-
The combustion of fossil fuels is the main source of nitrogen oxide (NOx) emissions[1,2], which have led to serious environmental problems. Recently, ammonia (NH3) has been proposed and used to mitigate the impact of greenhouse gas (GHG) as a promising carbon-free fuel, which further exacerbates nitrogen oxide emissions[3]. To control NOx pollution, selective catalytic reduction[4,5] (SCR) and selective non-catalytic reduction[6] (SNCR) stand out prominently for the treatment of flue gas after combustion. Both methods can convert NOx to harmless N2 through chemical reactions. Compared with SCR, SNCR does not require the use of expensive catalysts and therefore has a relatively low operation cost.
Since the SNCR of NO by NH3 was proposed by Lyon in the 1970s[7], DeNOx chemistry has been intensively studied over a long period. NH3 and urea have been widely used as reducing agents[8−10]. Extensive experimental and kinetic studies have confirmed that NO removal by NH3 and urea relies mainly on the reactions of NH2 + NO. However, the traditional SNCR has a relatively high and narrow temperature window (1,100−1,500 K), which limits its application at lower temperatures. Moreover, fluctuations in the oxygen concentration have significant adverse effects on the DeNOx efficiency and further elevate the temperature window[9].
Hydrazine (N2H4), another reducing agent, is widely used in rocket fuels, antioxidants, and pesticide materials[11,12]. Many studies have focused on the decomposition of N2H4[13−17], whereas few have reported NO removal by N2H4. In 1967, Sawyer & Glassman[18] first reported the gas-phase reactions of N2H4, NO2, and NO in a flow tube under oxygen conditions. They proposed that the cleavage of the N-N bond is a prerequisite to react with NO, which confirmed the ability of N2H4 to remove NO. In 1985, Azuhata et al.[19] explored the applicability of N2H4 in NOx removal and reported that N2H4 can remove NO in the temperature range of 773−873 K. Similarly, Lee & Kim[20] reported that the optimum reaction temperature for NO removal by N2H4 was 873 K, and the best normalized stoichiometric ratio (NSR) was 4.0. Based on the above studies, it is proven that N2H4 can lower the temperature window and could be a better reducing agent than ammonia.
However, unlike DeNOx studies of NH3, kinetic modeling studies on the NO removal by N2H4 are insufficient, which hinders practical applications. Konnov & Ruyck[21] developed a kinetic model of N2H4 to describe its thermal decomposition and flame chemistry. They indicated that the initial decomposition reaction of N2H4 and the subsequent reaction of NH3 + NH2 had high sensitivities to the overall decomposition rate of N2H4 under highly diluted conditions. Later, Hong et al.[22] reported that N2H4 has a four-stage NO removal phenomenon and established a kinetic model to predict it. Moreover, their study revealed that the NO removal efficiency of N2H4 can bear an increase in oxygen concentration within a certain range. However, previous models[21,23] cannot accurately predict the NO removal efficiency, and a detailed explanation of DeNOx chemistry by N2H4 is also inadequate.
In this work, a kinetic model of N2H4/NO/O2 is developed based on recent NH3 models to predict the experimental data[23] of N2H4/NO/O2 over 673−1,523 K. Through the kinetic modeling analysis, the four-stage NO removal phenomenon observed in the experiments is thoroughly explained, and the competitive relationship between the NO formation and DeNOx pathways is revealed. In addition, the effects of the oxygen content on NO removal by N2H4 are also discussed in detail.
-
This study reports a detailed kinetic model for the N2H4/NO/O2 system, which is based on a recent NH3/H2 model from Zhu et al.[24]. Zhu et al. recently developed their model based on a widely used H2/O2 model from NUIGMech1.3[25], and the NH2 related mechanisms, including pyrolysis, oxidation, and DeNOx reactions, were evaluated and thoroughly incorporated[26−28]. In addition, N2H4 and NOx-related reactions were included in their model, which is based on the work of Glarborg et al.[29] and Sahu et al.[30], respectively. Their model is applicable for predicting the validation targets at 500−3,200 K and 0.08−75 atm. N2H4 can generate both N1 and N2 pathways. Specifically, the N1 pathway involves primarily NH2 and NH radicals, whereas the N2 pathway includes a variety of N2 radicals (e.g. N2H3, N2H2, and H2NN). The present model updated the rate constant of some critical reactions related to those radicals based on previous studies[21,31−35]. Important reactions discussed later are summarized in Table 1.
Table 1. Important reactions in the N2H4/NO/O2 system. The parameters for use are in the modified Arrhenius expression k = ATβexp[-E/(RT)].
Reactions A
(cm3·mol−1·s−1)β
(unitless)Ea
(cal·mol− 1)Ref. R1 N2H4 (+ M) = 2NH2 (+ M) 5.0E14 0.000 251.22 [21] Low/ 1.5E15 0.000 39000 N2/ 2.4/ NH3/ 3.0/ N2H4/ 4.0/ R2 N2H4 + OH = N2H3 + H2O 4.0E13 0.000 0.0000 [35] R3 N2H4 + NH2 = N2H3 + NH3 3.8E01 3.440 −574.00 [40] R4 N2H3 + OH = H2NN + H2O 3.0E13 0.000 0.0000 [41] R5 N2H3 + HO2 = N2H2 + H2O2 2.8E04 2.690 −1600.0 [41] R6 N2H3 + OH = N2H2 + H2O 1.2E06 2.000 −1192.0 [41] R7 N2H2 = H2NN 1.0 E01 0.000 0.0000 [41] PLOG / 1.0E-01 9.2E38 −9.010 67727/ PLOG / 1.0E+00 2.0E41 −9.380 68452/ PLOG / 1.0E+01 1.3E45 −1.013 70757/ R8 H2NN = H2 + N2 5.0E13 0.000 52785 [34] R9 NH2 + NO = NNH + OH 4.3E10 0.294 −866.00 [43] R10 NH2 + NO = H2O + N2 2.6E19 −2.369 870.00 [43] R11 NNH = N2 + H 3.0E08 0.000 0.0000 [41] R12 NNH + O2 = N2 + HO2 5.6E14 −0.385 −13.000 [44] R13 N2H2 + NO = N2O + NH2 4.0E12 0.000 11922 [41] R14 N2O + H = N2 + OH 6.7E10 0.000 5390.0 [45] Duplicate 4.4E14 0.000 18900 R15 NH2 + NO2 = H2NO + NO 1.1E12 0.110 −1186.0 [31−33] Duplicate −4.3E17 −1.874 588.00 R16 H2NO + NO2 = HNO + HONO 3.0E11 0.000 2000.0 [46] R17 H2NO + O2 = HNO + HO2 2.3E05 2.190 18000 [46] R18 HNO + O2 = HO2 + NO 2.0E13 0.000 14000 [41] R19 H2NN + O2 = NH2 + NO2 1.5E12 0.000 5961.0 [41] R20 NH2 + OH = H2O + NH 3.3E06 1.949 −217.00 [47] R21 NH + O2 = HNO + O 3.3E09 1.034 11420 [3] R22 NH + O2 = NO + OH 4.5E08 0.790 1195.0 [48] Important reactions related to the N2 pathway
-
The important reactions of the N2 pathway include the unimolecular decomposition reaction of N2H4 and bimolecular reactions, such as H-abstraction reactions of N2H4 and other N2 radicals. Among them, the rate constant of R1 has been investigated by several groups[16,17,21,29,36−39]. Konnov & Ruyck[21] estimated the rate constants of N2H4 decomposition from updated thermodynamic data and successfully predicted the experimental data of N2H4 decomposition. Therefore, the present model adopted the rate constant of R1 from theirs. The H-abstraction reaction of N2H4 by OH (R2) was adopted from the kinetic model of Chen et al.[35] to better predict the DeNOx process by N2H4. Gao et al.[40] measured the rate constant of R3 through laser photolysis/laser-induced fluorescence (LP-LIF) and computationally investigated the rate constant via quantum chemistry, which was adopted in this model. Additionally, the rate constants of H-abstraction reactions of N2H3 by different radicals (i.e. R4−R6) were adopted from Dean & Bozzelli[41] via direct hydrogen transfer (DHT) estimation. Meanwhile, the isomerization of H2NN (R7) was also adopted from Dean & Bozzelli[41] via quantum Rice-Ramsperger-Kassel (QRRK) calculations. The decomposition reaction of H2NN (R8) was adopted from Hwang & Mebel[34] via theoretical calculations. The rate constants for other reactions associated with H2NN, N2H3, N2H2, and NNH were adopted from previous studies[10,32,42].
Important reactions related to the DeNOx process
-
The reactions of NH2 and N2H2 with NO play crucial roles in NO removal by N2H4. Through R9 and R10, NH2 converts NO to NNH and N2, respectively, and NNH can further be converted to N2[44] (i.e. R11 and R12), effectively leading to NO removal. Song et al.[43] measured the rate constants of R9 and R10 through shock tube experiments between 1,716 and 2,507 K, and fitted their measurements to cover 300−2,500 K based on previous results at lower temperatures. The final fitted results were adopted in this model to predict the experimental data under wide temperature conditions. Unlike NH3, N2H4 proceeds through N2 pathways to produce N2H2, which combines with NO to produce N2O. R13 was adopted from the evaluated results of Dean & Bozzelli[41]. N2O then reacts with H to ultimately produce harmless N2[31] (i.e. R14). The above reactions constitute the DeNOx mechanism in the present model.
Important reactions related to the NOx formation process
-
NH2 consumes NO2 via the chain-propagation reaction R15 and simultaneously generates NO and H2NO, which can further be converted to NO via H-loss reactions (i.e. R16−R18). Glarborg[32] re-evaluated the rate constant of this reaction, which aligns with the theoretical calculation results of Klippenstein et al.[33], and hence was adopted in this model. Stagni & Cavallotti[46] investigated a series of H-abstraction reactions from H2NO (i.e. R16 and R17) via CASPT2 calculations and their calculated results were adopted in this model. The reaction between H2NN and oxygen to form NO2 (i.e. R19) was referred to the comprehensive review by Dean & Bozzelli[41]. The rate constant of R20 was adopted from theoretical calculation results from Klippenstein et al.[47], which are consistent with experimental measurements[47,49]. In addition, the NOx formation pathways also involve the oxidation of NH under high-temperature conditions (i.e. R21 and R22). The rate constant of R21 was adopted from the evaluated value from Elishav et al.[3].
Simulation method
-
In this study, the perfect stirred reactor (PSR) module in ANSYS CHEMKIN-PRO[50] software was used for the simulations. The simulation conditions are consistent with those of Guan et al.[23]. The detailed simulation conditions are summarized in Table 2. Since NOx mainly comes from the combustion of hydrocarbon fuels, which simultaneously produces H2O and CO2 as major products. CO2 and H2O, accompanied with NOx were adopted to stimulate flue gas components. The complete kinetic mechanism comprises 43 species and 313 reactions, and the kinetic, thermodynamic, and transport files of this model can be found in the supplementary information.
Table 2. Validation conditions[23] of the present model.
Parameters Condition 1 Condition 2 Condition 3 T (K) 673−1,523 773−1,398 773−1,348 P (atm) 1 1 1 τ (s) 0.3 0.3 0.3 NO (ppm) 450 500 500 N2H4 (ppm) 450 1,000 1,000 NSR 2 4 4 O2 (%) 18−4.74 7.4−16.5 9.9−16.8 CO2 (%) 5 5 5 H2O (%) 10 10 10 N2 (%) Balance Balance Balance -
To better interpret the NO removal efficiency by N2H4, the simulated mole fractions of NO are converted to the NO removal ratios (η). It is calculated via Eq. (1), where NOinlet and NOoutlet represent the NO inlet and outlet concentration, respectively. Figure 1 shows the comparison between the measured and predicted NO removal ratios of the present model, as well as those of previous models[23,24,32,51,52]. Among previous models, three of them[23,24,32] include recent kinetic models of NH3 oxidation and DeNOx chemistry by NH3 or N2H4. Guan et al.[23] conducted experimental and simulation results for NO removal by N2H4. Chen et al.[51] developed a kinetic model to predict the laminar burning velocities of NH3/O2/Ar. Glarborg[32] updated critical reactions in NH3 ignition and N2O formation and presented a model for the NH3/NO2/O2 system. Thomas et al.[52] developed an NH3 model to predict the extinction of NH3/H2 flames and the formation of NO. Zhu et al.[24] developed a comprehensive NH3/H2 kinetic model over a wide range of temperatures and pressures. These literature models are named as Guan 2019, Konnov 2023, Glarborg 2023, Shrestha 2023, and Zhu 2024, respectively. The experiment results of Guan 2019 and the simulation results of the present model show that NO removal by N2H4 is dependent on temperature, resulting in a four-stage NO removal phenomenon.
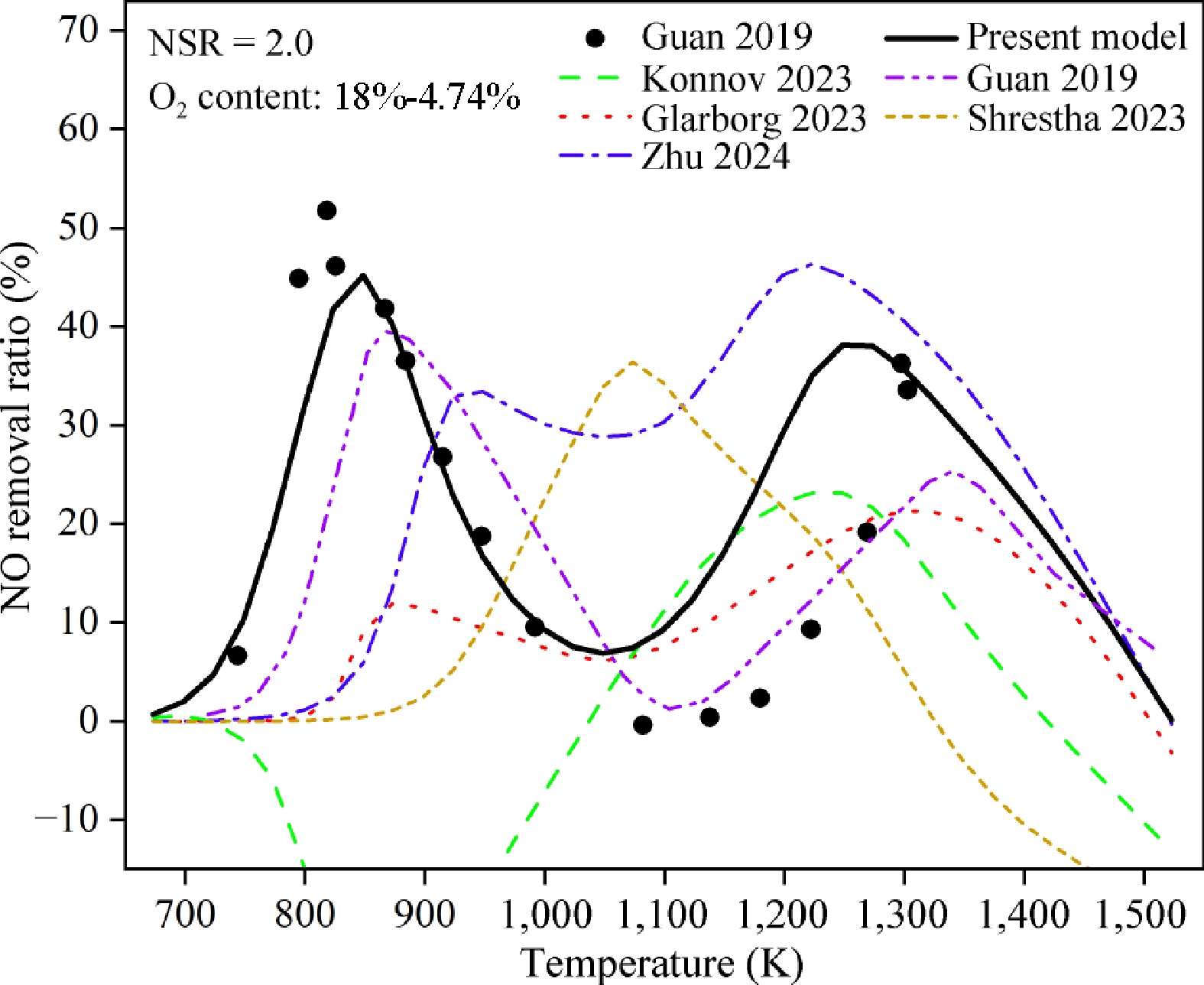
Figure 1.
NO removal ratios of N2H4/NO/O2 under condition 1. The symbols are experimental data from previous work[23]. The solid, dash dot dotted, dashed, dotted, short dashed, and dash dotted lines are the predicted results by the present, Guan 2019[23], Konnov 2023[51], Glarborg 2023[32], Shrestha 2023[52] and Zhu 2024[24] models, respectively.
$ \mathrm{\eta }=\frac{{\mathrm{N}\mathrm{O}}_{\mathrm{i}\mathrm{n}\mathrm{l}\mathrm{e}\mathrm{t}}-{\mathrm{N}\mathrm{O}}_{\mathrm{o}\mathrm{u}\mathrm{t}\mathrm{l}\mathrm{e}\mathrm{t}}}{{\mathrm{N}\mathrm{O}}_{\mathrm{i}\mathrm{n}\mathrm{l}\mathrm{e}\mathrm{t}}} $ (1) Compared with other models, the model proposed in this study demonstrates greater accuracy in capturing this non-monotonous trend, as shown in Fig. 1. Specifically, recently published kinetic models related to ammonia oxidation, such as Konnov 2023[51], Glarborg 2023[32], Shrestha 2023[52], and Zhu 2024[24], show different trends from experimental data. In the temperature range of 800−973 K, the Konnov 2023 model predicts negative NO removal ratios, which is the opposite of experimental observations. To better present and compare the NO removal efficiency of different models, the NO removal ratios below −15% are not shown in Fig. 1. Compared with the Guan 2019 model[23], the present model performs better in predicting the peak NO removal efficiency. Additionally, simulations are conducted to predict the experimental data with different oxygen concentrations, as shown in Figs 2 and 3. The results indicate that compared with previous models[24,32,51,52], the present model can better predict the four-stage NO removal behavior, although it still needs to be improved over 1,100−1,300 K. Therefore, recent comprehensive kinetic models for NH3 oxidation are not applicable in the investigation of NO removal by N2H4, since the N2H4 sub-mechanism and its interaction with NO have not been well tested. The uncertainties of the present model may come from both the kinetic parameters and simulation methods. The rate constants of N2-related reactions are mainly from estimations. Future efforts involving experiments and theoretical calculations are highly needed. In addition, the simulations are based on the PSR module, while the experimental process[23] makes it difficult to achieve the complete mixing state. Therefore, the perfect-stirred assumption may introduce extra uncertainties to the simulation results. Future work on improving the simulation methods is required.

Figure 2.
NO removal ratios of N2H4/NO/O2 under condition 2. The symbols are experimental data from previous work[23]. The solid, dashed, dotted, short dashed, and dash dotted lines are the predicted results of the present, Konnov 2023[51], Glarborg 2023[32], Shrestha 2023[52], and Zhu 2024[24] models, respectively.

Figure 3.
NO removal ratios of N2H4/NO/O2 at condition 3. The symbols are experimental data from previous work[23]. The solid, dashed, dotted, short dashed, and dash dotted lines are the predicted results of the present, Konnov 2023[51], Glarborg 2023[32], Shrestha 2023[52], and Zhu 2024[24] models, respectively.
Reaction kinetics of the four-stage NO removal behavior
-
The NO removal efficiency is the result of the competition between NO formation and DeNOx pathways[53]. To elucidate the mechanism of NO removal by N2H4, rate of production (ROP) analyses were conducted in this work. As shown in Fig. 4a, N2H4 proceeds via both the N1 and N2 pathways to generate NH2 and N2H3 (yellow box), respectively. These two radicals further participate in both the DeNOx (green box) and NO formation (red box) pathways. The dashed arrows represent the pathways that are important only under high-temperature conditions. The mole fractions of critical species related to the DeNOx and NO formation pathways are shown in Fig. 4b & c. Both the experimental and simulation results of the present model reveal that NO removal exhibits a four-stage process. Based on the model analyses, the NO removal mechanism of each stage is analyzed in this section. Detailed reaction pathways of N2H4 at four typical temperatures corresponding to each stage are presented in Supplementary Figs S1−S4.
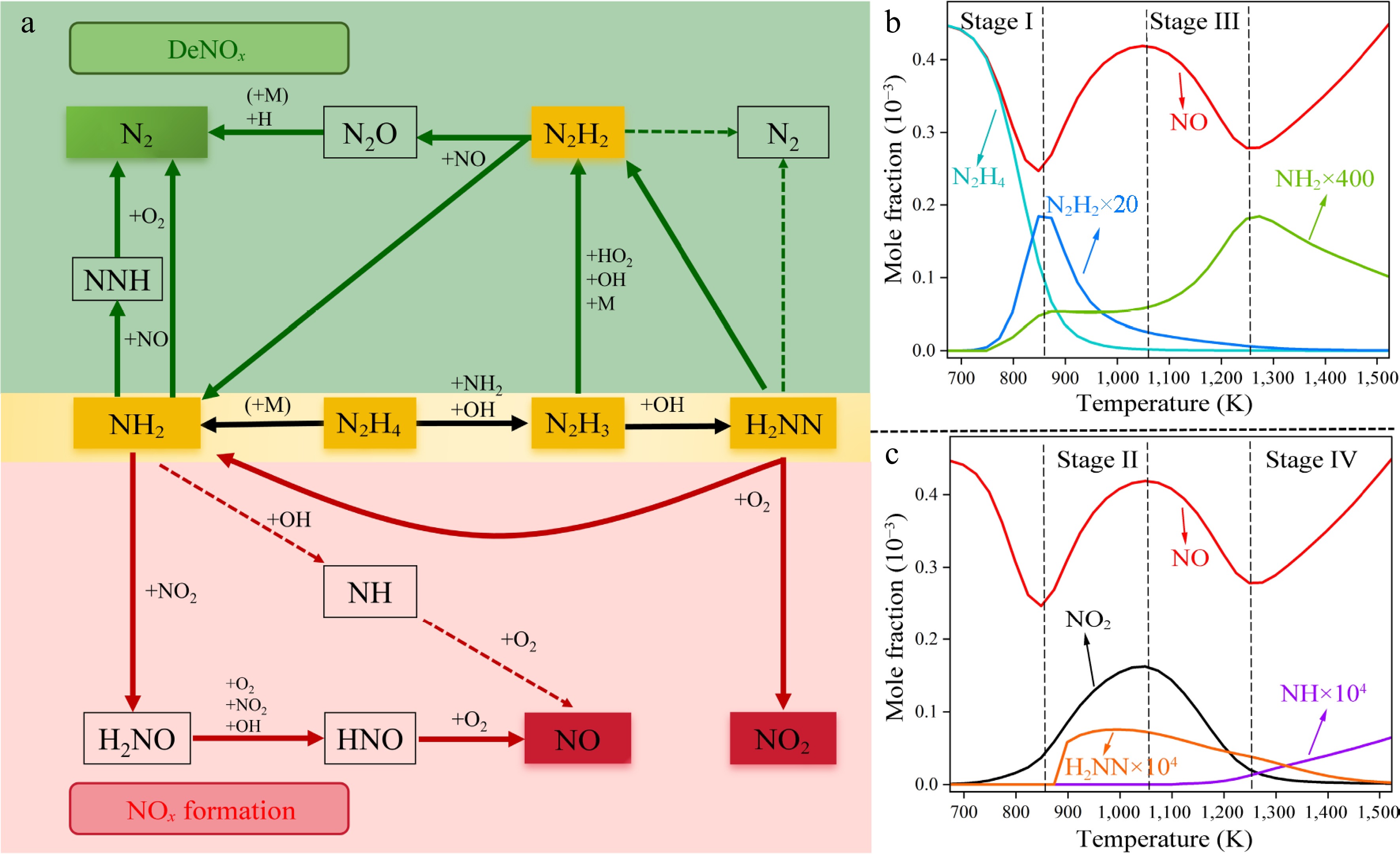
Figure 4.
(a) Reaction pathways of N2H4 under condition 1. The green and red boxes highlight reaction pathways involved in the DeNOx and NOx formation processes, respectively. The dashed arrows represent the pathways that are important only under high-temperature conditions. (b), (c) Simulated mole fractions of critical species in the N2H4/NO/O2 under condition 1.
NO removal mechanism in Stage I
-
Stage I (i.e. 673−848 K) can be considered as a DeNOx region, since the mole fraction of NO decreases. In this stage, more than 75% of the N2H4 is consumed, while N2H2 and NH2 radicals reach their first peak values, as shown in Fig. 4b. To study the NO removal chemistry of this stage, ROP analyses were conducted at 798 K. The results reveal that N2H4 is consumed mainly through the unimolecular decomposition reaction R1 to produce NH2, as well as H-abstraction reactions by OH (i.e. R2) and NH2 (i.e. R3) to generate N2H3. In this stage, the NH2 radical mainly reacts with NO to produce NNH and N2 via R9 and R10, respectively. While its reaction with NO2 to produce NO via R15 is not competitive, since the mole fraction of NO2 in this stage is very low, as seen in Fig. 4c. Therefore, the NH2-related pathway mainly results in the DeNOx-process, which can be confirmed by the sensitivity analysis results shown in Fig. 5. In contrast, N2H3 produced via R4 and R5 can be consumed by OH and HO2 to produce H2NN and N2H2, respectively. Both pathways are important in Stage I. N2H2 mainly reacts with NO to proceed the DeNOx pathways via R13, while H2NN is almost completely converted to NO2 via R19 in this stage. Therefore, the N2H3-related pathway contributes to both the DeNOx and NOx formation process. Combining the NH2 and N2H3 related pathways, the DeNOx pathway are more favorable than the NOx formation pathway in Stage I, leading to the increase of NO removal ratios, as seen in Fig. 1. In addition, since most of the NH2 radicals from N2H4 decomposition contribute to the DeNOx process, while only half of the fuel radicals N2H3 proceed DeNOx pathways via N2H2, as seen from Supplementary Fig. S1. DeNOx reactions related to NH2 (R9 and R10) present larger negative sensitivity coefficients than those of N2H2 (R13) for NO formation in this stage, as seen in Fig. 5. This finding indicates that the N1 pathways via NH2 are more effective than the N2 pathways in Stage I.
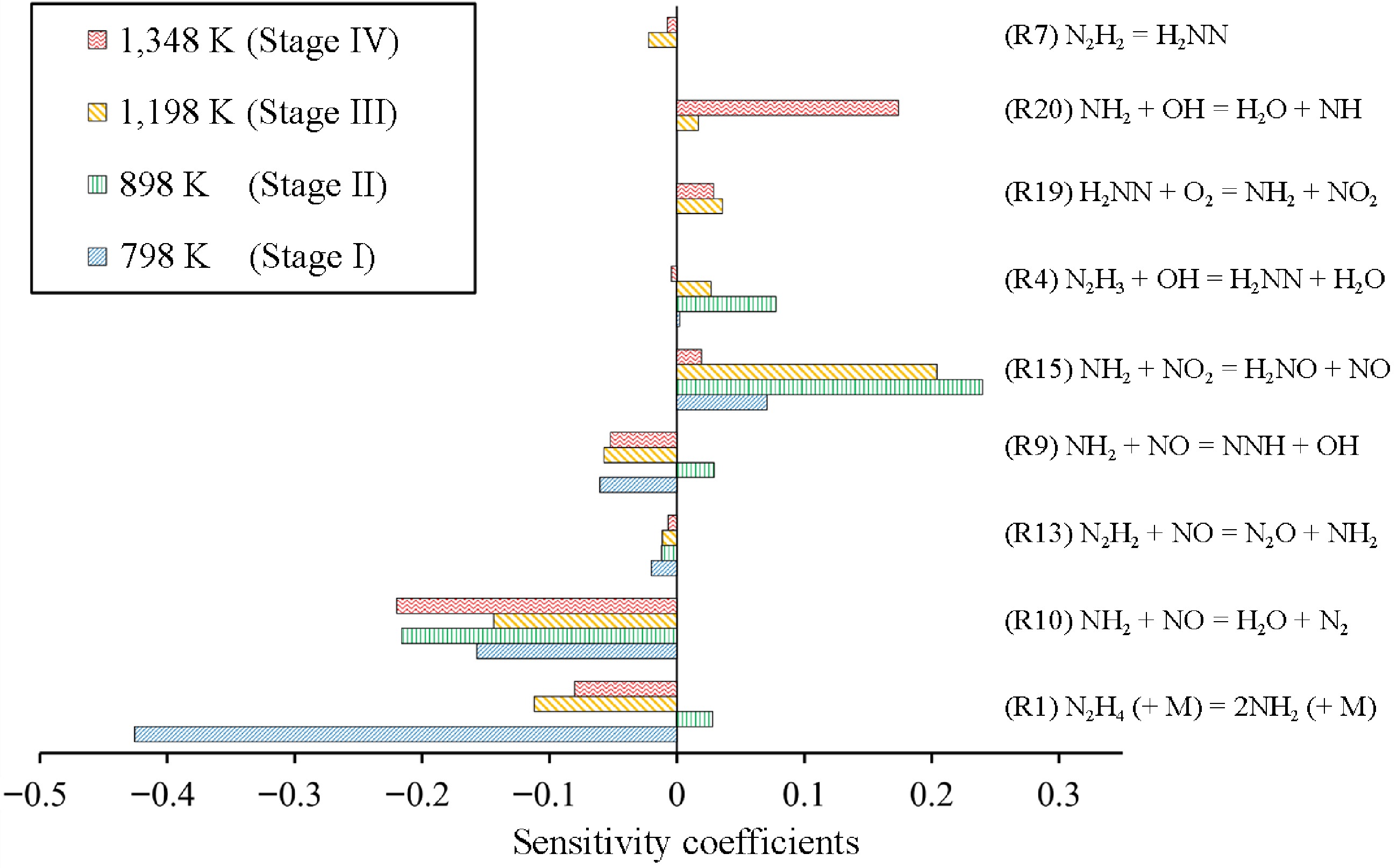
Figure 5.
Sensitivity analysis of NO at 798, 898, 1,198, and 1,348 K, corresponding to Stage I to IV, under condition 1.
NO removal mechanism in Stage II
-
As the temperature increases to Stage II (i.e. 848−1,048 K), the mole fraction of N2H2 starts to decrease, while those of H2NN and NO2 greatly increase (see Fig. 4c). The NO removal ratios decrease in this stage, as seen in Fig. 1. Compared with Stage I, significant changes in the reaction pathways of NH2 and N2H3 radicals occur in this stage. For the NH2 pathways, the branching fraction of R15 (NH2 + NO2 = H2NO + NO) increases, promoting the formation of NO. Meanwhile, H2NO can be converted into NO through a series of H-loss reactions (i.e. R16−R18). Therefore, R15 has a strongly positive sensitivity coefficient for NO formation in this stage, indicating that this reaction plays a key role in NOx formation, as shown in Fig. 5. For the N2H3 pathways, it is more inclined to generate H2NN via R4 rather than N2H2 via R5. As mentioned above, N2H2 and H2NN are two functionally distinct radicals. The former tends to participate in the DeNOx process, while the latter is more inclined to react with oxygen and generate NOx. Therefore, the reaction produces H2NN via R4 exhibits positive sensitivity coefficients for NO formation, as seen from Fig. 5. It is surprising that R9 (NH2 + NO = NNH + OH) presents positive sensitivity coefficient for NO formation in this stage. This is because this reaction produces OH radical, which is critical for converting N2H3 to H2NN via R4 (N2H3 + OH = H2NN + H2O). Since the flux of R4 increases significantly, the mole fraction of H2NN dramatically increases, which simultaneously promotes the formation of NO2 in this stage via R19 (H2NN + O2 = NH2 + NO2), as shown in Fig. 4c. In summary, in this stage, the reaction sequence N2H4 → N2H3 → H2NN → NO2 → NO and R15 prevail, leading to an increase in the NOx concentration.
NO removal mechanism in Stage III
-
In Stage III (i.e. 1,048−1,248 K), N2H4 is completely consumed, and an obvious downward trend in the NO content can be observed again. Besides, the mole fractions of H2NN and NO2 present decreasing trends, while that of NH2 starts to increase again as shown in Fig. 4b & c. In Stage II, H2NN is completely converted to NO2 via R19. However, in Stage III, the branching fraction of H2NN = N2H2 increases, as shown in Fig. 4a. This change leads to less NO2 formation (see Fig. 4c), and further reduces NO formation via R15 (NH2 + NO2 = H2NO + NO). Therefore, the flux of H2NN →NO2 → NO decreases. Meanwhile, the content of NH2 increases so that the DeNOx process via R9 and R10 becomes favorable. Therefore, R1 as the main source of NH2, shows negative sensitivity coefficient again compared with Stage II, as shown in Fig. 5. On the other hand, this reaction successfully converts H2NN to N2H2, which can effectively promote NO removal via R13 (N2H2 + NO = N2O + NH2). The sensitivity analysis further demonstrates that R7 (N2H2 = H2NN) has a negative sensitivity coefficient for NO formation and thus promotes NO removal. Therefore, the DeNOx pathway plays a dominant role again.
NO removal mechanism in Stage IV
-
In Stage IV (i.e. 1,248−1,523 K), the NH2 radical tends to consume, while the NH radical greatly increases, as shown in Fig. 4b & c. Under these high-temperature conditions, NH2 prefers to combine with OH to produce NH, which further reacts with O2 to generate NO via R20−R22. Therefore, R20 (NH2 + OH = H2O + NH) has a strongly positive sensitivity coefficient for NO formation, as shown in Fig. 5. For N2 species shown in Fig. 4c, H2NN remains and continues to be consumed. Compared with Stage III, the H2NN tends to produce N2 via the unimolecular decomposition reaction R8 (H2NN = N2 + H2), as shown in Fig. 4a (the dashed lines). Other pathways of H2NN, such as the formation of N2H2 and NO2, are less important, indicating that the N2 pathways in this stage have little influence on NO formation. Therefore, in this stage, NO formation is favorable due to the reaction sequence of NH2 → NH → NO.
Above all, compared with NH3, N2H4 can achieve SNCR of NO over wider temperature windows. The chain-initiation reaction of NH3 occurs at high temperatures (above 1,100 K) due to the strong N-H bond dissociation energy. However, much weaker N-N and N-H bonds in N2H4 make chain-initiation reactions of N2H4 easier to occur, resulting in a much lower DeNOx temperature window (i.e. 673−848 K). In addition, the special N2 pathways in N2H4 have two opposite effects: the production of N2H2 promotes the DeNOx process via R13, which is not found important in the NH3 DeNOx system; and the production of H2NN inhibits the DeNOx process due to the formation of NO2 via R19. The enhanced formation of NO2 further promotes NO formation via R15 (NH2 + NO2 = H2NO + NO).
Effects of the oxygen content on NO removal
-
The increased oxygen content enhances the oxidation reactions by increasing the mole fractions of oxygenated species such as O2, OH, and HO2. These radicals play important roles in the removal and formation process of NO[54]. The effects of the oxygen content on NO removal by N2H4 are simulated by the present model, and the results are compared in Fig. 6.

Figure 6.
Effect of O2 content on the removal of NO at [NO]initial = 500 ppm, NSR = 4.0 at 1 atm and τ = 0.3 s. Symbols are experimental data from Guan 2019[23], while lines are the predicted results by the present model.
Both the experimental and simulation results suggest that the NO removal ratio is not strongly affected by the oxygen content. However, at approximately 1,350 K, the experimental results show that an increase in the oxygen content leads to a significant decrease in the NO removal ratio, while the simulation results indicate that this effect is minimal. This could be attributed to the experimental uncertainties since only one point presents a large discrepancy. Based on model analyses, N2H4 initially generates NH2 radical via its unimolecular decomposition reaction R1 without oxidative pathways, while in the NH3 DeNOx system, NH2 is generated mainly via H-abstraction reactions by OH, O, and HO2 radicals[55]. Since NH2 is the main radical for NO removal in both systems, the N2H4 system is less influenced by the oxygen content. Nevertheless, the formation of N2H2 is related to oxygenated radicals such as OH and HO2, so the influence of oxygen content still exists. Above all, compared with NH3, it is testified that N2H4 has a wider range of oxygen fractions for NO removal during the SNCR process. Therefore, another advantage of using N2H4 for the SNCR of NO is that its NO removal efficiency is relatively less affected by fluctuations in the oxygen content. This characteristic enables N2H4 to play a more stable role in the SNCR process, which fits better with the practical flue gas treatment environment after combustion.
-
In this work, a kinetic model for NO removal by N2H4 was developed. The simulation results of this model were reasonably consistent with the experimental data over the temperature range of 673−1,523 K. Compared with recently published kinetic models, the present model was more accurate in predicting the temperature window and peak values of the NO removal efficiency. It has also been demonstrated that recent NH3 oxidation models are not accurate enough for predicting NO removal by N2H4, although they have been comprehensively validated against wide experimental targets of NH3. Both the experimental and simulation results of this model present a four-stage NO removal trend by N2H4.
Based on the model analysis, it revealed that N2H4 can generate N1 and N2 pathways to achieve DeNOx and the NO formation process. In the N1 pathway, NH2 can undergo the reactions of DeNOx and NO formation simultaneously. In the N2 pathway, N2H3 produces N2H2 and H2NN. N2H2 tends to participate in the DeNOx process, while H2NN tends to react with O2 to generate NOx. In Stage I (673−848 K), N2H4 generates NH2 and N2H2 through N1 and N2 pathways, respectively, both of which can promote NO removal via R9, R10, and R13, with N1 pathway being more effective. Since the chain-initiation reactions in the N2H4 DeNOx system have lower energy barriers than that in NH3, the DeNOx process by N2H4 occurs at lower temperatures. In Stage II (848−1,048 K), the reaction sequence N2H4 → N2H3 → H2NN → NO2 → NO and R15 (NH2 + NO2 = H2NO + NO) prevailed, increasing the content of NOx and decreasing the efficiency of NO removal by N2H4. When the temperature increased to Stage III (1,048-1,248 K), the flux of H2NN = N2H2 increased, leading to less NO2 and more N2H2 being produced, increasing NOx removal efficiency again. When the temperature exceeds 1,248 K, i.e. Stage IV, NH2 prefers to produce NH via H-abstraction reactions, and NH further reacts with O2 to generate NO. Furthermore, the simulation results suggested that NO removal by N2H4 is less affected by the fluctuations of oxygen content. The main reason is that N2H4 can be converted to NH2 directly via the unimolecular decomposition reaction R1, whereas in the NH3 DeNOx system, NH2 is generated mainly via H-abstraction reactions by OH, O and OH2. These findings are highly important for better understanding the chemistry of NO removal by N2H4.
The authors are grateful for the funding support from the National Natural Science Foundation of China (22403085) and the Natural Science Foundation of Anhui Province (2408085MB034).
-
The authors confirm contribution to the paper as follows: study conception and design: Zheng M, Zhang X; data collection: Zheng M; analysis and interpretation of results: Zheng M, Zhang X; draft manuscript preparation: Zheng M, Zhang X. Both authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
-
The authors declare that they have no conflict of interest.
- Supplementary Fig. S1 The rate of product (ROP) analysis of the N2H4/NO/O2 at [NO]initial = 450 ppm, NSR = 2.0, O2 content = 16.05% at T = 798 K, P = 1.0 atm, τ = 0.3 s.
- S4
- Supplementary Fig. S2 The rate of product (ROP) analysis of the N2H4/NO/O2 at [NO]initial = 450 ppm, NSR = 2.0, O2 content = 14.49% at T = 898 K, P = 1.0 atm, τ = 0.3 s.
- Supplementary Fig. S3 The rate of product (ROP) analysis of the N2H4/NO/O2 at [NO]initial = 450 ppm, NSR = 2.0, O2 content = 9.81% at T = 1198 K, P = 1.0 atm, τ = 0.3 s.
- Supplementary Fig. S4 The rate of product (ROP) analysis of the N2H4/NO/O2 at [NO]initial = 450 ppm, NSR = 2.0, O2 content = 7.47% at T = 1348 K, P = 1.0 atm, τ = 0.3 s.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zheng M, Zhang X. 2025. Insights into the NO removal mechanism by hydrazine. Progress in Reaction Kinetics and Mechanism 50: e003 doi: 10.48130/prkm-0025-0003
Insights into the NO removal mechanism by hydrazine
- Received: 07 November 2024
- Revised: 03 December 2024
- Accepted: 15 January 2025
- Published online: 13 February 2025
Abstract: Hydrazine (N2H4) can be a better reductant than ammonia (NH3) for NO removal during the selective non-catalytic reduction (SNCR) process due to its wider temperature window. To better understand the DeNOx chemistry by N2H4, this study conducted a kinetic modeling study for N2H4/NO/O2 based on recent NH3 oxidation models. Compared with previous kinetic models, the present model is more accurate in predicting a four-stage NO removal phenomenon over 673−1,523 K. In Stage I (673−848 K), N2H4 is more favorable to produce NH2 via N1 pathways, which mainly reacts with NO to proceed DeNOx pathways. In Stage II (848−1,048 K), the reaction sequence N2H4 → N2H3 → H2NN → NO2 → NO prevails, decreasing NOx removal ratios. In Stage III (1,048−1,248 K), the branching fraction of H2NN = N2H2 increases, resulting in less NO2 and more N2H2 being produced. The NO removal efficiency is further increased. When the temperature exceeds 1,248 K, i.e. Stage IV, NH2 is favorable to produce NH via H-abstraction reactions, which can be subsequently oxidized to produce NO. Additionally, the present model is also validated against experimental data at various oxygen contents, suggesting that the NO removal efficiency is less affected by the oxygen concentration.
-
Key words:
- Hydrazine /
- SNCR /
- Kinetic simulations /
- DeNOx mechanism














