-
Overpopulation in Egypt has led to an increases in the demand for food supply, which has focused the attention to raising crop production. This has led to an increase in the application of chemical fertilizers, especially in the cultivation of vegetable crops, where large amounts of chemical fertilizers are used to obtain the highest productivity[1]. The rates of chemical fertilizers applied in the cultivation of vegetable crops are considerably higher compared to other crops because of intensive cultivation and vegetative organs (leaves) as final products[2−4]. This in turn can lead to negative side effects on the environment and human health issues[5,6]. The addition of mineral fertilizers cannot replace the soil organic matter (SOM) that may be lost due to intensive crop production[7,8]. Maintaining appropriate levels of organic matter in soils is important for the sustainable management of light soils[9]. Instead, vermicompost (VC) and compost (CT) applications may improve the content of SOM. Compost and vermicompost could be alternatives to chemical fertilizers[9,10]. Vermicompost is the product of organic matter degradation through the interaction between earthworms and microorganisms[11,12]. Vermicompost is considering a biofertilizer with a varied microbial community[13−15]. Vermicompost contains nutrients such as P, K, Ca, and Mg[16,17]. Vermicomposting and composting are two distinct processes, and it is crucial not to confuse the two[18]. The vermicomposting process produces a high diversity and number of microorganisms because the temperature during VC production is suitable for worms[19]. A decrease in the C/N ratio of the vermicompost was testified than the compost[20].
Zucchini (Cucurbita pepo L.) is one of the most important vegetable crops grown in Egypt. Zucchini plants are very diverse and popular for human consumption throughout the world[21]. However, zucchini fruit contains many nutrients, bioactive compounds (antioxidants, flavonoids, vitamins) and have a high amount of dietary fiber, which is very low in calories[21]. Intensive zucchini production in Egypt requires higher rates of chemical fertilizers.
Soil degradation and its expansion are considered the greatest problem in Egypt, directly affecting food security and crop production[22,23]. Soil nutrients are necessary for crop growth and development and are critical factors for soil fertility along with adequate soil moisture, which is considered a key factor for crop growth and yield[24]. Therefore, manipulating nutrient release is an advanced and effective way to maintain sustainable crop production including zucchini[25,26]. Farmers assume that the extensive use of CF leads to better yields of various crops without considering the hazardous effects on the environment. The continuous use of chemical fertilizers only has a negative impact on soil fertility[27,28].
To ensure a healthy diet and minimize environmental risks of chemical fertilizer, and in line with sustainable development programs that call for a return to nature, this study was conducted to assess replacing chemical fertilizers with the vermicompost and compost on soil fertility, growth, and yield of zucchini cultivation under field conditions. It was hypothesized that the compost and vermicompost application would help produce zucchini fruit free from the residual effects of the chemical fertilizer and improve sandy soil properties under the arid conditions of Egypt.
-
This field experiment was carried out in 2021 and 2022 seasons at a commercial vegetable farm in Assiut City, Egypt (27°12'16.67" N; 31°09'36.86" E). Soil samples (0−20 cm) were collected, air-dried and ground to pass through a 2-mm sieve. Particle size distribution was determined according to the pipette method[29]. Soil pH and EC were determined in a 1:2.5 suspension using pH and EC-meter, respectively. The soil organic matter (OM) was determined by using the method of Jackson[29], while total calcium carbonate in the soil was determined by Collin's calcimeter method[29]. Available soil P was determined[30]. While available soil N and available K were determined according to Kompała-Bąba et al.[31]. The soil characterization before cultivation is presented in Table 1. Compost was produced from plant residues, obtained from the Nile Company, Egypt, while vermicompost was brought from the Agricultural Research Center (ARC), Egypt. Some chemical characteristics of the compost and vermicompost are presented in Table 2.
Table 1. Some physical and chemical properties of the soil before experiment.
Property Unit Value Sand g·kg−1 457 ± 10.5 Silt g·kg−1 327 ± 6.90 Clay g·kg−1 216 ± 5.40 Texture − Silty loam CaCO3 g·kg−1 32.7 ± 2.57 EC (1:2.5) dS·m−1 0.63 ± 0.10 pH (1:2.5) − 7.90 ± 0.05 OM g·kg−1 11.3 ± 1.39 Available N mg·kg−1 60.4 ± 5.40 Available P mg·kg−1 14.7 ± 1.30 Available K mg·kg−1 397 ± 5.30 Table 2. Characteristics of the compost and vermicompost.
Property Unit Compost Vermicompost EC (1:5) dS·m−1 4.39 ± 0.07 3.89 ± 0.82 pH (1:5) − 7.77 ± 0.07 7.88 ± 0.05 (OC) g·kg−1 203 ± 3.12 237.6 ± 2.89 Total N g·kg−1 16.4 ± 2.31 16.9 ± 6.76 Total P g·kg−1 6.80 ± 1.43 12.9 ± 0.84 Total K g·kg−1 18.7 ± 0.80 10.9 ± 0.90 C/N ratio − 12.4 ± 0.70 14.1 ± 1.20 Each value represents a mean ± standard error (SE) of five replicates. Field experiment
-
The experimental plot dimension was 4.5 m × 3.0 m with three terracing at a distance of 90 cm with ridge spacing of 40 cm in a row (18 plants·plot−1 equal to 13,330 plants·ha−1). The treatments were: CO = control, CF = chemical fertilizer, CT = compost, and VC = vermicompost, and were arranged in a complete randomized design with five replicates. For the CF treatment, the recommended NPK fertilizers were applied at the rate of 178.5 kg N·ha−1, 71.4 kg P2O5·ha−1, and 119 kg K2O·ha−1. Compost and vermicompost were applied as full dose before planting, nitrogen fertilizer as urea (46% N) was added in three doses: 20% as basal application, 40% 15 d after sowing (DAS), and 40% 30 DAS. Potassium fertilizer as potassium sulfate was applied in three splits: 40% at 30 DAS, 30% 30 d from the first addition, and 30% 30 d after the second addition; while the phosphorus fertilizer (superphosphate) was applied in one split as a basal application before sowing. The application rates of compost and vermicompost were 7.15 t·ha−1 according to the Egyptian Ministry of Agriculture and were applied as full dose before sawing. The compost and vermicompost and chemical fertilizer was mixed well with soil by raking to a depth of 10 cm. Seeds of zucchini (Cucurbita pepo L.) were planted directly in soil on Feb 15th 2021 and 2022 growing seasons.
Data collection
-
The zucchini plant samples were collected 50 DAS and the fresh and dry weights were determined using an electronic balance. In addition, the total chlorophyll index (SPAD) of the leaves was measured using (502-m Konica Minolta, Inc., Tokyo, Japan) taking four measurements per leaf. The zucchini fruit were harvested 90 DAS and yield parameters such as length of fruit, fruit diameter, fruit number, fruit weight, and fruit yield were recorded.
Plant analysis
-
The zucchini fruit samples were cleaned, and washed with distilled water, oven-dried (70 °C) for 48 h and then the digested by methods adopted by Page et al.[32] and finally the total concentrations of N, P, and K were measured according to previously described methods described[32,33].
Soil analysis at harvesting
-
After harvesting, soil samples were taken from each pot, air-dried, crushed, passed through a 2 mm sieve and then available, N, P, and K were determined according to methods described by Page et al.[32].
Nutrient use efficiency (NUE) is defined as the fruit yield obtained per amount of applied fertilizer and was calculated as:
$ NU E = (Y_{t} - Y_{0})_{ }/ N $ where, Yt = fruit yield of treatment (kg); Y0 = fruit yield of control (kg) and N is the amount of applied fertilizers (kg).
Plant uptake = Dry weight × Nutrient concentration.
Statistical analysis
-
Statistical analyses were run using analysis of variance technique by using statistics 8.10 software packages. Means of treatments were compared using the Duncan tests (p < 0.05). Principal component analysis (PCA) were run by Past software, version 4.06 and also the correlations among the soil properties and plant traits were calculated.
-
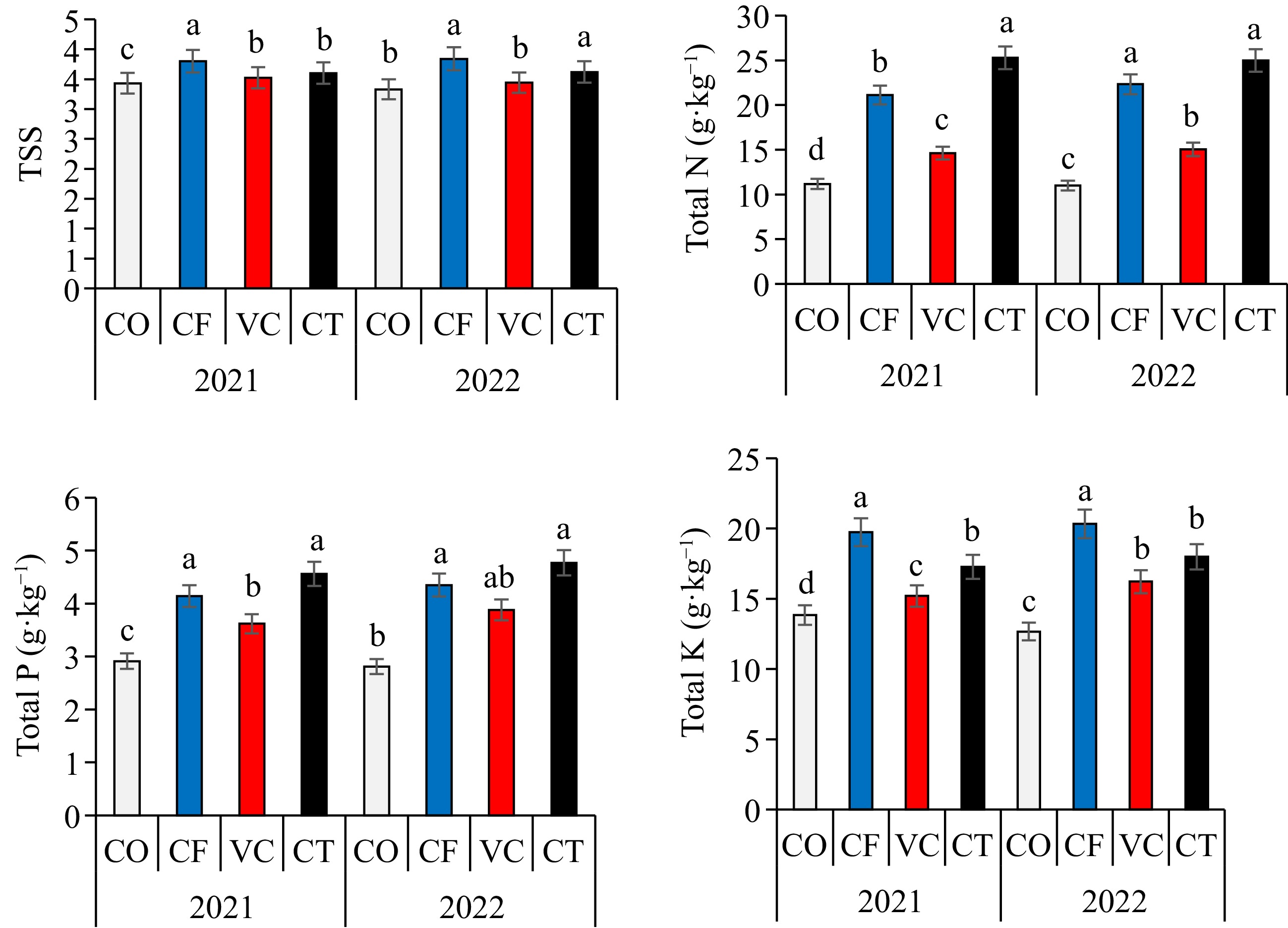
Application of CT, VC, and CF treatments significantly (p < 0.05) increased the fresh and dry weights of zucchini plants compared with CO treatment in the 2021 and 2022 seasons (Fig. 1). The fresh weight of the treatments could be arranged in a descending order: CT > VC > CF > CO, while in the dry weight can be arranged in a descending order: CT > CF > VC > CO. The highest leaf SPAD value was recorded in CT treatment. Significant differences were found in the fruit number, fruit length, fruit diameter, and zucchini yield among treatments (Fig. 2). Compared to the CO treatment, the fruit number per plant increased in the CF, VC, and CT by about 21%, 10%, and 37%, respectively. Fruit weight increased by about 1%, 5%, and 8%, respectively, in the CF, VC, and CT treatments, while fruit length increased by about 24%, 10%, and 13%, respectively. Compared to the CO treatment, the fruit diameter increased by about 21%, 6%, and 10%, respectively, while CF, VC, and CT treatments increased fruit dry weight by about 13%, 9%, and 5%, respectively. The highest yield of zucchini fruit was recorded in CT treatment in the two seasons. Significant difference was found in the total soluble solids and total concentration of N, P, and K in zucchini fruit (Fig. 3). The CF treatment, followed by VC recorded the highest total soluble solids and total NPK contents.
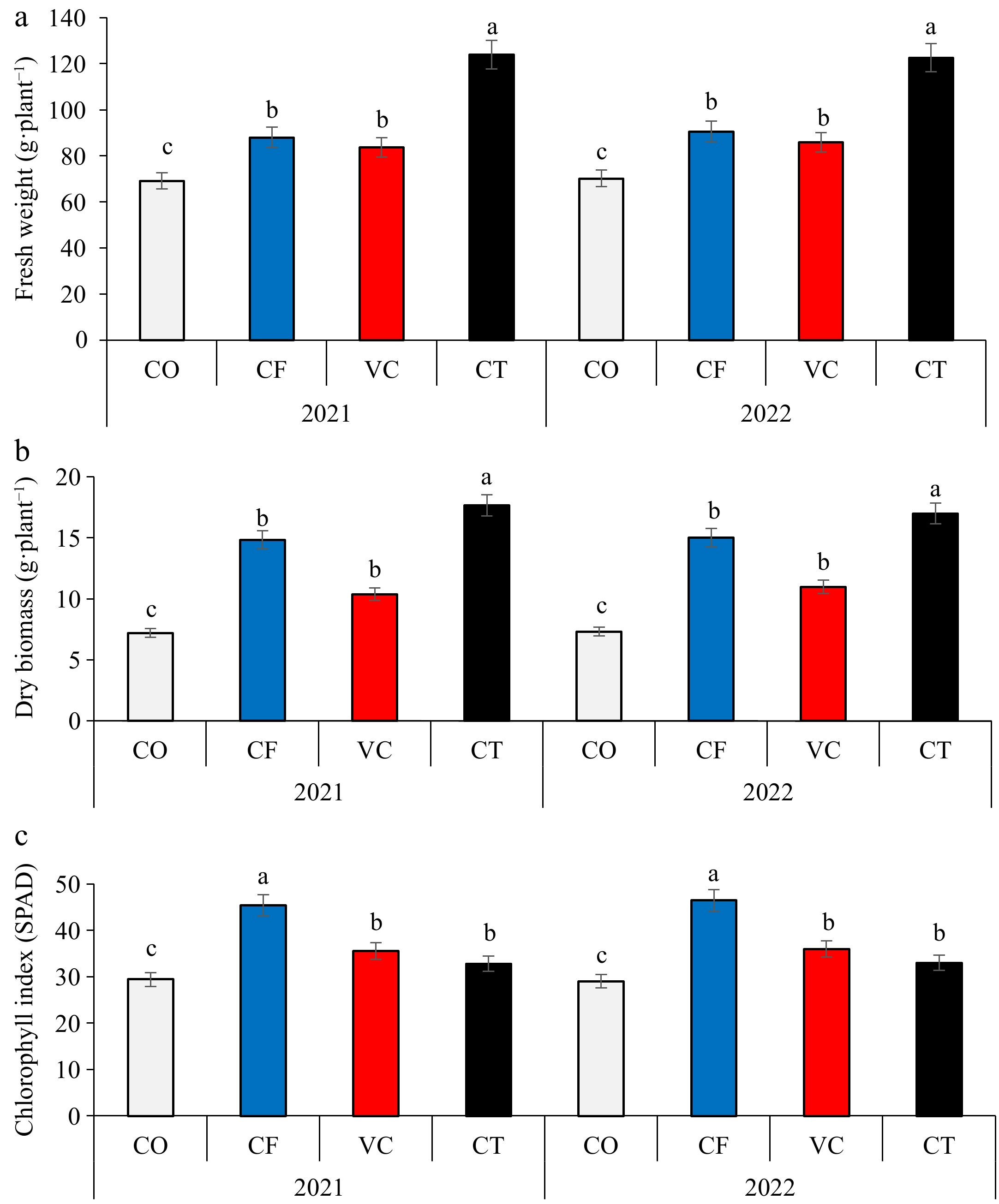
Figure 1.
Effect of compost and vermicompost application on (a) fruit fresh biomass, (b) fruit dry biomass, and (c) chlorophyll index of zucchini plants.
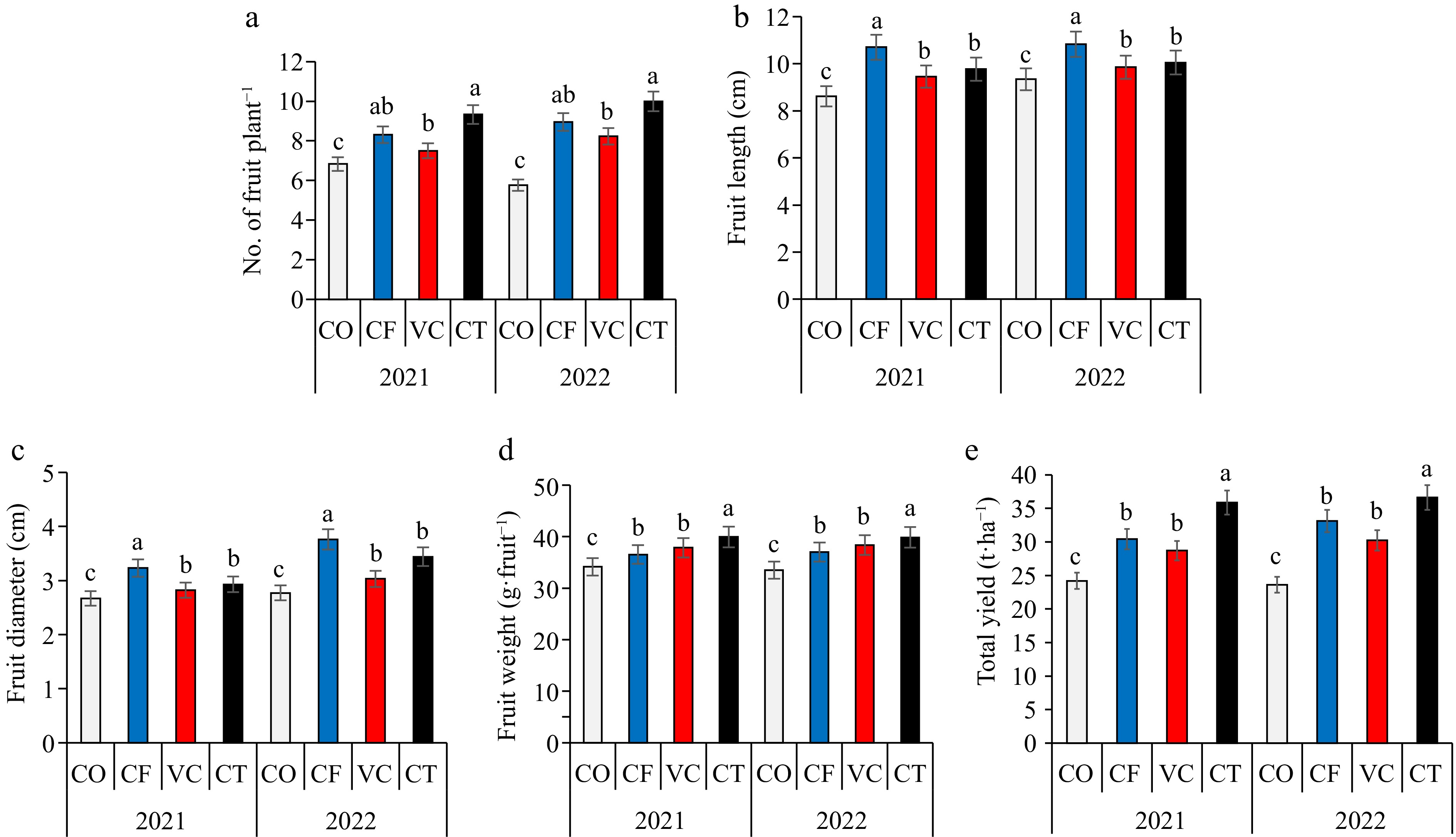
Figure 2.
Impact of different fertilizers treatment on (a) fruit number, (b) fruit length, (c) fruit diameter, (d) fruit weight, and (e) yield.
Zucchini uptake and nutrient use efficiencies
-
A significant difference was found in the uptake of NPK by zucchini fruit between the treatments (Table 3). The CT treatments significantly increased the uptake of NPK. Consequently, the uptake of NPK could be arranged in the descending order: CT > CF> VC > CO treatments in the 2021 and 2022 seasons. Significant differences were found in the agronomic NUE, PUE, and PUE by zucchini fruit among the treatments. In general, the CT treatment significantly increased the agronomic NUE, PUE, and PUE by zucchini fruit (Table 3).
Table 3. Effect of different fertilizer treatments on total NPK uptake and nutrients use efficiency.
Year Treatments N uptake (kg·ha−1) P uptake (kg·ha−1) K uptake (kg·ha−1) NUE (kg·kg−1) PUE (kg·kg−1) KUE (kg·kg−1) 2021 CO 27.83 ± 0.36d 7.25 ± 0.75d 34.46 ± 1.32c − − − CF 108.25 ± 1.23b 21.22 ± 1.21b 101.24 ± 3.12a 34.9 ± 0.87b 87.2 ± 2.12b 52.3 ± 1.45b VC 52.46 ± 1.02c 12.98 ± 1.54c 54.55 ± 2.54b 25.2 ± 0.56c 63.1 ± 1.88c 37.8 ± 0.98c CT 154.24 ± 2.33a 27.85 ± 0.97a 105.43 ± 2.64a 65.3 ± 1.34a 163.3 ± 3.21a 98.0 ± 1.98a 2022 CO 27.89 ± 0.54d 5.93 ± 0.54d 32.10 ± 0.43c − − − CF 115.99 ± 2.43b 22.60 ± 1.03b 105.60 ± 1.55a 53.2 ± 0.88b 133.0 ± 3.65b 79.8 ± 1.56b VC 57.16 ± 1.54c 14.74 ± 0.87c 61.61 ± 0.98b 37.2 ± 0.98c 92.9 ± 0.78c 55.8 ± 0.78c CT 146.99 ± 2.32a 28.06 ± 1.32a 105.82 ± 1.01a 72.8 ± 1.23a 182.1 ± 3.11a 109.2 ± 0.54a Means within a column followed by the same letter do not differ significantly (p < 0.05) according to DMRT. Soil chemical properties
-
After harvest, significant differences were recorded in pH, EC, OM, and availability of soil NPK contents among the different fertilizers (Table 4). At harvest, the CT and VC treatments slightly reduced the soil pH, while, the pH value in the CF treatment increased to 7.83 and 7.85 in 2021 and 2022, respectively. CF treatment recorded the highest pH value, while CT treatment recorded the lowest in the 2021 and 2022 growing seasons. Compared to the CO treatment, the CF, VC, and CT significantly (p < 0.05) increased the EC value by 39%, 17%, and 53%, respectively. A significant difference was observed in soil available N and K between the treatments. Soil available N and soil available K was significantly enhanced by the CT and VC treatments. Consequently, soil available P and soil available K could be arranged in descending order: CT > CF > VC > CO treatments.
Table 4. Effects of different fertilizer treatments on some selected soil properties.
Year Treatments pH EC (dS·m−1) OM (g·Kg−1) Available N (mg·kg−1) Available P (mg·kg−1) Available K (mg·kg−1) 2021 CO 7.80 ± 0.02a 0.38 ± 0.01c 10.9 ± 0.21c 50.4 ± 1.12c 8.71 ± 1.43c 221.7 ± 1.76c CF 7.83 ± 0.01a 0.53 ± 0.01a 10.7 ± 0.02c 60.2 ± 1.23b 16.4 ± 0.92a 466.4 ± 4.12b VC 7.76 ± 0.02b 0.45 ± 0.02b 13.5 ± 0.10b 67.8 ± 1.23a 12.7 ± 1.32b 452.7 ± 4.98b CT 7.71 ± 0.01c 0.58 ± 0.02a 16.1 ± 0.12a 71.9 ± 1.04a 18.8 ± 0.23a 689.2 ± 3.23a 2022 CO 7.77 ± 0.05b 0.36 ± 0.02c 10.6 ± 0.88c 54.8 ± 2.02b 9.20 ± 1.34c 201.9 ± 2.54c CF 7.85 ± 0.03a 0.58 ± 0.02a 10.6 ± 0.76c 58.0 ± 1.98b 17.7 ± 1.43a 426.5 ± 1.76b VC 7.74 ± 0.01b 0.53 ± 0.01b 14.5 ± 0.78b 64.9 ± 2.32a 11.4 ± 0.98b 462.9 ± 1.34b CT 7.68 ± 0.03c 0.62 ± 0.03a 17.2 ± 0.98a 68.7 ± 1.32a 17.6 ± 1.23a 609.3 ± 4.44a Means within a column followed by the same letter do not differ significantly (p < 0.05) according to DMRT. Correlations between soil characteristics and zucchini traits
-
The first two principal components contributed 93.7% of the differences between soil properties and zucchini parameters (Table 5). PC1 contributed about 65.1% of the differences and was significantly and positively correlated with soil electrical conductivity (EC), organic matter (OM), available N (AN), available P (AP), available K (AK), average fruit number (AFN), fruit weight (FW), fresh biomass (Fb), dry biomass (Db), nitrogen uptake (NUp), and phosphorus uptake (Pup). While PC2 contributed about 20.8% of the differences and were correlated positively with fruit length (FL), fruit diameter (FD), fruit dry matter (DM), total chlorophyll (TCh), total soluble solids (TSS), potassium uptake (KUp), and total yield (TY), and negatively correlated with the soil pH. The addition of CT and CF treatments positively increased the nutrient availability and zucchini growth indicators.
Table 5. Correlations coefficient among soil properties and zucchini traits.
Variables pH EC OM AN AP AK AFN FW FL FD DM TCh Fb Db TSS NUp Pup KUp TY pH 1.0 EC −0.46 1.0 OM −0.98 0.59 1.0 AN −0.88 0.68 0.95 1.0 AP −0.58 0.99 0.70 0.74 1.0 AK −0.69 0.94 0.81 0.88 0.97 1.0 AFN −0.56 0.99 0.67 0.71 1.00 0.95 1.0 FW −0.93 0.67 0.98 0.99 0.75 0.88 0.73 1.0 FL 0.19 0.75 −0.01 0.21 0.64 0.57 0.65 0.14 1.0 FD 0.29 0.71 −0.12 0.08 0.59 0.48 0.60 0.02 0.99 1.0 DM 0.30 0.49 −0.11 0.19 0.36 0.39 0.35 0.07 0.89 0.84 1.0 TCh 0.53 0.44 −0.36 −0.10 0.29 0.23 0.30 −0.19 0.92 0.93 0.93 1.0 Fb −0.78 0.91 0.85 0.83 0.96 0.96 0.96 0.87 0.41 0.35 0.14 0.03 1.0 Db −0.45 1.00 0.58 0.65 0.99 0.93 0.99 0.65 0.75 0.71 0.46 0.43 0.91 1.0 TSS 0.31 0.69 −0.15 0.05 0.58 0.46 0.59 −0.01 0.98 1.00 0.82 0.93 0.33 0.70 1.0 NUp −0.45 0.99 0.57 0.63 0.99 0.92 0.99 0.64 0.72 0.69 0.42 0.40 0.91 1.00 0.68 1.0 Pup −0.47 1.00 0.61 0.71 0.98 0.96 0.98 0.70 0.76 0.70 0.53 0.45 0.90 0.99 0.69 0.98 1.0 KUp 0.18 0.79 −0.02 0.16 0.69 0.57 0.70 0.11 0.98 0.99 0.78 0.87 0.46 0.79 0.99 0.78 0.78 1.0 TY −0.35 0.63 0.51 0.75 0.59 0.73 0.56 0.65 0.63 0.50 0.77 0.50 0.52 0.59 0.47 0.54 0.69 0.50 1.0 Electrical conductivity (EC), organic matter, (OM), available N (AN), available P (AP), available K (AK), fruit number (AFN), fruit weight (FW), fresh biomass (Fb), dry biomass (Db), N uptake (NUp), P uptake (Pup), fruit length (FL), fruit diameter (FD), fruit dry matter (DM), total chlorophyll (TCh), total soluble solids (TSS), P uptake (KUp) and yield (TY). -
The current study showed that compost and vermicompost application significantly improved soil fertility through increases in OM content, soil availability of N, P, and K. Therefore, these changes in soil characteristics led to improvement in the growth, yield, fruit quality, and nutrient uptake of zucchini. At harvest, the compost and vermicompost treatments slightly reduced the soil pH probably due to the production and release of hydrogen ions, organic acids, and carbon dioxide gases as suggested by previous studies[34−36]. The increase in soil salinity that accompanies the application of composts is a major environmental concern[37,38]. However, the data obtained indicated that the compost and vermicompost application has increased the soil EC slightly. These results may be due to the higher nutrient uptake by zucchini. Significant difference was observed in the availability of N and P contents among treatments. These results could be attributed to the higher P and K contents in compost and vermicompost. The application of compost and vermicompost significantly increased soil OM content. Organic matter addition directly through the use of compost and vermicompost could enhance the zucchini growth, and yield. In addition, the residual impact of compost and vermicompost increased the zucchini yield[39−42]. Organic matter provides substrates for decomposing microbes, which led to improved soil structure and increased soil holding capacity[43−46]. The application of compost and vermicompost recorded the highest values of N, P, and K soil availability and uptake confirming their ability to increase the efficient use of N, P, and K applied fertilizers. The growth, quality, and yield of zucchini were improved by the application of compost and vermicompost. Positive effects of compost and vermicompost amendments on the growth and fruit yield of zucchini may be related to the supply of mineral nutrients during the mineralization of compost and vermicompost[47,48]. Increases in dry weight of the zucchini plant can be explained through increased nutrient release through direct application of compost and vermicompost and increase the soil microorganisms, which enhance sand soil fertility[49,50]. Soils under semi-arid conditions, in Egypt, are highly alkaline and consequently, reducing the soil pH by using compost and vermicompost is important to increase the soil fertility.
-
The research findings highlight the significant positive effects of compost and vermicompost application on growth, yield of Zucchini, fruit uptake, as well as some soil fertility properties. Notably compost and vermicompost applications significantly improved soil organic matter, availability of soil nitrogen, phosphorus, and potassium and could be applied partially with chemical fertilizers in zucchini cultivation under field conditions. The application of compost and vermicompost resulted in enhancements across various parameters, including fruit growth and fruit yield. Compost and vermicompost modified the soil properties which increased zucchini grain yield.
-
The authors confirm contribution to the paper as follows: study conceptualization, methodology, investigation, formal analysis, visualization, and writing the original draft: Rekaby SA, Ghoneim AM, Gebreel M, Ali WM, Yousef AF, Mahmoud E. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors express their gratitude to the Faculty of Agriculture, Al-Azhar University (Assiut Branch), Egypt, for their assistance during this work. The authors would like to express their gratitude to the Field Crops Research Institute, Agricultural Research Center, Egypt.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Rekaby SA, Ghoneim AM, Gebreel M, Ali WM, Yousef AF, et al. 2024. Impact of some organic fertilizers on nutrients uptake, yield of Zucchini (Cucurbita pepo L.) and soil fertility properties. Technology in Agronomy 4: e030 doi: 10.48130/tia-0024-0029
Impact of some organic fertilizers on nutrients uptake, yield of Zucchini (Cucurbita pepo L.) and soil fertility properties
- Received: 27 May 2024
- Revised: 19 August 2024
- Accepted: 27 September 2024
- Published online: 26 November 2024
Abstract: Maintaining appropriate levels of organic matter is important as it ensures efficient nutrients, which contributes to the sustainable management of sandy soil and crop production. Compost and vermicompost can improve soil fertility and zucchini (Cucurbita pepo L.) production. This study aimed to determine the effects of compost and vermicompost on the yield, and nutrient uptake of zucchini as well as some soil properties under field conditions. The treatments were: control without fertilization (CO), chemical fertilizer (CF), compost (CT), and vermicompost (VC) and were arranged in a randomized complete block design with five replications. The results showed that the compost and vermicompost application significantly increased the yield of zucchini by about 7% and 53%, respectively, in comparison with the chemical fertilizer treatment. In addition, compost and vermicompost treatments significantly increased the soil organic matter, and availability of NPK compared with those in the control and with the chemical fertilizer treatments. The application of the compost and vermicompost amendments increased the total uptake of NPK compared with the control and chemical fertilizer treatments. The highest values of N, P, and K use efficiency were found in the compost treatment. Compost and vermicompost application increased the zucchini yield compared with other treatments. Fruit weight increased by about 1%, 5%, and 8%, respectively, in the chemical fertilizer, vermicompost, and compost treatments, while fruit length increased by about 24%, 10%, and 13%, respectively.
-
Key words:
- Compost /
- Chemical fertilizer /
- Fruit quality /
- Nutrient uptake /
- Vermicompost