-
Coffee is one of the most widely consumed beverages worldwide. The average consumption varies from country to country as does the type of coffee and the style of consumption, as well as the brewing procedures. In the last decade, coffee beverages with higher amounts of milk, like latte macciato or cappuccino, became more popular. The brewing techniques include the classical manual or machine brewing with paper or gold filter, French press, fully automated machines, capsule machines or espresso machines with portafilter. The degree of roast determines the sensory characteristics to a certain degree. Light and medium roasted types are popular in some countries as are coffee crema or espresso type coffees e.g. in some Mediterranean countries.
Coffee contains antioxidants (chlorogenic acids) which are believed to be beneficial to human health and also process contaminants due to the thermal treatment. Coffee consumption in 2016 was re-evaluated by the IARC (international agency for research on cancer) and judged as 'not classifiable as to its carcinogenicity to humans (Group 3)'. An earlier classification from 1991 was that coffee drinking is probably carcinogenic to humans[1].
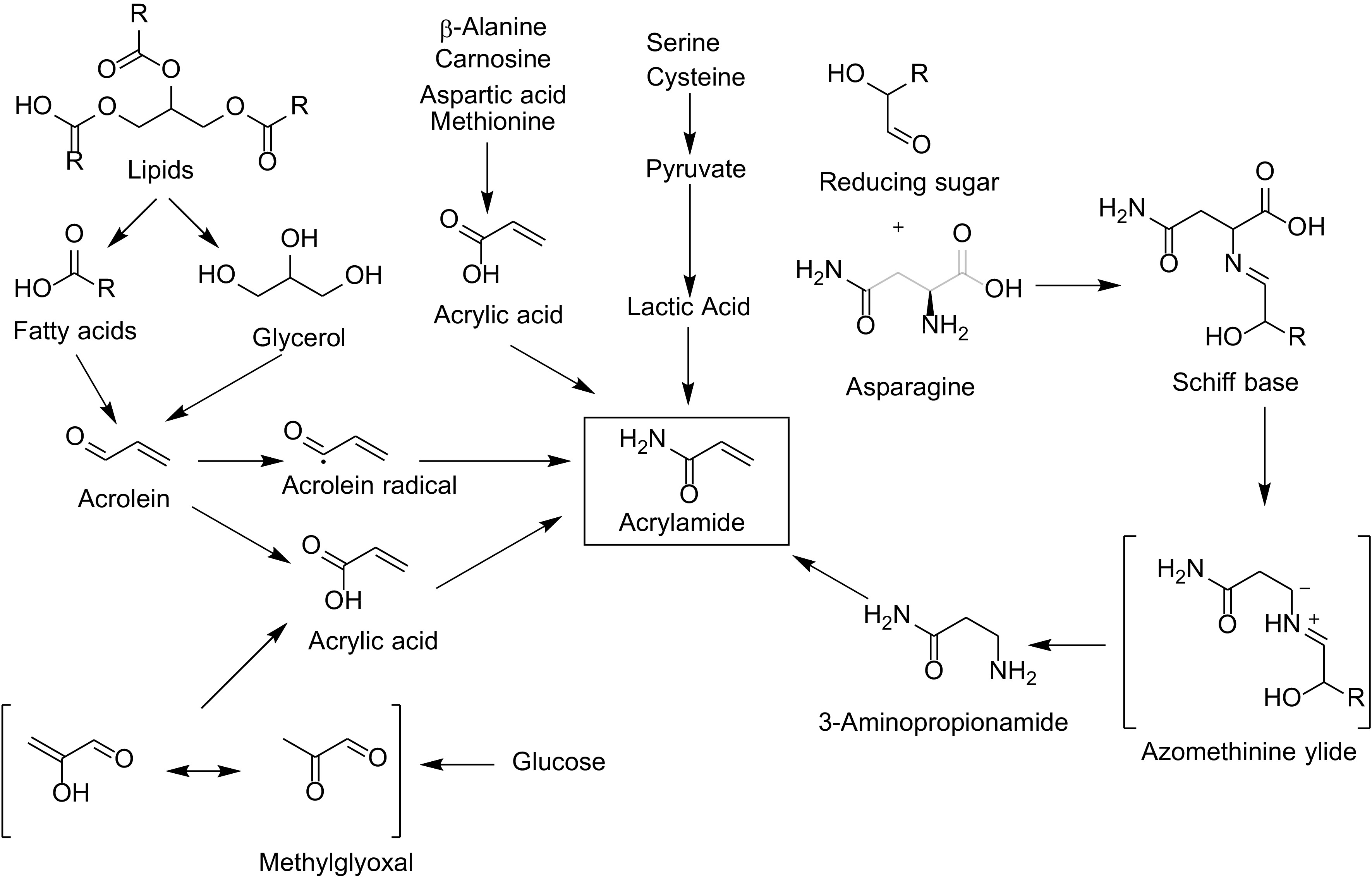
Both acrylamide and furan/methylfurans are in the group of food borne toxicants, also named process contaminants or food processing contaminants[2]. Acrylamide is classified by the IARC as probably carcinogen to humans (group 2A), while furan is in group 2B (possibly carcinogenic to humans)[3]. The occurrence of acrylamide and furan in coffee has beeni known for a number of years[4−6]. Coffee is one of the major sources of acrylamide intake, depending on the country[7]. This was also shown in a greater detail for European countries and regions, where it was also found that coffee, together with bread, was the major contributor of acrylamide[8]. Within the European Union a benchmark value of 400 µg/kg applies for roasted coffee[9] while there is currently no limit for furan itself and methylfurans. The EFSA made a call for data on methylfurans in foods including coffee and published statements on the risks of furan and methylfurans in foods[10]. Acrylamide is generated by the Maillard reaction during roasting. Figure 1 shows the possible formation pathways of acrylamide[11,12].
The precursors are amino acids, foremost asparagine, however, there are further pathways of formation. Mitigation strategies for acrylamide have been reviewed recently[13].
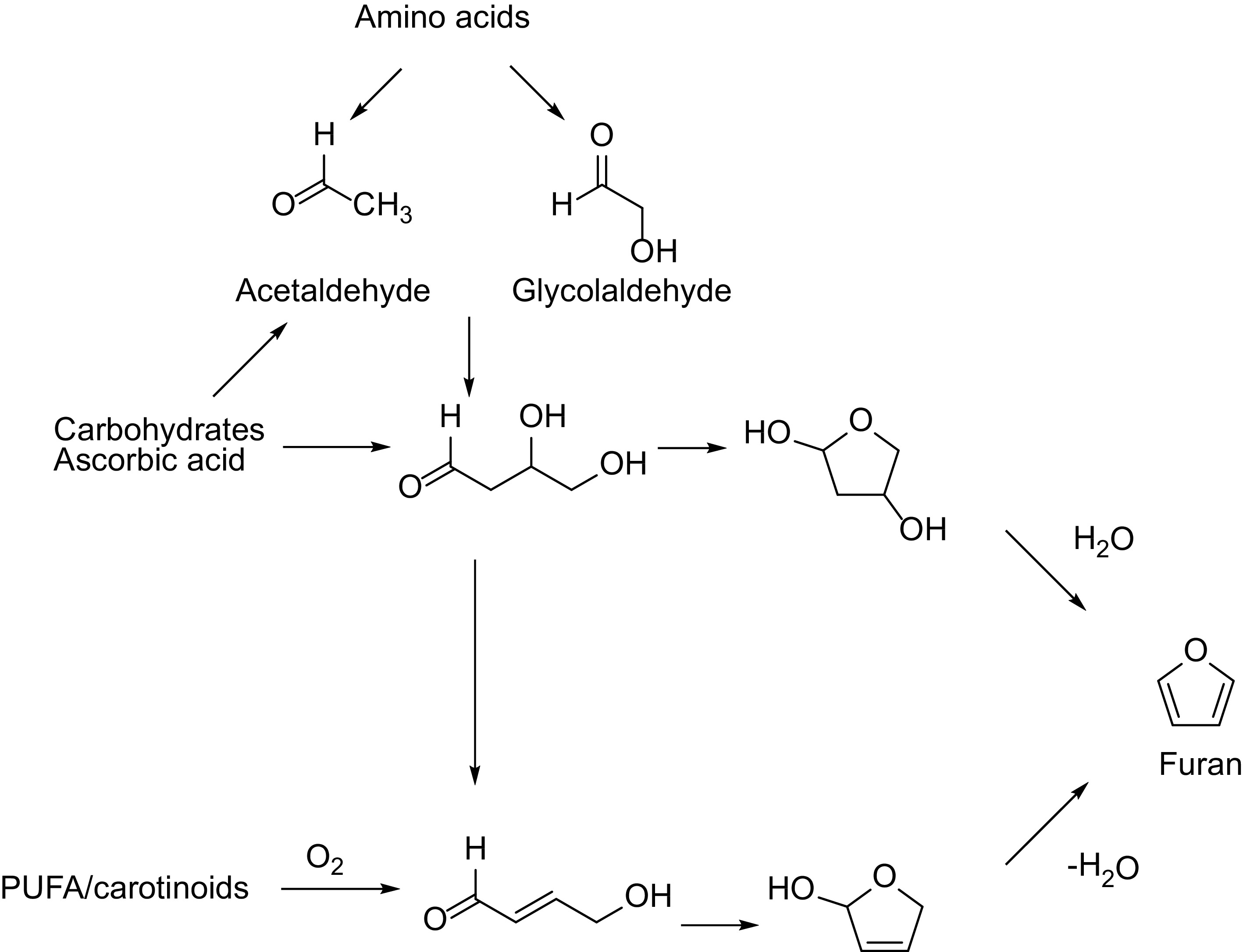
In Fig. 2 the formation of furan is shown according to the literature[14, 15]. Data for methyl furanes concentrations in coffee are more scarce as the latter, even in more recent studies, are not determined and only data for furan are provided[16]. In model systems a formation of 2-methylfuran by the degradation of furfuryl alcohol was found[17].
Other process contaminants, such as furfuryl alcohol are currently not in focus, however, their formation has been studied[18, 19].
The aim of this study was to evaluate mitigation options for both acrylamide and furan/methylfurans by varying the roasting parameters, including special procedures. As the roasting was the focus of the work, two green coffees were selected for the experiments including pretreatments such as decaffeination. The extraction of the contaminants under different conditions using various techniques are not included in the study.
Preliminary results of the projects have been published in our previous study[20].
-
A Vietnam Robusta grade 2 and an unwashed Brazil Arabica were used as the starting material. Different production areas and treatments can give rise to a variation of coffee constituents and consequently also the formation of food born toxicants. This was not the subject of our study. For some experiments a Kenya Arabica speciality coffee was analyzed. The coffees were roasted on a smaller scale (1 kg and 4 kg) in two different industry plants using drum and tangential roasting or hot air roasting devices. Tangential roasts were used for short roasting times as the drum roaster was not capable to work with shorter roasting times.
This approach was to ensure the possibility of a scale-up because using roasting on the lab scale will not allow this. To understand the influence of decaffeination, the samples were also decaffeinated using dichloromethane and some samples with water in an industrial plant. Another treatment analyzed was steam treatment, which is also carried out industrially. The following parameters were employed:
Reference roast
-
The coffees were roasted according to three different profiles[20]. Briefly, profile 1 includes a linear temperature increase, while roasting profile 2 the temperature is higher in the early phase of roast. Profile 3 is characterized by a small increase of the temperature in the beginning and a fast increase at the end. Figure 3 shows the different profiles.
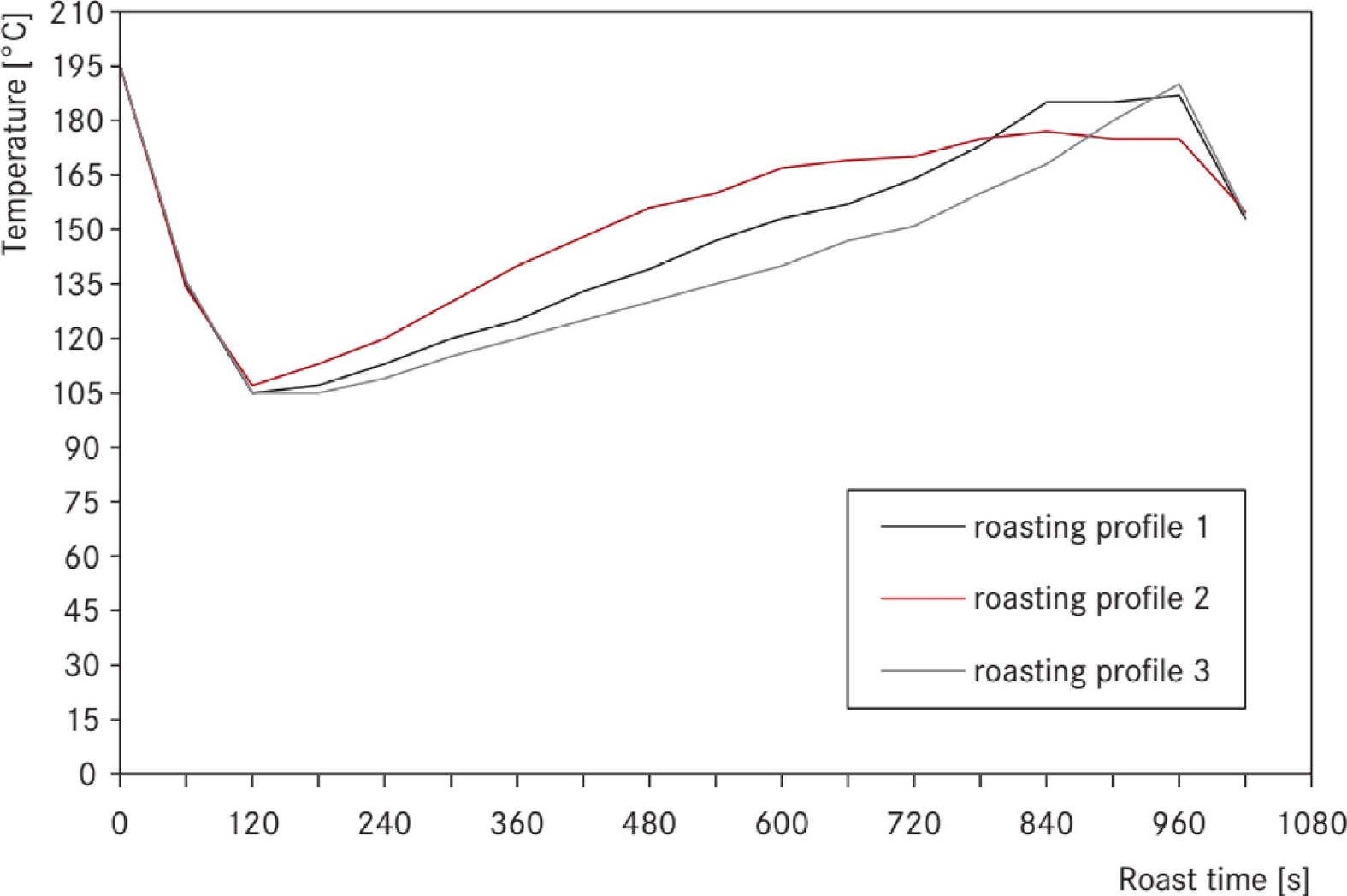
Figure 3.
The roasting profiles of the first series[[20]].
The coffees were roasted to a light, medium and dark roast by varying the roasting time (5, 10, 15 and 20 min) within the individual profiles. The roasts were characterized by evaluating the color by measuring the light reflectance on two different devices (see below). Overall in the first roasting series, 72 samples of Brazil Arabica and 71 Vietnam Robusta samples were produced from each green coffee.
Double roast
-
The coffees were roasted on day 1 until 150 °C according to the corresponding roasting profile and completely cooled down (ambient air). On the next day the samples were roasted to the final degree of roast according to the profiles.
Roasting with sudden temperature change
-
Within the capabilities of the roasters a more rapid rise of the temperature compared to the normal profiles was employed.
Decaffeination
-
Both coffees were decaffeinated in an industrial plant using dichloromethane and roasted according to all three profiles. For the Robusta samples, a water decaffeination was also employed in an industrial plant .
Steam treatment
-
Steam treatments are used to remove coffee wax. This treatment is believed to improve the tolerability of coffee even in sensitive subjects. The steam treatment was also carried out under typical industrial conditions (NKG Kala, Hamburg, Germany).
Color measurements of ground coffee to determine the degree of roast
-
The ground samples were characterized using a Colorette 4 (reflected light is measured). Data are given as Colorette scale units (0–200 / 0 = dark; 200 = light), also the L* a* b*- color data have been recorded. For some samples, the roasting loss/organic roasting loss was determined gravimetrically.
Chemicals
-
The solvents were of HPLC or MS grade, gases and all reagents used were of analytical grade. Acrylamide was obtained from VWR Int. S.A.S. (Darmstadt, Germany). Acrylamide-d3, sodium tetraborate decahydrate, sodium thiosulfate pentahydrate, triethylamine, hydrobromic acid, furan (99%), furan-d4 (≥ 99.9%), 2-methylfuran (99.1%), 3-methylfuran (99.1%), 2,5-dimethylfuran (99%) were supplied by Sigma Aldrich (Steinheim, Germany). 2,5-dimethylfuran-d3 (≥ 95%), 2-methylfuran-d3 (≥ 95%) and 3-methylfuran-d3 (≥ 95%) were supplied by Toronto Research Chemicals (Toronto, Canada). Bromine and methanol were purchased from Merck KGaA (Darmstadt, Germany). Other chemicals came from Carl Roth (Karlsruhe, Germany).
Sample preparation for acrylamide determination
-
The coffee samples were stored at –18 °C to avoid losses of acrylamide, furan and methylfurans as initial experiments had shown a degradation of acrylamide during storage at ambient temperature (see also below). DIN EN ISO 18862 was used for the quantification of acrylamide by GC-MS/MS after derivatization[21]. Briefly, the coffee beans were ground in a mill with nitrogen cooling. To the homogenized sample (2 g) 100 µL acrylamid-d3 standard solution (c = 10 µg/L), 2 ml n-hexane and 20 ml deionized water was added to a 50 ml centrifuge tube and extracted in an ultrasonic bath at 40 °C for 15 min. After that, the tube was centrifuged (4,000 rpm). Ten ml of the aqueous phase was taken in a 15 ml centrifuge tube, and 1 ml carrez I and 1 ml carrez II solution were added, followed by mixing and centrifugation at 4,000 rpm for 4 min. The supernatant was purified by SPE (using a Chromabond ABC18 SPE, Machery-Nagel, Düren, Germany). The residue was washed with 3 ml deionized water, centrifuged and again purified by SPE. For quantification an external calibration plot was used with stable isotope dilution analysis (SIDA). Concentrations were 0.0025–0.1 µg/L.
Sample preparation for the determination of furan and methylfurans
-
The method is based on a German standard method for furan[22] and a thesis on the determination of methylfurans[23]. Briefly, the samples were ground in a mill with nitrogen cooling and 1 g of the homogenized sample was added to a 20 ml headspace vial (baked out before use) and overlaid with 9 ml of water. Under the surface, 40 µl of the deuterated standards solution was added using a syringe. After that the headspace vial was sealed with a gastight cap and measured by HS-GC-MS. The concentrations used were as follows:
Furan 50–2,000 µg/L; furan-d4 400 µg/L; 2-methylfuran 100–4,000 µg/L, 2-methylfuran-d3 500 µg/L; 3-methylfuran 10–400 µg/L, 3-methylfuran-d3 80 µg/L; 2,5-dimethylfuran 10–400 µg/L, 2,5-dimethylfuran 80 µg/L.
GC-MS: (used for both acrylamide and furans determination) (Table 1) :
Table 1. GC-MS settings for both acrylamide and furans determination.
GC Trace 1300 GC (Thermo Fisher Scientific, Dreieich, Germany) PTV mode CT split Inlet temperature 240 °C Column VF-WAXms, 30 m, ID 0.25 mm, 0.25 μm (Agilent J & W Columns, Waldbronn, Germany) Carrier Helium 5.0 Mass spectrometer TSQ DUO (Thermo Fisher Scientific, Dreieich, Germany) Data processing Thermo TraceFinder GC 3.2, Thermo Xcalibur 3.0 (Thermo Fisher Scientific, Dreieich, Germany) Ionisation mode EI+, 70 eV MS transfer line/ion source temperature 250 °C Collision gas Argon Mode SRM (acrylamide) and SIM (furans) Autosampler Acrylamide: TRIPLUS RSH with 10 μl Syringe,
57 mm (Thermo Fisher Scientific, Dreieich, Germany)
Furans: TRIPLUS RSH, Temperature
70 °C, with 5 mL syringe, 65 mm; (Thermo Fisher Scientific, Dreieich, Germany)Split flow 12.0 ml/min (acrylamide) and splitless (furans) Flow 1.2 ml/min (acrylamide) and 1.0 mL/min (furans) Carrier mode Constant flow Ions used for identification and quantification of acrylamide[24] (Table 2):
Table 2. Ions used for the identification and quantification of acrylamide.
2-Bromopropenamide Identification + quantification: m/z 149 [C3H479BrON]+
→ 106 [C2H379Br]+ (identification)D2-Bromopropenamide Identification and quantification: m/z 153 [C32H21H381BrON]+
→ 110 [C22H21H181Br]+ (identification)Ions used for identification and quantification of furans[25] (Table 3):
Table 3. Ions used for the identification and quantification of furans.
Analyte Ions [m/z] Furan 68 [C4H4O]+ (identification and quantification) 2-methylfuran/
3-methylfuran82 [C5H6O]+ (identification and quantification)
81 (identification)2,5-dimethylfuran 95 [C6H7O]+ (identification and quantification)
96 (identification)D4-furan 72 [C4D4O]+ (identification and quantification) D3-2-methylfuran/
D3-3-methylfuran85 [C5H3D3O]+ (identification and quantification)
84 (identification)D3-2,5-dimethylfuran 98 [C6H4D3O]+ (identification and quantification)
99 (identification)Statistical analysis
-
Principal Component Analysis was used to evaluate the possible correlations between the concentrations of process contaminants and roasting parameters. The results are not presented in detail here but can be withdrawn from Bahar and Delker[24, 25].
All samples were evaluated by a sensory panel (in-house, 10 panelists) to check whether or not the samples are within consumer's expectation. As there were no deviations the results are not given here[24, 25].
-
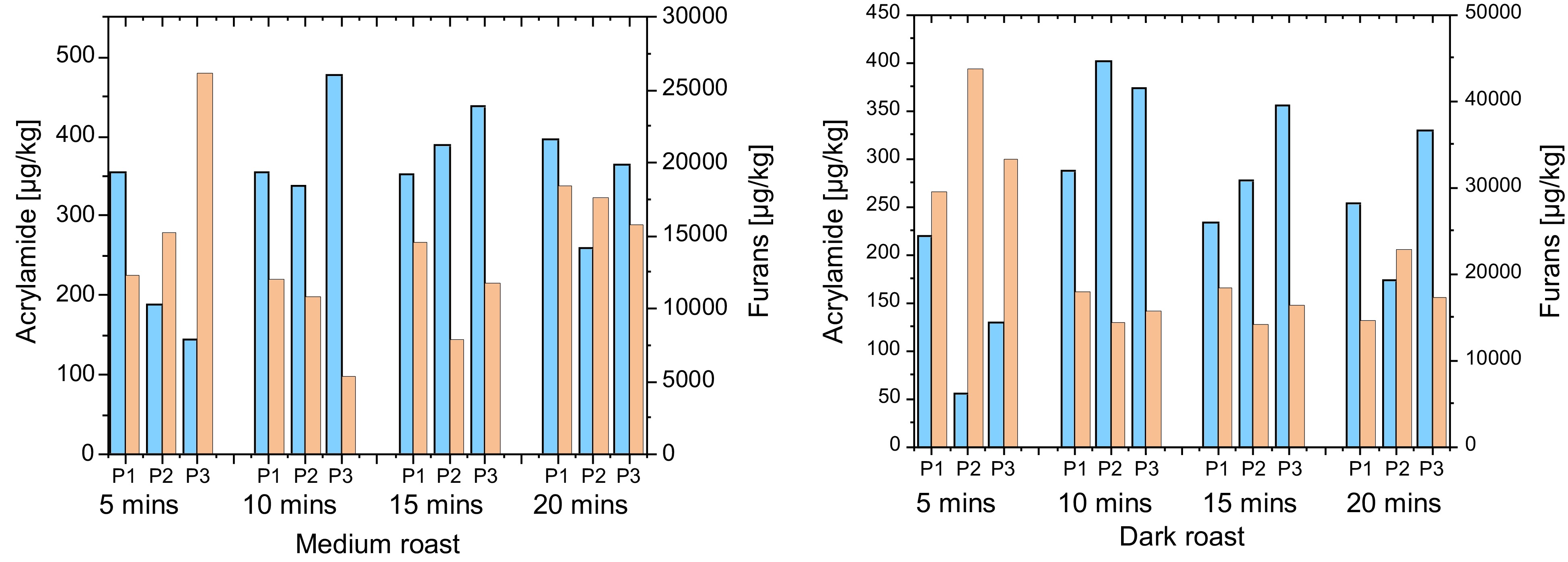
The first roasting series showed, that acrylamide was highest in the light roasted samples, and decreased after that. This is true for both Arabica and Robusta samples and also for the different roasting technologies (tangential, drum and hot air roasting). As an example, Fig. 4 gives data for a Brazil Arabica after tangential or drum roasting. The sum of the furans detected was higher the darker the roasts and the longer the roasting time. This is inline with other studies[26].
Another finding of this series was that in the furan fraction, 2-methylfuran was always the most abundant compound. This is also in line with the most recent literature[27]. Furan was higher in light roasts compared to the dark roasts while the opposite was true for 2-methylfuran. This might be due to the higher volatility of furan.
The overall concentrations of the sum of furans in the Arabica samples were in light roasts in the order of magnitude of 10.000 µg/kg and as high as 40.000 µg/kg in longer and/or darker roasted samples.
The bottom line is that the opposite behavior of the formation of acrylamide and the furans was confirmed by this roasting series. The roasting time and the profile do affect the concentration of process contaminants. All data used in the current studies[24,25] can be found in Supplemental Tables S1−S18. The individual data of the first roasting series can be found in Supplemental Tables S1 (drum roasting Vietnam Robusta), S2 (hot air roasting Vietnam Robusta), S8 (drum roasting Brazil Arabica) and S9 (hot air roasting Brazil Arabica).
Based on that findings a number of treatments were employed to achieve a reduction of process contaminants.
All individual results can be found in the electronic supplementary material.
Double roasts
-
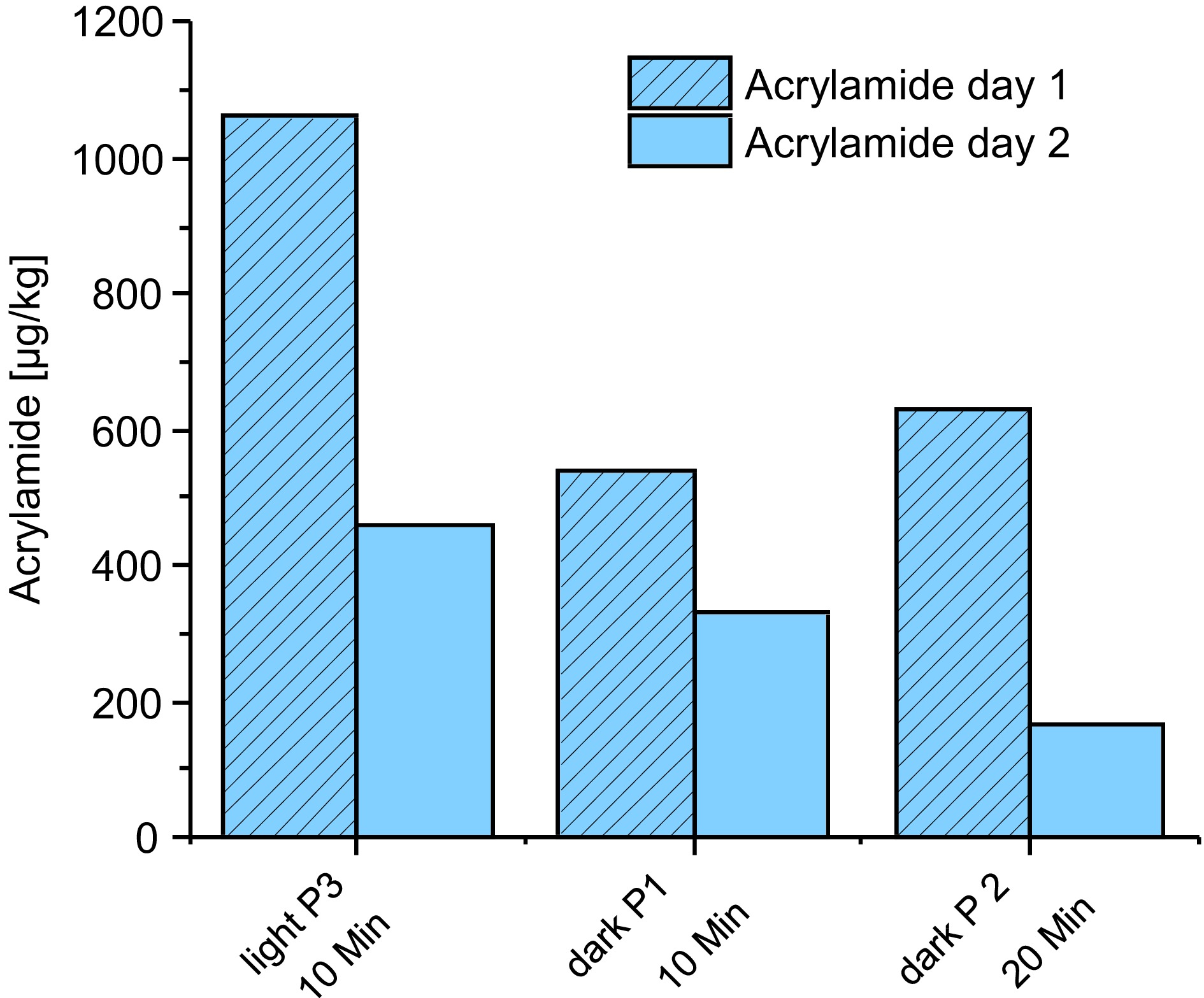
After the first roasting series, special roasts were evaluated, firstly double roasting/DR). Selected profiles from series 1 were tested. Figure 5 shows the content of Brazil Arabica in comparison. As can be seen for tangential and drum roasting, in light roasts the concentration of acrylamide is reduced up to 36%, while this trend was not true in dark roasts. In the 5 min DR sample (not shown in Fig. 5) the content is higher compared to the reference. This is probably due to the use of a tangential roaster. As regards the furans, no decrease but a trend to an increase is observed. Furhter details can be drawn from Fig. 5 in that acrylamide is high after the first roast/day 1 while the 2nd roast yields a degradation.
Data for furans are not shown here as on the first day maximum temperature was 150 °C and under these conditions no formation is expected (below detection limits).
Hot air roasting
-
Similar trends are found here. The furans are higher in dark roasts (48%−192%), in medium roast the increase is smaller (6%−35%).
The same trend was observed with the Vietnam Robusta samples in the tangential and drum roasts. An acrylamide reduction by double roasts seems to be possible, while no reduction of furans was observed. The hot air roasted samples did not show a significant acrylamide reduction.
Roasting with sudden change of temperature
-
For these samples, the tangential roaster was used as the drum roaster could not realize such rapid temperature changes. In Brazil Arabica, acrylamide is reduced in the samples between 22% and 76%. The furans are higher compared to the reference. Both results are in line with the assumption that higher temperatures accelerate the degradation of acrylamide and the formation of furans. Similar results are true for hot air roasts. It is worth mentioning that the medium and dark 5 min samples have much higher (88% and 102%) compared to the reference. This can at least in part be explained by the different color (lighter) which means a lower degradation of acrylamide.
It was shown for the Vietnam Robusta that smaller changes of temperature resulted in a decrease of acrylamide degradation. The content was higher in coffees which were initially roasted with lower and later with higher thermal energy.
The special roasting profiles are consequently no option for the simultaneous mitigation of the process contaminants.
Dichloromethane decaffeinated samples
-
In drum roasted Brazil Arabica samples, acrylamide is higher. This might be in part due to a higher asparagine concentration in the decaffeinated sample. This is in principle also true for the hot air roasted samples. The possible reasons and the comparison to literature data can be found in the discussion.
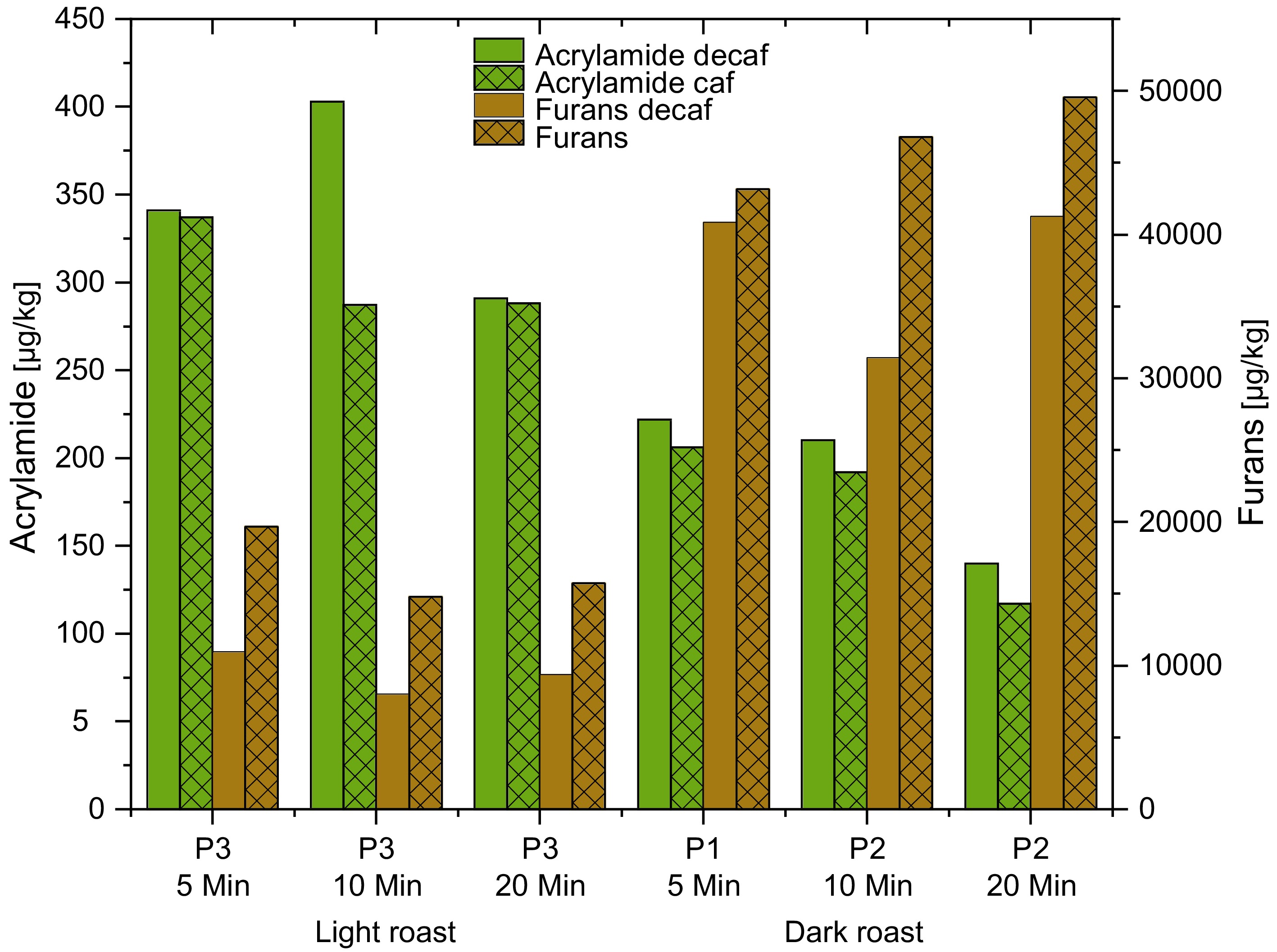
The content of asparagine was higher in decaffeinated green Vietnam Robusta coffee (781 vs 652 mg/kg). Again, in decaffeinated samples, acrylamide was higher in both tangential, drum and hot air roasted samples. With the exception of the short light roasted coffees, furans are higher in caffeine –containing (caf) samples.
Water decaffeinated samples
-
Only water decaffeinated Vietnam Robusta samples were available. Acrylamide was often higher in decaffeinated (decaf) samples, while furans were higher in caffeine-containing (caf) samples. This is shown in Fig. 6 for the Vietnam Robusta. This might be due to a possible loss of precursors during the decaffeination. The water decaffeinated green coffee had 37 µg/kg acrylamide. The decaffeinated coffee was darker: slightly different roasting conditions are possible as the endpoint of roasting was determined by the color.
A more comprehensive study on the food borne toxicants with a wider variety of coffees is underway and will be published in due course.
Steam treated coffees
-
The samples were treated in an industrial plant at elevated temperature and pressure. Details of the process are not available. As can be seen from Fig. 7, the color of the untreated and treated coffee is different. This certainly affects the results as the roasting is controlled by the color. Consequently, the treated samples are roasted with less thermal energy than the reference samples (see the discussion).
Drum roasted Brazil Arabica samples had a lower content of acrylamide, which was more significant in light roasted samples (6%–30% less) compared to dark roasts (max 17%). The furan content is slightly lower, only in the dark roast profile 1/10 mins is it slightly lower. Hot air roasting also gave a tendency to lower acrylamide concentrations, with the exception of light roast profile 1/10 mins, where the concentration was significantly higher. Furans are also lower, foremost in light roasts, while in dark roasts the reduction is not significant.
The steam treated Vietnam Robusta unroasted sample had already an acrylamide content of 73 µg/kg. Acrylamide content was higher in tangential and drum roasted untreated coffees (111–403 µg/kg) compared to treated (113–319 µg/kg). The hot air roasted samples did not show this trend. Furans were higher in untreated coffees (tangential and drum 10,608–43,714 µg/kg; hot air: 15,201–49,688µg/kg) compared to treated samples (tangential and drum 1,709–33,266 µg/kg; hot air: 3,671–20,907 µg/kg). Interestingly, the proportion of furan is higher in the steamed samples while 2-methylfuran is lower.
Quenching vs air cooling
-
Quenching means a fast cooling down of the coffee after roasting using water. It is also used to adjust the desired moisture in the final product. A comparison was made between air cooling and quenching with respect to the process contaminants.
The acrylamide content of Brazil Arabica samples correlates with the moisture content, while Furan/methylfurans did not show a trend.
Quenching led to higher content of acrylamide in Vietnam Robusta, e.g. a volume of 250 ml yielded 543 µg/kg in the light roast sample compared to 371 µg/kg in the air cooled sample. The furan content was slightly lower in the water cooled samples. Dark roasted coffees did not show significant content, with the exception of the 800 ml sample. The reason for that is not known.
Other furan related compounds were also determined by a HPLC-DAD method adapted from the literature[28]. 5-hydroxymethylfurfural and 5-hydroxymethylfurfuryl-2-carboxylic acid decreased with the degree of roast and time, while furfuryl alcohol and 2-furoic acid content increased. The results are only available for the Vietnam Robusta and more detail can be found in Supplemental Tables S1−S8.
-
The overall conclusion from the data in this study is the confirmation that a simultaneous mitigation of acrylamide and furan/methyl furans by varying the roasting parameters is not possible. This proved to be true for both the analyzed Brazil Arabica and Vietnam Robusta samples. For lower content of acrylamide. darker and longer roasts can be recommended. On the other hand, the characteristics of the coffee will be completely changed and most consumers will not accept that due to lower acrylamide content resulting in only dark roasted (espresso type) coffees on the market. Moreover, furans will be higher in darker roasts. For the time being, guiding values only apply for acrylamide while no such limits exist for furan. However, it is not unlikely that this might change in the future.
In the literature for acrylamide, similar formation kinetics compared to this study were described[29]. Under the conditions used the maximum value of acrylamide was reached after 10 min. After that a degradation took place[29]. Another study tried to optimize the roasting conditions and a decrease in acrylamide content was achieved[30].
In Chinese coffee products, levels of furan were n.d. –6,569 μg/kg and 2-methylfuran 2–29,639 μg/kg[31], which is comparable to the results of this study with respect to both the order of magnitude or the furan concentration and the proportions of the individual furans. In a study from Belgium, it was also stated that coffee and coffee products were relevant contributors to the overall intake and that the ratio of 2-methylfuran/furan was 3.71 in average, which is in tune with the findings of the present study[32].
The extraction procedures and techniques are relevant in case of the furans, however, it was not included in this study. It was shown that coffees brewed with a fully automatic machine had the highest levels of furan and furan derivative concentrations (9,905 µg/L furan, 263.91 µg/L 2-methylfuran, 13.15 µg/L 3-methylfuran and 8.44 µg/L 2,5-dimethylfuran) while instant coffee had neglectable concentrations only[33]. Another study stated that furan concentrations in ground coffee brews from an espresso machine were higher (43–146 µg/L) compared to brews from a home drip coffee maker (20 and 78 µg/L). The furan level from decaffeinated coffee (14–65 µg/L) were similar to the brews from a home drip coffee maker[34].
Studies are also available for the extraction efficiency of acrylamide and furan in coffees, e.g. cold brews[35]. It was shown that the temperature (here 5–20 °C) and time (5 min – 24 h) had a strong influence on the content of the process contaminants.
A risk assessment for coffee and coffee related products for adolescents indicated that for some of the products health problems might occur due to the fact that the MOE was below 10.000[36]. Methyl furans were not included in that research.
Studies on a asparaginase treatment of coffee indicate that a reduction of acrylamide is possible without relevant changes of other constituents[37].
It was shown in a model study that mitigation options for HMF and other furan related compounds could not be transferred from the model to the coffee[38].
-
It can be finally concluded that Arabica and Robusta samples behaved similar to each other. The acrylamide content was negligible in green coffee and increased rapidly in the early stage with a maximum in light roast. After that, the acrylamide content decreased with increasing degree of roast. Furan and methylfurans content increased during roasting and was highest in dark roasted coffees.
It can be concluded that mitigation options are available for acrylamide or furan/methylfurans, however, a simultaneous mitigation seems to not be possible. This has to be taken in account if limits or guiding values are be set in the future.
This IGF project (AIF 200049 N) is supported via AIF within the program for promoting the Industrial Collective Research (IGF) of the German Ministry of Economics and Energy (BMWi), bases on a resolution of the German Parliament. We thank all members of the project accompanying committee for their support. Special thanks are due to Coffein Compagnie for delivering the coffee and decaffeination and also to NKG Kala for delivering the steam treated samples. The support of Probat Werke and Neuhaus Neotec is gratefully acknowledged.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Vietnam Robusta grade 2 – 1st roasting series with tangential or drum roasting.
- Supplemental Table S2 Vietnam Robusta grade 2 first hot air roasting series.
- Supplemental Table S3 Vietnam Robusta grade 2: drum or tangential roasting series with extreme profiles; T1: first partial roast, T2: final roast.
- Supplemental Table S4 Vietnam Robusta grade 2: hot air roasting series with extreme profiles; T1: first partial roast, T2: final roast.
- Supplemental Table S5 Vietnam Robusta pretreated coffees tangential or drum roasting series.
- Supplemental Table S6 Vietnam Robusta grade 2. Pretreated coffees hot air roasting series.
- Supplemental Table S7 Vietnam Robusta grade 2 quenching experiments tangential or drum roasting series.
- Supplemental Table S8 Vietnam Robusta grade 2 quenching experiments hot air roasting series.
- Supplemental Table S9 Brazil Arabica drum and tangential roasting series.
- Supplemental Table S10 Brazil Arabica drum/tangential roasting series with extreme profiles; T1: first partial roast, T2: final r.
- Supplemental Table S11 Brazil Arabica pretreated coffees tangential or drum roasting series.
- Supplemental Table S12 Brazil Arabica drum and tangential roast-Quenching.
- Supplemental Table S13 Kenia Arabica drum and tangential roast series.
- Supplemental Table S14 Summary Brazil Arabica hot air roasting series.
- Supplemental Table S15 Brazil Arabica hot air roasting series alternate profiles.
- Supplemental Table S16 Brazil Arabica hot air roasting of pretreated samples.
- Supplemental Table S17 Brazil Arabica hot air roasting - quenching.
- Supplemental Table S18 Kenia Arabica – hot air roasting.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Engelhardt UH, Bahar I, Delker U. 2023. Food borne toxicants in coffee: Acrylamide and furan derivative content in Arabica and Robusta coffees with different roasting profiles and varying degrees of roast. Beverage Plant Research 3:8 doi: 10.48130/BPR-2023-0008
Food borne toxicants in coffee: Acrylamide and furan derivative content in Arabica and Robusta coffees with different roasting profiles and varying degrees of roast
- Received: 08 January 2023
- Revised: 05 February 2023
- Accepted: 09 February 2023
- Published online: 03 April 2023
Abstract:
-
Key words:
- coffee /
- food borne toxicants /
- acrylamide /
- furan /
- methylfurans