-
Nowadays, the demand and consumption of vegetable oils worldwide have risen expeditiously from 2016 to 2021, increasing by approximately 30 million tons in only 5 years[1,2]. The increment rate of vegetable oil consumption has outpaced the growth of world population. Based on the data retrieved from the Food and Agriculture Organization of the United Nations[3], it was observed that not only the amount of vegetable oils consumed, but the origin has changed over the last few decades. Various consumption patterns are claimed to be caused by different cultural aspects and religious traditions with economic, political, technological and social factors[4]. In fact, the consumption of vegetable oils is undeniably linked to the overall WCO produced in each country without considering any further reprocessing of WCO.
Owing to increased consumerism and a growing economy alongside urbanization, the production of WCO has increased dramatically giving rise to various environmental issues including blockages of drains and sewers as well as pollution of water sources. As a result, promulgation of environmental regulations related to the disposal of WCO in different countries is critical, either to comprehensively mitigate the issues arising from WCO disposal or to potentially utilize WCO as a renewable source in producing biofuels and other value-added products[5]. For example, with the intention to reduce the amount of WCO being disposed of into the sewage system, an effective WCO collection system should be introduced with financial support from the local government, to gather all possible WCO sources originating from hospitality, industrial sectors and restaurants. Similarly, in term of utilization of WCO, Renewable Energy Directive (RED II), the European Union (EU) had implemented the double-counting practice to effectively promote and further enhance the production of waste-based biofuels[6]. In short, the safety control of WCO can be carried out via deterring WCO dumping behaviour of inhabitants and fully utilizing the potential of hazardous WCO as a green material.
The sustainable characteristics of WCO are the main interest of the current energy sector due to its biodegradable, renewable and abundant properties. However, the physical and chemical properties are largely dependent on the origins of WCO[7]. In general, repeated cooking immersion may lead to a darker colour and higher viscosity accompanied by elevated acidity and specific heat as well as an unpleasant odour of WCO[8]. In this sense, this article summarizes the various techniques utilized in the WCO pretreatment process to ease further valorisation after recognizing the large annual production of WCO but with a low valorisation rate. Besides, the transformation of WCO into value-added products is also reviewed broadly. Relevant recommendations are provided in different sections accordingly.
-
The data reported under this section was tabulated by Teixeira et al.[9] which refer to oils that are applicable for valorisation after being utilised as cooking oils. All collected data originate from journals, web-based documents, grey literature, and reports. The concerns here are the homogeneity of detail and quality of the obtained information, methods used in quantifying vegetable oils as well as the lack of information about the techniques in acquiring the estimations for the consulted document which caused uncertainties on the results[9].
Liang et al.[10] reported that China produces 5.6 million tons of WCO annually while Borugadda and Goud[11] presented that nearly 1.135 million tons of WCO are produced in India every year. Thus, indicating that China is the largest WCO producing country worldwide[10,11]. Undoubtedly, China and India are the largest WCO producing countries due to their huge population and vegetable oil production. For instance, China produced about 14% of the total world production of vegetable oils, making it the world's second largest producer in 2015[12]. Italy, Canada, and South Korea are considered as intermediate producers as their WCO production ranges between 0.1 and 0.3 million tons per year[13−15]. Germany and Ireland are the two countries with low WCO production. WCO production in Germany is the lowest, ranging from 70,000 – 105,000 tons per year[16], whereas Ireland has the WCO production of 153,000 tonnes per year[17].
Teixeira et al.[9] have examined the relationship between the total consumption of vegetable oils (TVOC) and the amount of WCO produced in each country. It was found that there was no significant correlation between these parameters when per capita values were used. Nevertheless, a linear relationship was observed between TVOC and WCO produced which could be indicated by production factor, α = 0.32, after plotting the two parameters for 23 different investigated countries. This indicates that 320 kg of WCO will be produced for each ton of TVOC[9]. In conclusion, deteriorating impacts will arise if the disposal of WCO into sewage is not solved due to the huge increase of WCO production annually. From another point of view, it may be an opportunity to fully explore the potential of WCO as an oleochemical feedstock in eliminating and valorising hazardous waste.
Valorisation of WCO
-
The extent of the valorisation of WCO can be directly linked to the amount of WCO produced or vegetable oil consumed in that country. For example, Teixeira et al.[9] utilized two parameters, Human Development Indices (HDI) and Gross Domestic Product (GDP), to quantify and analyse the relationship between them with TVOC and valorisation of WCO (VWCO). By investigating the relationship of VWCO with GDP per capita and HDI, the study concluded that a country with high GDP per capita and HDI will own additional advanced valorisation systems which led to more WCO being valorised. Germany was discovered to have roughly 100% WCO valorisation while India has only 0.1%. An empirical analysis was proposed for the relationship of VWCO versus TVOC for different countries which are separated into three classes: high, intermediate and low-performance groups, according to their conversion factor (δ)[9]. Among the countries, the United Kingdom, Spain, and China were listed under the low-performance group while Denmark was placed under the high-performance group due to the high values of β and δ. It was observed that a similar δ could be obtained by the product of α and β for the better performing country and underperforming country.
In general, specific legislation for WCO management is necessary to achieve a higher conversion factor which increases the efficiency of the valorisation system. Two approaches of WCO management have been identified, first is to provide subsidies for the biofuel industry by following regulations to incorporate biofuels into petroleum-based fuels (Brazil and USA), while the second method is in the form of environmental taxes established on third generation economic instruments which are added on to consumer products (Japan, European Union and Argentina)[9]. It was found that the former can attain a higher valorisation rate for a country. WCO collection, transportation and legal/policy framework facilitation are also playing an important role in affecting the WCO valorisation[18,19].
-
Pretreatment methods of WCO, prior to further processing WCO into various useful products such as alkyd resin, green solvent, soap, plastics, etc., are essential to remove or reduce the FFA content or any impurities present in WCO. Pretreatments are required in refining and upgrading WCOs, which help to further produce suitable WCO-based feedstocks for value-added applications in oleochemical industries. Aforementioned, the physicochemical properties and nature of WCOs vary and thus largely affect the required pretreatment processes. Majorily, the variables affecting the physicochemical properties of WCOs are the origin of cooking oil and the parameters of the frying process such as the temperature, duration and method used. In biodiesel production, the WCOs have to achieve certain quality requirements with respect to the acidity, moisture content and iodine value[20,21]. In the production of solvents and lubricants, the performance and efficiency of catalysts in successive operations are strongly dependent on the composition of WCO feedstock, which must be free from sulfur and metal elements. On top of that, bleaching is necessary as a pretreatment prior to the fabrication of WCO-based monomers and additives for resins and polymers as a light-toned colour is the key aspect. The bleaching process can be done using bleaching earths as adsorbents, oxidizing agents for chemical bleaching or treatment with solvents via liquid-liquid extraction[22]. In the case of WCO-based surfactants production, it is crucial to eliminate toxic or hazardous components during the pretreatment processes[23]. During the process of synthesising plasticizers from WCOs, the content of volatiles must be reduced to avoid generating vapour and bubbles during the entire process. In contrast, the pretreatment process required is less restricted when WCO is employed as drop-in additives for asphalts and concrete manufacture. This is because WCOs as drop-in additives have more flexibility and tolerance to the undesirable characteristics such as odour and appearance compared to other oleochemical production[24]. At present, several pretreatment techniques are either being implemented commercially or under investigation.
Removal of solid impurities
-
Initially, WCOs are treated via physical routes to remove the suspended solid impurities, oligomers and gums which lead to fouling and pipeline hindrance. Generally, WCOs contain roughly 10−20% of solids, depending on the cooking process and waste management methods utilized. The carbohydrate and protein components in WCOs assist the oil emulsification with water by acting as surfactants, while promoting the initiation of aggregates and solid suspension. Since highly saturated oils appear solid at room temperature, they tend to form larger aggregates and capture other solid residues[24]. Hence, it is important to destabilize the water-in-oil emulsions to stimulate oil extraction and solid removal, as well as to melt the solid lipid fraction completely to set free the trapped water and residues[25]. At the first stage of filtration, fabric bags or stainless strainers with sieve diameter of 150−200 µm are used to filter coarse particles. Next, fine filtration is proceeded by utilizing 70−100 µm stainless steel meshes, together with heating at 60−65 °C via the addition of steam. Heating the oil not only helps to melt the solid fats, it is also advantageous to reduce the viscosity and ease the transportation process through pumping. The liquid will be stored in heated tanks at 60 °C after fine filtration as this can help to maintain the viscosity of oil[25,26]. Moreover, centrifugation is a common method to separate water and remove solid particulates from the WCOs. Due to higher capital and operating costs, it is usually utilized in large-scale operations which work continuously[24]. According to Skelton[27], the contamination standard for solid separation of WCOs is 24 mg kg−1, while the water content is limited to 500 ppm, as established in EN 14214 by the European Committee[27].
Reduction of free fatty acid
-
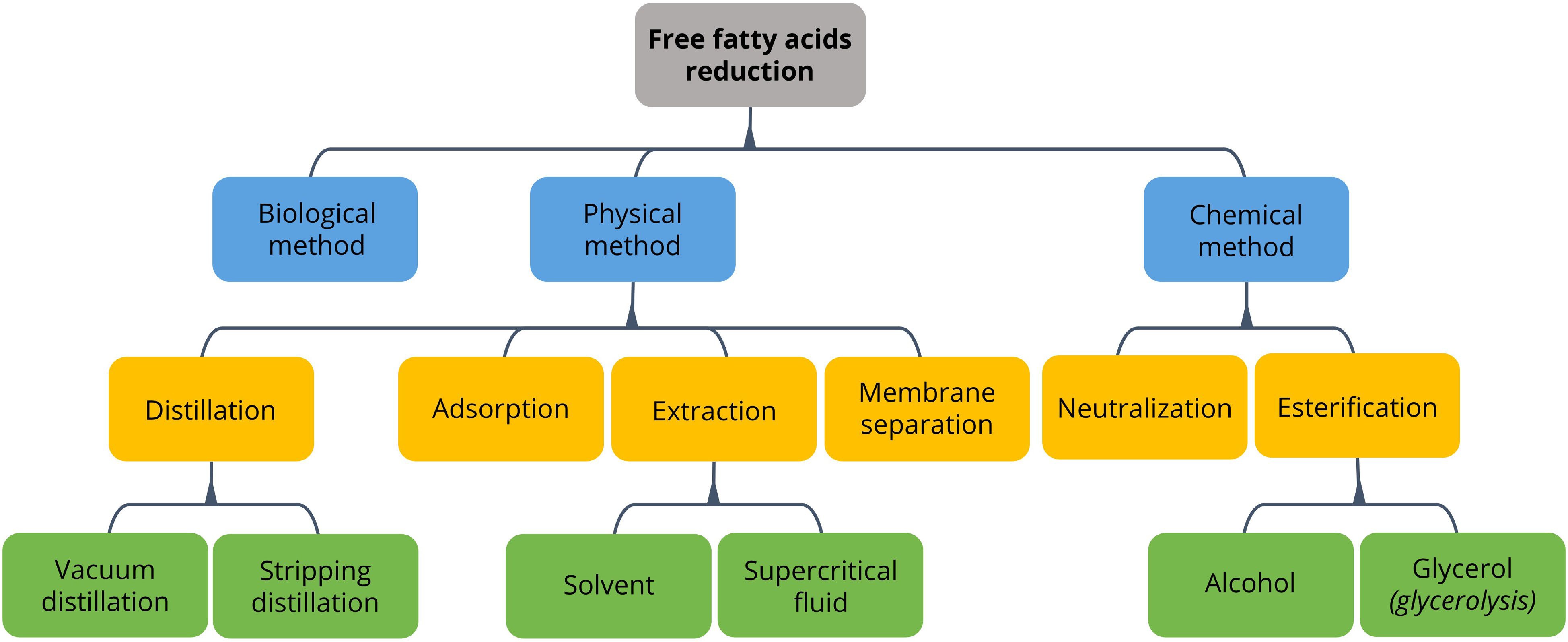
In addition, the acid value in the WCOs is found to be noticeably high as compared to virgin vegetable oils, which is mainly due to the hydrolysis of acyl glycerides into free fatty acids[28,29]. In fact, the maximum acidity content acceptable is at 5 wt%. Thus, FFA reduction is a crucial pretreatment process to ensure the acidity does not cause negative impacts during valorisation processes of WCOs[24]. There are several conventional approaches, including neutralization and esterification as chemical methods, while distillation, extraction with solvent or supercritical fluid, adsorption and membrane separation as physical means. Neutralization is carried out with alkaline solutions to neutralize acids by precipitating FFA as soaps while producing water as by-products. Sodium hydroxide and calcium hydroxide are commonly used alkaline solutions in the treatment and the soap produced will be removed via centrifugation or water washing methods[30]. Moreover, esterification of FFA in WCOs with alcohols to produce esters is a different approach to reduce the FFA content. The common esterification process uses methanol as the alcohol to produce methyl ester and water. By using glycerol, the FFA can be converted into mono-, di- or triglycerides[24,30]. These reactions can be catalyzed by homogeneous acid catalysts, heterogeneous base catalysts and other acid catalysts[24,31]. Al-Sakkari et al.[32] compared the conversion efficiency of FFA into methyl esters by using two type of catalysts, which are sulphuric acid as the homogeneous acid catalyst and cement kiln dust (CKD) as a heterogeneous catalyst. Comparatively, esterification catalyzed by sulphuric acid had achieved a conversion of 96.2%, whereas 98.8% for the economical CKD catalyst[32]. Abidin et al.[31] also found that the high conversion of FFA into methyl ester at 92%, was catalyzed by Purolite D5081 as the ion-exchange resin[31].
On the other hand, glycerolysis that uses glycerol to break the chemical bonds between FFA into glycerides as stated, is advantageous in reducing FFA content. Previously, glycerolysis was found to be relatively inefficient due to the requirement of high temperature, long reaction time and high energy consumption. As a solution, Gopinathan et al.[33] investigated an alternative that uses continuous flow microwave irradiation for glycerolysis reaction. This technique, with lower energy intensity, was able to reduce FFA from 3.95% to 0.34% under the operating conditions of 1:1 oil to glycerol molar ratio, 10 min reaction time and pump speed of 70 rpm[33]. Additionally, FFA removal using biological methods is currently under investigation. It was discovered that Pseudomonas sp. has the capability to utilize FFA as a carbon source to promote their growth. However, the inability to utilize the short chain fatty acids has been the major drawback[30]. Recently, Bacillus thermoamylovorans was discovered to have the potential to reduce FFA content through the conversion into polyhydroxyalkanoate (PHA) where WCOs act as the substrate. At the laboratory scale, the pretreated WCOs had a FFA content of 0.99%, which was largely reduced from 3.5% initially. Meanwhile, 3.47 g/L of PHA was produced under optimal conditions of 45 °C, agitation speed of 200 rpm and 50 g/L WCOs[34]. This biological pretreatment technique is promising for industrialization as the FFA content is found to be lower than 1% after pretreatment, and value-added products like PHA can be produced.
Furthermore, distillation as a physical method is a common approach which is used to reduce FFA content, mainly vacuum and stripping distillations. Vacuum distillation is simple, with high applicability as it is able to produce desired glycerides by separating them from FFA and water. Compared to glycerides, acidic compounds and water have higher volatility. Hence, able to be removed from crude WCOs in a simpler way. Moreover, other volatile components can be removed simultaneously through distillation, including hydrocarbons, aldehydes, ketones etc[24,35]. As a result, FFA content can be reduced from 6.2% to 4.3% via distillation[25]. In addition, FFA removal can be done through solvent extraction by using solvent as the separation agent to extract FFA selectively, which is the solute of interest in this case. The mechanism behind is the solubility difference of FFA between WCOs and solvent which acts as a driving force for this selective separation. It can be performed in single or multiple stages to improve the contact. The effectiveness of the extraction process is strongly influenced by the type of solvent used. In this regard, Hansen solubility parameter functions as an elementary parameter to estimate the affinity of FFA, by evaluating the similarity of solvent as well as the loading required[24,36]. Ethanol has been a conventional solvent used for the extraction process and able to reduce the acid content below 0.1% with multistage liquid film contactor as the contact device and solvent to oil ratio of 2:1[24]. Likewise, extraction using supercritical fluid such as carbon dioxide at low temperature and high pressure is advantageous as well. This is because the characteristics of carbon dioxide such as high availability, low toxicity and low cost, have made it a superior solvent which is simple to utilize and safe. In fact, the extraction performance is enhanced using ethanol as co-solvent[24,37].
As mentioned, FFA content can be reduced conventionally via an adsorption process where covalent bonds are formed between the active materials and the carboxylic constituents of acid compounds. Not only being used for acidity adsorption, it can also get rid of the coloured compounds to enhance the colour of refined WCOs[24,38]. It was conducted by heating the WCOs at a reduced pressure before undergoing a dual-stage adsorption with activated carbon and adsorbent with hydrophilic properties such as activated alumina or silica gel[35]. By using column chromatography with magnesium silicate and aluminium oxide, Lee et al.[39] were able to reduce FFA content from 10.6 wt% to 0.23 wt%[39]. Alternatively, agro-waste like rice husk can be an adsorbent that achieves a reduction capacity of 63%, which is similar to the conventional activated carbon. Notwithstanding the similar capacity of FFA reduction in WCOs, the utilization of rice husk is more favourable comparatively. For example, rice husk as an adsorbent is able to achieve its maximum capacity in a shorter time without catalysts, which leads to higher energy and cost efficiencies[40]. On top of that, using membrane technology to separate FFA from WCOs is promising due to the relatively low energy consumption and its ability to retain nutrients after separation. Membrane separation is basically driven by the pressure gradient established, as well as the differences of components in terms of molecular weight, size, shape and physicochemical interactions with the membrane. As compared to triglycerides with molecular weight greater than 800 g/mol, FFA only has a molecular weight of less than 300 g/mol. Owing to the difference, undesired components in WCOs such as FFA and low molecular weight hydrocarbons are able to be separated selectively[24,41]. In general, there are two major categories of materials used to fabricate membranes, which are polymers including polyamide, cellulose acetate and polysulfone; as well as metals and ceramic materials such as stainless steel and alumina. As reported by Mannu et al.[41], polyethersulfone membrane modified with hexamethyldisiloxane is able to retain 35.3%−40% of FFA after operation[41].
Fig. 1 outlines current FFA reduction techniques, while Table 1 summarizes the pros and cons of the techniques discussed along with the proposed acidity removal efficiency from past research. Neutralization and esterification are the most commonly used methods in removing FFA contents of vegetable oils due to their high effectiveness in reducing the acid value and are applicable for a wide range of WCOs properties. Additionally, esterification can also be associated simultaneously with transesterification of WCO in biodiesel production. However, major disadvantages also present in these methods which complicate the operations in scaling up for commercialization.
Table 1. Pros and cons between the existing techniques for FFA removal from WCO.
Techniques Process Pros Cons Acid removal References Chemical methods Neutralization Lesser energy usage Suitable for WCOs with different origins Higher removal selectivity Produce soaps and wastewater Reduce triglyceride content Large amount of water needed during washing process Up to < 1 wt% Pinzi[42],
Rodrigues and Meirelles[43], Shahidi[44], Tanzer et al.[45]Esterification Higher efficiency Suitable for WCOs from different origins Flexible working principle (simultaneous esterification-transesterification in biodiesel production) Higher processing costs Produce wastewater Cause corrosion if homogeneous acidic catalysts are used Able to achieve 100% acid conversion Mendecka et al.[21], Vaisali et al.[30], Divakar and Manohar[46], Elias et al.[47], Saravanan et al.[48] Physical methods Distillation Simple and universal applicability Simultaneous removal of other volatile components Less amount of waste generated Good quality of FFA High capital and operating costs Large energy consumption Vacuum system operates with steam jets and can generate higher wastewater Up to < 1 wt% Cárdenas et al.[24], Maddikeri et al.[35], Rodrigues and Meirelles[43], Shahidi[44], Yuan et al.[49] Extraction with solvent or supercritical fluid No generation of by-products Low energy consumption Low loss of WCO during extraction High capital and equipment costs Selection of solvent with low reactivity and high thermal stability is critical From 2−4 wt% to < 0.1 wt% Cárdenas et al.[24], Rodrigues and Meirelles[43], Bhosle and Subramanian[50], Meirelles[51] Adsorption Enhance the color of WCO by removing α and β unsaturated carbonyl compounds Applicable even under the presence of different impurities in WCO Large amount of absorbents are required Solid waste generation High separation effectiveness is required prior to industrial implementation Roughly 65%–80% efficiency Cárdenas et al.[24], Rahayu et al.[38], Rincón et al.[52], Sumnu and Sahin[53] Membrane separation Able to achieve selective FFA separation Lower energy consumption Improved FFA quality Higher yield of WCO Cost of membranes is very high High generation of solid waste Solvent is required to achieve separation Usually associated with solvent extraction with similar removal efficiency Cárdenas et al.[24],
Vaisali et al.[30], Bhosle and Subramanian[50],
Ladhe and Kumar[54]Removal of moisture, sulfur, phosphorus, and nitrogen containing compounds
-
Moisture removal is crucial to prevent adverse impact in subsequent oleochemical processes and the occurrence of microbial or enzymatic degradation during operation and storage. The presence of water will cause hydrolysis of glycerides into FFA, which is undesirable. In general, water is being removed together with acid compounds when undergoing the various pretreatment methods stated. For instance, steam stripping, adsorption, membrane separation and solvent extraction are able to eliminate water content during operations. Other than that, heating WCOs at 60 °C for 10 min by microwave is found to be efficient in removing water content at low energy intensity[24]. The polar compounds in WCOs can also affect the quality through degradation when the amount is greater than 25% by mass[55]. During pretreatment with distillation, polar compounds with high volatility will be removed along with FFA while polar compounds with larger size can be eliminated during the adsorption process. Presently, the combination of distillation and adsorption is implemented industrially to eliminate polar compounds. Solvent extraction with water or aqueous acid solutions are capable of removing these compounds also. They also can be removed through polar membrane separation although low effectiveness in commercialization was observed. Even so, it was found to have potential in achieving 40% reduction of polar compounds after being processed[24].
The quality of WCOs as oleochemical feedstocks is also dependent on the sulfur, phosphorus and nitrogen containing compounds. According to the requirement established by EN 14214, the maximum acceptable content for sulfur is 10 ppm, while for phosphorus is 4 ppm[56]. Sulfur and phosphorus have the ability to deactivate the active sites of catalysts in subsequent operations and corrode the operating equipment. As a solution, adsorption, solvent extraction, precipitation and oxidation reaction are the most common methods used to get rid of sulfur compounds in WCOs. Besides, hydration of phosphatides using saline solutions or dilute acids is used to remove phosphorus compounds in WCOs, along with the removal of waxes, gums and other impurities through degumming process[24]. Some compounds in WCOs such as aldehydes, ketones, FFA, sulfur and phosphorus with unpleasant odour might be transferred to oleochemical derivatives during subsequent processes. Fortunately, these unpleasant odours are being removed predominantly during the pretreatment with steam distillation[30]. Further deodorization and flavour enhancement processes can be performed at the final stage. This is done by evaporation and distillation of refined WCOs using superheated steam entrainment that operates under vacuum conditions at elevated temperatures around 150−160 °C[24]. Eventually, the final end product after a series of pretreatment processes will be high quality refined WCOs which are odourless and light in colour[30]. As a result, the enhancement of the sensory properties of WCOs after removal of problematic impurities has enlarged the possible applications of WCOs.
-
Alkyd resin is a unique family of polyesters which is produced via polycondensation of fatty oils or fatty acids, dibasic acids or acid anhydrides (polycarboxylic acids) and polyols with hydroxy functionality greater than 2[57]. Traditionally, vegetable oils such as soybean and sunflower are used to synthesise alkyd resin, demonstrating excellent characteristics such as biodegradable, great durability and flexibility, good adhesion as well as economically feasible. Alkyd resins are generally applied in coating applications such as paints, inks, lacquers, enamels and varnishes[58].
A patented technology has been reported which utilizes the WCO to replace virgin vegetable oils with polyol and polycarboxylic acid in synthesising alkyd resin via polycondensation[59]. The invention presented that weight percent of WCO in the reaction mixture could be varied from 10% to 65% while others were polycarboxylic acids and polyols. The weight percent of these components in the reaction mixture could be varied accordingly. Different polyols and polycarboxylic acids were reported to be suitable for this invention. The proposed method was said to exhibit a suitable hydroxyl number of at least 10 to 20 while in some embodiments, the alkyd resins might have a hydroxyl number up to 150. Also, suitable acid numbers were exhibited to be at least 2 to 5 while some could be up to 40. Removal of water produced during formation of alkyd resins by distillation process was necessary to improve the reaction. Side reaction should be inhibited to avoid formation of acrolein from glycerine due to the contaminants present in WCO that affect the properties of alkyd resin such as colour, molecular weight, hydroxyl number, viscosity, and acid number. Additional additives such as pigments and crosslinkers could be combined with the invented alkyd resins to be utilised in coating formulations[59].
Green solvent
-
Likewise, glycerol as the major by-product of WCO transesterification reaction for biodiesel synthesis is regarded as a promising green solvent for various industrial applications, particularly organic synthesis. In this sense, Díaz-Álvarez and Cadierno[60] proposed a study in examining the potential role of glycerol to act as both solvent and hydrogen source in metal-catalysed transfer hydrogenation (TH) reaction. The replacement of glycerol to 2-propanol which was the common hydrogen source used in TH reaction is cost-effective due to its wide availability and lower environmental impact than petroleum-based hydrogen sources. Additionally, the authors claimed that glycerol can be used in the formation of metal nanoparticles as it is more convenient in terms of cost and toxicity[60]. Other investigations were also carried out, many by Liu et al.[61] who introduced a novel integrated solvent system consisting of glycerol and ethanol for lignin fractionation[61].
WCO can also be directly utilized as a green solvent for wastewater treatment or other pollutant-extracting technologies. For instance, Sujatha et al.[62] presented an investigation on the preparation of green emulsion liquid membrane (GELM) for lead extraction with the use of WCO as the process diluent. Approximately 97.39% of maximum lead extraction efficiency was achieved in the study when WCO was used as the diluent. Also, the prepared GELM could be reused up to seven times without the reduction of extraction efficiency. On the other hand, Shokri et al.[63] proposed the use of WCO as a low-cost, easily available, non-toxic organic solvent in GELM process for water decolorization. In the study, methyl violet 2B (MV) dye was employed to examine the extraction efficiency. The maximum achieved MV extraction efficiency was 99.1% under optimized process conditions without the use of carrier agents. Similarly, Wahab et al.[64] studied the characterization of WCO on its application in liquid-liquid extraction (LLE) for wastewater treatment. In short, the use of WCO or WCO-derived compounds as solvents is possible after fully understanding the working principles and conditions of WCO in the process with varied WCO origin and physicochemical properties.
Soap
-
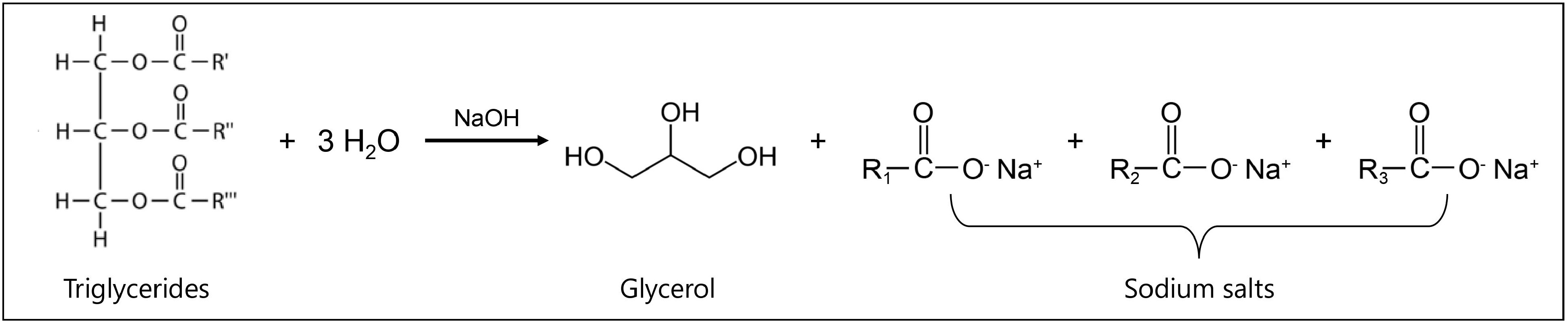
Soap is typically made via saponification, where triacylglycerols (major constituents of WCO) react with a strong mineral base like sodium hydroxide forming sodium salts of the hydrolysed FFA (the opaque soap) and glycerol, as shown in Fig. 2[65]. Soaps can be presented in solid or liquid form depending on the raw materials used in the process. For example, soaps in solid form are generally produced from fatty acid sodium salts while soaps in liquid form are produced from fatty acid potassium salts[66]. Félix et al.[65] proposed soap, from a green perspective, could be produced by reusing waste materials including almond shells, orange peel and WCO. In fact, they concluded that these waste materials are generally able to produce a good quality and cheap soap. However, the produced soap aroma would be largely dependent on the addition of additive types such as orange essential oil to increase the acceptability of the soap[65].
Adane[66] recently carried out a study on the synthesis of laundry soap from WCOs to replace the use of competitive palm oil, for the preparation due to the increasing utilization of palm oil in the biodiesel industry. The use of WCOs in synthesising laundry soap has provided two main advantages by substituting palm oil and animal fat, avoiding environmental issues and reducing soap production cost[19]. Three samples of WCOs were obtained, each from restaurants and hotels to be reacted with sodium hydroxide in producing soap. After the saponification process, the obtained physicochemical properties were pH value ranging between 9.31 to 10.56, 6.67% to 14.47% moisture content, 0.19% to 0.22% of free caustic alkali, 0.19% to 0.22% of chloride, 0.12% to 0.20% of total alkali content values and total fatty matter of 75.42% to 88.53%. The observed differences for soap properties were mainly due to the different nature of WCOs. Comparison was performed by evaluating these properties between WCO-based laundry soaps and commercial soaps purchased from the local market. The research concluded that the proposed method of synthesising laundry soaps from WCOs had similar physicochemical properties to that of commercial soaps and could be produced in small and even large scales[66].
In addition, Maotsela et al.[67] presented a study of the synthesis of toilet (bath) soap from waste cooking vegetable oils and beef tallow as an additive for the saponification reaction. Firstly, it was proposed that a brine solution should be used to purify WCO and the purified WCO would be bleached with hydrogen peroxide (H2O2) before mixing with beef tallow and coconut oil. Then, the presence of triglycerides in WCO and beef tallow underwent saponification with sodium hydroxide (NaOH) via hydrolysis to obtain the final product. It was reported that disparate triglycerides ratios and storing conditions would provide improvements in physical properties for cold process soap[67]. Kazuo[68] has invented a patented technology for the formation of soap or detergent from WCO by using alkanol amine as an amine derivation with surface-active agents such as salcosine salt[68]. A safe handling mild liquid soap was produced from the mentioned invention. Besides, the author stated that the peroxides could be eliminated from WCO via this invention, thus removing unpleasant odour of the soap[68].
Plastics
-
Plastic is a synthetic polymeric molecule which is made from petroleum and natural gas, exhibiting attractive features like good strength to weight ratio, heat seal ability, transparency and softness[69]. Petrochemical-based plastics including polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC) and polystyrene (PS) are widely used in daily life as they exhibit outstanding thermal and rheological properties, lightweight as well as inexpensive cost. However, synthetic polymeric materials are non-degradable which cause serious environmental problems to marine habitats and natural terrestrial over the years as they are mostly non-biodegradable and release toxic components during uncontrolled combustion[70,71]. Hence, there has been introduction of bioplastic which is made up of biomass and renewable sources such as agriculture waste, rice straw, cellulose and starch, even vegetable oil[71]. In this regard, vegetable oil or WCO has been examined in the synthesis of biopolymers via various approaches such as fermentation or direct conversion.
In the first place, Bayer et al.[72] performed research on the formation of bioplastics from direct transformation of edible vegetable waste via interaction between edible vegetable waste with trifluoroacetic acid (TFA) in extracting biopolymers from lignocellulosic biomass. Although the regeneration of cellulose from edible vegetable wastes and cereals could be conducted through chemical purification steps, extra time and cost are required to be utilized in industrial applications in the future. In this case, TFA was used as a special solvent in edible vegetable residues as cellulose could be co-solubilized with other contained organic matter when TFA was used to produce cellulose-based bioplastics. Therefore, the steps for cellulose regeneration were no longer required. It was also concluded that this type of bioplastic exhibited comparable mechanical properties to that of non-degrading plastic, and opened up new applications of cellulose in packaging and biomedicine, as well as designing new feedstock in biofuel production[72].
In fact, to date, various studies have proposed the use of WCO to synthesize various type of polyhydroxyalkanoates (PHAs), an important substance in the production of bioplastics. Tamang and Nogueira[73] utilized WCO in the two-step processes, enrichment of PHA-accumulating bacteria (first) and enrichment of PHA (second) by a mixed culture. However, the development of filamentous bacteria in the first step which caused foam formation and bulking in the sequencing batch reactor indicated the unsuitability of WCO as the substrate. As a result, the authors continuously fed the mixed culture with nonanoic acid as the second substrate and the results showed successive enrichment of PHA-accumulating bacteria which subsequently accumulated scl- and mcl-PHA (short-chain-length-PHA and medium-chainl-length-PHA). To boot, both of the synthesized PHAs exhibited the advantageous properties such as high melting point and low degree of crystallinity which are relatively attractive for soft bioplastic production. Likewise, Ruiz et al.[74,75] also presented their study regarding the conversion of WCO into mcl-PHAs by using different bacterial strains. These two studies achieved PHA productivity of 1.93 g mclPHA/L/h and 0.29 g mclPHA/L/h. Other than that, Kim et al.[76] also successfully converted WCO into sophorolipids by a fed-batch fermentation of Starmerella bombicola which would be further used to synthesize methyl hydroxy branched fatty acids (MHBFAs). Thereafter, MHBFAs were incorporated with polymer to formulate bioplastics in which the authors concluded that even with 1 mol% of MHBFAs was able to improve the mechanical properties of the formulated plastics. To put it briefly, the potential of WCO as a substrate for production of PHAs or other substances is vast and attractive due to its suitability to be applied in fermentation media as carbon source. The produced bioplastics, indeed, contribute to tremendous environmental benefits.
Plasticizers
-
Plasticisers which are the important agents for the synthesis of polyvinyl chloride (PVC) can also be made from WCO via sustainable approaches. In actuality, plasticisers are known as an auxiliary additive during PVC processing due to its ability in improving the flexibility, durability and workability of PVC plastic[77]. Over the years, approximately 5 million tons of plasticisers are consumed every year by which 75% of these plasticisers were made from fossil resources which commonly known as dioctyl phthalate (DOP)[78]. Over the years, due to the depletion of fossil oils, researchers have started switching their interest of study towards renewable sources such as agricultural by-products and wastes with the intention of bio-based plasticisers production[79]. For this reason, a short review was done by Jia et al.[80] in regards to the utilisation of biomass resources including vegetable oils in the synthesis of different types of bio-based plasticisers with their respective chemical structures and functions[80]. In addition, Greco et al.[81] presented their study by introducing the use of cardanol in the synthesis of bio-plasticiser for PVC formulations[81]. Generally, cardanol is an industrial grade oil that can be extracted from cashew nut shell liquid (CNSL) via vacuum distillation[82]. Nevertheless, to date, most of the proposed bio-based plasticizers are yet to be treated as an alternative to conventional plasticisers due to the limitations in commercialisation and poor comprehensive performance. For example, the use of cardanol derivatives in synthesizing bio-based plasticizers had contributed to poor compatibility and precipitation resistance[79]. As a result, currently, the exploration of other renewable sources is still significant so as to identify possible sources which fulfil the requirements of performance, sustainability and possibility in commercialisation.
In this sense, WCO has been proposed and utilized in the synthesis of bio-based plasticizers in order to examine the feasibility of WCO as a raw material in synthesising plasticizers. In the first place, Feng et al.[79] demonstrated a study on the synthesis of a bio-based plasticizer, namely acetylated-fatty acid methyl ester-citric acid ester, AC-FAME-CAE from WCO and citric acid via user-friendly epoxidation and ring opening reaction. Indeed, due to the presence of higher ester bonds formation during epoxidation, the proposed PVC plasticiser was confirmed to have higher molecular weight which could subsequently reduce volatility and extraction loss, thus resulting in an improvement on the compatibility and stability of PVC films. The glass transition temperature of PVC films was found to be decreasing when the content of AC-FAME-CAE increased. Also, the overall mechanical properties of the synthesised PVC films were actually better than those PVC films epoxidized by vegetable oil, and as good as those epoxidized by DOP owing to the formation of hydrogen bonds[79]. Likewise, Liu et al.[83] studied the use of WCO and trimellitic acid ester (TAE) in the formation of efficient plasticizer (AC-FAME-TAE). In the study, AC-FAME-TAE was confirmed to enhance the flexibility and thermal stability of the PVC films. To add on, PVC film plasticised by AC-FAME-TAE also showed significant improvement in the volatility and non-polar solvent resistance[83]. In reality, similar studies were also introduced by Feng et al.[84] and Liu et al.[85] on the synthesis of plasticizers from WCO and terephthalic acid, adipic acid and benzoic acid as well as malic acid ester. These studies successfully demonstrated the outstanding performance of the bio-based plasticizers produced from WCO[84,85]. In summary, WCO-based plasticizer is not only beneficial in terms of sustainability, abundancy, economic feasibility, but at the same time ensuring the comprehensive performance of the plasticizer.
-
Despite the fact that WCO represents a promising raw material with a broad spectrum of applications, there are still a number of challenges that need to be solved in order to attain a circular economy. Firstly, the management of supply chains, from raw materials to products, is inevitably crucial to industrial production from both environmental and economic perspectives. In fact, WCO collection remains the most challenging aspect other than the technical limitations during production. To date, most countries are yet to have appropriate collection schemes, and this would be more challenging as the WCO disposed from households are generally having very low recovery rates[86]. Hence, it is important for governments to establish policies and regulated practices in order to mature the recycling supply chain and make it more efficient and sustainable. Not only to increase the efficiency of collection schemes, but also to optimize the overall resource consumption including energy and financial aspects.
In addition, the large variability of WCO properties from different sources is also a challenge in terms of valorisation. The diversified nature of WCOs is mainly due to cooking and waste management practices. In short, the oil sources, food types, cooking temperature, processing time and fryer type will affect the oils or fats to suffer changes in their compositions. This has resulted in highly varying physicochemical properties, sensory characteristics and occurrence of impurities in WCOs[52]. Hence, pretreatment is a key step to enhance the standards of WCOs used in subsequent production or drop-in usages. As mentioned, there are several pretreatment techniques available to remove specific undesired components and to enhance the sensory properties. In this regard, specific pretreatment techniques should be chosen wisely to maximize the effectiveness and efficiency to produce desired results. The occurrence of trace impurities after pretreatment will restrict the use of WCOs as raw materials in some applications such as pharmaceutical, food and cosmetics industries[86]. Thus, it is promising to investigate more advanced technologies in WCO purification so as to expand the utilization in the sensitive products market. Also, intensified technologies can be developed to permit the use of waste lipid products discarded during the processes, such as trap grease, rancid oils etc.
Furthermore, another challenge faced by WCO-based oleochemicals is the market itself. People are more interested in biofuels such as biodiesel, compared to oleochemical products. To boot, most of the regulations and policies established for renewables are focused on energy and biofuels. For instance, Renewable Fuel Standard in the United States, Renewable Energy Directive (RED II) in the European Union, National Biofuels Policy in Brazil, National Biodiesel Mission and National Policy on Biofuels in India, National Biofuel Policy in Malaysia and such are all focused solely on biofuels[48,86,87]. Therefore, it is important for governments to enact policies, including both regulations and subsidies, in order to stimulate the utilization of WCO in oleochemical industries. In essence, WCOs as third-generation raw materials are readily available in large quantities at relatively low cost. It is auspicious to valorise WCO into various oleochemicals to develop a circular economy by establishing environmental and economic feasibility simultaneously.
-
Briefly, the accelerating consumption of cooking oil without proper management and regulations prompts environmental problems such as contamination of sea water and groundwater due to the direct disposal of WCO into water drainage. Hence, regulations related to the utilization of WCO-based products or fuels in certain sectors should be promulgated in each country in order to eventually actualize the ideal model of the current business world, which is a circular economy. Preliminarily, the collection procedures or system enforced by the local government must be relatively matured in order to resolve the current issue on low recovery rate of WCO which hardens the commercialization of WCO-based products. Indeed, the heterogenous characteristics of WCO is mainly due to the different oil origins as well as the extent of cooking process. Therefore, characterization of WCO prior to its valorisation is significant to thoroughly examine the suitability of WCO in a certain application. Generally, high FFA content in WCO which leads to higher viscosity can be pre-treated via neutralization (chemical approach) or distillation (physical approach) by transforming FFA into soaps or desired glycerides respectively. Removal of solid impurities and unwanted compounds such as sulphur and nitrogen-containing compounds are required prior to the valorisation of WCO. In essence, oleochemicals such as alkyd resin, green solvent and plastics as well as plasticizers are indeed possible to be produced using WCO as explained in this article. Each of the investigations have successfully proven that WCO is not only improving the current-existed products, but also reducing the dependency of the present-day industrial activities on petroleum sources. Last but not least, although the use of WCO in the production of biodiesel seems permissible nowadays, the exploration of other potential of WCO is still promising in the future so as to broadly utilize the overwhelm of WCO in different sectors.
This work is supported by Xiamen University Malaysia Research Fund (Grant number: XMUMRF/2021-C7/IENG/0033) and Hengyuan International Sdn. Bhd.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Foo WH, Koay SSN, Tang DYY, Chia WY, Chew KW, et al. 2022. Safety control of waste cooking oil: transforming hazard into multifarious products with available pre-treatment processes. Food Materials Research 2:1 doi: 10.48130/FMR-2022-0001
Safety control of waste cooking oil: transforming hazard into multifarious products with available pre-treatment processes
- Received: 18 November 2021
- Accepted: 23 December 2021
- Published online: 06 January 2022
Abstract: The increase in worldwide vegetable oil consumption has produced a large increase in hazardous waste cooking oil (WCO) production. The improper disposal of WCO has been a significant problem from both an environmental and economic perspective. Therefore, it is advantageous to transform WCOs into valuable products efficiently and effectively in order to contribute towards the establishment of a circular economy. In this review, the current state of WCO is discussed in relation to WCO production and valorisation. The valorisation rate of each country can be categorised into three groups related to the consumption of vegetable oil, production and valorisation of WCO, as well as the production, conversion and valorisation factors. Before any valorisation and processing of WCO can be carried out, pretreatments are crucially needed in refining and upgrading WCOs, specifically to reduce their free fatty acid (FFA) contents. This could help to produce refined WCOs with appropriate feedstock properties suitable for value-added applications in oleochemical industries. Hence, several pretreatment methods (e.g., solid impurities removal, FFA reduction, moisture removal) have been summarized and evaluated in depth. The transformation of WCO into valuable products including alkyd resin, green solvent, soap, plastics and plasticizer are also reviewed. Recent technological advances have made WCO feasible as sustainable feedstocks for oleochemical production, but not limited to biofuel production which in turn maximizes the value of this hazardous waste and turns WCO into a sustainable source.
-
Key words:
- Esterification /
- Free fatty acids /
- Valorisation /
- Alkyd resin /
- Green solvent /
- Soap