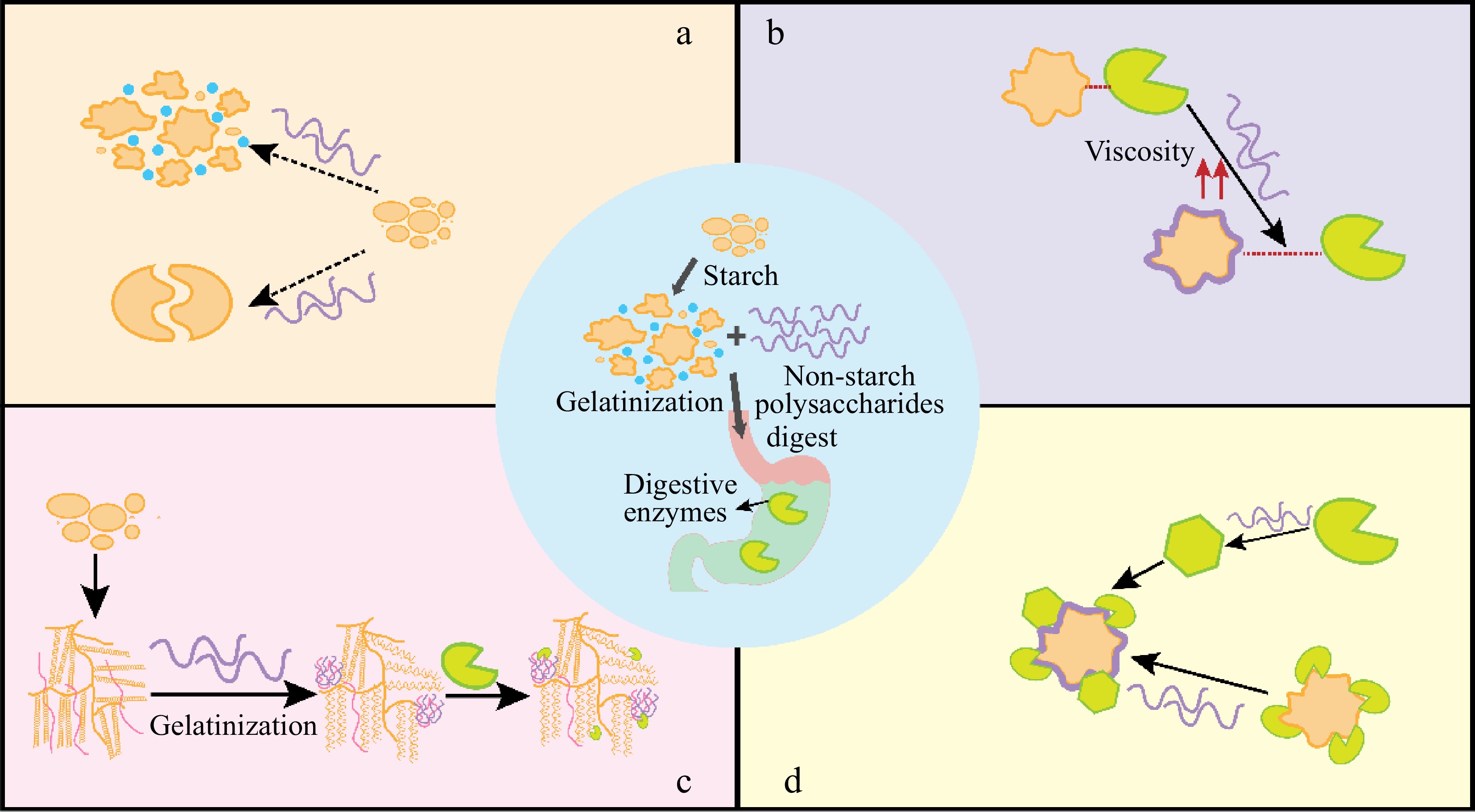
-
Starch plays a considerable role in offering energy to humans mainly in digestible carbohydrate form, and is exerting numerous uses in versatile food recipes[1]. However, relying solely on starch-based foods may not satisfy the diverse needs of consumers. Moreover, the intake of foods that are predominantly composed of rapid digestion starch causes a fluctuation in blood glucose, and in the long term a decrease in insulin sensitivity, which is not suitable for individuals with certain diet-related chronic diseases[2]. To address these challenges, non-starch polysaccharides (NSPs) are incorporated into starch-based foods. NSPs refer to the nonstructural complex polysaccharides joined through glycosidic linkages, in addition to starch, which consists of numerous monosaccharide units. Given their safety, good biocompatibility, and biodegradability, NSPs have been utilized for stabilizing, rheological improvement, and emulsifying in the food industry[3]. When combined with starch, NSPs can modify the basic properties of starch, which improves the benefits of the mixture system.
Gelatinization is an instrumental procedure in the fabrication of starchy foods, involving the swelling process, amylose dissolution, birefringence disappearance, and transformation from ordered structure to disordered[4]. When the binary system is formed (non-starch polysaccharide with starch), the gelatinization process of starch will be undoubtfully affected. Many reports have reported the role of NSPs in interfering with starch gelatinization[5,6]. NSPs can cause variations in starch gelation temperature and changes in endothermic enthalpy. The difference depends highly on the NSPs' characteristics, the origin of starch, and their interaction manner. Most hydrocolloids exert an adverse role on the starch gelatinization profile, resulting in incomplete gelatinization or hindered process, which ultimately affects its functional properties such as digestibility.
In terms of the digestion properties, the influence of NSPs on gelatinization directly relates to the breakdown and absorption of starch in food. During digestion, starch is degraded into oligosaccharides by amylase, which is then absorbed into the bloodstream through the villi of the small intestine. Various NSPs play a role in modulating starch hydrolysis, resulting in reducing rapidly digestible starch (RDS) and accordingly raising the content of slow and resistant starch[7]. However, variations were encountered with starch and hydrocolloids from various origins. Thus, NSPs' addition resulted in a different distribution between starch fractions. Researchers have explored the underlying mechanism by which NSPs affect starch digestibility. Most share the opinion that viscosity plays a critical role[8,9] as it can retard starch swelling, hinder the collapse of the starch structure, and induce different changes in the crystalline regions based on the source and type of colloids[10]. However, it is important to note that the viscosity of hydrocolloids alone does not solely determine starch hydrolysis, as the interaction between the composition of the colloid and starch or enzymes also plays a crucial role[11].
This paper aims to present a comprehensive summarization of the effect of NSPs on starch gelatinization and digestion properties. The NSPs covered different origins and functions. The underlying mechanisms of NSPs played on starch gelatinization and hydrolysis were also discussed. Therefore, this manuscript provided an overview of the NSP's role in starch properties to promote healthy starchy-based food development.
-
Gelatinization is a crucial step occurring in many starchy food operations, for example, the extrusion of cereal-based products and baking, etc. A profound understanding of starch in terms of the fundamental molecular interactions of the gelatinization process is vital for its industrial applications[12].
Gelatinization gives rise to the irreversible changes occurring in the starch structure associated with loss of birefringence, starch granules swelling, amylose leaching, and viscosity changes[13]. It is a molecular transition process with the underlying mechanism described as the amorphous growth rings once contact with excess water molecular will swell firstly (breaking of hydrogen bonds), and then the semi-crystalline lamellae change accordingly, which leads to the decrease of crystallinity of the granule[14,15]. Slade & Levine believe that the dissolution of amylose leads to increasing viscosity, which is not before the amorphous region's glass transition or melting is completed[16]. Moreover, Waigh et al. suggest that the gelatinization process involves two steps in high water circumstances. The slow dissociation can take the place of the helix-helix in molecular evidenced by the crystalline smectic-nematic test parameters first. The second stage is related to the transition from helix to coil which is accompanied by the helices unwinding[17].
In fact, during food industry utilization, starch is not merely a component, and kinds of hydrocolloids exist to overcome the gap between research and application. Moreover, the influences of NSPs on starch gelatinization appear to vary due to multiple factors. For example, the native characteristic of polysaccharides such as their origin, structures, molecular weight ranges, ionic charge, and flexibility can influence their role. Plenty of research focused on the gum on starch gelatinization[18]. In this review, we examine the effects of NSPs from different origins, based in part on the classification proposed by Kumar et al.[19], which is mainly categorized based on chemical structures. Moreover, this paper not only focused on botanic-origin non-starch polysaccharides but NSPs derived from animal and microbial origin were also included.
NSPs
-
NSPs are supposed to be the nonstructural complex polysaccharides except for starch, which is made up of various monosaccharide units, which mainly form on the linkage through β-glycosidic bonds (Table 1)[19]. In this review, the term NSPs refer to gums, which are generally considered safe for human consumption, and widely used for versatile functionalities such as thickening, gelling, stabilizing, or emulsification[20]. Based on the reaction with water, NSPs can be characterized into two groups. Soluble NSPs such as pectin, inulin, konjac glucomannan, and β-glucan, often increase viscosity. While insoluble NSPs can serve as water-binding reagents for their fecal-bulking capacity[21]. Though many kinds of criteria are used to classify the term of NSPs, based on Bailey's recommendations, the most preferred classification method was chosen to organize NSPs here to avoid ambiguity, and at the same time take into account chemical structure[22]. Firstly, we classified the NSPs into three categories (according to the origins), in terms of the botanical category (namely plant cell wall structural polysaccharide), the NSPs are divided into three sections, namely the cellulose and non-cellulosic polymers and pectic substances, according to their function in the cell wall. Cellulose is the fiber polysaccharide in the cell wall which acts as the fiber microfibrils, non-cellulosic polymers function as fiber matrix and pectic polysaccharides serve as the intercellular cement. Lastly, based on different chemical structures, the non-cellulosic polymers fall into two main groups (pentosans and hexosans that are pentose-free). Figure 1 outlines the detailed classification scheme.
Table 1. Summary of important molecular characteristics of some common non-starch polysaccharides used in foods.
Origin Name Solubility Major composition Molecular weight (kDa) Main function Reference Botanical Cellulose derived molecules Methyl Cellulose Soluble β (1,4) D-glucose 20~1,000 Thickening, gelling, stabilizing, emulsification [71,73] Cellulose derived molecules Carboxy methylcellulose Soluble β (1,4) D-glucose 95~1,100 Thickening [74] Cellulose derived molecules Hydroxypropyl methylcellulose Soluble β (1,4) D-glucose 20~1,000 Thickening, gelling, stabilizing, emulsification [74] Plant tissue extracts Pectin Soluble α-(1–4)-linked D-galacturonic and mannuronic acid. 50~150 Stabilizing, gelling [71,73] Tree gum exudates (Acacia Sap) Gum Arabic Soluble Galactose 200~800 Emulsification, film forming [71,73] Roots of chicory (Asteraceae) Inulin Soluble β-D-fructose 0.5~13 Prebiotic, thickening [71] Tubers Konjac-glucomannan Soluble D-glucose and D-mannose, 10~2,000 Thickening, gelling, texturing, water binding [73] Viscous plant substances (Seeds mucilages) Locust bean gum Soluble D-mannose and D-galactose 500~1,000 Stabilizing, thickening, [71,73] Viscous plant substances (Seeds mucilages) Tara gum Soluble D-mannose and D-galactose ~1,000 Stabilizing, thickening, gelling [71] Plant tissue extracts β-glucan Soluble D-glucose 10~1,000 Stabilizing, thickening, emulsification [74] Seed endosperm of Cyamopsis tetragonolobus Guar gum Soluble Linear chain of Galactomannan unit 100~2,000 Stabilizing, thickening [73] Tree gum exudates (Dried sap of several legumes of the Astragalus, including A.
adscendens, A. gummifer,
and A. tragacanthus)Tragacanth gum Soluble: tragacanthin; Insoluble: bassorin Tragacanthin and tragacanthic acid ~840 Stabilizing, thickening, emulsification [73,74] Viscous plant substances (mucilages) Psyllium Soluble Arabinoxylan 35~3,800 Thickening, gelling [74] Brown seaweeds Alginate Soluble β-D-Mannuronic Acid 32~400 Stabilizing, gelling [71] Red seaweeds (Sphaerococcus euchema) Agar Soluble in hot water β-D-Galactopyranose 80~140 Stabilizing, gelling [71,73] Red seaweeds Carrageenan (kappa-, lambda- and iota-) Soluble Sulphated D-galactose and L-anhydrogalactose 400~700 Stabilizing, gelling, thickening [71,73,75] Animal Crustaceans, Invertebrates Chitosan Soluble in acetic aqueous solutions 2-amino-2-deoxy-β-D-glucose 4~500 Gelling [73,77] Microbial Aureobasidium
pullulansPullulan Soluble α-D-glucan 40~600 Thickening, gelling, foaming, flocculating, stabilizing, binding [10,75] Fermentation gums (Xanthomonas campestris exudate) Xanthan Gum Soluble β-D-glucose u, two mannose and one glucuronic acid 1,000~50,000 Structure formation, thickening, stabilizing [71,73] Fermentation gums (Pseudomonas elodea) Gellan gum Soluble in hot water The basic unit is composed of 1,3- and 1,4-linked 2 glucose residues, 1,3-linked
1 glucuronic acid
residue, and 1,4-linked
1 rhamnose residue~500 Gelling, film forming [74−76] Fermentation gums
(of microbial origin)Curdlan Soluble in an alkaline aqueous solution linear glucan D-glucose 53~5,800 Gelling [77] Fermentation gums
(of microbial origin)Dextran Soluble Composed of D-glucose, the main chain is α-1,6 bonds, and there are also branched chains with
α-1,4 or α-1,3 bonds40~70 Stabilizing, thickening, emulsification [74] 
Figure 1.
The classification scheme of non-starch polysaccharides (NSPs). Italics represent the NSPs chosen under each category to depict the effect on starch. Cellulose serves as the fiber microfibrils, non-cellulosic polymers serve as cell walls or fiber matrix, and pectic substances function as intercellular cement.
-
Carboxymethyl cellulose (CMC) is a homopolysaccharide that has been extensively used in food research and industry. Zhou et al. reported that CMC with the wheat starch mixture was accompanied by a higher To and Tc and endothermic enthalpy, which may be the result of the association of NSPs with starch, thus changing the mobility of the starch chain[23]. Nixtamalization maize dough with CMC was made by Andres et al., whose research suggests similar results, namely, the addition of CMC brought about a surge of thermal parameters of maize starch though the gelatinization enthalpies values decreased when the NSPs concentration increased[24].
Non-cellulose categories
β-glucans
-
β-glucans as a category of non-starch polysaccharides that can be obtained from many cereals, such as oats, barley, and wheat, mainly through the β-glycosidic linkage in different ratios of β-1,3 and β-1,4. The β-glucans, which have various chemical structures, can serve as gelling and stability agents in food recipes[25]. Satrapai & Suphantharika stated that the thermal properties of mixtures (rice starch/β-glucan ) switch to a higher level, while ΔH declined with the increasing amount of NSPs[26]. This may be explained as limiting water mobility. While Rawiwan & Suphantharika found nearly no effect of β-glucans on rice starch[27].
Inulin
-
Luo et al. estimated three kinds of inulin on wheat starch thermal properties[28]. As inulin increased, there was a slight increasing trend in terms of To, and the effect may be more evident when the additives are at higher levels due to the hydration of NSPs. Peak temperatures (Tp) increased with the addition of concentrations of inulin, while Te varied depending on the degree of polymerization. As inulin has a lower degree of polymerization (DP), it plays a more significant role in ΔH because the smaller polysaccharide could easily interfere with the orderly assembled crystallized region and double-helical architecture.
Arabinoxylans
-
Arabinoxylans' effectiveness in starch gelatinization keens on the molecular weights[29]. Low molecular weight, water-extractable arabinoxylan plays a more evident role in the inhibition of amylose leaching. Corn fiber gum exhibits a similar influence on the maize starch, accompanied by the concentration increase. It can interact with the amylose molecules, which would hinder starch granule breakdown[5]. This interaction occurs through entanglements and hydrogen bonds, thus stabilizing the system[30].
Tamarind
-
Tamarind seed polysaccharide can increase three kinds of corn starch thermal transition temperature (Tp), which shows a negative effect on starch gelatinization. This effect is attributed to the binding capacity between tamarind seed polysaccharide and starch granules and changes that occur in the molecular conformation of starch[31]. Consequently, the starch/non-starch polysaccharide systems exhibit higher ΔH.
Fenugreek gum
-
Fenugreek gum lifted the onset temperature of viscosity and a reverse trend was observed when the starch concentration was lower[32]. Moreover, when the concentration of starch is higher (15%), the endothermic enthalpy value remains unchanged[33]. The discrepancy is because of the larger volume effect at higher concentrations on the rheological properties than the molecular associations.
Konjac glucomannan
-
Konjac glucomannan (KGM) brings a surge in parameters (To, Tp, Tc) with no changeable enthalpy[34]. Schwartz et al.[35] reported the effect on potato starch depends highly on the KGM concentration and water content. To was unchanged and Tc increased as more KGM occurred. It is often assumed that the enthalpy decreases with the increase of KGM and declined water content, which is mainly caused by the unable fully gelatinization when limited water exists[35].
Guar gum
-
Guar gum has been frequently investigated by researchers in the past years in case of interfering with starch gelatinization. Torres et al. suggested guar gum reduces the availability of water, which owing to its hydrophilic nature, leads to lower starch hydration and consequently lower associated enthalpy when the gum concentration is 0.5%. Guar gum delays chestnut starch gelatinization[36]. The parameters related to the second peak both shifted higher with the increasing guar gum. Moreover, guar gum can also limit granule swelling and constrain amylose leaching[37]. In terms of acorn starch, guar gum retard the gelatinization and decreases the ΔH[38], which gives rise to the reduction in the hydration capacity of the mixture systems[39]. Though some exceptions were detected, as Mali et al. reported, the guar gum had a negligible effect on yam starch either transition temperature or enthalpy[40].
Carrageenan and Alginate
-
NSPs derived from algae, such as carrageenan and alginate, are commonly used as polyhydroxy compounds to enhance the properties of starch slurries. This approach is considered safe and effective, offering advantages over chemical modification and enzymatic hydrolysis methods. Sodium alginate and stearic acid can raise the starch onset temperature, which suggests the hydrocolloid would delay the gelatinization process while decreasing the enthalpy by 5.7−6.7 J/g[41]. Carrageenan, on the other hand, protects starch granules and contributes to achieving the desired texture for the starch-based formulation. Carrageenan shows different impacts on the aqueous starch gelatinization profile, mainly because the thermodynamic incompatibility of the polysaccharide with the amylose and phase arrangements occurs[42].
Pectin substances categories
Pectin polysaccharides
-
Pectin polysaccharides can be classified into high and low-methoxylated kinds, based on the degree of esterification[43], which makes their difference in properties. However, it seems that both high and low methoxylated pectin can raise the temperature of cornstarch gelatinization and decrease the ΔH[44]. It seems that the concentration of pectin is more important than variety. When a higher level of pectin exists, the transition temperature shifts to a higher trend, especially for potato starch. In contrast, inulin has a different tendency, which depends on its DP. As reported by Teresa et al. the medium DP inulin exerted a prominent role in interfering with potato starch gelatinization and the inferior role played by the lowest DP[45].
Extracts or mucilage
-
Arranz-Martínez et al. did not find the effect of NSPs in both waxy rice and non-waxy rice starch, as well as the enthalpy[9]. The same result was conveyed by Liu et al. who found the yellow mustard mucilage had no inferring effect on wheat or rice starch gelatinization temperature only causing a slight increase in melting enthalpy[46]. However, Alamri et al. studied the okra extract with starch blends. The NSPs namely okra extract retards the starch gelatinization by raising the peak temperature. Moreover, the okra extract can perform an indirect role through interaction with water molecules[47]. In terms of Mesona chinensis polysaccharide, it relies heavily on structure associated with extraction methodology when interacting with starch[48].
-
When it comes to polysaccharides of animal origin, chitosan serves as the most representative example. In acidic media, chitosan acts as a cationic polysaccharide. When comparing the starch thermal properties in the presence of positively charged polysaccharides, researchers found that chitosan can increase the DSC onset gelatinization temperature and show more effectiveness in terms of the lab-made maize starch than the commercial one[49]. Different results have been given where researchers suggest that the effect depends on the amount of polysaccharide. Specifically, when the chitosan level is below 5%, there seems to be no significant effect[50]. Interaction between starch and polysaccharide solution was stronger, giving rise to increasing gelatinization parameters, conversely, when starch interacts with water molecules predominately, the effect will tend to reverse[51].
-
Viturawong et al. reported that xanthan did not modify the rice starch thermal parameters besides the enthalpies were significantly decreased[52]. The effects were more pronounced when there was xanthan gum with higher molecular weight. The decline in ΔH owing to the incomplete gelatinization in the condition where water mobility is restricted[53].
Moreover, xanthan with sodium alginate through the interaction with the maize starch granules formed a hydration film via hydrogen bonding cross-linking and/or coating retard normal corn starch gelatinization[54].
Therefore, we summarized the results of NSPs from different origins on starch gelatinization properties in Table 2. Most studies give the results that hydrocolloids lead Tc increased or unchanged while To remain unchanged or increased. Among most research, ΔH value was found to be decreased while the phase-transition temperature range was varied across the literature.
Table 2. Effect of non-starch polysaccharides on starch gelatinization.
Type of non-starch polysaccharide Type of starch To Tp Tc ΔH Reference Botanical Arabinoxylans Wheat starch ↑/↓ (depends on arabinoxylans molecular weight) ↑/— ↑/↓/— — [28] β-glucans Rice starch ↑ ↑ ↑ ↓ [25] Corn fiber gum Wheat starch — — — ↑ [29] Carboxymethyl cellulose Wheat starch ↑ N ↑ ↑ [23] Carboxymethyl cellulose Nixtamalization maize dough ↑ ↑ ↑ ↓ [24] Fenugreek gum Corn starch ↑/↓(depends
on starch
nitrationation)N N — [31] Guar gum Chestnut starch ↑ ↓/—(depends
on guar concentration)↑ ↑ [38] Guar gum Acorn starch ↑ ↑ ↑ ↓ [40] Inulin Wheat starch ↑ ↑ ↑/—(depends
on inulin DP)↓/—(depends
on inulin DP)[27] Konjac glucomannan Corn starch ↑ ↑ ↑ — [34] Konjac glucomannan Potato starch — ↑ ↑ ↓ [35] Konjac glucomannan Maize starch/potato starch — — —/↑(depends
on starch origin)↓ [36] Mesona chinensis polysaccharide Waxy maize starch/normal maize starch ↑ ↑ ↑ ↓ [44] Okra extract Wheat starch/corn starch ↑ ↑ N ↑(wheat starch)/ ↓(corn starch) [43] Pectin/Inulin Potato starch ↑(pectin)/↓(inulin) ↑(pectin)/↑(inulin) ↑(pectin)/ —(inulin) ↓(pectin)/ —(inulin) [37] Sodium alginate Wheat starch ↑ N N ↓ [48] Tamarind Waxy/normal/high amylose corn starch N ↑ N ↑ [30] Yellow mustard mucilage Wheat starch/rice starch N — N ↑ [42] Animal Chitosan Maize starch ↑ ↑ ↑ ↓ [45] Microbial Xanthan Rice starch — — — ↓ [50] Multiple types β-glucans (curdlan, oat, barley and yeast β-glucans) Rice starch — — — ↓ [26] Guar gum/xanthan Tapioca starch ↑(guar) —(guar)/
↑(xanthan)— ↓ [51] Guar gum /CMC/Xanthan gum/tapioca extracts/tamarind seeds extracts Waxy rice starch/non-waxy rice starch — — — — [8] Konjac glucomannan/ CMC/chitosan Corn starch — —(konjac glucomannan, CMC)/↑(chitosan) — ↓(konjac glucomannan)/ ↑(CMC)/ —(chitosan) [46] Xanthan gum/Guar gum Yam starch — — — — [41] To, Tp, and Tc represent the gelatinization beginning, highest, and end temperatures, respectively. The ΔH represents enthalpy (the heat energy required by the test starch during the endothermic transition). The arrow (↑, ↓, —) represents an increase/decrease or a no change in temperature, respectively. The letter "N" represents the corresponding parameters not mentioned in the research. -
NSPs play a role in reducing water activity during starch gelatinization. When polysaccharides are added to starch, there is an observed increase in gelatinization temperature, especially with higher concentrations of polysaccharides. This delayed gelatinization occurs because the polysaccharides limit water availability[55], decreasing the number of water molecules accordingly. Water molecules' access to the starch interior is hindered, directly impacting the hydrogen bonds between them, thus restricting starch swelling ability[48].
NSPs can interact with starch
-
NSPs, for example, glucomannan and xanthan will decrease the starch fluidity while β-glucans show a relatively weak influence[56]. Gelatinization endotherm refers to the energy required for starch granules to collapse and disassemble the molecular structure. The increase of ΔH owing to the starch chain limitation. Sodium alginate decreased ΔH of rice starch indicating a restricted gelatinization process and partial gelatinization of starch granules[57]. During heating, polysaccharides can act as a protective membrane, thus inhibiting starch expansion. However, at higher hydrocolloid concentrations, the hydrophilic chain between NSPs and starch might be conducive to the increase of ΔH. From the molecular scope to interpret this phenomenon, though the short-range order may not be changed by the NSPs, there are fewer double helix structures formed, which may be owing to the partly disruptive effect NSPs played on the original double helix structure in starch[58]. Consistent with the FTIR results, the NSPs can reduce the crystallinity of porous maize starch (XRD)[59]. The 13C NMR test also suggests that the single and double helix structure of the original starch changed differently according to the types of NSPs added[58]. Luo et al., also verified that NSPs modify the rearrangement of amylose especially the linear chains around the gelatinization molecules[48]. Therefore, the interactions between polysaccharide molecules and starch play a vital role in determining the gelatinization profile[56].
NSPs show different effects on starch gelatinization temperatures and endotherm enthalpy, which delays the progress and incomplete gelatinization in most conditions, which will exert various significant influences on starch susceptibility to enzymatic digestion. As the gelatinization degree increased, the starch hydrolysis degree increased and vice versa[4]. Therefore, the next part will focus on the NSPs' role in the digestion of starch.
-
As mentioned above, NSPs can interact with starch so undoubtedly, they can play a critical role in determining starch digestibility. We have summarized the articles associated with non-starch polysaccharides on starch hydrolysis in recent years in Tables 3 & 4. Table 3 presented macroscopic profiles of starch hydrolysis caused by NSPs while Table 4 mainly focused on the changing trends in specific starch digestion parameters (such as rapidly digestible starch content, slowly digestible starch content, resistant starch content, starch equilibrium hydrolysis concentration, and hydrolysis reaction rate). From the Tables, it is clear to see, that most hydrocolloids cause a significant inhibition on kinds of starches, though their structure and functional abilities vary. Most non-starch polysaccharides reduced the RDS, except chitosan. Reports of the opposite trend were also given that for corn starch, NSPs, such as xanthan and guar gum, raised the content of RDS. In terms of RS, NSPs increased their amount, except for xanthan gum and chitosan. The impact of NSPs on starch digestion parameters (C∞ and k) was generally reduced, thereby bringing about the digestion inhibition effect on starch and the lowering effect on the glycemic index. From the above mentioned, NSPs undoubtedly strongly interfere with starch digestion, leading to a lower glycemic index. We summarize the main mechanism of action as follows (Fig. 2).
Table 3. Effect of non-starch polysaccharides on starch digestibility.
Type of non-starch polysaccharide Type of starch Some findings and conclusions Reference Psyllium (Gluten-free bread) Rice PSY reduces the chickpea flour-based bread glycemic response. [86] Gellan gum Rice Gellan gum reduced starch digestion and GI index. [87] Guar gum/sodium alginate xanthan gum/ Waxy rice The NSPs decreased the starch digestion rate. [88] Xanthan gum Rice Xanthan increased the glycemic index of the mixture. [89] Nano-cellulose Corn Higher nano-cellulose amounts slow down the initial glucose release rates. [90] Carboxymethyl cellulose/ xanthan gum/ guar gum Fried-natural fermented
rice noodles (rice)NSPs improve digestion. [72] psyllium Rice /cassava The psyllium decreased starch digestion. [91] Nano-fibrillated cellulose Corn NSPs reduced the level of hydrolysis glucose. [92] CMC/ guar/ xanthan gum High amylose rice NSPs decreased the surge of blood glucose. [93] Pectin Corn Pectin hindered starch digestion. [62] Chitosan Waxy maize Chitosan modification altered starch digestion. [94] Guar/ xanthan gum/ sodium alginate Wheat/buckwheat The hydrocolloid's addition reduced starch hydrolysis. [95] Xanthan/ guar gum/ pectin/ konjac-glucomannan Gelatinized potato NSPs hindered starch digestion and the extent perform on blood glucose depends highly on the types. [96] Locust bean/ guar/ fenugreek/ xanthan/
flaxseed gumCorn The XG showed a prominent effect in interfering with glucose. [97] Extracted malva nut gum Wheat bread (wheat) MNG-containing breads showed low glucose levels. [98] Table 4. Non-starch polysaccharides influence on RDS, SDS, RS and digestion parameters.
Type of non-starch polysaccharide Type of starch RDS SDS RS C∞ (equilibrium concentration) k (kinetic constant) Reference Xanthan Rice ↓ ↑ — ↓ ↓ [78] Creeping fig seed polysaccharide Potato ↓ ↓ ↑ ↓ ↓ [79] Pectin Corn ↓ ↑ ↑ ↓ ↓ [80] Arabic/ xanthan/ guar gum Corn ↓ ↓/↑(xanthan) ↑/↓(xanthan) N N [7] Guar gum Rice ↓ ↑ ↑ ↓ ↓ [8] Chitosan/ xanthan/
sodium alginateWet sweet potato ↑(chitosan)/
↓(xanthan, SA)↑(chitosan, xanthan)/
↓(SA)↓(chitosan)/
↑(xanthan, SA)N N [81] Guar gum Lotus seed ↓ — ↑ N N [68] Pullulan/pectin Fried potato ↓ ↑ ↑ ↓ ↓ [60] Konjac glucomannan Quinoa/maize ↓ ↓ ↑ N N [82] Chitosan Lotus seed ↓ ↑ — N N [68] Hydroxypropylmethyl cellulose (HPMC)/ carboxymethyl cellulose/ xanthan gum (XG)/ apple pectin (AP) gluten-free potato steamed bread(potato starch) ↓ ↓ ↑ ↓ ↓ [83] Pectin Corn ↓ ↑ ↑ N N [84] Cellulose nanocrystals Corn /pea /potato ↓ ↓ ↑ N N [85] Pullulan Rice ↓ ↑ ↑ ↓ ↓ [10] High methoxylated pectin/ guar gum/ carboxymethyl cellulose/ xanthan gum/ hydroxypropylmethyl cellulose Corn /potato ↑/↓(guar gum in terms of potatoes starch) ↓(corn starch)/ —(potatoes starch) —/↓(xanthan and HPMC in terms of corn starch)/
↑(potatoes starch exception of HPMC)↑(corn starch by adding CMC, potatoes starch by adding guar gum and pectin)/ ↓(xanthan in corn starch) N [11] The arrow (↑, ↓, —) represents an increase/decrease or a no change in temperature, respectively. The letter "N" represents the corresponding parameters not mentioned in the research. Restricting starch granule breakdown and delaying starch gelatinization, retaining more intact structures for the protection of enzymatic digestion[60]
-
The starch digestion rate is influenced by starch gelatinization which has been widely reported. NSPs such as galactomannan restrict starch expansion leaving granule ghosts in the paste. The unable to fully gelatinization of granules in the presence of hydrocolloids is also linked to the limited water availability. This gives rise to resistance toward enzymes[61]. Tester & Sommerville illustrated that the inhibition profile was always greater at the gelatinization temperature for each kind of starch and at higher starch-to-water ratios, where higher temperatures promote extensive gelatinization and mask the decreasing effect of NSPs on starch hydrolysis[55].
The bulk viscosity of hydrocolloids reduces starch accessibility to the enzyme[62]
-
NSPs raise the bulk viscosity of the substrate, limiting the enzyme's accessibility. For example, guar gum as a kind of thickening agent can decrease glucose levels[63]. One of the most important reasons is that NSPs increase the viscosity of the food matrix which can result in slowing gastric emptying, restricting the diffusion of substrate[61,64]. However, mixing at high speeds can negate the hindering effect[64]. Kim & White reported oat starch hydrolysis decreased as the β-glucan molecular weight increased[65]. Apart from the viscosity factor, the NSPs may perform another physical effect during starch digestion progress. The structural modifications to the food matrix may also be a response to the change in starch digestibility[66]. The NSPs can coat the granules by forming a physical barrier as evidenced by the CLSM technique which protects the starch from hydrolysis[67]. Different levels of additional inulin also caused a different matrix structure leading to modified starch hydrolysis. A denser gluten network appears for the 5% inulin of degree of polymerization 12−14 enriched sample, while the starch digestion increased with a higher level of inulin, causing an easily disrupted protein architecture[67].
Interact with starch molecules to assemble more ordered structures[68]
-
The effect of NSPs interacting with the leaching of amylose is indicated by Ramirez et al.[69] by the change of the complex index. This means the molecular interactions occurred when NSPs occurred. NSPs changed the crystalline structure of starch. The increasing inulin strengthens the XRD peak. The higher crystallinity may be due to the preferable digestion of amorphous regions or the formation of more ordered areas during hydrolysis. The more perfect crystalline with the addition of inulin may also lower the digestibility of starch[67].
Hydrocolloids interact with enzymes thus changing the enzyme conformation and/or hindering its accessibility[62]
-
NSPs exemplified as cellulose or nanocrystalline cellulose can interact with α-amylase, their binding role on the enzyme, leading to an inhibition of the enzyme activity which relies highly on the hydrocolloid surface, packing density, and its entrapment on the enzyme[70,71].
Apart from above mentioned, different phenomena also occur when hydrocolloids exist. High methoxylated pectin, carboxymethyl cellulose, and xanthan gum lead to an increased trend of RDS as opposed to guar gum, while CMC can decrease the RS of corn starch. A similar result was observed when guar gum, as well as pectin added to potato starch[11]. The researchers suggest that the hydrocolloid's origin plays a critical role in determining starch digestion and some polysaccharides may retard starch retrogradation, especially amylose as a result of the NSPs-amylose interaction. The basis lies in the composite network between the participants, and phase separation may occur. Besides, xanthan added to rice noodles brings air cells thus leading to higher water absorption and can promote the digestive enzymes' contact with starch inside areas and increase the rate of starch[72].
-
The role of NSPs in the gelatinization properties of starch varies across the characteristics of NSPs and the types of starches. We classified NSPs into three major categories. In plant and animal sources, most NSPs increase the To and Tp of starch gelatinization. However, polysaccharides derived from microorganisms, such as xanthan gum, did not show an evident effect, while the mixture was more sensitive to salt. Most non-starch polysaccharides reduced the RDS, except chitosan. Reports of the opposite trend go for corn starch, NSPs, for example, xanthan and guar gum, raised the content of RDS. NSPs origin from botanica increased RS amount, except for xanthan gum and chitosan which are animal resources. First, some NSPs reduce water activity, due to their excellent hydration properties, thereby limiting starch gelatinization. Secondly, gums can interact with starch molecules (amylose or amylopectin), affecting the thermodynamic properties of the latter. As a consequence, the digestion of incompletely gelatinized starch-based foods is altered. From the perspective of reaction kinetics, the hydrolysis rate and final digestion starch concentration are altered, potentially influencing the physiological role of starch-based food by reducing glucose released into the bloodstream and affecting insulin levels. NSPs, with different origins, will exert distinct effects due to various properties (polysaccharide concentration, molecular weight, water holding capacity, charge, etc.) and used levels. In addition to the above-mentioned factors, the formation of interpenetrating network structure or the phase separation between NSPs with starch emerges, and the combination between NSPs and enzyme molecules affects the hydrolysis of starch as well.
The current review only macroscopically summarizes the impact of various NSPs on starch gelatinization and digestibility, without intricately refining the structural characteristics of each colloid such as molecular weight, branching degree, molecular flexibility, charge positive or negative, and charge amount effect on starch. The critical or fundamental mechanisms by which NSPs affect starch properties are not identified, while aspects of mechanisms are generally covered. Furthermore, food, as a complex system, does not merely contain NSPs. The appearance of other components will also interfere with the starch, such as salt, protein, lipids, phenolic compounds, etc. To achieve a more comprehensive understanding of starch-based foods, the comprehensive effects of these aspects need to be further evaluated. Further research is necessary to deepen our understanding of these complex interactions and their implications for utilization.
-
The authors confirm contribution to the paper as follows: writing-original draft: Li S; writing-review & editing: Li S, Zongo AWS, Chen Y, Liang H; resources: Li B; visualization: Li S, Chen W; validation: Li S, Chen Y; data curation: Li S, Chen W; form analysis: Li S, Li J, Liang H; project administration: Li J, Li B; supervision & funding acquisition: Li B. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by the National Key R&D Program of China (2022YFD2101300) & National Natural Science Foundation of China (Grant No. 32172200).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li S, Chen W, Zongo AWS, Chen Y, Liang H, et al. 2023. Effects of non-starch polysaccharide on starch gelatinization and digestibility: a review. Food Innovation and Advances 2(4):302−312 doi: 10.48130/FIA-2023-0029
Effects of non-starch polysaccharide on starch gelatinization and digestibility: a review
- Received: 20 July 2023
- Accepted: 10 October 2023
- Published online: 08 December 2023
Abstract: Non-starch polysaccharides have been given wide consideration for their use in starch-based food due to their ability to improve texture, sensory attributes, and functional properties of the end product. In a binary system (starch and non-starch polysaccharides), the characteristics of starch, exemplified as gelatinization and digestibility undergo significant changes. This review article, through a combination of origin and chemical structure-based classification approach, explores the impact of non-starch polysaccharides on starch behavior, concretely for gelatinization and hydrolysis. The underlying mechanism to retard gelatinization gives rise to some colloids that can reduce water accessibility and interact with starch molecules, which vary with the origin. The interfering role of starch hydrolysis attributed to polysaccharides restrict starch swelling, the bulk viscosity, and more ordered structures occur in the mixture. Besides, the role of non-starch polysaccharides on enzymes is another factor. Therefore, this paper gives an overview of how non-starch polysaccharides interfere with starch gelatinization and digestion, which provides a comprehensive understanding of starchy products.
-
Key words:
- Non-starch polysaccharide /
- Starch /
- Gelatinization /
- Digestion