naturally present in the soil.
Statistical analysis revealed a significant treatment effect over different periods, with plots that received AM fungi inoculation displaying significantly higher root colonization compared to non-inoculated plots (control). These fungi are part of the soil's intrinsic microbial community and sustain themselves over time through natural processes.
-
The rhizosphere incidence of AM fungi varied with the number of fungal populations in treated and non-treated plants. The occurrence and distribution of many fungal species were reduced or totally suppressed in the rhizospheric soil of AM fungi-inoculated plants. The beneficial effect of mycorrhizae is not only attributed to nutritional factors but also to the protection of roots against soil pathogens. Agarwal[30] reported that mycorrhizal fungi utilize the surplus of nutrients from soil which becomes a limiting factor for other soil fungi. He also reported that mycorrhizal fungi may trigger the production of antifungal substances which may reduce the chances of infection by soil pathogens. In the present experiments also, the parasitic fungi were almost eliminated from the rhizosphere soil of AM fungi-mediated plants.
It was observed the AM fungal species diversity and denticity in sugarcane field soil samples of 10 different locations from Pusa, Samastipur, Bihar, India (Table 1). Rhizosphere fungal populations depend on the micro-environment and nutrient status, which depends on the physiological state of the plant[31] and the pattern of its root exudation[32]. Since the physiological condition of plants and the pattern of their root exudation are expected to vary in different seasons, and stages of plant growth, the fungal population in the rhizosphere varies accordingly (Table 2). Although the occurrence of fungal species was significantly different compared to the control, the present results are in conformity with the observations made by Bagyaraj & Menge[15]. The harshness of mycorrhizal root attachment and the population of AMF spores in rhizospheric soil vary from season to season, due to plant age and plant physiology. However, these findings are similarly presented in previous research work, where root exudation of rhizospheric fungi and their fluctuations in environmental conditions[12−15].
AM fungal species were commonly identified in rhizosphere soils of all studied sugarcane fields. Previous research[33−35] have reported that plants previously inoculated with AMF exhibited resistance toward soil-borne diseases like wilt and root. Zambolin & Schenck[36] observed the interaction of G. mosseae and M. phaseolina species that the colonization in the host root of soybeans and inhibits pathogens, and enhances the growth in the host. Similarly, Caron et al.[37] studied that root colonization by Glomus species was not affected by the presence of Fusarium. The number of campaigns of Fusarium sp. plants were consistently absent when inoculated with a mycorrhizal endophyte. In this study, the occurrence and diversity of G. fasciculatum totally inhibited the disease-causing pathogens viz., F. moniliforme and C. sacchari in the rhizosphere soil of AM fungi-mediated sugarcane plants (Table 2). Thus, the findings of the diversity results of maximum fungal species found in the winter (November–February), monsoon (July–October), and summer (March–June). The AM fungal population of spores in the rhizosphere soil and the concentration of mycorrhizal attachment in the roots of sugarcane plants also fluctuated in different months.
Mycorrhizal colonization rate showed a significant difference between treated and untreated plants (Table 2). These results are correlated with the study conducted on maize sorghum, where AMF inoculation considerably increased soil nutrient availability[12]. A few previous researchers have revealed that the variation of AM fungal species intensity, their host plants' age, phonology, soil nature, root morphology, and environmental factors also may be affected. Previous studies indicated that the intraradical development of AM fungi is highly influenced by plant species, soil pH, and phosphorus content[12−14,36,38−40].
Different diversity of soil fungal communities was observed in the sugarcane soil samples at different locations of the sampling sites. Among these identified AM fungal species, R. fasciculatus, G. aggregatum, R. intraradices, F. mosseae, and G. constrictum and G. macrocarpum; two species of Gigaspora, namely Claroideoglomus claroideum and G. gigantean and two species of Acaulospora, namely Acaulospora tuberculate and A. laevis and one species of Sclerocystis spp. However, AM fungal community variations and frequency being higher especially Glomus species compared to the control sample (Tables 1−5). The results of the AM fungal diversity study are very similar to previous reports where there is a correlation between diversity and abundance[11−13,37]. Thus, this study suggests that sugarcane root serves as a suitable host for these AM species to form a mutualistic symbiosis.
Hence, the study presents clear and significant results, demonstrating the significant impact of AMF on sugarcane growth and yield, along with a noticeable differences between the seasonal abundance of fungal species. However, the findings were limited to the specific conditions and cultivars used in this study, and their applicability to other regions or sugarcane varieties could be addressed.
-
The author confirms sole responsibility for all aspects of this article.
-
The data that support the findings of this study are available on request from the corresponding author.
-
Author is thankful to the Director, Sugarcane Research Institute, Pusa, Samastipur for Sugarcane seeds and Prof. J.J. Deploey, Pennsylvania University, Pennsylvania, USA for his encouragement and his valuable guidance and comments on the manuscript.
-
The author declares that there is no conflict of interest.
-
Received 28 May 2024; Accepted 20 August 2024; Published online 11 October 2024
-
Different types of fungal species were obtained in the rhizosphere soil of AM fungus-infected and non-infected sugarcane plants.
Outcome of our isolation process, abundance of rhizosphere fungi isolates was lower in plants infected with AM fungi than in plants without mycorrhizal infection.
The occurrence and distribution of different rhizosphere fungi varied from plants infected with AM fungi compared to uninfected plants.
We observe that parasitic fungi are virtually eliminated from the rhizosphere soil of plants infected with AM fungi.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Prasad K. 2024. Occurrence and interactions of arbuscular mycorrhizal fungi (Rhizophagus fasciculatus) and rhizospheric fungi in Saccharum officinarum L. Tropical Plants 3: e033 doi: 10.48130/tp-0024-0035
Occurrence and interactions of arbuscular mycorrhizal fungi (Rhizophagus fasciculatus) and rhizospheric fungi in Saccharum officinarum L.
- Received: 28 May 2024
- Revised: 09 August 2024
- Accepted: 20 August 2024
- Published online: 11 October 2024
Abstract: Arbuscular Mycorrhizal fungi (AM fungi) promote plant growth and enhance nutrient uptake under unfavorable conditions. The present study investigates the interactions between AM fungi and rhizospheric fungi in sugarcane soil samples from sugarcane Pusa, Samastipur, districts, India. Sugarcane is the major crop mainly cultivated in these regions. Mycorrhizae and rhizosphere fungi were examined by isolating fungi from the soil of AM fungal infected and uninfected sugarcane crop field, 14 fungi belonging to nine genera isolates were obtained from the soil sample of AM fungi infected plant, on the other hand uninfected sugarcane yielded, 22 fungi belonging to 16 genera. In all, 10 fungi were common in both the rhizospheric soil of AM fungi-infected and uninfected sugarcane plants. The frequency and abundance of rhizospheric fungi were lower in plants infected by AM fungi than in plants without mycorrhizal infections. Furthermore, the presence of wilt-causing organisms and parasitic fungi was significantly lower in the soil of AM fungi-infected plants, while saprophytic organisms were more abundant. The frequency of different rhizosphere fungi during different months in a year fluctuated from 175 to 794 in AM fungi-infected plants, while the occurrence of different soil fungal isolates in the soil of non-infected plants fluctuated from 199 to 1,041. Parasitic fungi were almost eliminated from the soil of AM fungi-mediated plants. The results showed a significant interaction between AM fungi intensity and rooting time colonization compared to the control sample. Therefore, the present study data provides detailed knowledge on AM fungal inoculum and its effect on plant growth in a specific area.
-
Key words:
- AM fungi /
- Rhizospheric soil /
- Colonization /
- Plant growth.