-
Hydrogels have hydrophilic 3D network structures generated by cross-linking polymer chains that can hold a considerable amount of water[1]. In recent years, hydrogels have received increasing attention in agriculture, medicine and food areas[2]. Furthermore, hydrogels have the ability to change the structures and textures of food matrices and operate as colloidal systems for bioactive ingredient delivery. Hydrogel components can be derived from a variety of sources, including microbes, animals, plants, and algae[3]. κ-Carrageenan (KC) is a red seaweed-derived sulfate linear anionic hetero-polysaccharide. It is made up of one sulfate group per repeating unit and a repeated disaccharide unit of (1-4)-3,6-anhy-dro-D-galactose and (1-3)-D-galactose[4]. KC is frequently employed as a gelling agent. Through structural rearrangement, a thermally reversible hydrogel can be produced[5]. However, hydrogels made only of KC have a high degree of brittleness, poor elasticity, and poor water retention ability[6].
For uses in food, a variety of strategies have been used to enhance the mechanical characteristics of biopolymer gels, including particle/fiber-reinforced hydrogels[7], double network hydrogels[8], dual cross-linking hydrogels[9], and solvent replacement hydrogels[10]. These strategies generally aimed to enhance the interactions between molecules or colloidal particles[11]. For example, alginate-polyacrylamide (PAAm) hydrogels strengthened by cross-linking fibers and divalent/trivalent cations exhibited greater resistance to deformation by increasing fiber content[12]. Cereal β-glucan solutions (1%–3% w/v) treated by 12 cycles of freeze-thawing resulted in stronger gels[13]. Currently, most research on hydrogel enhancement have been focused in material fields, while not many have been carried out in relation to the food industry.
Solvent replacement is an easy, efficient and facile approach to enhance the macroscopic mechanical characteristics of hydrogels. To encourage cross-linking in the system, the gels are submerged in solutions which contain high concentrations of ions, cross-link agents (such as genipin or phytate) or antisolvents (such as alcohols). In solvent replacement, the rheological behavior of gel is altered by the addition of a new component to the traditional gel. This new component may result in novel interactions, which in turn give the network a new structure. When a polyol solution is used to immerse a gel, the polyols displace the water molecules and bind with the gel substrate, resulting in stronger hydrogen bonds[14,15]. Only a few papers on employing this method to create biopolymer hydrogels are currently available. Zhou et al.[16] simply soaked premade weak, non-stretchable and brittle protein hydrogels in glycerol and obtained tough protein hydrogels. The original hydrogels could only support 0.3 kg, whereas the tough hydrogels could support 2 kg. In addition, a considerable improvement in the tensile strain, elastic modulus and fracture energy of the gelatin gels upon solvent replacement was observed with increased solvent replacement time. In addition to glycerol, sorbitol is also a common food additive. It shows good moisturizing properties and is not converted to glucose in the blood after consumption[17]. These results served as the inspiration for our proposal to develop strong KC hydrogels through solvent replacement with sorbitol.
In this research, the hydrogels were prepared with different contents of KC and the structures were enhanced via a solvent-replacement method. The effects of KC content and solvent replacement on the textures, rheology, freeze-thawing stability and swelling property of the hydrogels were investigated. The protection role of the hydrogels on a model bioactive, i.e., epigallocatechin gallate (EGCG), was also tested. The aim was to understand the structure-functionality relationship in the strengthened hydrogels. The information gained would be helpful for potential hydrogel applications in the nutraceutical and biomedical fields.
-
κ-Carrageenan (food grade) was purchased from Beijing Zhongbai Chuangye Chemical Products Company (Beijing, China). Sorbitol (food grade) was obtained from Shandong Yousuo Chemical Technology Company (Shandong, China). EGCG (purity ≥ 99.5%) was bought from Beijing Beishi Zongheng Technology Development Company (Beijing, China).
Preparation of KC hydrogels
-
Distilled water was mixed with 0.50, 0.75, 1.00, 1.25 and 1.50 wt% KC. The mixtures were heated to 70 °C, agitated for 10 min, and then cooled to 25 °C to create the hydrogels. Then, the hydrogels were soaked in sorbitol (70 wt%) with a mass ratio of 1:4 for 1 h. Hydrogels loaded with EGCG were prepared by adding EGCG powder (0.15 wt%) into the KC solution. The hydrogels before solvent replacement were termed KC-BR, and the hydrogels after solvent replacement were termed KC-AR. The hydrogels with 0.50, 0.75, 1.00, 1.25 and 1.50 wt% KC before solvent replacement were termed 0.50% KC-BR, 0.75% KC-BR, 1.00% KC-BR, 1.25% KC-BR and 1.50% KC-BR, respectively. Similarly, the hydrogels after solvent replacement were termed 0.50% KC-AR, 0.75% KC-AR, 1.00% KC-AR, 1.25% KC-AR and 1.50% KC-AR.
Characterization of KC hydrogels
Scanning Electron Microscope (SEM)
-
A SU8020 scanning electron microscope (HiTachi Inc., Japan) was used to examine the microstructures of hydrogels. Samples were freeze-dried for 72 h after being pre-frozen at −18 °C for 24 h. Finally, at a magnification of ×50 and an acceleration voltage of 15.0 kV, the microscopic morphology of samples was recorded[18].
Fourier transform infrared spectroscopy (FTIR)
-
FTIR spectra of samples were captured in the range of 400~4,000 cm−1 by using a Spectrum 100 Fourier transform infrared spectrometer (PerkinElmer, UK) with a resolution of 4 cm−1 and 64 scans[19].
Low field - nuclear magnetic resonance (LF-NMR) measurement
-
A previously described technique was modified to determine the hydrogen proton status in hydrogels[20]. The magnetic field strength of a LF-NMR analyzer (VTNMR20-010V–I, Niumag, China) was 0.5 T and the radio frequency coil was 40 mm. The transverse spin-spin relaxation time (T2) was calculated using the Carr-Purcell Meiboom-Gill (CPMG) sequence with the following testing parameters: proton resonance frequency (SF) 21 MHz, spectral width (SW) 100 kHz, repeated sampling waiting time (TW) 3,000 ms. Data were collected from 18,000 echoes at 4 scan repetitions with a 1.5 s gap between each scan. The MultiExp Inv analysis program (NIUMAG, Shanghai, China) was used to invert the data after all measurements were made in triplicate. The distribution of the multi-exponential fitting of the CPMG decay curves was given by the following Eqn. (1):
$ M(t) = \displaystyle\sum\limits_{i = 1}^N {{P_i}} \exp \left( - \dfrac{t}{{{T_{2i}}}}\right) $ (1) where Pi standed for the amplitude size (a.u.) of the ith component; t was the decay time (ms); and T2i was the transverse relaxation time (ms) of the ith relaxation.
Magnetic resonance imaging (MRI)
-
The proton density-weighted images (PDWI) of composite hydrogels were acquired via a VTNMR20-010V-I Analyzer. The parameters were adjusted to 500 ms for the repetition time, 20 ms for the echo, 1 mm for the slice gap, 5 mm for the slice number, and 2 mm for the slice width. The third layer of the sample was used to produce the image after unified mapping and pseudo-color processing[21].
Textural evaluation
-
Textural properties of hydrogels were determined by a TMS-Pro texture analyzer (CT3, Brookfield, USA). Samples for the measurement were cut into small pieces (1 cm × 1 cm × 1 cm). The testing conditions were initial stress 0.05 N, test speed 1 mm/s, and deformation was set to 50% of the total sample height[22].
Rheological measurement
-
Rheological properties of the hydrogels were measured using a HAAKE IQ AIR rheometer (Thermo Scientific Inc., Germany). To estimate the linear viscoelastic range (LVR) of the samples, strain sweeps were conducted with a frequency of 1 Hz and a strain range of 0.1% to 100%. Frequency sweeps were carried out between 0.1 and 10 Hz, 1% strain (within LVR), and 25 °C. From 25 to 90 °C, temperature ramps were conducted at a rate of 5 °C/min, 1% strain, and 1 Hz[23].
Swelling analysis
-
A conventional approach was used to evaluate the swelling characteristics of hydrogels[24]. Samples were dipped into solutions of different pH (pH 3, 7 and 11) and taken out at predetermined intervals. After using blotting paper to remove extra moisture, samples were weighed. The following equation was used to compute the swelling ratio (SR, %):
$ {\text{SR}}\left( {\text{%}} \right) = \dfrac{{{M_s} - {M_D}}}{{{M_D}}} \times 100 $ (2) where MS and MD represented the masses of swollen and initial hydrogels.
Syneresis rate
-
A usual gravimetric method was applied to examine the syneresis rate of hydrogels[25]. Samples were kept at 25 °C and weighed for four successive days. The following equation was used to obtain the syneresis rate (S, %):
$ {\text{S }}\left( {\text{%}} \right) = \dfrac{{{W_0} - {W_d}}}{{{W_0}}} \times 100 $ (3) where W0 and Wd represented the masses of initial hydrogels and hydrogels after storage (d = 1, 2, 3, 4), respectively.
Freeze-thawing stability
-
Freeze-thawing stability of the hydrogels was assessed using a modified version of an earlier method[26]. In brief, hydrogels were kept in a refrigerator (−18 °C) for 24 h and thawed at 25 °C for 4 h. Then, samples were stored at 25 °C and weighed for four consecutive days. The freeze-thawing stability of KC hydrogels was determined by the syneresis rate using Eqn (3).
Determination of EGCG content
-
Samples were stored at 25 and 4 °C for 3, 6, 9, 12, 15 d. EGCG was extracted with distilled water. The supernatants were mixed after three iterations of the extraction. The absorbance at 273 nm was measured using an ultraviolet-visible spectrophotometer (UV 1800, Shimadzu, Japan) to ascertain the content of EGCG in the hydrogels following the experiments. The retention rate (ER, %) of EGCG was obtained using Eqn (4):
$ ER\left( {\text{%}} \right) = \dfrac{{{C_d}}}{{{C_0}}} \times 100$ (4) where C0 represented the initial content of EGCG and Cd represented the content of EGCG after storage.
Statistical analysis
-
Except when otherwise noted, each measurement was carried out three times. The software program SPSS 20.0 was used for analyzing the data. The significance of differences between the mean results of each test was assessed using a one-way analysis of variance (ANOVA), followed by Tukey's test. Throughout the investigation, a significance level of p < 0.05 was applied.
-
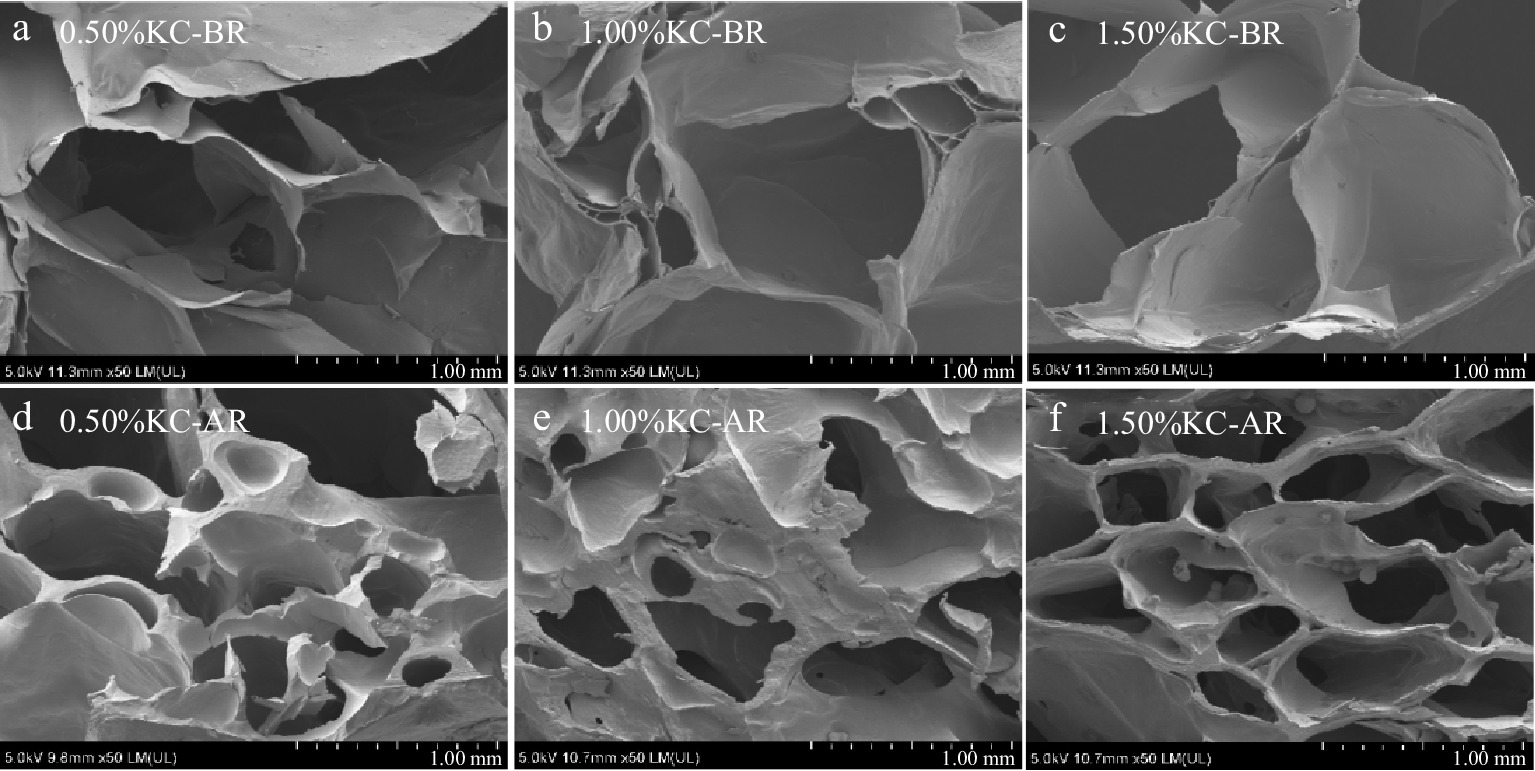
The SEM micrographs revealed the structural changes of KC-BR and KC-AR. As shown in Fig. 1a−c, KC-BR presented honeycomb-like structures, with thin wall layers that had many folds. In contrast, KC-AR (Fig. 1d−f) had denser holes and smoother wall layers. The KC hydrogels with sorbitol had good hydration. Sorbitol was added, which decreased the average amount of water molecules which surrounded the KC chain. As a result, the KC chain stopped attaching to itself and started connecting to the neighboring chain, weakening the KC-water connection.[27]. Also, sorbitol is a low co-solvent that can resist low temperatures. Thus, during the lyophilization process, it was difficult to form ice crystals[28]. Consequently, the 3D network structures of hydrogels exhibited more continuous skeletons. Furthermore, an increase in the content of KC improved the compactness of network structures.
FTIR
-
The FTIR spectra of KC hydrogels are presented in Fig. 2. The absorption peaks of KC molecules were mainly the sulfate group at 1,253 cm−1, the 3,6-dehydration-galactose group at 928 cm−1, the D-galactose-4-sulfuric group at 847 cm−1, and the largest and widest hydroxyl vibration peak of KC at 3,437 cm−1[10]. Figure 2 exhibits that the absorption peaks near 3,367 cm−1 and 1,080 cm−1 presented in both KC-BR and KC-AR[29]. The absorption peaks near 3,367 cm−1 were indicative of C-H stretching in KC, while the absorption peaks near 1,080 cm−1 were due to the stretching of carbonyl groups in KC[30]. A wide band can be seen between 3,200 and 3,600 cm−1, which was caused by the O-H stretching vibration of water molecules[31]. The hydroxyl groups in sorbitol were abundant, and the hydrogen bonds between KC and sorbitol grew stronger after solvent replacement, causing the peak at 927 cm−1 to move to a higher wavenumber. Additionally, a minor deflection of the peak at 846 cm−1 to 853 cm−1 also demonstrated the establishment of intermolecular hydrogen bonds. The formation of hydrogen bonds was the main cause of the dispersion and junction of polysaccharides[32].
T2 relaxation time
-
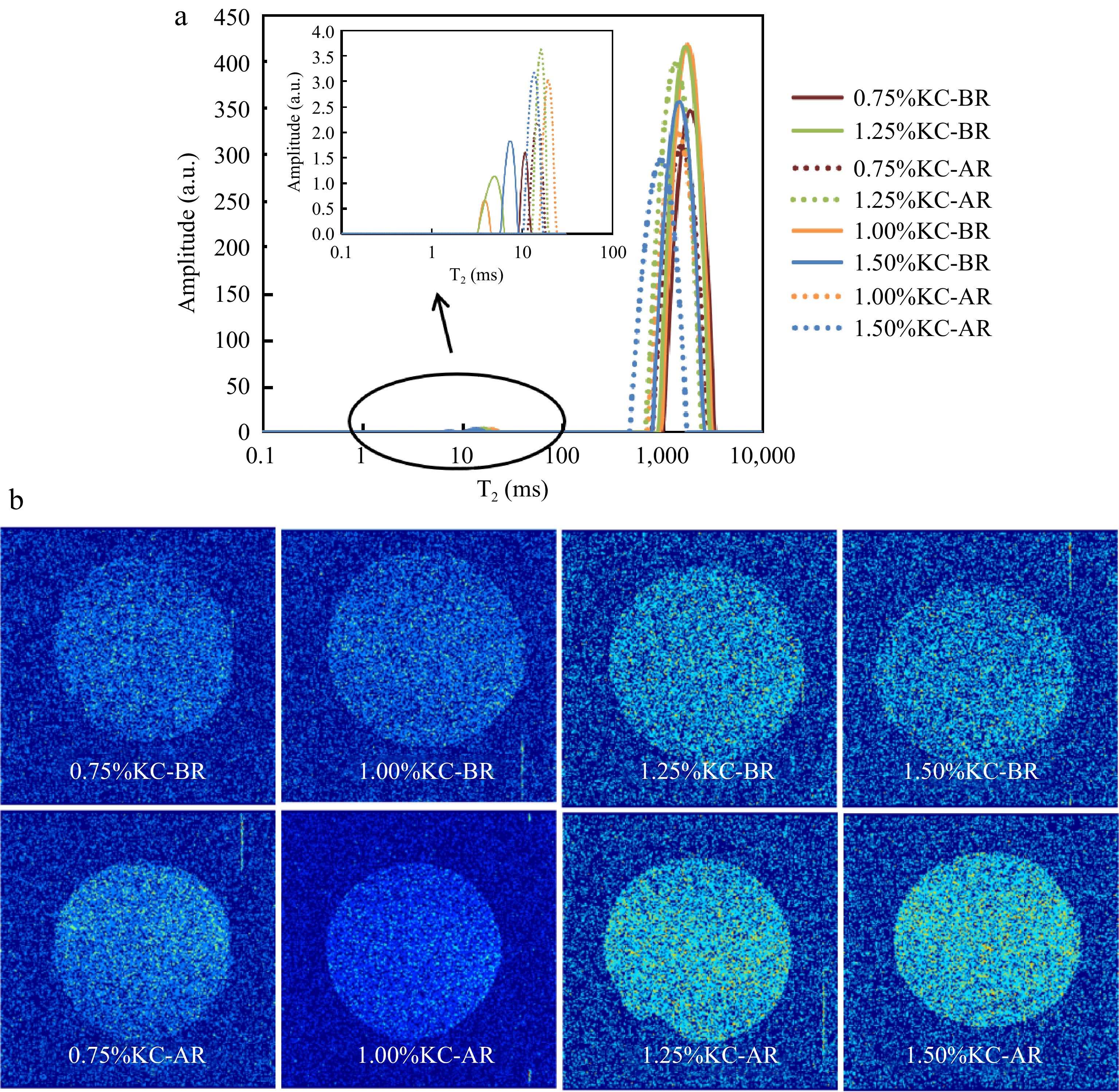
LF-NMR is an efficient method for analyzing the distribution of water in cross-linked networks[33]. Compared to other methods, LF-NMR offers advantages such as higher accuracy, quantitative determination of the relationship between molecules and water molecules[34], classification of mesh size in the hydrogel network according to different relaxation times, and categorization of different groups in the effluent system based on the relaxation time[35]. In this study, the location and movement of hydrogen protons in KC hydrogels were assessed using the transverse spin-spin T2 relaxation time[36]. Figure 3a illustrates that the hydrogels had two peaks in T2 relaxation time curves. T21 of KC-BR (1.00, 1.25 and 1.50 wt%) appeared within 1−10 ms, while T22 of them appeared beyond 100 ms. T21 of KC-BR (0.75 wt%) and KC-AR appeared within 10−100 ms, while T22 of them appeared beyond 100 ms. The peak within the range of 1−10 ms corresponded to protons strongly bound to the gel matrix. The peak within the range of 10−100 ms represented immobilized water presented within the networks, while the peak beyond 100 ms was free hydrogen proton in the gel framework[33]. Table 1 indicates that the peak area of T22 (P2) constituted a sizeable component of each group, indicating that the hydrogel predominantly consisted of free hydrogen protons. In KC-AR, T22 and the corresponding peak area (P2) were decreased while T21 and the corresponding peak area (P1) were increased. Also, with the increase in KC mass fraction in KC-AR, P2 were decreased while P1 were increased. Different NMR1H relaxation times can be acquired either from sorbitol or water in a multi-component gel system due to the superimposition of their resonance. P2 may have decreased as a result of limited mobility of protons due to the ability of sorbitol to form hydrogen bonds with KC. More hydrogen protons were firmly maintained in the gel matrix, as seen by the larger percentage of T21 peak area[36] .
Table 1. The peak area proportion of KC hydrogels.
Sample Proportion of peak area P1 (%) P2 (%) 0.75% KC-BR 0.119 99.881 1.00% KC-BR 0.138 99.862 1.25% KC-BR 0.160 99.840 1.50% KC-BR 0.194 99.806 0.75% KC-AR 0.247 99.753 1.00% KC-AR 0.284 99.716 1.25% KC-AR 0.304 99.696 1.50% KC-AR 0.409 99.591 MRI
-
The pseudo-colored MRI images of hydrogels are shown in Fig. 3b. The images of KC-AR were yellower compared to KC-BR. Generally, a section of the pseudo-image that was yellower often had a stronger resonance signal and more hydrogen proton density. Conversely, a bluer color indicated lower proton density[37]. The total response signal of the 0.75% KC-BR was the poorest. Sorbitol was added to the KC hydrogels, which increased signal strength and caused the image color to change to yellow. The hydrogen proton density was risen after solvent replacement, which was consistent with the FTIR results. The distinctive peaks for KC were the significant signals at 845 and 930 cm−1. These peaks can be observed in Fig. 2a. These two bands were absent from sorbitol-containing hydrogels, suggesting that the sorbitol binding caused these KC groups to vanish[38]. The OH-group was essential in molecular characterization by regulating the interaction between sorbitol and KC through the preferential hydration and the intermolecular hydrogen bonding[27]. Furthermore, as the KC mass fraction was increased, the signal intensity in KC-AR was intensified and the image color changed to yellow. This outcome might be explained by signal enhancement caused by increased hydrogen bonds between KC molecules and sorbitol.
Textural analysis
-
Figure 4a reflects that solvent replacement could improve the hardness of hydrogels. For example, 0.75% KC-BR had a hardness value of 1.48 ± 0.19 N, and the value was increased to 1.95 ± 0.28 N when the hydrogel was replaced by sorbitol. These results proved that sorbitol replacement could significantly improve the hardness of hydrogels. According to the microstructural observation, KC-AR had denser holes and more continuous skeletons. Therefore, KC-AR had higher hardness. This outcome was in line with the discoveries made by Kozlowska et al.[39], who found that the mechanical characteristics of KC hydrogels were greatly impacted by the sorbitol concentration. KC hydrogels which contained more sorbitol exhibited more stiffness. The two main processes in the KC gelation process were the conversion of coils to helix in junction zones and the accumulation of double helix, which was accompanied by gel formation at proper polymer concentration. A greater number of bonds and double helix aggregates were created during the change of helix structural as the concentration of KC increased. Additionally, by creating intermolecular cross-linked bonds between KC and polyols, the helix-shaped combination of polyols and KC encouraged the accumulation of cyclic junctions and generated bigger junctions[27].
Cohesiveness indicated the comparative ability of the hydrogel to resist the second compression[39]. As shown in Fig. 4b, increases in cohesiveness could be observed in KC-AR. The energy needed to bite solid foods was interpreted as chewiness[20]. In Fig. 4c, an increase in chewiness could be observed in KC-AR, just the same as cohesiveness. As a result of the direct binding of sorbitol to KC, there were more junction zones per unit volume with shorter average lengths. This was explained by the development of intermolecular cross-linking hydrogen bonds between the OH-groups of sorbitol and KC, which stabilized the distinctive intermolecular hydrogen bonding between individual KC stranded at a typical intersection[40]. With the increase in KC mass fraction, the hardness of KC-AR was gradually increased. The enhanced interactions between KC and sorbitol resulted in a more compact gel, leading to an increase in hardness[41].
Rheological analysis
-
The equilibrium between the interactions of various structural elements, including KC, sorbitol, and water molecules, may be reflected in the rheological characteristics of the KC hydrogels. Linear viscoelastic region (LVR) referred to the region in which the storage modulus (G') and loss modulus (G") of the sample remained constant by changing stress or strain[42]. According to Fig. 5a & b, 0.01%−0.1% was included in the linear viscoelastic zone of all KC hydrogels samples. After sorbitol replacement, G' of the KC hydrogels were increased. The KC-AR network was closely interconnected. Due to its low molecular mass and excellent water-holding capacity, sorbitol disrupted the stiff framework between the polymer molecules and expanded the molecular free-moving space. As a result, the G' was higher and mechanical properties of KC-AR were improved[27].

Figure 5.
Rheological behavior of (a) KC-BR and (b) KC-AR during strain sweep; rheological behavior of (c) KC-BR and (d) KC-AR during frequency sweep; (e) rheological behavior of KC hydrogels during temperature sweep.
In Fig. 5c & d, G' of KC hydrogels was always higher than G", demonstrating that the samples possessed gel-like viscoelastic properties[43]. After sorbitol replacement, both G' and G" of the KC hydrogels were increased, which revealed the strengthening of the network structures. The decreased water weakened the interactions between KC and water, causing KC strands to group together towards adjacent strands. Sorbitol promoted junction zone formation and gelation by forming cross-linking hydrogen bonds with KC strands through equatorial-OH groups[40]. As sorbitol replacement created hydrogen bonds between KC and sorbitol and further altered the gel networks, these outcomes corroborated the conclusions of the textural analysis. Additionally, G' of KC hydrogels remained almost unchanged as the frequency was increased, showing weak frequency dependency, which was a characteristic of the strong gel[44]. Besides, as the KC mass fraction increased, G' of KC-AR increased, suggesting that a higher KC mass fraction led to a tighter network structure in KC-AR[45]. This denser structure might be attributed to an increase in helix aggregate density and/or helix junction size[46].
At high temperatures, the organized network structure of some hydrogels would disintegrate and change into a random solution state. Thermal response of the KC hydrogels was also assessed by monitoring the evolution of G' and G" in a heating procedure (25 to 90 °C) (Fig. 5e). Both G' and G" were steadily reduced as the temperature increased. In the gel state, KC-sorbitol binding was an important factor governing KC gelation. According to the experimental data, the OH-groups of sorbitol were significant molecular characteristics that controlled the interactions between the sorbitol and KC in both solid (through preferential hydration) and gel (by intermolecular hydrogen-bonding) states. Since an increase in the quantity of equatorial-OH groups on sorbitol led to an increase in the amount of intermolecular hydrogen bonds between the sorbitol and each KC strand in the gel state, the enhanced thermal stability of KC hydrogels was consistent with sorbitol-KC binding[40]. Huang et al.[47] reported the effects of polyols on KC hydrogels. It was found that the addition of sorbitol led to higher gelling temperature. The enthalpy of gelation reduced as the concentration of sorbitol grew, which may be due to the replacement of polymer-solvent hydrogen bonding with polymer-polymer hydrogen bonding. The equatorial-OH groups on the polyols were important molecular features that might control the interactions between KC and polyols in the solid state and were advantageous for hydration. The number of equatorial-OH groups also had an impact on the thermal stability and gelation of KC hydrogels. Higher polyol concentrations could result in the formation of glasses with highly diverse physical characteristics that, in turn, had varied impacts on KC gels[47].
Swelling property
-
During the swelling property test, hydrogels were cut into small pieces. However, 0.50% KC-BR had a texture that was too soft to retain its shape after being cut into small pieces. Therefore, KC-AR with KC concentrations between 0.75 wt% and 1.5 wt% were chosen for the swelling test. Figure 6a & b demonstrates that KC-AR exhibited a rapid swelling rate in a neutral solution. The swelling rate of hydrogels was increased significantly within the first 10 min of swelling, then slowed down between 10 and 120 min, eventually reaching a state of equilibrium. After 40 min of swelling, the mass of 0.75% KC-AR was decreased. Therefore, the swelling experiment for this particular group was over. When the swelling time was 10 min, the swelling ratios of the hydrogels with 0.75, 1.00, 1.25 and 1.50 wt% KC were 6.02% ± 0.71%, 6.84% ± 0.85%, 7.59% ± 0.62% and 11.30% ± 1.17%, respectively. The swelling ratio of hydrogels and their swelling performance were gradually increased with the increase in KC mass fraction, which agreed with the conclusions of Bai et al.[48]. The increased KC mass fraction made the intermolecular interaction of polymers stronger and additional hydrogen bonds were established [49]. As a result, the capacity for swelling of hydrogels was reinforced by their increased toughness and stronger network structure[50].
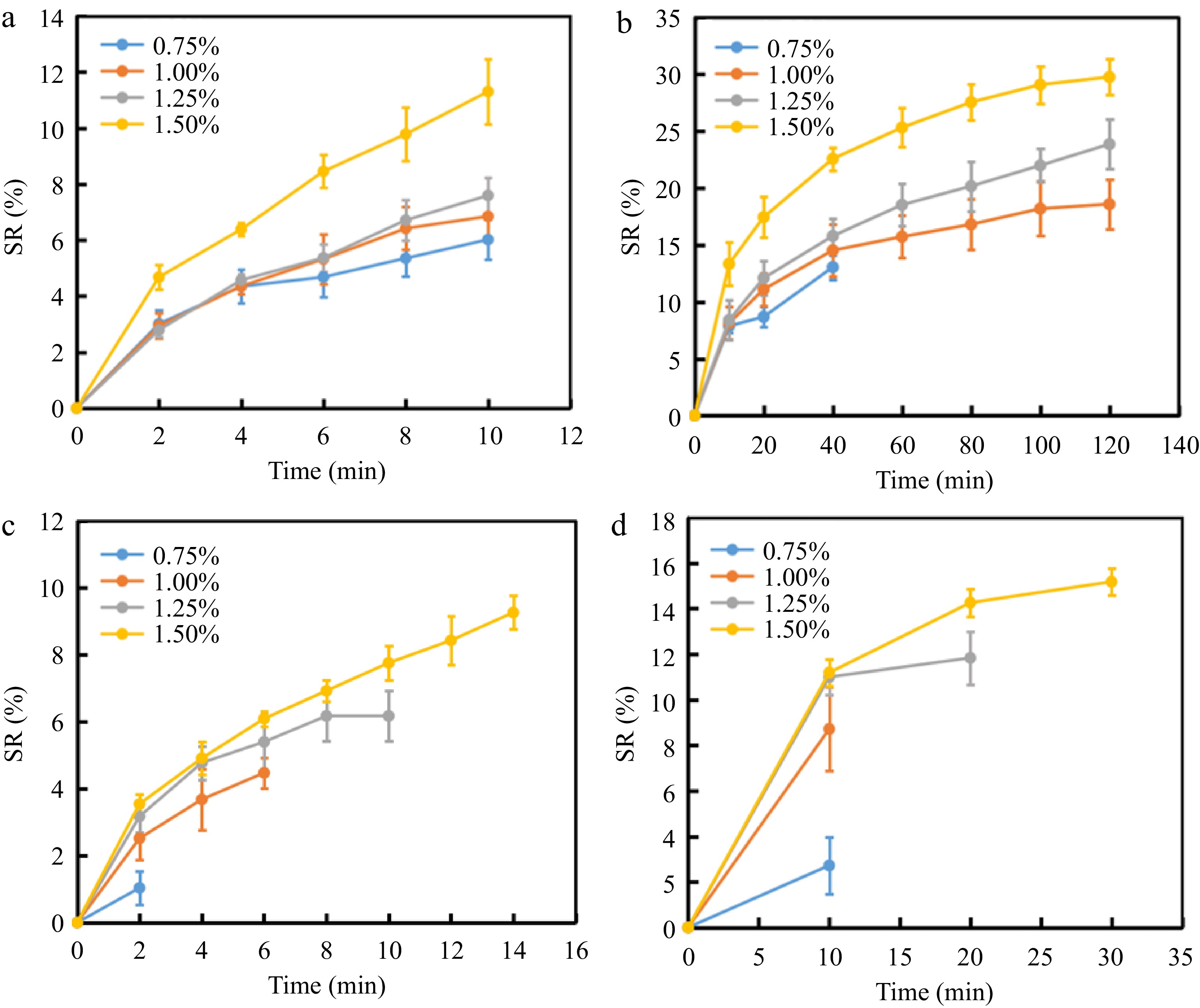
Figure 6.
Swelling ratio of KC-AR. (a) 0−10 min in solution (pH = 7). (b) 0−120 min in solution (pH = 7). (c) Solution (pH = 3). (d) Solution (pH = 11).
According to Fig. 6c & d, the swelling property of KC hydrogels in acidic and alkaline solutions was generally weak, with the most instability observed in the acidic solution. Figure 6c exhibits that in the acidic solution, the mass of 0.75% KC-AR was decreased after 2 min of swelling, while the mass of 1.50% KC-AR was decreased after 14 min of swelling. When the swelling time was 10 min, the swelling ratio of 1.25% KC-AR was 6.17% ± 0.74%, and that of 1.50% KC-AR was 7.75% ± 0.52%. This was due to the hydrolysis of KC at low pH, and the 3,6-dehydration-d-galactose bond was broken[48]. Figure 6d demonstrates that in the alkaline solution, the mass of 0.75% KC-AR and 1.00% KC-AR was decreased after 10 min of swelling, while that of 1.50% KC-AR was decreased after 30 min of swelling. When the swelling time was 10 min, the swelling ratios of hydrogels with 0.75, 1.00, 1.25 and 1.50 wt% KC were 2.72% ± 1.24%, 8.72% ± 1.83%, 11.01 ± 0.78% and 11.19% ± 0.59%, respectively. After 10 min of swelling, the swelling ratios of KC hydrogels with 1.25 wt% and 1.50 wt% KC was still increased, revealing that the network structure of KC-AR became more stable in the alkaline solution with increasing KC mass fraction. KC is an anionic polysaccharide. The anions of KC could react with the H+ in acidic conditions, leading to the hydrolysis of KC. There was more H+ in acidic solutions than in alkaline solutions[51]. Overall, KC-AR were relatively stable in neutral solutions, followed by alkaline solutions, and were most unstable in acidic solutions[52].
Stability of KC hydrogels
Syneresis rate analysis
-
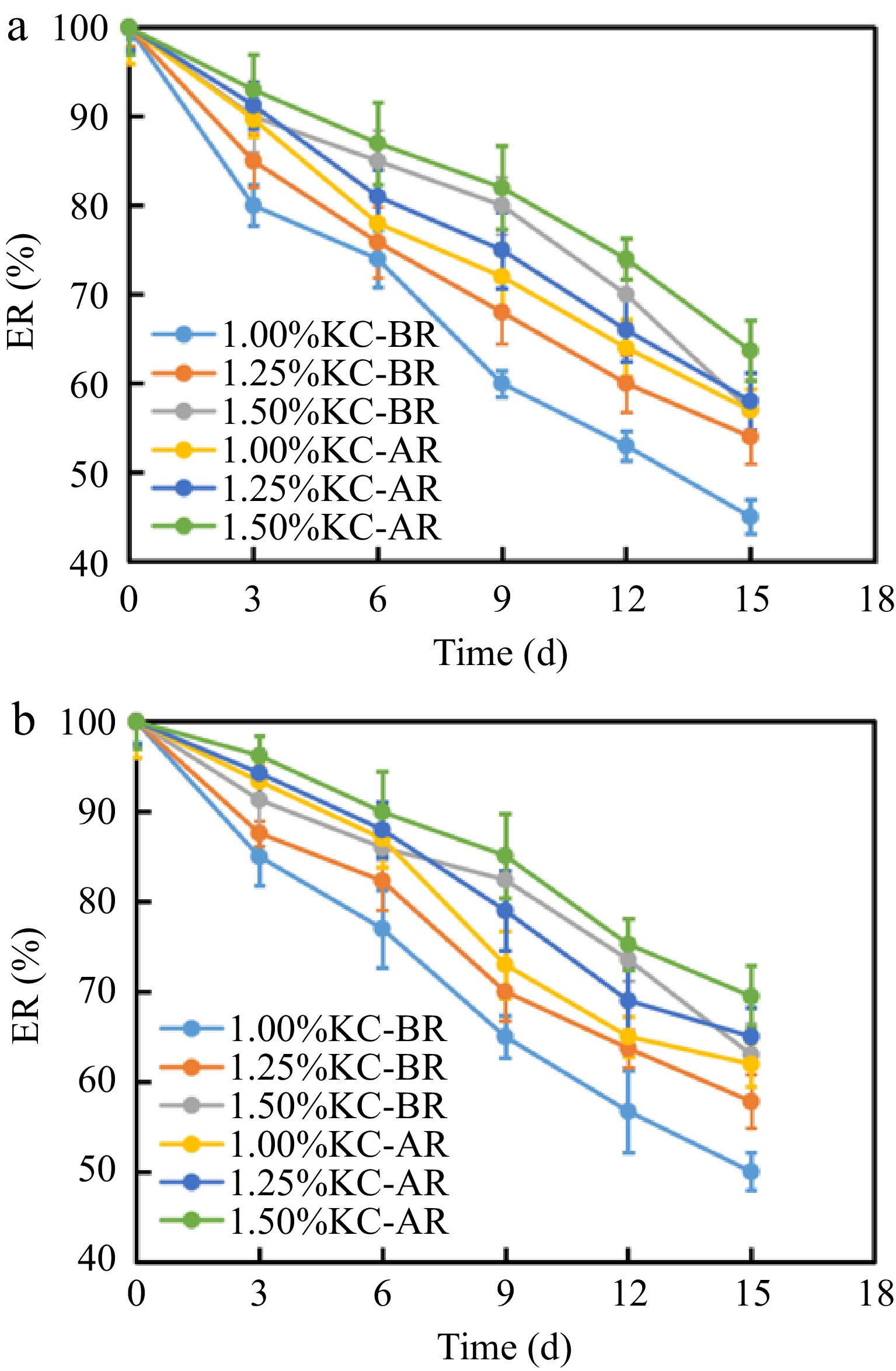
A remaining key challenge of hydrogels that needs to be addressed is their long-term stability. With the extension of storage time, water was gradually leaked out from the hydrogel, resulting in an increased syneresis rate. Figure 7a & b demonstrates that the syneresis rate of KC-AR was lower than KC-BR. One reason for this discovery was that during the replacement process, the hydroxyl group in sorbitol partially replaced the hydroxyl group in the water molecule in KC hydrogels. Due to the existence of abundant hydroxyl groups and long molecular chains in sorbitol, the ability of KC hydrogels to retain solvent (water in this study) was strengthened[53]. As seen in Fig. 7b, as the KC mass fraction was increased, the syneresis rate of KC-AR was gradually decreased, while the water retention ability of hydrogels was increased. Increased KC reduced the water fraction between KC and water molecules, weakening the interaction between them. At the same time, the KC chains interacted more closely, leading to a denser structure[53]. This conclusion corresponded with the results of LF-NMR, where the flexibility of hydrogen protons was decreased as KC mass fraction was increased.

Figure 7.
Syneresis of (a) KC-BR and (b) KC-AR. Syneresis of (c) KC-BR and (d) KC-AR after freeze-thawing cycle.
Freeze-thawing stability
-
Water molecules were converted into crystals during the freezing process, and the formation and expansion of ice crystals could harm the gel structure. Free water may seep from the network after defrosting, impairing the quality of gels[31]. Due to its abundant hydroxyl groups, sorbitol might have a variety of uses: (1) created powerful hydrogen connections with the KC framework that promoted the gelation process; (2) by creating hydrogen bonds with the water molecules, these bonds broke the connections between water molecules, preventing the crystallization of ice, and decreasing the freezing point[54]. Syneresis is commonly used for the evaluation of the restorability and stability of hydrogel systems[55]. Figure 7c shows that after one freeze-thawing cycle, the syneresis rates of KC-BR on the thawing day were generally significant. The syneresis rate of 0.50% KC-BR on the thawing day was 76.56% ± 1.02%. With the extension of storage time, water continued to be leaked out from the hydrogel, causing an increase in the syneresis rate. After 4 d of storage at room temperature, the syneresis rate of 0.50% KC-BR reached 95.39% ± 0.95%. KC hydrogels formed a spongy structure after repeated freezing and thawing, causing water to be leaked out from the main body of hydrogels, resulting in dehydration condensation[56].
Figure 7d illustrates that the syneresis rate of 0.50% KC-AR was 33.55% ± 2.93% on the day of thawing, and after 4 d of storage at room temperature, the syneresis rate of 0.50% KC-AR was 48.11% ± 2.14%. In Fig. 7c & d, it could be observed that after one freeze-thaw cycle, the syneresis rates of KC-AR were lower than those of KC-BR. The flexible structure of sorbitol competed with KC for the chelating moisture molecules, which could cause the conversion of free water into bound water. In this sense, the proper inclusion of sorbitol improved the gelation. Additionally, the production of ice crystals became more challenging due to the rise in the bound water content, together with an increase in resistance to the break force and deformation brought on by the freeze-thawing process[57]. As the mass fraction of KC was increased, the syneresis rates of KC-AR were gradually decreased. The increase in KC could limit the growth of ice crystals and water evaporation, thus reducing the fluctuation of breaking force and gel strength during freeze-thawing cycle. Additionally, KC, a powerful cryoprotectant, could reduce water movement to enhance the functional qualities of hydrogels throughout freeze-thawing process[58]. Furthermore, sorbitol is a low co-solvent that can resist low temperatures. Therefore, sorbitol replacement could significantly improve the interior structure of hydrogels and weaken the damaging effect of the freeze-thaw cycle on their structure[54].
Stability of EGCG in functional KC hydrogels
-
EGCG is a naturally-occurring bioactive substance and may have antineoplastic and chemopreventive effects. However, its widespread use in food was constrained by its vulnerability to environmental stressors[59]. Polysaccharides have been combined with EGCG to create hydrogels[60]. As a result, it was selected as the bioactive component in the manufacture of gels. Thermal stability (25 and 4 °C) of EGCG within KC hydrogels was investigated (Fig. 8). Generally, EGCG was more retained at 4 than at 25 °C. Low temperature could slow down the degradation of EGCG due to its thermal instability[61].
During storage, EGCG was degraded to different degrees. With the extension of storage time, the retention rate of EGCG was decreased. After 15 d of storage, the retention rate of EGCG was increased in KC-AR with an increase in KC mass fraction. At 25 °C, it was discovered that 1.50% KC-AR was able to preserve the most EGCG (63.7% ± 3.4%). On the contrary, 1.00% KC-BR had the lowest retention. Similarly, at 4 °C, 1.50% KC-AR retained the highest level of EGCG (69.5% ± 3.2%). For 1.00% KC-BR, the least amount of EGCG was retained, with up to 50.2% of it degrading after the test. Some EGCG was placed on the surface of the hydrogel and the porous structure inside when the hydrogel was loaded with EGCG. Numerous hydroxyl groups in the chemical structure of EGCG allowed it to create hydrogen bonds with sorbitol and KC[62]. Thus, sorbitol helped the structure of the hydrogel become more rigid. The interaction between internal molecules was improved, and the embedding effect was accomplished. Wang et al.[63] developed functional nano-hydroxyapatite/carboxymethyl konjac glucomannan/gelatin (TA@n-HA/CKGM/G) hydrogels and studied the sustained release of EGCG. They found that the TA@n-HA enhanced pH sensitivity, biodegradability and entrapment efficiency of the hydrogels. These findings suggested that TA@n-HA hydrogels have a considerable chance of serving as a delivery vehicle for the intestinal site-specific administration of small drugs.
-
The present study reported a new approach to strengthen the structures of KC hydrogels through sorbitol replacement. The hydroxyl group in sorbitol combined with KC to form hydrogen bonds, thereby strengthening the structures of the hydrogels. The findings demonstrated the swelling property, gel strength, and water retention ability of the gels after replacement were enhanced with the increase in KC mass fraction and sorbitol replacement. Furthermore, the strong gel networks favored a higher retention rate of EGCG during storage. This study offered fresh perceptions into the structure-function relationship of KC hydrogels from a bioactive perspective, and the findings contributed to wider application of KC hydrogels in the development of functional food.
-
The authors confirm contributions to the paper as follows: study conception and design: Wang Y, Mao L, Yuan F, Gao Y; data collection: Wang Y, Zhang X; analysis and interpretation of results: Wang Y, Mao L, Zhang X; draft manuscript preparation: Wang Y, Mao L. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This research was funded by National Natural Science Foundation of China (No. 32272471).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang Y, Zhang X, Gao Y, Yuan F, Mao L. 2023. Development of κ-carrageenan hydrogels with mechanically stronger structures via a solvent-replacement method. Food Innovation and Advances 2(4):313−323 doi: 10.48130/FIA-2023-0031
Development of κ-carrageenan hydrogels with mechanically stronger structures via a solvent-replacement method
- Received: 05 September 2023
- Accepted: 17 November 2023
- Published online: 18 December 2023
Abstract: Strong κ-carrageenan (KC) hydrogels were fabricated via solvent replacement with sorbitol, and the effects of KC mass fraction and solvent replacement on the structural characteristics encapsulation capability of the hydrogels were evaluated. Microstructural observation showed that the 3D network structures of hydrogels exhibited a complete and continuous skeleton. FTIR spectra of KC hydrogels revealed the formation of intermolecular hydrogen bonds after sorbitol replacement. The stability against heating and freeze-thawing of hydrogels was enhanced due to the addition of sorbitol and the rise in KC mass fraction. The hydrogel with 1.5 wt% KC after sorbitol replacement presented the best stability. Frequency sweep tests suggested that storage modulus of the samples were influenced by sorbitol replacement and KC concentration. Swelling tests revealed that the hydrogels after replacement with a higher KC content (1.25, 1.50 wt%) presented higher swelling capacity, and they were more stable in alkaline and acidic solutions. When epigallocatechin gallate (EGCG) was incorporated within the hydrogels, the hydrogels after sorbitol replacement offered higher protection capability. The information obtained in this study indicated that sorbitol replacement strengthened KC hydrogels, and they could act more appropriately as accountable carriers for bioactives.
-
Key words:
- κ-carrageenan /
- Hydrogel /
- Solvent replacement /
- Sorbitol /
- EGCG