-
The global prevalence of diabetes mellitus has surged in parallel with rapid socioeconomic development and shifts in lifestyle, positioning it as one of the most pressing public health challenges of the modern era. Diabetes, including type 1 diabetes (T1DM), type 2 diabetes (T2DM), and gestational diabetes mellitus (GDM), are mainly characterized by hyperglycemia. The deleterious influence of diabetes on reproductive health have been demonstrated by previous studies[1,2]. The offspring of diabetic patients have a high risk of metabolic syndrome[3], and that can be transmitted to subsequent generations via germ cells[4]. Therefore, the prevention of the deleterious effects of diabetes on reproductive and offspring health is an important issue. It has been demonstrated that chronic diseases are closely associated with lifestyles, and healthy lifestyles may be the most effective method to treat and prevent chronic diseases[5].
Tea, made from tender shoots and leaves of Camellia sinensis has become the most popular beverage worldwide (with the exception of water) and has been consumed in China for thousands of years. The benefits of tea extract on health, such as anti-obesity, anti-diabetes, anti-bacteria, anti-ageing, antioxidant, and anti-cardiovascular diseases, have been demonstrated by numerous studies[6]. Active components in tea extract reduce the risk of developing diabetes and the complications through multiple pathways, such as restoring glucose metabolic homeostasis, protecting pancreatic β-cells, improving insulin resistance, inhibiting inflammatory responses, ameliorating oxidative stress, and modulating gut microbiota[6]. Tea extract can also alleviate the adverse effects of chronic diseases on reproduction[6]. We have found that tea polyphenols alleviate the deleterious influence of maternal diabetes on oocyte quality[7]. Here, we would like to review the advances in the benefits of tea extract in improving the reproduction of diabetes.
-
Type 1 diabetes mellitus (T1DM), also termed as insulin-dependent diabetes, is characterized as hyperglycemia and insulin deficiency. Studies have shown that T1DM has severe effects on male fertility (Fig. 1). Men with T1DM have a decreased sexual function and a lower rate of children (natural fertility)[8]. Holstein et al. found that men with T1DM had a lower number of children compared with the background population, and over 50% of them were childless[9]. Male onset of T1DM at an earlier age had a significantly lower rate of children[10]. However, paternal T1DM has no significant effects on the biochemical pregnancy, clinical pregnancy, or live birth of embryos produced by assisted reproductive technologies (ARTs)[11]. These suggest that the subfertility/infertility of men with T1DM may be associated with lower sperm quality. A previous study shows that sperm morphology, progressive motility, and semen volume are decreased in men with T1DM[12]. Although the testicular volume is not significantly affected in patients with T1DM, the diameters of the caput and tail of epididymis after ejaculation are higher compared with controls[13]. Hyperglycemia and insulin deficiency, the hallmark of patients with T1DM, may be a reason for affected male gonadal function. The role of insulin in gonadal function and spermatogenesis has been demonstrated by animal studies[14]. It has been reported that there is a high level of advanced glycation end products and oxidative stress in testicular tissues of men with T1DM. The glucose uptake and metabolism in the testis of men with T1DM are disrupted[15]. Reproductive hormones are also essential for spermatogenesis. It has been reported that T1DM decreased the levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in men, but some studies suggest that the levels of testosterone, LH, and FSH are not influenced by T1DM[13]. Although the effects of diabetes on reproductive hormones are inconsistent in studies, these indicate that sperm quality may be influenced by the disrupted secretion of hormones. In addition, the disrupted intestinal microecology may also be associated with the deleterious effects of T1DM on male reproduction[14]. The low sperm quality induced by T1DM is also observed in animal models, and that can be transmitted to the next two generations[16]. T2DM is mainly featured as hyperglycemia and insulin dysfunction. Previous studies have demonstrated that T2DM has a detrimental effect on paternal reproduction. Zhu et al. reported that T2DM had a significant causal relationship with erectile dysfunction and male subfertility/infertility[17]. Another study reported that about 35.1% of infertility is associated with male T2DM[18]. Decreased sperm quality is a reason for the subfertility/infertility of men with T2DM. Facondo et al. showed that T2DM decreased the sperm concentration, progressive motility, and the percentage of spermatozoa in humans[12]. The serum levels of FSH and testosterone are, respectively, increased and decreased by T2DM in men[13,19], which indicates that the disrupted hormone levels may be a reason for the low sperm quality of T2DM. Furthermore, the concentration of mitochondrial superoxide is higher in men with T2DM, which may be associated with higher sperm DNA fragmentation rates[20]. Sperm DNA methylation is also disrupted by T2DM in humans[21]. In addition, the adverse of T2DM on male reproduction could be transmitted to offspring[22]. These results suggest that the reduced sperm quality induced by T2DM may play a key role in the subfertility/infertility of males.
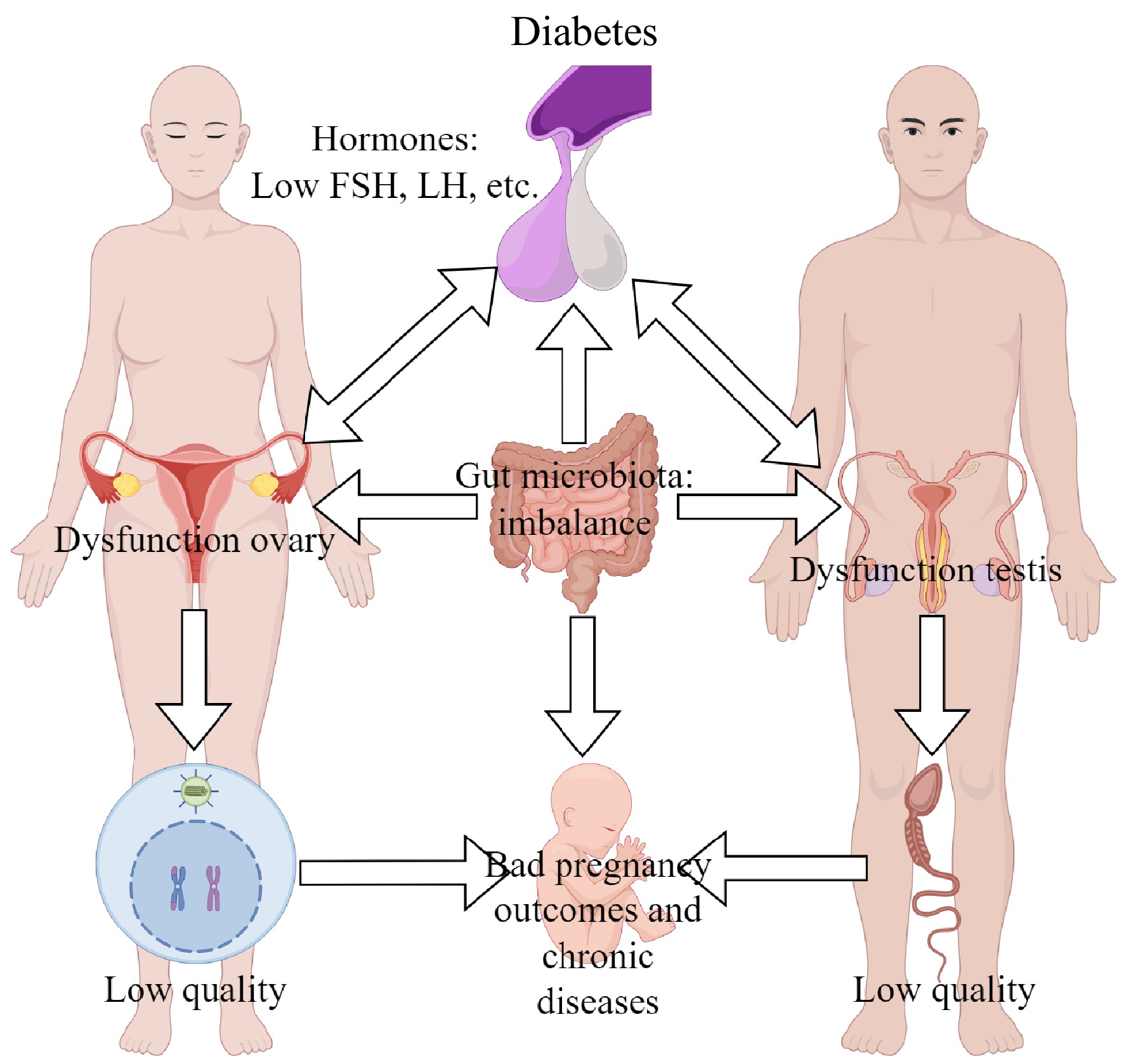
Figure 1.
Influence of diabetes on reproduction of patients. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Nevertheless, the mechanisms underlying the impaired reproduction males with T1DM and T2DM are not well understood, although the disrupted secretion of hormones, excessive ROS, and gut-testis axis may be involved.
Diabetes and female reproduction
-
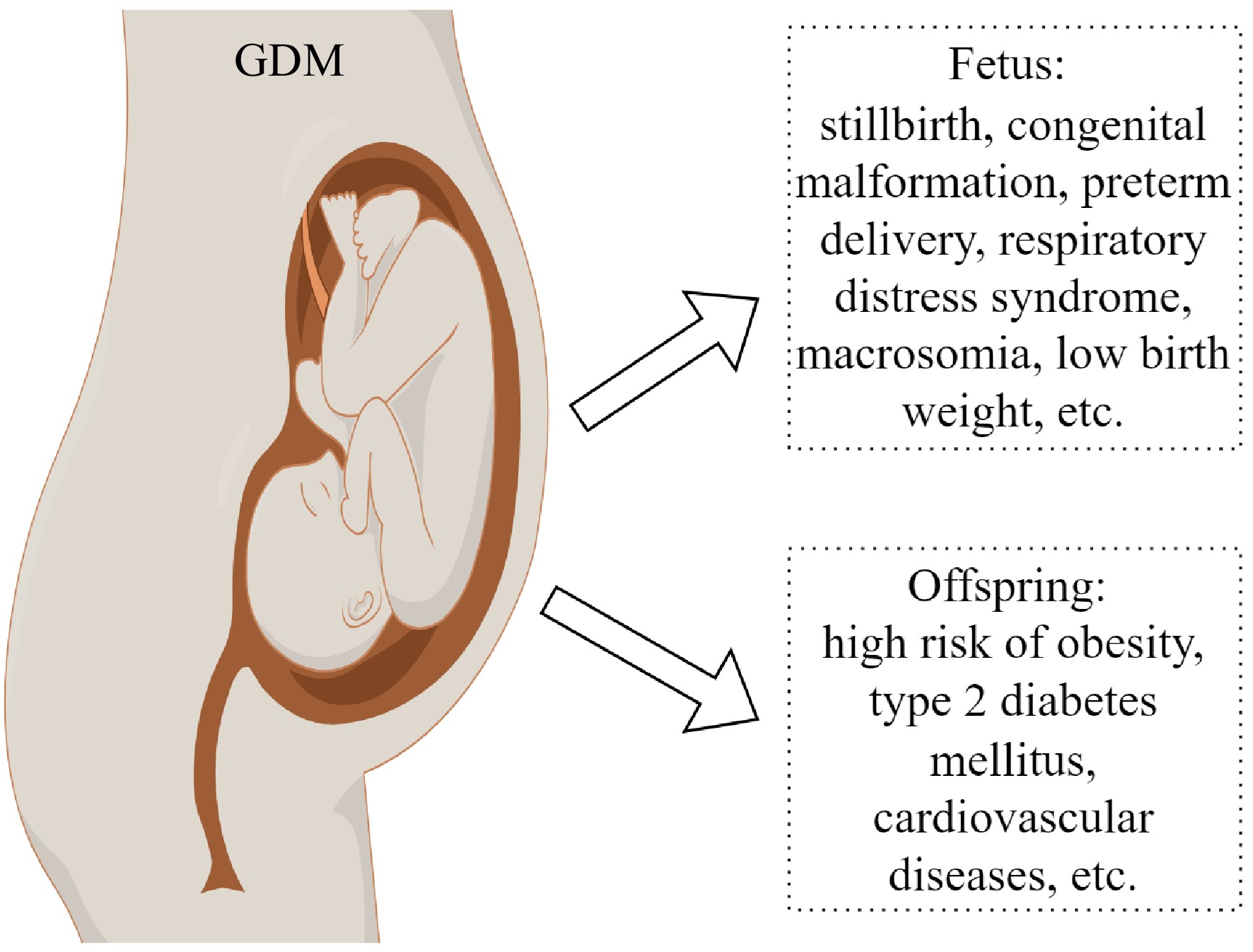
Approximate 16.7% of live births worldwide in 2021 were associated with maternal diabetes, of which 10.6% are associated with pregestational diabetes including T1DM and T2DM, 89.4% are associated with gestational diabetes (GDM) including T1DM and T2DM first detected in pregnancy and hyperglycemia developing in the second trimester[23]. The effects of maternal diabetes on pregnancy outcomes are reported by previous studies[24]. Both pregestational and gestational diabetes (Fig. 2) increase the risk of miscarriage, preterm birth, congenital anomalies, pre-eclampsia, neonatal hypoglycemia, macrosomia, stillbirth, Apgar score, and respiratory distress syndrome[24]. The offspring of maternal diabetes have a higher risk of metabolic disorders, congenital, and cardiac malformations[25]. Pregestational diabetes has more serious effects on pregnancy outcome and offspring health[24], which may be associated with ovarian dysfunction and low oocyte quality. Diabetes increases the ovarian length and reduces the ovarian response to leuprolide in adolescent girls[26]. In mouse models, diabetes decreases the growing follicle number, delays follicular development and oocyte maturation, and increases apoptosis in follicles[27]. The gap junction is important for material transportation and signal transmission during follicular development, but the communication between oocyte and cumulus cells is impaired in diabetic mice[28]. These results suggest that diabetes decreases ovarian function. The influence of maternal diabetes on oocyte quality has been reviewed previously by Wang & Moley[2]. It has been demonstrated that maternal diabetes disrupts mitochondrial function, spindle assembly, and chromosome separation, and increases apoptosis in oocytes[29]. Li et al. reported that oocytes from diabetic mice have a thin zona pellucida and abnormal genomic DNA methylation[30]. It has been found that maternal diabetes disrupts the transcriptome of oocytes[31], and the DNA methylation of imprinted genes in oocytes[32]. High glucose concentration also increases the DNA methylation level of Peg3 in vitro-matured human oocytes[33]. To explore the possible mechanisms, Xin et al. reported that the deficiency of sirtuin 3 (SIRT3) in oocytes induced by maternal diabetes might be involved in regulating the oocyte quality by the SIRT3-GSK3β deacetylation pathway[34]. Loss of pyruvate dehydrogenase kinase 1 (PDK1) is associated with meiotic defects in oocytes of diabetic mice[35]. However, the molecular mechanism underlying the reduced oocyte quality of diabetes is complex, and further studies are required. In addition, the low oocyte quality induced by maternal diabetes may be associated with the abnormal development of embryos and the disrupted metabolism of offspring[2]. Chen et al. reported that the reduced expression of ten-eleven translocation 3 (TET3) in oocytes induced by pregestational diabetes caused hypermethylation of paternal alleles including glucokinase gene (GCK) in zygotes, which is associated with the abnormal embryo development and the metabolic disorders in offspring[4]. These results suggest that maternal diabetes not only has serious effects on oocyte quality but also on offspring health.
Effects of maternal diabetes on offspring health and its inheritance
-
Since the hypothesis that adult diseases are initiated from the environment of the uterus and early postnatal life was proposed in 1989[36], it has been demonstrated by lots of studies[37]. Nowadays, it has been demonstrated that the reduced quality of oocytes also plays a key role in adult diseases[38]. In humans, offspring exposed to gestational diabetes have a high risk of metabolic disorders and hypertension[39] (Fig. 1). The metabolic syndrome in offspring caused by maternal diabetes is also observed in animal studies[39]. More importantly, adult chronic diseases induced by environmental factors of females can be transmitted to subsequent generations[37,38]. The impaired memory in offspring exposed to hyperglycemia during gestation can be also transmitted through two generations[40]. The occurrence and inheritance of metabolic disorders induced by maternal diabetes may be associated with the changes in epigenetic modifications. Our study indicates that pregestational diabetes changes the methylation and expression levels of imprinted genes Peg3 and H19 in dpc10.5 placentas[41]. The changed DNA methylation levels of Igf2/H19 and Gck in offspring islets induced by maternal diabetes are transmitted to the next two generations, which may be associated with the inheritance of the disrupted metabolism[4]. Additionally, histone 3 lysine 9 acetylation (H3K9ac) is reduced by gestational diabetes in the placenta[42]. Elevated H3K4me3 caused by gestational diabetes may be involved in mediating the inflammatory and oxidative phenotype in adolescents[43]. These studies suggest that epigenetic changes caused by maternal diabetes may play a key role in the high risk of offspring for chronic diseases and their inheritance. However, the mechanisms underlying the inheritance of metabolic disorders induced by maternal diabetes and its relationship with epigenetic changes are not completely elucidated.
Effects of paternal diabetes on offspring health and its inheritance
-
Besides maternal diabetes, paternal diabetes also contributes to the health of offspring. If fathers have the onset of diabetes before 35 years, their offspring are thin and present an earlier decrease of insulin level than those from healthy fathers[44]. Offspring of men with the onset of T2DM at preconception have a lower birth weight, and increased BMI, T2DM incidence and triglyceride level[45]. Paternal T2DM disrupts metabolism, increases the abnormal sperm rate, and reduces the fertility of offspring in mice, which can be transmitted to the second generation[46]. Altered epigenetics may be a reason for the metabolic disorders in offspring from diabetic fathers. Wei et al. reported that prediabetes altered sperm methylome, which may be associated with metabolic disorders in offspring[22]. These suggest that paternal diabetes have bad effects on offspring health, which may be mediated by epigenetics. However, more studies are needed to further elucidate the underlying mechanisms.
-
Diabetes not only has deleterious effects on male and female reproduction, but also on offspring health. Offspring exposed to diabetic patients have a high risk of non-communicable diseases, which can be inherited by subsequent generations. Therefore, it has become an urgent problem to improve the reproductive capacity and offspring health of patients with diabetes. A cohort study shows that treatment of diabetic fathers with insulin in preconception has no effects on the birth defect frequency, and that it is increased in the treatment of metformin and sulfonylurea[47]. Another study[48] indicates that metformin used during the period of sperm development does not increase the congenital malformations in offspring. Antidiabetic drugs are also used to treat maternal diabetes including pregestational and gestational diabetes, but there are no consistently improving outcomes on female reproduction and offspring health. Chronic diseases are closely associated with lifestyle. Therefore, lifestyle has become an important strategy to prevent the deleterious effects of diabetes on reproduction and offspring health. Tea has become the most popular beverage worldwide (with the exception of water). There are many bioactive components in tea, which are beneficial to health such as anti-diabetes, anti-inflammation, anti-cancer, anti-obesity, anti-virus, and anti-oxidation[6]. These indicate that tea may be a good way to prevent the damage of diabetes on reproduction and offspring health. Here, we mainly discuss the benefit of tea in improving the impaired reproduction induced by diabetes (Fig. 3).
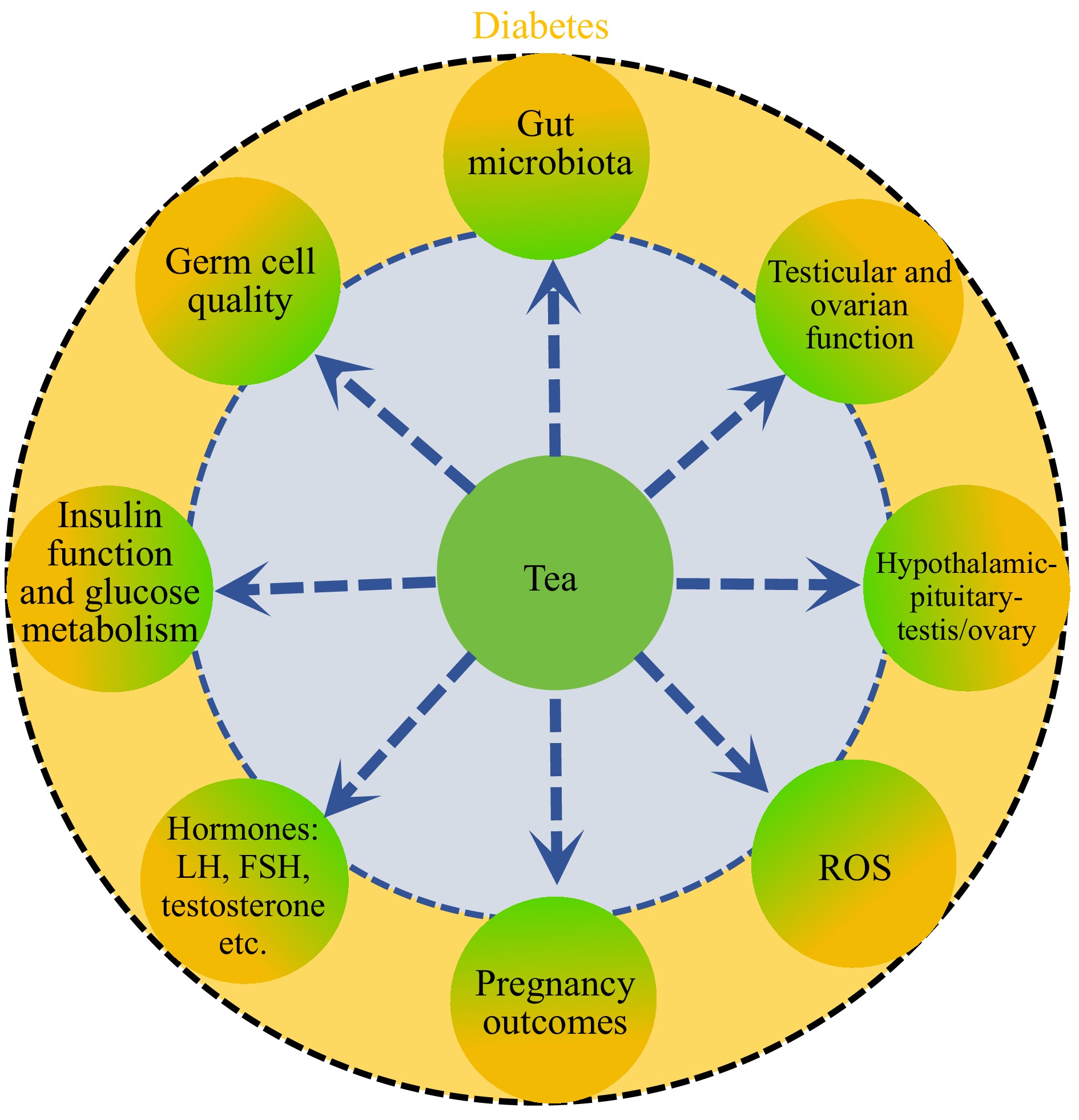
Figure 3.
Schedule of tea extract improving diabetic reproduction. Yellow, negative effects of diabetes on diabetic reproduction; green, the alleviation of tea extract on diabetic reproduction.
Role of tea in anti-diabetes
-
Chinese people have consumed tea for thousands of years. There have been records on tea used as a medicinal herb since the Tang and Song dynasties[49]. The role of tea in treating diabetes is also presented in Chinese folklore. Currently, the benefit of tea on diabetes has been demonstrated by numerous studies. A case-cohort study researches the association between the incidence of T2DM and tea consumption. Individuals consuming more than four cups of tea per day have a lower incidence of T2DM. There is a reducing trend in the incidence of T2DM while 1–4 cups of tea are consumed every day[19]. Yang et al. reported that the incidence of T2DM was reduced by 4.6% with the increase of two cups of tea consumption per day, and the benefit of tea on the incidence of T2DM is dose-dependent[50]. Green tea consumption significantly reduces the bodyweight, body mass index, and body fat of T2DM patients. A long-term intake of green tea at a low dose is also beneficial to T2DM patients[51]. Patients with T2DM consuming green tea at > 800 mg/d for more than 8 weeks have a low serum triglyceride. Green tea consumption at < 800 mg/d for more than 8 weeks significantly reduces the serum total cholesterol in T2DM patients[52]. T2DM induces hypoadiponectinemia, but that can be alleviated by green tea supplementation. An epidemiologic study in the Mediterranean Islands shows that tea consumption (black tea and green tea) at 1–2 cups/d reduces the fasting blood glucose of T2DM patients[53]. A multi-centre study in China shows that daily tea drinkers have a lower risk of diabetes compared with non-tea drinkers[54]. Furthermore, tea and its components can improve diabetic complications. Drinking green tea every week for one year significantly protects women with diabetes from retinopathy[55]. Green tea has beneficial effects on the blood pressure of patients with T2DM[56]. It is reported that black and oolong teas have benefits on improving complications of patients with diabetes[57]. Intake of catechin for 12 weeks significantly decreases the waist circumference and increases the adiponectin level in patients with T2DM[58]. Nevertheless, some studies show that tea has no benefit in reducing the incidence of diabetes. A prospective cohort study in the Singapore Chinese Health study shows that black tea consumption is not significantly associated with the risk of T2DM in Asian men and women[59]. It is reported that consumption of oolong tea (≥ 2 cups/d) over the long-term increased the risk of T2DM in Japanese[60]. This may be associated with many reasons, such as tea types, drinking method, tea origin, age, sex, and individual patient differences. Although some studies indicate that tea consumption has no significant influence on reducing the risk of diabetes in humans, more studies support the positive role of tea, especially green tea, in anti-diabetes.
To clarify the role of tea on diabetes and elucidate the underlying mechanisms, studies have been performed using animal models. (+)-Catechin, an important compound in green tea, alleviates renal injury by inhibiting endoplasmic reticulum and renal inflammation by suppressing the expression of NLR Family Pyrin Domain Containing 3 (NLRP3), absent in melanoma 2 (AIM2), and interleukin 18 (IL-18) in streptozotocin (STZ)-induced diabetic mice[61]. Renal injury caused by diabetes in mice can also be improved by tea polypeptide and theaflavins[62]. Zhang et al. reported that black tea polysaccharides reduced blood glucose levels in T2DM mice via the PI3K/Akt/GLUT2 pathway[63]. Fu brick tea aqueous extract improves the disrupted intestinal microecology and metabolism in T2DM mice and protects from T2DM by the IRS1/PI3K/Akt and AMPK-mediated gluconeogenesis signaling pathways[64]. EGCG reduces the incidence of neural tube defects in embryos exposed to gestational diabetes and DNA hypermethylation by decreasing the expression of DNA methyltransferase 3a (DNMT3A), and DNMT3B[65]. Theaflavins, a tea polyphenol derivative, improve hyperglycemia and hyperlipidemia, and regulates carbohydrate and lipid metabolism by influencing the gut microbiota in diabetic mice[66]. Although studies using animal models have discussed the mechanisms underlying the benefit of tea on anti-diabetes and improving the complications, it is still not completely elucidated.
GDM is a common gestational disease and affects 1% to 14% pregnant women in the US. The prevalence of GDM in China is approximately 8%, and in Europe, its prevalence is up to 52%[67]. Lifestyle may be a better way to protect pregnant women from GDM[68]. A study in the Danish National Birth Cohort investigates the influence of tea on GDM in the first trimester, and results show that tea intake decreases the risk of GDM, but the difference is not significant. Among smokers, tea intake has no consistent pattern in GDM risk[69]. Mu et al. showed that tea intake had no significant effects on GDM in a Mendelian randomization analysis[70]. However, a study among pregnant women in Greece shows that tea intake significantly increases the risk of GDM in the adjusted analysis, although the risk is not reached to significance in the rude analysis[68]. These indicate that more studies are required in the future to elucidate how tea influences GDM.
Influence of tea on reproductive hormones of patients with diabetes
-
Appropriate reproductive hormone levels secreted by the hypothalamic-pituitary-gonadal axis (HPG) are crucial for gametogenesis in mammals. Nevertheless, insulin receptor knockout in male mice brains decreases the secretion of luteinizing hormone (LH) and impairs the function of Leydig cells and spermatogenesis[71]. Insulin level is associated with leptin levels that correlate to HPG function[72]. Low LH, follicle-stimulating hormone (FSH), and testosterone levels are also observed in men with T2DM[73]. Hyperglycemia exposure causes low LH levels in serum resulting in the decrease of serum testosterone level, which reduces the sensitivity of hypophysis to gonadotropin-releasing hormone (GnRH) via negative feedback regulation[74]. These suggest that diabetes damages the function of HPG. It has been shown that the bioactive compounds of tea affect hypothalamic and pituitary functions[75]. This means that tea extract may restore the dysfunction of HPG. The low plasma level of testosterone in diabetic rats can be improved by green tea supplementation at 100 mg/kg body weight[76]. It is reported that green tea decreases the high level of leptin induced by depot medroxyprogesterone acetate in female rats[77]. Polyphenols enhance the insulin function in the brain[78], and green tea reduces the high levels of LH, FSH, and testosterone in women with polycystic ovary syndrome[79]. These indicate that tea may have a positive role in improving the HPG function in diabetic patients, but the evidence is not enough, and the underlying mechanisms are not well elucidated. Therefore, more studies are needed to investigate the role of tea in improving the HPG function of diabetic patients.
Tea and oxidative stress in germ cells of diabetes
-
Oxidative stress due to the excessive accumulation of reactive oxygen species (ROS) is an important pathogenic factor in diabetes and its complications[80]. Excessive ROS impairs the lipid bilayer on the membrane of sperm and disrupts its fluidity by affecting the polyunsaturated fatty acids on the membrane, which influences the material transport, energy fluid, and immune defense[81]. The DNA integrity in sperm can also be impaired by high levels of ROS[81,82]. Otherwise, elevated ROS destroys the testicular function via abnormal autophagy induced by the inhibited PI3/AKT/mTOR pathway. Antioxidants are one of the most important properties of tea because of the compounds of polyphenols including flavonoids, flavandiols, and flavols, which constitute up to 30% of the dry matter of green tea[83]. Catechins are the main flavonoids in green tea, including EGCG, EGC, ECG, and EC[84]. The antioxidant capacity of polyphenols is based on their specific chemical structure including a minimum of five hydroxyl groups[84]. However, few studies are focusing on the role of tea in improving oxidative damage in reproduction. Oliveira et al. produced a prediabetes rat model, and then the rats were treated with the water extract of white tea for 2 months. After that, the ROS level in testicular tissues is reduced, but the glutathione is not affected. The decreased ROS level may be a reason for the improved epididymal sperm quality of prediabetic rats[85]. Dias et al. reported a similar benefit of white tea ingestion on testicular function and sperm quality[86]. While diabetic rats induced by STZ are given green tea infusion by gavage at 100 mg/kg body weight for 42 d, the testicular damage and sperm quality are alleviated. Nevertheless, the activities of superoxide dismutase (SOD) and glutathione S-transferase are not significantly changed[87]. Another study reported that the decreased testicular function, sperm quality, and the activity of SOD in diabetic rats was improved by the treatment of methanolic extract of green tea for 28 d, and the disrupted expression of Bcl-2-associated X protein (Bax) and Bcl-2 was alleviated[76]. Kaplanoglu et al. showed that green tea combined with vitamin E was more effective in improving the damage of diabetes on testicular function[88]. These indicate that tea extract can improve the damage of diabetes on testis function in animals, but few studies have focused on humans, and the underlying mechanisms are still not well known.
Maternal diabetes also has deleterious effects on follicular development and oocyte maturation. Previous studies have reported the role of tea in oogenesis, but there are few studies focusing on the influence of tea on oogenesis of females with diabetes[89]. In our previous studies, we produced diabetic mouse models using STZ and investigated the effects of tea polyphenols on oocyte maturation in vitro. Tea polyphenol significantly increases the oocyte maturation rate of diabetic mice at 50 μM and alleviates the adverse influence of diabetes on spindle assembly and chromosome alignment. The mitochondrial dysfunction in oocytes of diabetic mice is also improved by tea polyphenols. Maternal diabetes results in excessive accumulation of ROS in oocytes by reducing the expression of SOD1 and SOD2, while tea polyphenols significantly decrease the level of ROS and increase the expression of SOD1 and SOD2 during oocyte maturation in vitro. Tea polyphenol also improves the DNA damage in oocytes induced by maternal diabetes[7]. EGCG is one of the main components of tea polyphenols. Therefore, we further examined the role of EGCG in improving the oocyte quality of diabetic mice. Results show that EGCG significantly increases the oocyte maturation and mitochondrial function, and reduces the ROS level, DNA damage, and the incidence of chromosome mismatch[90]. These indicate that tea extract can improve the impairment of maternal diabetes on oocyte quality. Nevertheless, the evidence of the benefits of tea extract on oocytes of maternal diabetes in vivo and in humans is insufficient, and the underlying mechanisms are not well understood.
Tea and glucose metabolism during gametogenesis in diabetic patients
-
Glucose is the main energy source during gametogenesis. Sperm relies on glucose metabolism to maintain basic cellular activity and specific functions[91]. Glucose is converted into pyruvate by 6-phosphofructokinase (PFK1) in the testis, which is further transformed into lactate and alanine by lactate dehydrogenase (LDH) and glutamic pyruvic transaminase (ALT), respectively. Glucose also plays a key role in oocyte growth and maturation[92]. Nevertheless, the glucose metabolism is disrupted by diabetes[91]. Prediabetes in male rats decreases the protein expression of GLUT2, PFK1, LDH, and ALT in cauda epididymis, but this can be alleviated by the ingestion of white tea extraction for 2 months[86]. The ingestion of white tea also restores the decreased lactate content in the testis of prediabetic rats. Although the expression of ALT in testis is decreased by white tea ingestion in prediabetic rats, the alanine content is not affected[86]. Another study shows that green tea extract significantly decreases the high level of seminal vesicular fructose in diabetic rats at doses of 50, 100, and 200 mg/kg[76]. Similarly, the tea active component protocatechuic acid (PCA) increases the glucose uptake in visceral adipose tissues by upregulating the phosphorylation of p38 mitogen-activated protein kinase[93]. Maternal diabetes also impairs the glucose uptake in oocytes, which results in low oocyte quality[28]. The expression of pyruvate dehydrogenase kinase 1 (PDK1), a crucial glucose metabolism enzyme, in oocytes is decreased by maternal diabetes[35]. However, studies that focus on the effects of tea on glucose metabolism during oocyte maturation of diabetes are few. These results suggest that tea extract can reduce the damage of diabetes on glucose metabolism during gametogenesis.
Influence of tea on gut microbiota
-
The imbalance of gut microbiota induced by diabetes plays a key role in the impairments of germ cell quality, embryo development, and offspring health. These suggest that the impaired reproduction of diabetes can be alleviated by improving the balance of gut microbiota. The aqueous extract and polysaccharides of Fu brick tea can relieve T2DM in rats by reshaping the gut microbiota[64,94]. Alleviated gut microbiota dysbiosis mediates the anti-T2DM of EC in rats[95]. Zhou et al. showed that green tea or black tea can improve the composition and diversity of the gut microbiota in diabetic mice[96]. Tea polyphenols alleviate the impairment of T2DM on the memory of rats by the microbiota-immune-synaptic plasticity axis[97]. These suggest that tea can improve the gut microbiota dysbiosis of diabetes, but there are few studies focusing on how tea alleviates the reproduction of diabetes by gut microbiota.
Tea and offspring of diabetic patients
-
Diabetes not only has a deleterious influence on the reproduction of patients but also increases the risk of noncommunicable diseases in offspring. It has been demonstrated that the offspring of parents with diabetes have a high risk of obesity, diabetes, and cardiovascular diseases, and those can be inherited by subsequent generations[38,39]. These findings suggest that it is very important to explore ways to improve the damage of diabetes on offspring health and block its inheritance. To discuss the benefit of tea on the offspring health of diabetic parents, we first need to know the safety of tea intake during pregnancy. The 2020 Dietary Guidelines Advisory Committee in the US examined this important public health question and shows that there is no sufficient evidence to determine the relationship between tea consumption (overall tea) during pregnancy and birth weight outcomes[98]. Lu et al. have shown that tea consumption (green tea, oolong tea, and black tea) during early pregnancy is not associated with an increased risk of preterm birth or abnormal fetal growth[99]. An umbrella review shows that tea consumption reduces the risks of total mortality, cardiac death, and chronic diseases with increments of 2 to 3 cups/d. Although tea intake is harmful to health, the benefits seem to be more[100]. These indicate that tea consumption should be safe for offspring health. Therefore, we further discuss the benefit of tea on the offspring health of diabetic parents. EGCG from green tea reduces the incidence of embryos with neural tube defects in diabetes from 29.5% to 2% at the dose of 10 μM. The hypermethylation in diabetic embryos is improved by EGCG treatment via decreasing the expression of DNMT3A and DNMT3B. The DNA methylation levels of CpG islands located in the neural tube closure contain essential genes, including grainy head like transcription factor 3 (Grhl3), paired box 3 (Pax3), and TUB like protein 3 (Tulp3), which are increased by diabetes, but are improved by EGCG treatment[65]. Chen et al. precipitated the water extract of green tea with ethanol, which was further deproteinized with trichloroacetic acid, neutralized with NaOH, and fractionated by DEAE-cellulose DE-52 column chromatography, and then obtained three unexplored polysaccharide-conjugate fractions. These three compounds protect the human umbilical vein endothelial cells from the impairments of high glucose at doses of 50, 150, and 300 μg/mL[101]. These suggest that the compounds of tea have protective effects on the embryos of diabetic mothers. However, studies focusing on the benefit of tea on diabetic embryos and offspring are limited. More studies are necessary in the future.
-
Many studies have demonstrated the deleterious influences of diabetes on reproduction[30,38,41], but there have been no ways to prevent it to date. To resolve this, it is necessary to elucidate how diabetes impairs the reproductive system. Firstly, oxidative stress is essential for the damage of diabetes on reproduction. Hyperglycemia disrupts glucose metabolism and mitochondrial function resulting in the excessive accumulation of ROS in cells, which induces DNA damage and apoptosis[2]. The impairment of excessive ROS on testis and oocytes has been demonstrated[54]. Furthermore, the oxidative stress induced by the parental environment is bad for embryo development and offspring health. These suggest that oxidative stress may be an important reason for the impaired reproduction of diabetic patients. Secondly, the dysfunction of HPG may be associated with impaired reproduction in individuals with diabetes. GnRH regulates the secretion of gonadotropins in adenohypophysis, such as FSH and LH. For females, FSH regulates follicular development, and LH is crucial for ovulation. FSH and LH also regulate spermatogenesis and the secretion of testosterone in Leydig cells. The neural injury, including the impairment of hypothalamus-pituitary-target gland axis, caused by diabetes has been demonstrated[102]. Therefore, the disrupted HPG function may be crucial for the impaired reproduction of diabetic patients. Thirdly, the imbalance of gut microecology induced by diabetes may play a key role in reproductive damage. Gut microbiota regulates oocyte maturation and embryo development via bile acid metabolism and plays an important role in regulating the reproductive endocrine system[103]. Otherwise, gut microbiota regulates spermatogenesis by the gut-testis axis[104]. The imbalance of gut microbiota induces the low-quality germ cells and impairs embryo development and offspring health[103]. Gut microbiota dysbiosis in diabetic patients has been demonstrated. Fecal microbiota transplantation significantly improves sperm quality in young men with T2DM by increasing the intestinal Allobaculum[104]. These indicate that the imbalance of gut microbiota induced by diabetes may be an important reason for the impaired reproduction. It has been demonstrated that the offspring of diabetic parents have a high risk of metabolic syndrome[39]. In our previous study, we found that maternal diabetes alters the DNA methylation in oocytes[32]. The disrupted DNA methylation in oocytes can be partly transmitted to subsequent generations by females and may be associated with metabolic disorders in offspring. The abnormal DNA methylation of sperm induced by prediabetes can also be inherited by the offspring, which may be associated with metabolic disorders[22]. These suggest that abnormal epigenetic modifications are associated with the gametogenesis and offspring health of diabetic parents.
The health benefit of tea includes antioxidant and regulating gut microbiota and neural function. Epigenetic modifications can also be affected by tea extracts[65]. Therefore, tea may be an important candidate to improve the reproduction of diabetic patients. Although there are some studies focusing on this possibility, more studies are necessary in the future to elucidate how tea protects the reproduction of diabetic patients. Future studies should focus on: 1) the molecular mechanisms of tea improving the oxidative stress induced by diabetes in reproduction; 2) how tea regulates the function of HPG in diabetes; 3) how tea alleviates the reproduction of diabetes through gut-testis/ovary axis; and 4) the effects of tea on the glucose metabolism and insulin function in the reproduction of diabetes. We also cannot ignore the toxicity of tea to health. Therefore, we need to clarify the beneficial and harmful components of tea. It is also important to find out what is the best method of improving the reproduction of diabetic patients. More studies are necessary in the future to elucidate how tea improves the reproductive health of patients with diabetes.
We thank Dr. Shen Yin (Qingdao Agricultural University) for editing the language, and Dr. Ming-Hui Zhao and Gui-An Huang (Qingdao Agricultural University) for their helpful suggestions. This work is supported by the National Natural Science foundation of China (32470566, 32272775), the Breeding Plan of Shandong Provincial Qingchuang Research team (Innovation Team of Farm Animal Cloning, 012-1622001), the Technology System of Modern Agricultural Industry in Shandong Province (SDAIT-19-14), and the Project of Laoshan Tea Industry Innovation Group (LSCG2023002132).
-
The authors confirm contribution to the paper as follows: study conception and design: Ge ZJ, Zhao L; data collection: Lin J, Li L, Ge ZJ; analysis and interpretation of results: Lin J, Li L, Ge ZJ; draft manuscript preparation: Ge ZJ, Lin J, Zhao L. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Lei Zhao, Jing Lin
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao L, Lin J, Li L, Ge ZJ. 2025. Benefits of tea on the reproductive health of diabetes mellitus. Beverage Plant Research 5: e019 doi: 10.48130/bpr-0025-0007
Benefits of tea on the reproductive health of diabetes mellitus
- Received: 25 November 2024
- Revised: 09 February 2025
- Accepted: 22 February 2025
- Published online: 07 July 2025
Abstract: The global prevalence of diabetes mellitus has elevated it to a critical public health concern. Diabetes mellitus and its complications pose a serious threat to reproduction. Furthermore, the offspring of diabetic patients have a high risk of chronic diseases, which can be transmitted to subsequent generations. Therefore, identifying effective strategies to prevent the damage of diabetes on reproduction and offspring health is imperative. A healthy lifestyle may be a better way to prevent chronic diseases as chronic diseases are closely associated with lifestyles. Tea has become the most popular beverage worldwide besides water and has been consumed in China for thousands of years. The benefits of tea on health, such as antioxidants, anti-diabetes, anti-inflammation, and anti-obesity, have been demonstrated in research studies. These indicate that tea may serve as a potential intervention to ameliorate diabetes-induced reproductive dysfunction and improve offspring health outcomes. To better understand the benefit of tea in reproductive health, the present study reviews the effects of tea on reproductive and offspring health of diabetes mellitus.
-
Key words:
- Tea /
- Diabetes /
- Reproductive health /
- Offspring health