-
Salt stress is one of the important abiotic stresses that restricts plant growth and development[1,2]. The symptoms of salt damage to plants are mainly manifested in leaves, such as changes in leaf color, brown-yellow necrosis at the leaf edge, and leaves becoming fleshy[3]. Long-time salt stress inhibited the growth of turfgrass. It significantly inhibited leaf weight, branch dry weight, and branch length of turfgrass, increasing dry weight ratio of underground and aboveground biomass[4]. Salt stress inhibited the growth and biomass of tall fescue (Festuca arundinacea) and seashore paspalum (Paspalum vaginatum)[5,6].
Plants have developed a series of adapting mechanisms to survive under salt stress. Under high salinity treatment, plants active the synthesis or accumulate organic (pro-line, soluble sugar, betaine, and glycerol, etc.) or inorganic substances (Na+, K+, Cl−, Ca2+, SO42−, Mg2+, and NO3−, etc.) in cells to enhance the dehydration tolerance and regulate the osmotic stress[7]. Plants can also activate their antioxidant system and the synthesis of stress-related hormone (abscisic acid) response to salt stress[8,9]. Under salt stress, plants have to modulate Na+/K+ homeostasis. High-affinity potassium transporter, HKT1, provides Na+ exclusion and maintains a high K+/Na+ ratio in leaves during salinity stress[10]. The SOS pathway also maintains the Na+ homeostasis and transports excess Na+ from the cytosol to the apoplast to prevent the accumulation of Na+ to toxic levels[11].
Iron (Fe), one of the nutrient microelements, is essential to plant growth and development, it is involved in many physiological and biochemical reactions in living organisms[12,13]. Iron not only plays an irreplaceable role in photosynthesis, cellular respiration, and electron transport but also participates in plant growth and stress response[13]. To ensure the efficient utilization of iron nutrition, chelated iron (such as Fe-EDTA) is widely used in agricultural production based on its ease of solubility and stability[14]. Iron deficiency will affect the growth and development of plants, and will cause significant economic losses[15]. However, it is also suggested that excess iron is toxic to plants[16]. Thus, regulating ions homeostasis to avoid both iron deficiency and toxicity is crucial for plants[17].
Salt stress causes osmotic stress in plants, and the homeostasis of ions in cells is destroyed. High concentrations of salt ions reduce the absorption of other nutrient ions, leading to the imbalance of nutrient elements, cell metabolism disorder, and death[3,18]. Excessive Na+ accumulation in plant roots limited the absorption of iron and resulted in the imbalance of iron under salt stress[19,20]. Now, it is well documented that excess iron could alleviate salt toxicity in plants under salt stress[12,21]. Iron could enhance the activity of antioxidative enzymes, scavenge reactive oxygen species (ROS) and thereby enhance cell defense mechanisms against salinity[22−24]. Under salt stress, appropriate excess iron reduce the accumulation of Na+ in chamomile and improve the absorption of K+[12]. Another study showed that excessive iron increased the contents of Fe2+, Zn2+, and K+ in shoots of tomato, and increased the activities of catalase (CAT), and ascorbate peroxidase (APX) in leaves under salt stress[24]. These results indicate that excessive iron can alleviate the adverse effects of salt stress on plants to a certain extent.
The objectives of the present study were to select an appropriate excess iron (Fe (II) EDTA) concentration for improving salt tolerance of seashore paspalum plants, analyze tissue-specific responses of ion contents, and detect the expression level of salt tolerance regulating genes in seashore paspalum plants exposed to salt stress and excess iron.
-
Plants of seashore paspalum ('Sea Isle 2000') were vegetatively propagated from erect stems cultivated in the grass germplasm resource nursery at Nanjing Agricultural University (Nanjing, Jiangsu Province, China). Ten erect stems were randomly selected and fixed with a sponge on a circular foam board and the plants were fixed with six sponges. The plants were then cultivated in hydroponic culture in plastic containers (10 cm in height and 15 cm in diameter) filled with 1 L half-strength Hoagland's nutrient solution[25] in a growth chamber. The nutrient solution was replaced once every 3 d and not ventilated during the cultivation and treatment. Plants were established in the growth chamber for 20 d with 30/25 °C day/night temperatures with a 14 h photoperiod with 6,000 LX light intensity. The plants cultivated in one plastic container represented an independent biological repetition, and three independent biological repetitions were used in each different treatment in this study.
Screening of iron concentration threshold
-
Seven different Fe (II) EDTA concentrations were used in the experiment: 0 μmol, 10 μmol, 20 μmol, 30 μmol, 50 μmol, 80 μmol, and 100 μmol. After 10 d of treatment, plant phenotypes were observed and photographed.
Experimental design of excessive iron on salt stress
-
Four treatments were designed to explore the effect of iron nutrition on salt tolerance of seashore paspalum: The control group (C): 20 μM Fe (II) EDTA + 0 mM NaCl; salt treatment (CS): 20 μM Fe (II) EDTA + 250 mM NaCl; excessive iron treatment (E): 80 μM Fe (II) EDTA + 0 mM NaCl; excessive iron and salt treatment (ES): 80 μM Fe (II) EDTA + 250 mM NaCl. 1/2 Hoagland nutrient solution including NaCl or Fe (II) EDTA were added at once. Samples were taken on 0 d and 10 d of salt stress treatment for physiological and ion content analysis.
Leaf photochemical efficiency (Fv/Fm) and relative chlorophyll content (SPAD)
-
Leaf photochemical efficiency (Fv/Fm) was measured using a fluorescence induction monitor (OPTI-Sciences, Hudson, USA). Leaves were dark-adapted for 30 min before the measurement. Relative chlorophyll content (SPAD) was measured using the chlorophyll meter (SPAD-502, Konica Minolta, Japan).
Chlorophyll content
-
The chlorophyll content of leaves was measured according to the method of Hiscox & Israelstam[26]. Leaf chlorophyll was extracted by soaking 0.05 g fresh samples in 10 mL dimethyl sulfoxide (DMSO) and kept in the dark until full extraction. The absorbance was measured at 665 nm and 649 nm using a spectrophotometer (Ultrospec 2100 pro, Amersham, USA), and the concentration of chlorophyll was calculated according to the following equations: Ca = 13.95 × A665 − 6.88 × A649; Cb = 24.96 × A649 − 7.32 × A665; Chlorophyll (mg/g DW) = (pigment concentration × extraction liquid volume)/dry weight.
Electrolyte leakage of leaves (EL)
-
Electrolyte leakage (EL) of leaves was measured according to the method of Blum & Ebercon[27]. About 0.2 g of fresh leaves were weighed and placed into a 50 mL centrifuge tube containing 30 mL deionized water. The centrifuge tubes were placed on a shaker for 24 h at room temperature and the initial conductivity (C0) was measured. Afterward, leaf tissues were killed by autoclaving at 121 °C for 15 min, and the final conductivity (Cl) was measured after the tubes were placed back on the sharker for another 24 h. The EL was calculated as: EL = C0/Cl × l00%.
Net photosynthetic rate of leaves (Pn)
-
Net photosynthetic rate (Pn) of plant leaves was measured using a LI-6400XT portable photosynthesis system (LI-COR Inc., NE, USA) equipped with a standard 2 cm × 3 cm chamber with light-emitting diode light sources. All the measurements were taken under a light intensity of 600 μmol/m2/s and at a CO2 concentration of 400 μmol/mol with a constant flow rate of 300 μmol/s.
Root activity
-
Root activity was measured using the TTC method[28]. Five mL of 0.4% TTC solution (m/v) and 5 mL of phosphoric acid buffer (pH = 7.0) were added into 0.5 g root tip samples. The roots were completely immersed in the solution and kept in darkness at 37 °C for 1−2 h. Then, 2 mL of 1 mol/L sulfuric acid was added to terminate the reaction. The liquids were then removed and the remained root samples were ground thoroughly with 4 mL ethyl acetate. The supernatants were collected in a new test tube and the final volume was adjusted to 10 mL with ethyl acetate. The absorbance was measured at 485 nm using a spectrophotometer (Ultrospec 2100 pro, Amersham, USA), and root activity was calculated as follows: Root Activity = TTC reduction amount/(1,000 × root weight × reaction time) [mg TTF/(g·h)].
Ion content
-
Plant samples were taken on 0 d and 10 d after salt stress treatment and the shoots and roots were separated, oven-dried to a constant weight, and ground to a fine powder. About 0.1 g ground sample was decomposed for 45 min at 160 °C by a microwave (ETHOS ONE, Milestone, Italy), using 3 mL 65% nitric acid as the digestion solution. After that, the liquid was diluted to 30 mL with deionized water. The contents of K, Na, Fe, P, Ca, Mg, Mn, Zn, and Cu ions were determined by an ICP-OES (Optima 8000, Perkin Elmer, USA). The ranges of calibration were 0−60 mg/L (Na, K), 0−30 mg/L (P, Ca), 0−10 mg/L (Mg, Mn), 0−2 mg/L (Zn), 0−0.2 mg/L (Fe, Cu), respectively. Seven concentrations were diluted according to a halving gradient of each calibration, respectively.
Total RNA extraction, cDNA synthesis, and qRT-PCR analysis
-
Total RNA was extracted from seashore paspalum roots which were collected on the 10th day. Using Plant RNA Kit (R6827, Omega, USA), and then reverse-transcribed to cDNA using MonScript™ RTIII Super Mix with dsDNAse (MR05201, Monad, China). qRT-PCR analysis was performed with Roche LightCycler480 II machine (Roche Diagnostic, Rotkreuz, Switzerland) and the MonAmpTM ChemoHS qPCR Mix (MQ00401, Monad, China) as the intercalating dye to detect gene expression level. The operating procedure included 1 × qPCR mix, 0.2 μM primer, 10−200 ng/μL cDNA template, and nuclease-free water up to 20 μL. Thermocycling conditions for a PCR: 95 °C, 10 min; 95 °C, 10 s, 55−65 °C, 10 s, 72 °C, 30 s, 40 cycles.
Gene expression levels were calculated by the 2−ΔΔCᴛ method[29] with PvEF1α as the reference gene. Primer sequences are listed in Table 1.
Table 1. Primers of genes used for qRT-PCR analysis.
Gene Primer sequences 5'-3' (RT-F/RT-R) PvSOS1 GCTTGAAGAGGGACGAATAAA/ACGAAGAAATGCAGCACAGAT PvCIPK24 GGCTTAATGAGGTGTTGGCTG/TGGTAAACTCCTTTGCTGTGG PvCBL4 GCGCCGACATCAGACAAGA/CGAGCAATGCCAAGACCAT PvPHA CAGGAAGTACCCGAGAAATCA/CGTTAACACCAAGAACAAGAGC PvVHA CTCTCCTCCTGGTGGTGGTTT/CCTCACGTGCTTTTGTCCTAATAT PvHKT1 CCATCATCTACAACATTTGTGC/TCATTTCTGAGCCTTCTCCTC PvEF1α GCGGACTGTGCTGTGCTTATC/AGTGGTGGCATCCATCTTGTT Statistical analysis
-
All experimental data were statistically analyzed and processed by SPSS (Statistical Package for the Social Science, Chicago, IL, USA) and Excel. The analysis of data types used single factor analysis of variance.
-
To screen the iron absorption threshold of Sea Isle 2000, seven iron concentration gradients were designed. As shown in Fig. 1, plants provided with 0 and 10 μM Fe-EDTA showed obvious iron deficiency symptoms, with yellow leaves and stems. SPAD and Fv/Fm of leaves increased with the increase of iron concentration until 20 μM and tended to be relatively stable when iron concentration was higher than 20 μM, and SPAD content peaked at 80 μM Fe (II) EDTA (Fig. 1). Based on these results, the iron concentration threshold for normal growth of Sea Isle 2000 was set at 20 μM and 80 μM Fe (II) EDTA was selected for the excess iron treatment in the following experiment.
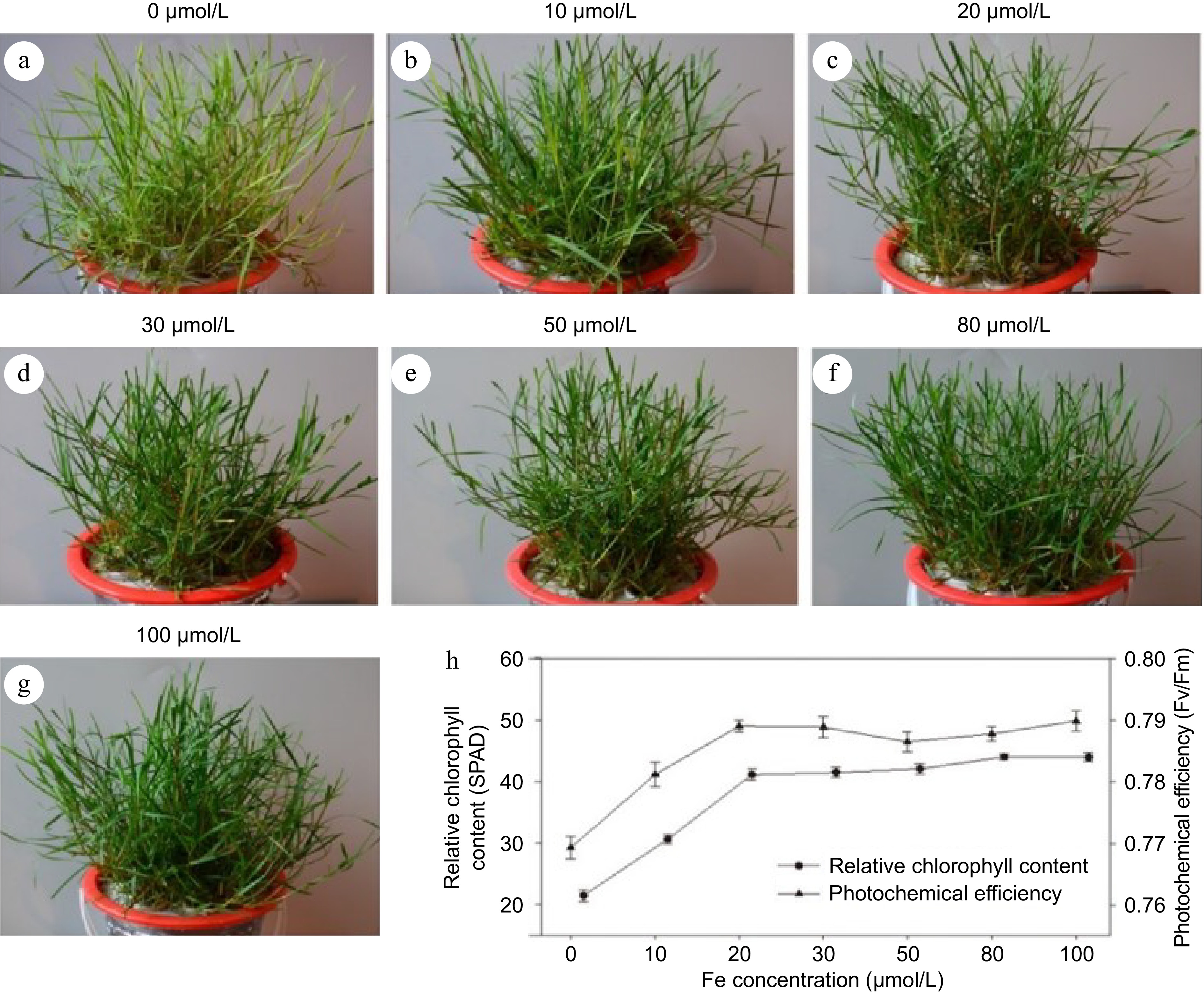
Figure 1.
Screening of optimum concentration of iron in seashore paspalum. (a)−(g) The phenotype of different concentrations iron in seashore paspalum. (h) Indexes of relative chlorophyll content (SPAD) and photochemical efficiency (Fv/Fm). The mean value and standard error were obtained from three biological replicates of every index.
Effect of excessive Fe (II) EDTA on salt tolerance of seashore paspalum
-
Under non-stress conditions, excessive iron (80 μM Fe (II) EDTA) had no significant effect on plant appearance, biomass, and root activity (Fig. 2). Under salt stress condition, plants with excessive iron (80 μM Fe (II) EDTA) showed greener appearance, significantly higher shoot biomass and root activity than plants with optimal iron (20 μM Fe (II) EDTA). Excessive Fe (II) EDTA had no significant effects on electrolyte leakage, chlorophyll content, Fv/Fm, and Pn under non-stress conditions, but helped to maintain significantly higher chlorophyll content, Fv/Fm and Pn and lower electrolyte leakage under salt stress (Fig. 2).
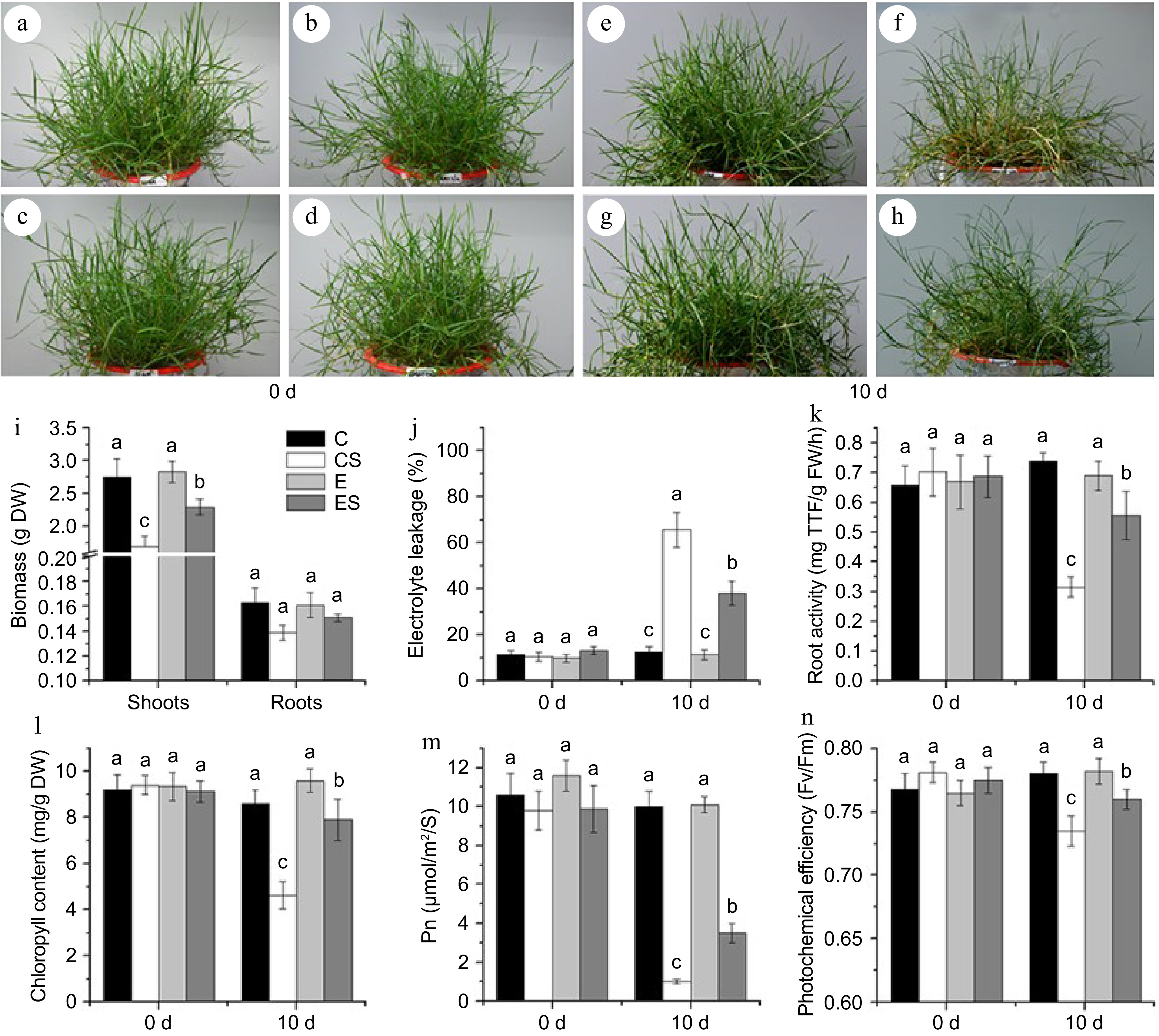
Figure 2.
The appearance and physiological indexes of seashore paspalum under different treatments. (a)−(d) The control group. (e) The treatment of 20 μM Fe (II) EDTA + 0 mM NaCl (C); (f) The treatment of 20 μM Fe (II) EDTA + 250 mM NaCl (CS); (g) The treatment of 80 μM Fe (II) EDTA + 0 mM NaCl (E); (h) The treatment of 80 μM Fe (II) EDTA + 250 mM NaCl (ES). (i)−(n) Physiological indexes of biomass, electrolyte leakage (EL), root activity, chlorophyll content, net photosynthetic rate (Pn), and leaf photochemical efficiency (Fv/Fm) successively, the mean value and standard error were obtained from 3 biological replicates of every physiological index, and the significance difference level p < 0.05.
Effect of excessive Fe (II) EDTA treatment on ion content of seashore paspalum under salt stress
-
The ion content of K+, Na+, P, Ca2+, Mg2+, Mn2+, Zn2+, Fe2+, and Cu2+, as well as Na+/K+ ratio in roots and shoots showed no significant difference before salt stress. Salt stress significantly increased the content of Na+ and the ratio of Na+/K+ in both shoots and roots. However, excess Fe (II) EDTA increased the content of K+, decreased the ratio of Na+/K+ in roots, reduced the content of Na+ in roots, and increased Na+ content in shoots under salt stress. Salt treatment significantly reduced the content of P in roots, while excess Fe (II) EDTA had no significant effect on P content. In addition, excess Fe (II) EDTA increased Ca2+ content under salt stress and significantly increased the content of Mg2+ in roots regardless of salt stress.
Excessive Fe (II) EDTA also affected the changes of trace elements in Sea Isle 2000 under salt stress. Salt stress decreased the content of Mn2+ in roots, but increased its content in shoots. Excessive Fe (II) EDTA significantly increased the content of Mn2+ in non-stress and alleviated the decreasing trend of Mn2+ under salt stress in roots. Excessive Fe (II) EDTA increased the contents of Fe2+ and Cu2+ under non-stress conditions and increased the Fe2+ content in roots under salt stress.
Excessive Fe (II) EDTA increased the expression of ion transporter in seashore paspalum under salt stress
-
To further clarify the molecular mechanism of excess Fe (II) EDTA on improving salt tolerance of seashore paspalum, the expression level genes of encoding ions transporter (PvSOS1, PvHKT1, PvCIPK24, PvCBL4), proton pump (PvPHA, PvVHA) in the roots of Sea Isle 2000 were analyzed. The result showed that salt stress decreased the expression level of PvSOS1, PvCIPK24, PvCBL4, PvHKT1, and PvPHA, while excessive Fe (II) EDTA significantly improved their expression under salt stress (Fig. 3a−e). Under normal conditions, the expression of PvCBL4, PvHKT1, and PvPHA were lower when iron was excessive (Fig. 3c−e). PvVHA didn't show significant changes in response to salt stress but significantly increased in response to excess Fe (II) EDTA under both control and salt stress (Fig. 3f).
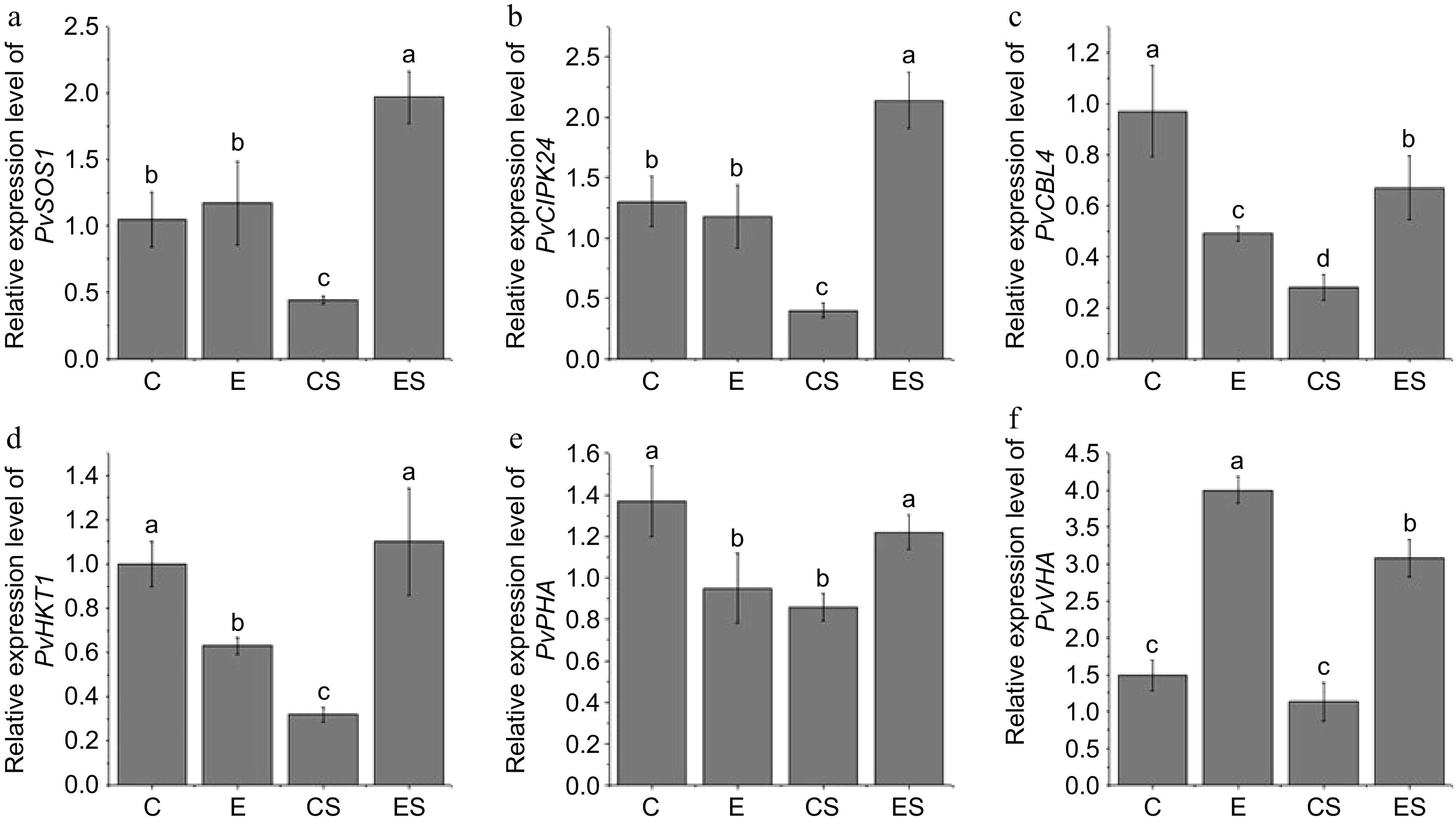
Figure 3.
Effects of excess iron on relative expression level of PvSOS1, PvCIPK24, PvCBL4, PvHKT1, PvPHA, and PvVHA in seashore paspalum roots under salt stress. (C), 20 μM Fe (II) EDTA + 0 mM NaCl; (CS), the treatment of 20 μM Fe (II) EDTA + 250 mM NaCl; (E), the treatment of 80 μM Fe (II) EDTA + 0 mM NaCl; (ES), the treatment of 80 μM Fe (II) EDTA + 250 mM NaCl. The mean value and standard error were obtained from five biological replicates, and the significance difference level p < 0.05.
-
Iron deficiency directly leads to decreased or even stopped chlorophyll synthesis, significantly decreased photosynthetic rate, yellowing of leaves, decreased biomass, and other symptoms[12]. In the process of exploring the iron nutrient threshold of seashore paspalum, it was found that the symptoms of iron deficiency occurred when the iron concentration was lower than required for plant growth. Nevertheless, there was no iron deficiency in seashore paspalum when the Fe (II) EDTA concentration was higher than 20 μM (Fig. 1). Therefore, 20 μM Fe (II) EDTA can be used as an optimum concentration of iron for studying iron absorption in seashore paspalum.
The report referred that a homeostasis and high availability of iron improved the biomass and quality of plants[15]. EDTA acts as a chelating function, increasing the solubility and stability of inorganic ions, while excess EDTA reduces the biomass of plants[30]. In the present study, salt stress inhibited the growth of seashore paspalum under 20 μM Fe (II) EDTA, while the biomass of samples treated by 80 μM Fe (II) EDTA was higher substantially compared with 20 μM Fe (II) EDTA under salt stress. It indicated that excessive iron changed the adverse effect of EDTA on biomass. Previous studies have shown that chlorophyll synthesis and photosynthesis in plant leaves are strongly inhibited when plants are exposed to high salinity, which limits plant growth and development[31,32]. In the present study, salt stress reduced chlorophyll content and photosynthesis of seashore paspalum, while excessive Fe (II) EDTA reduced chlorophyll degradation, and significantly increased chlorophyll content, and photosynthetic capacity of leaves (Fig. 2). Chlorophyll is the main pigment for photosynthesis located on the thylakoid membrane, but the stability of the thylakoid membrane is affected by stress[33]. Under salt stress, the activities of chlorophyll degradation enzymes are activated, resulting in chlorophyll degradation and a decrease in leaf photosynthesis[34]. Leaf photochemical efficiency (Fv/Fm) is the maximum photochemical quantum yield of PSII or the light energy conversion efficiency reflecting the maximum PSII, which has little change under abiotic stress and is independent of species and growth conditions, and it is the survival mechanism of plants to adapt to stress[35,36]. In the present study, Fv/Fm decreased under salt stress but increased by excessive Fe (II) EDTA treatment (Fig. 2). According to the above, excessive Fe (II) EDTA under salt stress can enhance the photosynthetic capacity of plants, maintain the viability and growth potential of seashore paspalum, and improve the salt tolerance.
Effects of excess Fe (II) EDTA on ion content in seashore paspalum under salt stress
-
Salt stress seriously affects the balance of inorganic ions in plants, and the sharp increase of Na+ inhibits the absorption of other ions in plants[37,38]. When plants were supplied with excessive iron, the accumulation of Na+ was reduced and increased the absorption of K+ and Fe2+[12]. In this study, excessive Fe (II) EDTA significantly increased K+ content in roots under salt stress, while the accumulation of Na+ and the ratio of Na+/K+ decreased significantly (Table 2). It is supposed that excessive Fe (II) EDTA can promote the absorption of K+, and reduce the content of Na+ in roots, thereby reducing the accumulation of harmful ions and maintaining the homeostasis of ions in roots, and improving the salt tolerance of plants.
Table 2. The ion content in seashore paspalum before and after 10 d under different treatments (mg/g DW).
Treatment days 0 day 10 day C CS E ES C CS E ES Roots Na/K 0.502a 0.557a 0.565a 0.583a 0.519c 6.344a 0.612c 4.232b K 4.634a ± 0.079 4.524a ± 0.121 4.341a ± 0.097 4.569a ± 0.174 4.236b ± 0.160 4.040b ± 0.079 4.677a ± 0.126 4.631a ± 0.079 Na 2.324a ± 0.092 2.517a ± 0.050 2.450a ± 0.099 2.648a ± 0.101 2.198c ± 0.100 25.622a ± 0.327 2.856c ± 0.391 19.561b ± 0.816 P 11.320a ± 0.059 10.860a ± 0.064 11.460a ± 0.818 11.830a ± 0.468 11.850a ± 0.092 8.180c ± 0.718 10.420ab ± 0.501 9.140bc ± 0.409 Ca 7.020a ± 0.449 7.575a ± 0.195 7.726a ± 0.184 7.152a ± 0.015 7.578b ± 0.283 7.752b ± 0.116 7.802b ± 0.339 9.388a ± 0.379 Mg 2.236a ± 0.187 1.943a ± 0.030 2.339a ± 0.l82 2.169a ± 0.099 2.129b ± 0.040 2.041b ± 0.003 2.355a ± 0.054 2.448a ± 0.068 Mn 3.762a ± 0.104 3.788a ± 0.127 3.684a ± 0.177 3.804a ± 0.067 3.630b ± 0.061 1.006d ± 0.062 4.002a ± 0.159 1.725c ± 0.085 Zn 0.501a ± 0.028 0.484a ± 0.009 0.508a ± 0.028 0.459a ± 0.029 0.514a ± 0.010 0.403bc ± 0.045 0.464ab ± 0.018 0.368c ± 0.011 Fe 0.0252a ± 0.0016 0.0247a ± 0.0018 0.0247a ± 0.0017 0.0272a ± 0.0015 0.0240bc ± 0.0034 0.0174c ± 0.0040 0.0738a ± 0.0017 0.0307b ± 0.0008 Cu 0.0109a ± 0.0011 0.0102a ± 0.0002 0.0091a ± 0.0007 0.0112a ± 0.0002 0.0100b ± 0.0004 0.0103b ± 0.0001 0.0193a ± 0.0024 0.0116b ± 0.0008 Shoots Na/K 0.011a 0.010a 0.009a 0.009a 0.007b 0.436a 0.007b 0.415a K 23.100a ± 0.514 22.360a ± 0.890 22.530a ± 0.334 23.740a ± 1.302 24.810a ± 1.096 19.380b ± 0.693 25.820a ± 0.055 21.730b ± 0.869 Na 0.265a ± 0.054 0.231a ± 0.015 0.205a ± 0.010 0.217a ± 0.022 0.176c ± 0.022 8.453b ± 0.310 0.169c ± 0.040 9.007a ± 0.474 P 8.676a ± 0.511 8.587a ± 0.395 8.782a ± 0.279 8.386a ± 0.389 8.313a ± 0.785 7.683a ± 0.186 8.190a ± 0.496 8.440a ± 0.154 Ca 3.357a ± 0.024 3.505a ± 0.086 3.320a ± 0.053 3.544a ± 0.091 3.458a ± 0.071 3.472a ± 0.167 3.721a ± 0.154 3.370a ± 0.130 Mg 1.440a ± 0.051 1.513a ± 0.020 1.461a ± 0.036 1.507a ± 0.020 1.476a ± 0.080 1.437a ± 0.117 1.583a ± 0.026 1.482a ± 0.086 Mn 0.419a ± 0.037 0.394a ± 0.023 0.405a ± 0.022 0.411a ± 0.023 0.386c ± 0.008 0.451ab ± 0.030 0.467ab ± 0.042 0.476a ± 0.011 Zn 0.277a ± 0.021 0.286a ± 0.015 0.254a ± 0.014 0.276a ± 0.018 0.265a ± 0.154 0.262a ± 0.013 0.238a ± 0.015 0.243a ± 0.017 Fe 0.0040a ± 0.0010 0.0034a ± 0.0004 0.0039a ± 0.0003 0.0031a ± 0.0008 0.0032a ± 0.0009 0.0027a ± 0.0008 0.0044a ± 0.0003 0.0033a ± 0.0007 Cu 0.0049a ± 5.805E-05 0.0047a ± 0.0001 0.0049a ± 9.293E-05 0.0047a ± 0.0001 0.0045b ± 0.0002 0.0043b ± 0.0002 0.0053a ± 0.0003 0.0048ab ± 0.0002 (C), 20 μM Fe (II) EDTA + 0 mM NaCl; (CS), the treatment of 20 μM Fe (II) EDTA + 250 mM NaCl; (E), the treatment of 80 μM Fe (II) EDTA + 0 mM NaCl; (ES), the treatment of 80 μM Fe (II) EDTA + 250 mM NaCl. Different lowercase letters represents the significance difference level p < 0.05 between C, CS, E and ES treatment. In this study, it was found that the Ca2+ content under salt stress was not significantly different from that under normal growth conditions, but it was significantly increased by excessive Fe (II) EDTA treatment (Table 2). Ca2+ is known as a second messenger in plants and is involved in the process of plant responses to stress[39,40]. A previous study has shown that calcium functions as a long-distance signaling messenger, carrying salt stress signals to induce the expression of salt tolerance genes in leaves[41]. In the present study, excessive Fe (II) EDTA stimulated the increase of Ca2+ in roots, thereby inducing the production of calcium signals under salt stress. Mg2+ is the main mineral element of chlorophyll, which mainly affects the photosynthesis of plants and the synthesis of sugar and protein[42]. The results of this study (Table 2) indicated that the excess Fe (II) EDTA under salt stress could contribute to the absorption of Mg2+, maintain the normal transport and distribution of Mg2+ in the plant.
As an essential trace element for plants, iron affects the growth and development of plants. Plants with iron deficiency all show green loss of leaves, become dwarfism, and even whole death[12]. In saline-alkali soil, absorption of iron in plants was inhibited, and excessive accumulation of Na+ in plants of stems reduced the content of Fe2+ and other nutrient elements in plants[19]. Under salt stress, the content of Fe2+ decreased in shoots and roots of seashore paspalum. It indicates that salt stress inhibited the absorption, transportation, and distribution of iron. However, excessive Fe (II) EDTA alleviated the inhibition effect of salt stress on Fe2+ and significantly increased the content in the roots (Table 2). The improvement is beneficial to promote the formation of chlorophyll in leaves and promotes normal metabolism, enhancing the salt tolerance of plants.
The function of Mn2+ in plants by affecting enzyme activity, and the content of Mn2+ is directly related to the formation of chloroplast membrane structure[43,44]. In this study, excessive Fe (II) EDTA treatment increased the content of Mn2+ in plants under salt stress, maintained the function of Mn2+ in the oxidation-reduction system, avoided serious damage of chloroplasts, and enhanced the photosynthesis of leaves under salt stress. On the other hand, a previous study reported that the free EDTA could chelate with Zn2+, Cu2+, and Mn2+ affecting the absorption of these ions in plants[30]. In this study, the contents of Zn2+, and Cu2+ were not significantly different whether normal iron (20 μM Fe (II) EDTA) or excessive iron (80 μM Fe (II) EDTA). It indicated that excessive iron increases the absorption of ions when injected with the same concentration EDTA under salt stress.
Effects of excess Fe (II) EDTA on salt-tolerance related gene expression in seashore paspalum under salt stress
-
Plant response to salt stress is a complex process, including induction and transmission of external salt signals at the molecular level, which stimulates the expression of various downstream physiological and biochemical genes or ion transporters to maintain ion homeostasis[45]. Under salt stress, excessive accumulation of Na+ in plants can cause ion toxicity, and exportation or regionalization of Na+ is one of the self-protection mechanisms to eliminate ion toxicity[46]. To maintain intracellular ions homeostasis under salt stress, Na+/H+ antiporter and proton pump are activated to maintain high K+ concentration and low Na+ concentration[47−49]. The SOS1 gene encodes Na+/H+ antiporter located on the plasma membrane, and functions to expel intracellular Na+ into the extracellular[3,48,50]. SOS2/CIPK24 and SOS3/CBL4 participate in the regulation of SOS1 gene, promoting the activity of SOS1 regulating Na+/H+ exchange[51]. In addition, CIPK24 regulates Ca2+ transport through the Ca2+ transporter CAX1 on the vacuolar membrane, and sensing the stimulated calcium signal, and specifically binds with CBL4 to form a complex to regulate salt stress and activate SOS1 activity, thereby increasing salt tolerance[51,52]. In this study, it was found that excessive Fe (II) EDTA significantly upregulated the expression of PvSOS1, PvCIPK24, and PvCBL4 in the roots under salt stress, suggesting that the upregulated expression improved the ability that PvCBL4 recruit PvCIPK24 to the plasma membrane to achieve efficient interaction with PvSOS1.
The transport protein requires energy to transfer Na+ to the extracellular or vacuole, while the proton pump (P-H+-ATPase (PHA), V-H+-ATPase (VHA)) can provide a proton driving force for this process by hydrolyzing ATP[53]. It has been suggested that V-H+-ATPase plays a pivotal role in salt stress, which provides proton driving force, and expels Na+ to extra-cellular milieu or into the vacuole[45,54,55]. P-H+-ATPase provides an active mechanism for Na+ extrusion to keep Na+ concentration in root cells below the threshold level of toxicity[56,57]. In the present study, the expression levels of PvVHA and PvPHA were significantly increased by excess Fe (II) EDTA treatment under salt stress, which could drive Na+/H+ antiporter protein in vacuole membrane to transport Na+ into vacuole or expel the Na+ from plasma membrane.
HKT is a high-affinity K+ transporter, which plays a decisive role in regulating intracellular Na+/K+ equilibrium[10,58,59]. Many studies have shown that HKT has the function of transporting other cations (such as Na+)[60,61]. Two types of HKTs exist in plants (HKT1 and HKT2). HKT1 is functional in transporting Na+ and K+ within plants and is linked to salt tolerance[62−64]. In the present study, excessive Fe (II) EDTA treatment upregulated the expression levels of PvHKT1 in roots under salt stress compared with normal and salt stress treatment (Fig. 3d). Combined with the decrease of Na+/K+ ratio by excess Fe (II) EDTA treatment under salt stress (Table 2), it is supposed that the increasing of PvHKT1 in roots regulated the transport of Na+ and K+ from root to shoot, sustained the Na+/K+ homeostasis under salt stress, and reduced the toxicity of salt stress.
-
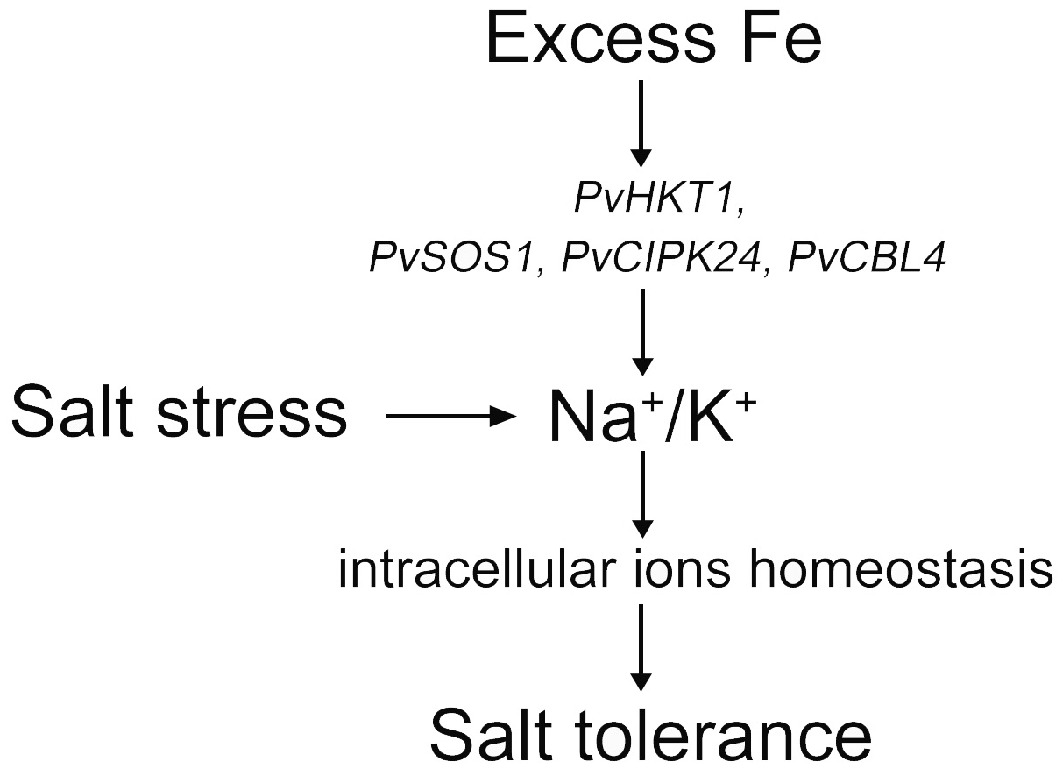
In the present study, salinity stress severely inhibited the growth and physiological function of seashore paspalum. The application of appropriate excess Fe (II) EDTA increased the photosynthetic capacity and improved the ion balance of seashore paspalum under salt stress. Excessive Fe (II) EDTA enhanced the outflow of Na+ by increasing the expression of PvSOS1, PvCIPK24, and PvCBL4, and reduced the accumulation of excess Na+. The up-regulation of PvHKT1 positively regulated Na+ and K+ flow, PvVHA provided energy for transporting excess Na+ to the vacuole, helped to maintain the balance of intracellular Na+/K+, and thus improved salt tolerance of seashore paspalum (Fig. 4).
This work was supported by the program of the National Natural Science Foundation of China NSFC (32071876, 32101437) and the China Postdoctoral Science Foundation (2018T110518, 2017M611842).
-
The authors confirm contribution to the paper as follows: study conception and design: Chen Y, Yang Z, Li Z; data collection, data analysis: Zheng Y, Tan Z; manuscript writing: Liu J, Hu J; manuscript review and editing: Liu Y, Zhang X. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zheng Y, Li Z, Tan Z, Liu Y, Zhang X, et al. 2025. Iron (II)-EDTA alleviate salinity injury through regulating ion balance in halophyte seashore paspalum. Grass Research 5: e002 doi: 10.48130/grares-0024-0029
Iron (II)-EDTA alleviate salinity injury through regulating ion balance in halophyte seashore paspalum
- Received: 29 October 2024
- Revised: 19 December 2024
- Accepted: 23 December 2024
- Published online: 10 January 2025
Abstract: Salt stress is one of the most important abiotic stresses that limits plant growth and development. In high salinity environments, plants adapt to stress mainly by changing their appearance and cell physiological metabolism. Iron is an important trace element in plant growth and development. It is not only an important factor affecting plant photosynthesis but also an ion to maintain plant homeostasis. To ensure the efficient utilization of iron nutrition, Fe (II) EDTA is usually used in iron supplementation of plants. In this study, the aim was to investigate the effects of excessive iron (Fe (II) EDTA) on physiological responses and expression levels of salt-tolerance-related genes in seashore paspalum under salt stress. The results showed that the salt toxicity could be alleviated by applying appropriate excess Fe (II) EDTA under salt stress. Plant biomass, chlorophyll content, net photosynthetic rate (Pn), photochemical efficiency (Fv/Fm), and root activity were improved by excess Fe (II) EDTA under salt stress. High concentration Fe (II) EDTA significantly reduced Na+ content, increased K+/ Na+, and significantly increased Ca2+, Mg2+, Mn2+, and Fe2+ contents in seashore pasplum roots under salt stress. The expression levels of salt-tolerance related genes (PvSOS1, PvCIPK24, PvCBL4 (Na+/H+ transporter), PvHKT1 (K+ transporter), PvPHA, and PvVHA (proton pump) were significantly increased by excess Fe (II) EDTA under salt stress. Therefore, the results of this study suggested that excess Fe (II) EDTA treatment can effectively enhance salt tolerance of seashore paspalum by maintaining ion balance.
-
Key words:
- Seashore paspalum /
- Salt stress /
- Excess Fe (II) EDTA /
- Gene expression