-
Neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD), belong to the chronic neurological disorders characterized by cognitive impairment and/or motor impairment, which pose a serious threat to human health[1]. Most neurodegenerative diseases are diagnosed in the elderly. AD is the most prevalent, and the number of older people suffering from AD is predicted to be approximately 58 million worldwide by 2050[2]. Therefore, the irreversible progression of neurodegenerative diseases threatens the health and lives of tens of millions of individuals globally, which imposes an immense social and financial burden. Despite the great efforts made to develop pharmacological drugs or treatments for the diseases, clinical trials have been less than satisfactory[3]. Studies have shown that the molecular basis of the devastating neurodegenerative diseases is related to changes in the native conformation of proteins, and subsequent accumulation of these misfolded amyloidogenic proteins in the central nervous system, resulting in progressive neurological impairment and neuronal dysfunction[2]. Amyloidosis refers to the aggregation and deposition of amyloid fibrils accompanied by the production of toxic oligomer intermediates and protofibrils en route[4]. Amyloidosis can appear at different sites of the body, for example, accumulation of amyloid fibrils have been observed in the brain, joints and pancreas[5]. Extensive evidence at different levels of genetics, pathology and biochemistry indicate that the accumulation of amyloid fibrils is associated with many severe neurodegenerative diseases[6].
The term 'amyloid' was originally used by the physician Rudolf Virchow to describe a visible tissue abnormality with a pale, waxy appearance that produces a positive result characteristic of starch-like materials in iodine staining reactions[7]. Eanes and Glenner made a landmark contribution to the study of human amyloid 50 years ago[4]. The study of amyloid over the past half century has indicated that numerous peptides (proteins) form amyloid fibrils under physiological or artificial conditions, some of which are pathological and some of which are functional[7]. Amyloid fibrils share a similar and typical conformation of a cross-β structure formed by cross networks of β-sheets running parallel to the fibril axis[6]. There are driving forces between β-sheets, such as van der Waals' force in the interface between the β-sheet layers, hydrogen bonds mainly from amide groups and hydrophobic interactions in the interior between β-sheets[8]. These structures make amyloid fibrils more stable and have higher mechanical strength via providing them with a rigid structure. In addition to these characteristics, amyloid fibrils have other properties, including high aspect ratio, which broaden the range of their potential applications[8]. At the same time, maybe due to the rigid structure of amyloid fibrils, that many of the related neurological diseases are difficult to treat. The formation mechanism of amyloid fibrils has attracted increasing attention in recent years.
Over the years, the amyloid cascade hypothesis has gained widespread traction and emerged as one of the most convincing causes for the pathogenesis of neurodegenerative diseases. Scientists have recognized a strong correlation between the neurodegenerative diseases and the presence of amyloid fibrils in the brain. For example, in AD, the amyloid fibrils are mainly composed of deposits formed by aggregated peptide fragments of various proteins, including the amyloid precursor protein (Aβ), tau and others[9]. The core of the amyloid cascade hypothesis is that it is the pathological accumulation of Aβ that acts as the root cause of AD and initiates its pathogenesis. The hypothesis could be recognized as a linear pathway that begins with Aβ formation and ends with dementia. In general, toxicity occurs during the formation and aggregation of amyloid fibrils, thus inducing neurodegenerative diseases. There are four hypotheses for toxicity during amyloid fibril formation and aggregation: (1) loss of protein functions after the conversion from native proteins to misfolded conformations, (2) disruption of cellular membranes, (3) disruption of mitochondrial function and generation of reactive oxygen species, (4) overload of the protein homeostasis regulatory network[10]. However, on the other hand, some studies have suggested that neurodegenerative diseases may not be caused by amyloid fibrils[4,11]. There was evidence that amyloid fibrils from different proteins were found in postmortem tissue of individuals who did not show any symptoms of amyloid disease before death[12]. Besides, therapeutic interventions that successfully cleared human amyloid plaques did not reverse or improve the clinical symptoms of AD[4,9].
Scientists are making efforts to develop drugs to treat neurodegenerative diseases with amyloids as the target, which can perform multiple tasks simultaneously, such as prevention of amyloid fibril formation and driving the disrupting of preformed fibrils[2]. However, no perfect drug or treatment for neurodegenerative diseases has been found so far. Recently, studies have suggested that a proper diet, especially polyphenol-rich foods, could have a preventative and alleviation effect on neurodegenerative diseases[7]. Polyphenols, as one of the dietary components, demonstrated the capability to upregulate the ubiquitin−proteasome system (UPS) to reduce protein aggregation and regulate gut microbiota, thereby reducing the development rate of neurodegenerative diseases[13]. Teresa et al. suggested that polyphenols could interact with other food components as a diet for neurological health benefits and revealed that polyphenols could regulate most of the molecular events involved in neurodegeneration including inflammation, redox status, proteostasis, synapsis homeostasis, glucose metabolism and blood-brain barrier[14]. Polyphenols have been found to inhibit the self-assembly of proteins related to neurodegenerative diseases. Dominik Szwajgier et al. also reviewed the inhibitory effect of phenolic acids on the formation of amyloid β-peptide (Aβ) fibrils and illustrated the beneficial effects of phenolic acids and their derivatives against Alzheimer's disease[15]. Different polyphenols inhibit amyloid formation by different mechanisms and they act on various assembly pathways. During the process of inhibition, polyphenols interact with different protein conformation status during amyloidosis, such as monomers, oligomers, or preformed fibrils. For example, epigallocatechin gallate (EGCG), the most abundant and bioactive polyphenol in green tea could redirect amyloid fibril formation to non-fibrillogenic oligomers or alter the properties of oligomers[16,17]. Therefore, polyphenols have the potential to become one of the ideal candidates in the treatment of amyloid-related diseases.
In this review, we firstly introduce the structure and related properties of polyphenols. After that the mechanisms of the interaction between proteins and polyphenols were discussed. We then briefly described the formation of amyloid fibrils and some amyloidosis or amyloid disease. We also discussed how polyphenols can potentially alter the structure and/or disturb the amyloid fibrils formation, hence, potentially playing a key role in the progression of protein misfolding disorders. In addition, we introduce the potential of polyphenols as therapeutic molecules in amyloidosis or amyloid disease.
-
Polyphenols widely distributed in plants, mainly vegetables and fruits, are natural ingredients in plant food. They usually protect plants against oxidative stress and ultraviolet radiation[18]. Therefore, polyphenols are often used commercially as natural antioxidants because of their functions on delaying lipid oxidation. In recent years, polyphenols have attracted increasing attention from many researchers due to their health benefits. In addition to their excellent antioxidant activity, polyphenols have many multifaceted functions, such as antiglycative activity, antimicrobial and anti-inflammatory properties[19]. Numerous studies have also demonstrated the favorable effects of polyphenols on preventing various diseases such as cancer, diabetes, obesity, neurodegenerative diseases, and others[20]. Polyphenols are naturally synthesized by the secondary metabolism in plants, characterized by one or more aromatic rings with two or more hydroxyl groups[18,19]. Polyphenols exist in free form or in combination with sugars, acids and other biomolecules. Currently, there are more than 8,000 naturally occurring polyphenols identified to date[21]. According to their structures, polyphenols can be divided into flavonoids and non-flavonoids. Flavonoids are the most common polyphenol in fruits and vegetables because they play an important role in plant color, flavor, synthesis of enzymes and vitamins, as well as in reducing lipid peroxidation[18,22]. Nevertheless, the content of flavonoids in plants depends on many factors, such as the plant species, growing environment, climate conditions and their maturity[23]. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which is composed of two aromatic rings (A and B) and a tetrahydropyran ring (C)[18,19]. This structure is usually abbreviated as C6-C3-C6. Because of the differences of the tetrahydropyran ring (C), flavonoids can be divided into six groups: flavones, flavonols, flavanones, flavanols, anthocyanins and isoflavones[18]. Non-flavonoids mainly include phenolic acid, tannic acid, and others[19]. Fig. 1 shows the chemical structures of several polyphenols belonging to the basic structures of flavonoids and non-flavonoids. Polyphenols have been utilized in many commercial applications, such as food dyes, bioactive packaging, curing agents and cosmetic products[18,24].

Figure 1.
The basic structures of several polyphenols including, (a) flavonoids and (b) non-flavonoids.
Among the many health benefits of polyphenols, their antioxidant properties are most frequently studied. Some studies have confirmed that polyphenols have strong antioxidant activities[25]. It is implied that the antioxidant properties of polyphenols are related to their chemical structure, especially the presence and site of the phenolic hydroxy groups[26]. In addition to their antioxidant properties, polyphenols also play important roles in anti-diabetes[27], anti-cancer[28,29], anti-cardiovascular disease[30], anti-inflammation[31], anti-hypertension[32] and anti-microbial[33]. In fact, most of these diseases are related to the imbalance between reactive oxygen species (ROS) and the body's antioxidant process[34]. Thus, these polyphenols properties against human diseases are mainly attributed to their significant antioxidant activity, such as scavenging of accumulated ROS[35]. However, low bioavailability limits the practical application of polyphenols. Bioavailability is a complex process that includes liberation, absorption, metabolism and other stages[36]. It usually refers to the success of ingested active compounds in reaching the systemic circulation and the specific sites where it can exert its biological action successfully[26]. Bioavailability is a key factor affecting the efficacy of polyphenols. Taking tea polyphenols as an example, its bioavailability is relatively poor, and its oral bioavailability is reported to be less than 5%[32]. It has been reported that after ingesting dietary polyphenols, 10% of polyphenols are absorbed in the small intestine and 90% of the total polyphenols ingested are converted into other metabolites by large intestine microbiota[13]. Large intestine microbiota can alter the biological properties of dietary polyphenols, such as bioavailability and their ability to reach the brain[13].
The blood-brain barrier (BBB) is a physical barrier composed of brain microvascular endothelial cells, basement membrane and astrocyte foot process, which plays an important role in transporting intravascular material into the brain and might inhibit the permeation of polyphenols to the brain[37]. It has been reported that some dietary polyphenols and their microbial metabolites can cross the BBB and influence the central nervous system (CNS) both directly and indirectly, thus achieving the purpose of protection or alleviation of neurodegenerative diseases[13]. The bioavailability of flavonoids in the brain may be affected by O-methylation and glucuronidation[17]. Besides, the uptake and efflux transporters of polyphenols (e.g. P-glycoprotein) play crucial roles in penetrating the BBB[13]. There are reports that naringin could not be detected in the rat brain in the absence of P-glycoprotein inhibitors, which indicates that the BBB penetration of naringin might be enhanced by P-glycoprotein inhibitor[38]. The transport of flavonoids across RBE-4 cells (an immortalized cell line of rat cerebral capillary endothelial cells) was evaluated[39]. All of the tested flavonoids (catechin, quercetin and cyanidin-3-glucoside) passed across the RBE-4 cells in a time-dependent manner, indicating that the tested flavonoids were capable of crossing the BBB. Chen et al. reported that the metabolites of flavan-3-ol and quercetin were detected in all regions of the brain in young swine model, suggesting that these polyphenols were able to cross the BBB[40]. Besides, Jersey blueberry anthocyanins were demonstrated to transit the BBB and were found in brain tissue 18 h after the last anthocyanin feeding to pigs[41]. Purple sweet potato color (PSPC), a class of naturally occurring anthocyanins, protected brain function against oxidative stress induced by D-galactose (D-gal)[42]. Fornasaro et al. found that after the intravenous administration, cyanidin 3-glucoside (C3G) could be detected in the brain of rats within 15 s[43]. Anthocyanins were found to inhibit activated astrocytes and neuroinflammation via suppression of various inflammatory markers including inducible nitric oxide synthase (iNOS) and tumor necrosis factor-alpha (TNF-α) in the hippocampus and cortex regions of D-gal-treated rats brain[44]. Several anthocyanins (cyanidin-3-O-β-galactoside, cyanidin-3-O-β-glucoside, cyanidin-3-O-β-arabinose, malvidin-3-O-β-galactoside, malvidin-3-O-β-glucoside, malvidin-3-O-β-arabinose, peonidin-3-O-β-arabinose and delphinidin-3-O-β-galactoside) were found in the cerebellum, cortex, hippocampus or striatum of the blueberry-fed rats, suggesting that polyphenolic compounds were able to go cross the blood brain barrier and localize in various brain regions important for learning and memory. In the brain, total anthocyanin content (blackberry anthocyanins and peonidin 3-O-glucoside) reached 0.25 ± 0.05 nmol/g of tissue in rats fed with a blackberry (Rubus fruticosus L.) anthocyanin-enriched diet for 15 d[45].
Here, the tea polyphenol EGCG is taken for an instance to illustrate the bioavailability of dairy polyphenols, which is shown in Fig. 2. Polyphenols first enter the body through the mouth. In the oral cavity, glycosides of flavonoids if applicable, especially glucose conjugates, are hydrolyzed by saliva to release aglycones, some of which have been reported to be hydrolyzed by β-glucosidase from oral bacteria and salivary epithelial cells, and then partially assimilated by oral epithelial cells[46]. EGCG could be catalyzed into epigallocatechin (EGC) by the catechin esterase in saliva, and both of the two compounds could be assimilated through oral mucosa[47]. Besides, it is commonly seen that polyphenols interact with salivary albumin and mucin, which could increase the solubility of polyphenols in saliva[48]. Polyphenols are frequently oxidized, degraded or converted in the gastrointestinal tract, leading to reduction in their bioactivity. In the stomach, polyphenols might be depleted as antioxidants and small amounts of polyphenols are assimilated as well[49]. Polyphenols are hydrolyzed to release aglycones for assimilation in the small intestine. Specifically, lactase-phlorizin hydrolase (LPH) is a β-glucosidase existing in the brushlike border membrane of the small intestinal epithelium that can hydrolyze a series of flavonol glycosides, flavone glutamate, flavanone glycosides, or isoflavone glycosides[50]. After that, the released aglycones pass through the small intestine epithelium mainly by passive diffusion. Some transporters are involved in the processes of aglycone transport, such as the monocarboxylic acid transporter (MCT), sodium/glucose cotransporter 1 (SGLT1)[26], multi-drug resistance protein 2 (MRP2) and P-glycoprotein[51]. After phenolic aglycones are assimilated by enterocyte, they may undergo further metabolism, such as glucuronidation, methylation, and sulphation, mainly in the small intestine and liver[52]. Most polyphenols are not absorbed in the upper digestive tract, and the unabsorbed polyphenols may enter into the colon where they are metabolized by enzymes secreted by intestinal microbiota[53]. EGCG was found to be hydrolyzed by intestinal microbiota to produce EGC and gallic acid (GA) in the large intestine. After that EGC could be further degraded into some ring-fission metabolites in the gut tract and 11 colonic microbial ring-fission metabolites of EGC were found, including 5-(3,5-dihydroxyphenyl)-γ-valerolactone (EGC-M5) and 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone (EGC-M7) that have been found as the main metabolites in mice, rat and human plasma, urine, and bile[54].

Figure 2.
The digestion, metabolism and absorption of polyphenols, taking green tea polyphenol EGCG as an example.
Polyphenols are also confirmed to have significant inhibitory effects on the formation of amyloid aggregates, which have been shown to either prevent toxic fibrillar oligomers during oligomerization or disrupt protein deposition and mature fibrils[55,56]. Ferreira et al. investigated the ability of curcumin and EGCG in inhibiting transthyretin (TTR) amyloid formation and found that EGCG and curcumin efficiently disaggregated pre-formed TTR amyloid fibrils[56]. Researchers revealed that the disruptive effects of tea catechins on lysozyme amyloid fibrils, which showed that tea catechins induced the conversion of lysozyme fibrils to amorphous aggregates and inhibited fibril-induced hemolysis[57]. Thapa et al. investigated the anti-amyloidogenic effects of mono-flavonoid and bi-flavonoid in Aβ1–42 fibrillogenesis and found that the bi-flavonoid could inhibit the Aβ1–42 fibrillogenesis more effectively than that of mono-flavonoid[58].
-
Proteins and polyphenols can interact with each other through non-covalent interaction or covalent bonding. The covalent interaction between polyphenols and protein could lead to the formation of protein-polyphenol conjugates. This can not only influence the functionalities and bioactive properties of proteins, but also change the gastrointestinal stability and biological activities of polyphenols.
Non-covalent interactions between proteins and polyphenols
-
Non-covalent interactions between proteins and polyphenols are usually reversible and weak, which often include hydrophobic interactions, hydrogen bonding and electrostatic interactions[59]. In general, hydrophobic interactions and hydrogen bonding are supposed to be mainly involved in the binding of proteins and polyphenols. Huang et al. studied the interaction between rutin and whey protein isolate using either a direct mixing or pH-driven approach[60]. The results indicated that the rutin-whey protein isolate complexes formed by the pH-driven method were more effective than those formed using the direct mixing method and the main driving force was ascribed to hydrophobic interaction. This might be due to the partial unfolding of the whey protein isolate molecules in the pH-driven method, leading to the whey protein isolate being more flexible with a higher surface hydrophobicity, meaning that they could bind more rutin molecules. The type of interactions between β-lactoglobulin and chlorogenic acid was also dominated by hydrogen bonding and hydrophobic interaction[61].
Hydrophobic interactions mainly occur between hydrophobic areas of proteins and aromatic rings of polyphenols. Hydrophobic areas of proteins mainly refer to some hydrophobic amino acids, such as leucine, isoleucine, glycine, methionine, alanine, phenylalanine, valine, tyrosine, cysteine, and tryptophan. For example, bovine lactoferrin could interact with A-type procyanidin dimer at Ala 42, Ile11, Val 57, Phe 190, Arg 296, Lys 301, and Arg 121 residues through hydrophobic interactions[62]. The benzene ring of the hydroxytyrosol formed three hydrophobic bonds with the hydrocarbyl side chains of the hydrophobic amino acids Ala 212, Leu 346, and Ala 349 of the bovine serum albumin, which maintains the three-dimensional structure of the hydroxytyrosol- bovine serum albumin complexes[63].
The phenolic groups contribute to the formation of hydrogen bonds with C=O groups of proteins as phenolic groups are excellent hydrogen donors[64]. Furthermore, hydroxyl groups in polyphenols also form potential hydrogen bonds with protein residues, especially hydroxyl (–OH) and amino (–NH2) groups of proteins[65]. Lv et al. found that hydroxytyrosol and bovine serum albumin formed two conventional hydrogen bonds (O···H−X), in which the hydroxyl oxygen of hydroxytyrosol and the amino hydrogen of Arg 208 formed a hydrogen bond. The hydroxyl hydrogen of hydroxytyrosol also formed a hydrogen bond with the carboxyl oxygen of the side chain of Glu 353[63]. There were five hydrogen bonds observed between the A-type procyanidin dimer and Phe 190, Glu 980, Arg 121, Glu 15, and Cys 9 of bovine lactoferrin[62].
Electrostatic interactions between proteins and polyphenols are not common in the protein-polyphenol complexes. Because most polyphenols are not charged at the pH of food, except for some phenolic acids[49]. Li et al. investigated the interactions between a milk protein (β-casein) and a number of phenolic acids: 3,4-dihy-droxybenzoic acid (DA); gallic acid (GA); syringic acid (SA); caffeic acid (CaA); ferulic acid (FA); and chloro-genic acid (ChA). They found that cinnamic acid derivatives (CaA, FA, and ChA) exhibited a stronger binding affinity with β-casein than benzoic acid derivatives (DA, GA, and SA). In addition, they also identified the driving force between β-casein and benzoic acid was the hydrophobic interaction while that between β-casein and cinnamic acid derivatives were electrostatic interactions[66]. Other interactions such as van der Waals forces may also play a secondary role in protein-polyphenol interactions, which usually occur together with other non-covalent bonds[67].
Covalent interactions between proteins and polyphenols
-
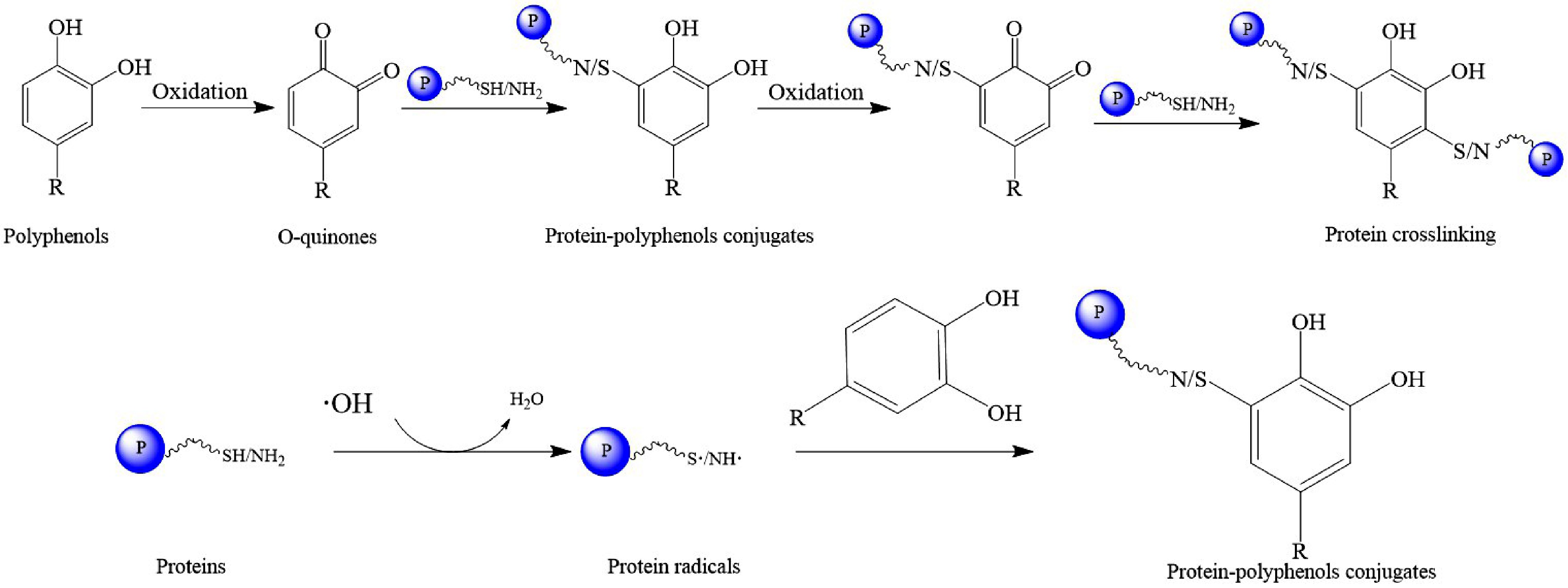
Covalent interactions between proteins and polyphenols are mainly caused by the formation of quinones. Polyphenols with a catechol ring are prone to undergo oxidation in different ways, such as metal ions[68], alkaline conditions[69], and enzyme catalysis[70]. Under these conditions, polyphenols are inclined to form quinones, which readily react with nucleophilic residues (thiol, amino, guanidine, or imidazole) in protein side chains or peptides (Fig. 3)[71]. Chlorogenic acid could react with the nucleophilic lysine amino (-NH2) or cysteinyl thiol group (-SH) of β-lactoglobulin to form a covalent compound via C–N or C–S bond[61]. Purified phenolic extract (PPE) prepared from Cinnamomum camphora seed kernel was oxidized to form the o-quinones under alkaline conditions and covalently bind to protein isolate (PI) from Cinnamomum camphora seed kernel. The results showed that thermal stability and antioxidant activities of PI-PPE conjugates were improved by the covalent modification of PPE[64]. Protein crosslinking may occur during covalent bonding of proteins to polyphenols. During this process, the phenolic group might be regenerated and then subsequently oxidized to form quinones which might react with a nucleophilic group on another protein, resulting in protein crosslinking[49]. Free radicals, such as hydroxyl radicals, may induce covalent interactions between proteins and polyphenols. Free radicals may attack proteins to form protein radicals which covalently bind to polyphenols at the ortho- or para-positions of their hydroxyl groups[72]. Li et al. successfully prepared four kinds of lactoferrin−EGCG (LF-EGCG) conjugates fabricated by enzymatic and nonenzymatic methods, including laccase-catalyzed oxidation, tyrosinase-catalyzed oxidation, free radical grafting and alkali treatment. The structures, functional properties, and allergenicities of LF-EGCG conjugates were investigated, which were compared with those of LF−EGCG physical complexes. The results showed that enzymatic cross-linking was more effective for promoting covalent attachment of EGCG to LF and reducing the allergenicity of the protein under the conditions used[70].
Factors influencing the interactions between proteins and polyphenols
-
The binding of proteins to polyphenols is usually a combination of non-covalent and covalent interactions. There are some factors influencing the interactions between proteins and polyphenols, such as pH, temperature, and type of proteins and polyphenols.
Protein-polyphenol conjugates can be formed over a wide range of pH (2.0–7.5)[73]. Different pH can change the conformation of proteins, the structure of polyphenols and the charges of proteins and polyphenols if applicable. At low pH (pH < 7), proteins would dissociate and expose more binding sites, thus binding to polyphenols via non-covalent interactions[74]. In alkaline environments (pH > 7), as previously mentioned, polyphenols are readily oxidized to quinones, which interact with proteins through covalent bonding in a non-enzymatic method[69].
Temperature is also one of the important parameters affecting the interaction between proteins and polyphenols. Generally, the temperature at which proteins interact with polyphenols is below 50 °C due to the thermal sensitivity of both protein and polyphenols[74]. Partial denaturation and conformational change of proteins can be induced by heating at an appropriate temperature, which caused more hydrophobic sites exposed to bind with polyphenols mainly via hydrophobic interaction between proteins and polyphenols[75]. In addition, polyphenols can be oxidized into quinones or quinone derivatives at higher temperature, which can interact with proteins through covalent bonding[76].
Moreover, the type and conformation of both proteins and polyphenols are the crucial factors affecting the interaction between proteins and polyphenols. Different proteins have different amino acid compositions, isoelectric points, and hydrophobicity, resulting in different ability to interact with polyphenols. It is reported that unfolded proteins have a higher affinity to polyphenols than compact and globular proteins due to the higher accessibility of amino acid residues of unfolded proteins[77]. Amino acid compositions, especially proline content, play a vital role in the interaction between proteins and polyphenols[65]. Some researchers have confirmed that α-casein had a lower affinity to polyphenols than β-casein while β-lactoglobulin had the lowest affinity to polyphenols among these three proteins because of the differences in proline contents and structures of proteins[78]. Zhang et al. found that the interactions between α-casein and cyanidin-3-O-glucoside were dominated by van der Waals forces and hydrogen bonding while that between β-lactoglobulin and cyanidin-3-O-glucoside was driven by electrostatic interaction[78]. Because of the differences in protein structure, Cong et al. reported that the strongest interaction appeared between grape seed procyanidins and fish protein, compared to chicken protein and pork protein[79]. Polyphenols with different structures and molecular weights can also affect the interaction with proteins. Polyphenols with larger molecular weights showed higher affinity to proteins due to more binding sites available for interaction compared to the polyphenols with smaller molecular weights[76]. Hydroxy groups on the polyphenols are the donors of hydrogen bonds, thus the number and location of the hydroxy groups have significant influence on the hydrogen bonding between proteins and polyphenols[49]. Besides, the hydroxylation, glycosylation, and methylation of polyphenols could also affect the interaction with proteins[66]. Ma et al. investigated the interaction mechanism of β-casein with five types of oligomeric proanthocyanidins, including B1, B2, B3, A2, and C1, using multi-spectroscopic and molecular docking methods[80]. The results showed that all five proanthocyanidins could interact with β-casein through hydrophobic interactions, van der Waals forces, and hydrogen bonding, and the corresponding binding ability was as follows procyanidins B1> B2> B3> C1> A2. Liu et al. successfully fabricated the conjugates of whey protein isolate (WPI) and each of the four dietary polyphenols (epigallocatechin gallate, quercetin, apigenin, and naringenin) through free-radical grafting. The binding capacities of the polyphenols to proteins were as follows: epigallocatechin gallate (EGCG) > quercetin (QC) > apigenin (AG) > naringenin (NG). Of the four conjugates, WPI-EGCG showed the strongest functional properties, followed by WPI-QC, and those of WPI-AG and WPI-NG were almost the same[72].
-
Protein folding is a complex process that can endow a protein the native structure to exert its specific biological functions. Under certain specific conditions, misfolding of proteins may occur, causing hydrophobic residues at the folded protein core to be exposed to the solvent. Then, the unfolded or at least partially unfolded proteins may self-assemble into a variety of aggregate structures, including the insoluble fibrillar one known as amyloids. Amyloid fibrils are self-assembled linear aggregates of proteins and there is extensive evidence from genetics, pathology and biochemistry that the accumulation of amyloid fibrils is associated with many neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases. Even though the proteins involved in amyloid fibrils differ in sequence and folding, the end products of their aggregation have spectacular structural similarities, including the cross-β fibrillar structure[4]. There is a common pathway to form amyloid fibrils for different proteins. Some classical theoretical folding models, such as the nucleation–propagation model and folding funnel model, have been proposed regarding protein folding and the credibility of the models has been verified by extensive experiments in order to better understand the complex protein folding process[81,82].
The process of amyloid fibril formation mainly occurs through three stages: lag phase (initially the protein misfolds), growth phase (the protein is rearranged to form the β-sheet) and saturation phase (the β-sheet matures to form stable fibrils or amyloid deposits)[83]. This process is usually called the nucleation–propagation (seeding-nucleation) model. In this model, the formation of amyloid aggregation is nucleation–dependent[7]. The soluble amyloid proteins or peptides are firstly partially unfolded, misfolded or disordered in nature to initiate protein aggregation. Then, several molecules must bind together to form a nucleus which is prepared for the growth of amyloid[7]. In this model, the soluble and small misfolded proteins are formed and act as seeds, recruiting more of the native proteins to be misfolded. The misfolded seeds are produced during the lag phase, which can nucleate the aggregation of the same protein (homologous seeding) or another amyloidogenic protein (heterologous seeding)[84]. After the formation of the first seed, it enters the growth phase where a rapid and exponential recruitment of soluble protein increases the population of misfolded units[84]. In the saturation phase, the stable fibrils or amyloid deposits are formed, during which the soluble misfolded proteins or monomers are almost exhausted. Simply, the formation of amyloid fibrils via the nucleation–propagation model involves the formation of dimers and small oligomers, then the growth of protofibrils and fibrils, through a complex mediated multistep-nucleated polymerization, form aggregates[16], shown in Fig. 4. The whole process is a transition from the soluble form to the insoluble form, which involves the formation of intermediates, for instance, oligomeric forms, Aβ derived diffusible ligands or amyloid beta derived diffusible ligands and protofibrillars[16]. Specifically, there are two distinct ways: one is reversible non-nucleation growth in which oligomers and semi-flexible fibrils are rapidly assembled, and ultimately amorphous aggregates are formed, the other way is nucleation-dependent, where rigid long-straight fibrils (amyloids) are formed through lag-phased nucleation and growth[85].
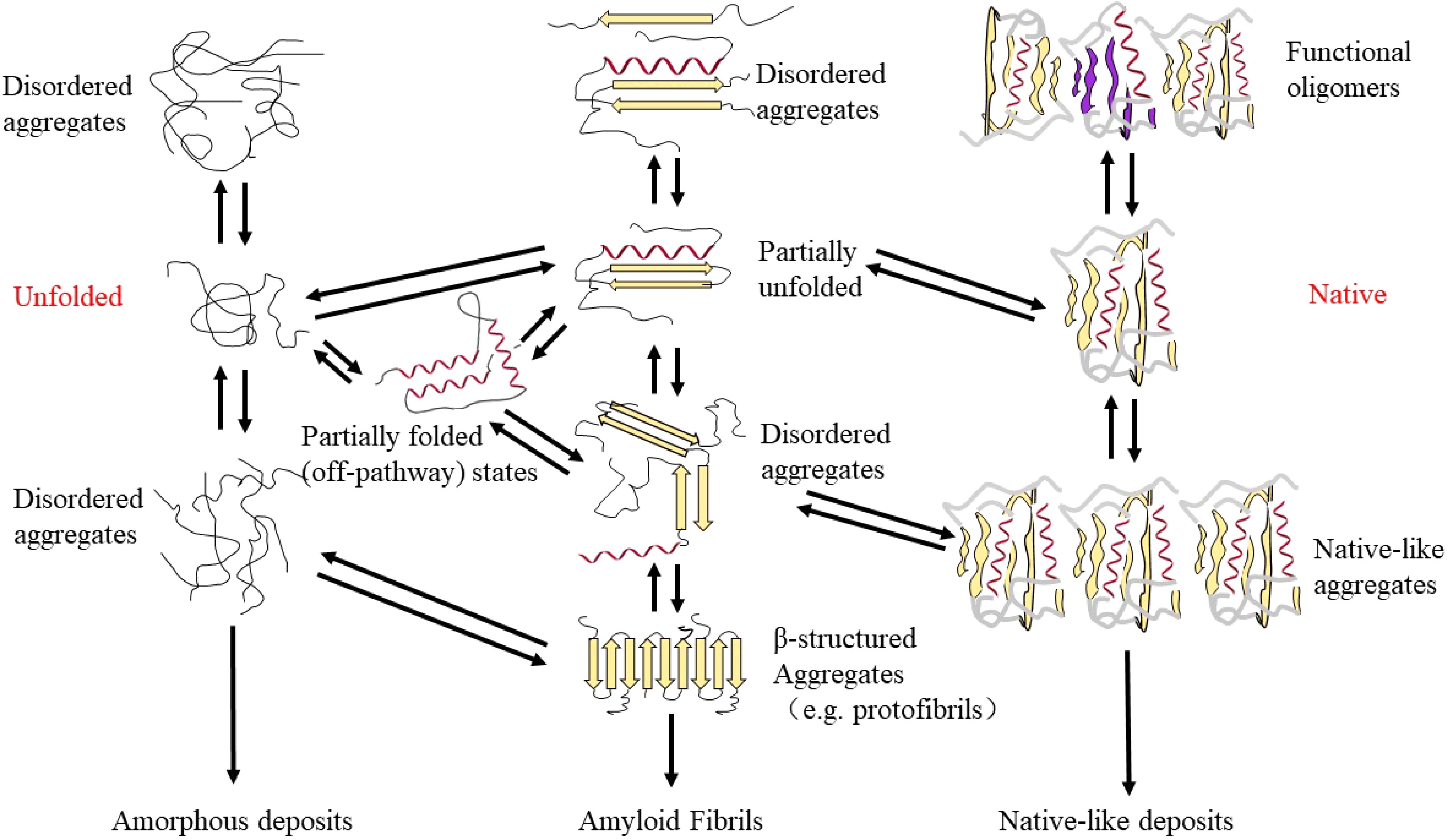
Figure 4.
The protein conformations possible in the on-way and off-way of protein aggregation to amyloid fibrils.
The folding funnel model describes the on-folding pathway of the protein folding process and represents the energy of all possible protein conformations. During protein folding, an unfolded polypeptide chain needs to pass through multiple folding intermediates to obtain a native folded state and the energy landscape toward thermodynamically favorable natural states is often rugged, meaning that molecules need to cross a large number of kinetic energy barriers[86]. The general protein folding energy landscape takes the shape of the picture shown in Fig. 5. It illustrates the folding funnel which is a simplified 2D picture. According to the energy landscape, it includes relatively stable structures of various states, such as unfolded state, folding intermediates, native state, amorphous aggregates, oligomers and amyloid fibrils. The broad top of the funnel represents a number of conformations about protein or polypeptide chains in an unfolded state while the narrow bottom of the funnel depicts the unique native structure of the protein. The separation of the top and bottom of the funnel is due to the contribution of other energies (solute enthalpy, solvent entropy, and enthalpy) to the protein conformation[4]. Generally, the conformation of proteins at the top of the funnel have a higher degree of entropy and free energy, during the folding process, the number of conformational states and free energy decrease in the direction of the minimum state occupying the lowest absolute energy, resulting in the conformational stability necessary for the folded state, which usually refers to the conformation of natively folded proteins in the case of on-folding pathway[86]. However, amyloid fibrils are assumed to be the most stable thermodynamic state in the energy landscape when it comes to the off-folding pathway. During the process of protein folding, the competition between intermolecular and intramolecular interactions plays a vital role for the reason that both the on-folding pathway and off-folding pathway vie with each other[87]. In order to prevent the off-folding pathway route, chaperone molecules have been studied to diminish interactions among different molecules prior to the completion of the folding process[88]. On the one hand, chaperone molecules can help the polypeptide chains fold into the native state by lowering the energy barriers separating folding intermediates in the intramolecular contacts (the on-pathway region of the energy landscape), while on the other hand, chaperone molecules can prevent abnormal intermolecular interactions between misfolded molecules and thereby promoting the on-pathway of the protein folding process when it comes to the off-pathway[86,89].
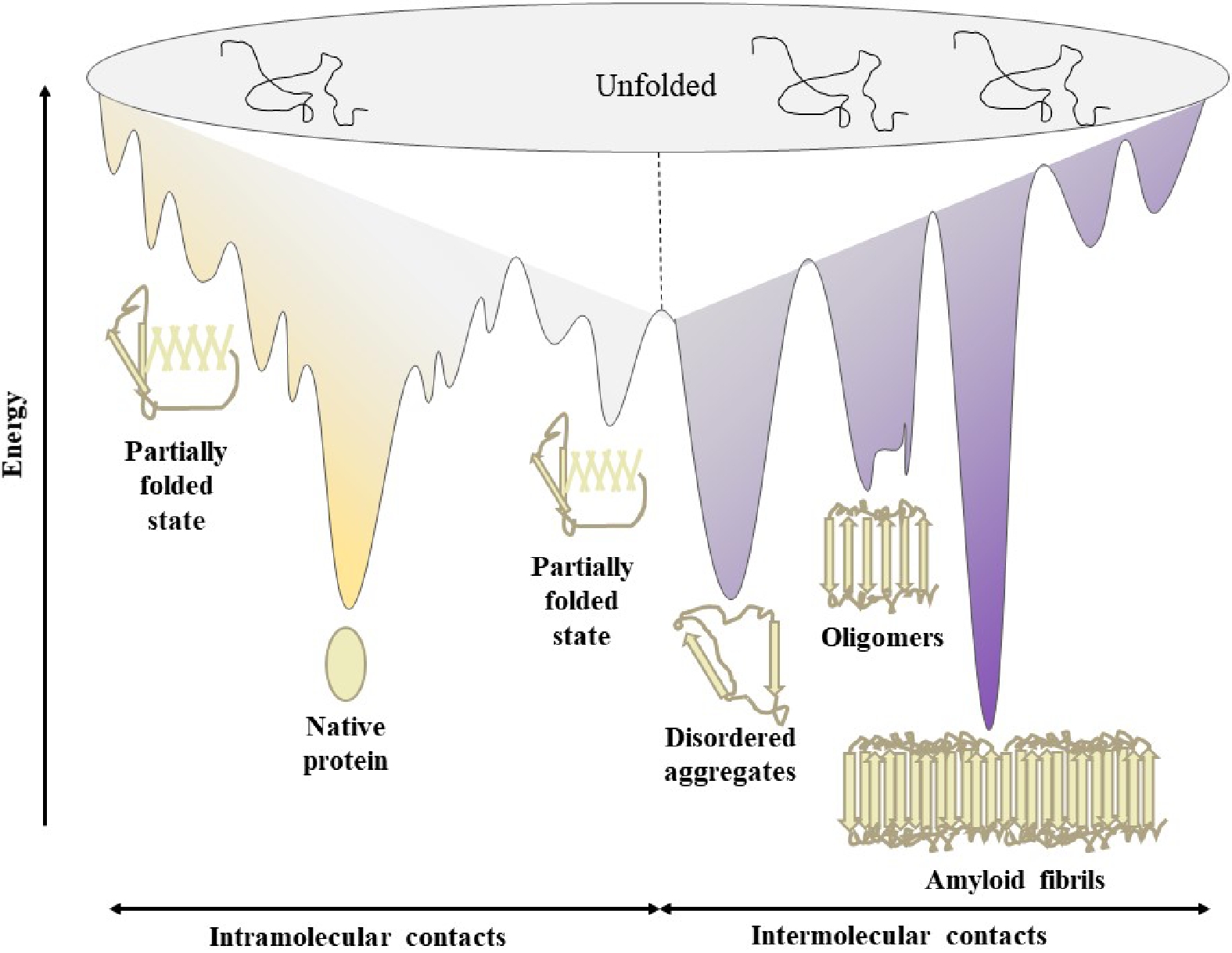
Figure 5.
The energy landscape of general protein folding and aggregation (redrawn from the previous literature)[86].
Amyloidosis or amyloid disease
-
Protein misfolding disorders (PMDs) or proteinopathies are a general term for degenerative diseases affecting the central nervous system or peripheral organs which are characterized by protein misfolding and aggregation into fibrillar deposits or amyloid fibrils[90]. PMDs often occur in conditions when at least one protein or peptide misfolds, aggregates and accumulates in damaged tissues[83]. The pathogenesis of several neurodegenerative disorders is associated with this, including AD, PD, HD, and type 2 diabetes (T2D). Different diseases are the accumulation of different protein amyloid fibrils, such as amyloid β (Aβ) and tau proteins aggregates in AD, α-synuclein in PD, poly-Q expanded the protein huntingtin (htt) in HD and the islet amyloid polypeptide (IAPP or amylin) in T2D[83,91,92].
AD is the most common type of neurodegenerative disease with the characterization of memory and cognitive functions lost occurring in the elderly. The pathogenesis of AD is related to two principal features: the abnormal aggregations of amyloid β (Aβ), forming amyloid plaque and hyperphosphorylation of tau protein, forming neurofibrillary tangles[16,93]. Aβ is produced by amyloidogenic metabolism of amyloid precursor protein, by the sequential action of β- and γ-secretases, leading to the liberation of peptide between 39 and 42 amino-acid residues[16]. Aβ40 and Aβ42 undergo conformational changes to form β-sheets resulting in increased aggregation[93]. Tau protein is a soluble micro-tubule protein in neuronal cells that plays a dominant role in axonal growth and neuronal development by stabilizing the micro-tubular assembly[94]. Upregulation of kinases and downregulation of phosphatases lead to hyperphosphorylation of tau protein, resulting in double-helical insoluble filaments and tangled clumps of neurofibrillary tangles under pathological conditions[95].
PD is also one of the most common neurodegenerative diseases in the world which has different symptoms, including static tremor, muscular rigidity, and bradykinesia[96]. The progressive degeneration of nerve cells in the substantia nigra is the cause of PD[97]. Pathologically, it is characterized by the progressive loss of dopaminergic neurons from the substantia nigra region of the brain. In addition, the presence of insoluble protein aggregates called Lewy bodies and Lewy neurites is also characteristic of PD[98]. The presence of Lewy bodies and Lewy neurites is due to abnormal protein folding and endoplasmic reticulum stress. α-Synuclein is a major fibrillar component of Lewy bodies and Lewy neurites[99]. Under physiological conditions, α-synuclein is soluble while it undergoes abnormal conformation and becomes insoluble, leading to toxic aggregates under pathological conditions[100].
Diabetes mellitus is one of the major diseases threatening human health worldwide, of which type 2 diabetes (T2D) is the main type. The main pathophysiologic driven factors of T2D are insulin resistance (IR) and pancreatic β-cell dysfunction, of which IR is proposed to be the primary driver and β-cell dysfunction a later manifestation[83,84]. There are many factors leading to IR, for instance, a defect in insulin signalling and a defect in glucose transporters or lipotoxicity[83]. Dysfunction of β-cells caused by the accumulation of islet amyloid polypeptide (IAPP) fibrils or amylin in the pancreatic islet's cells, resulting in increased oxidative stress and membrane permeability. Both of these features are critical to the pathogenesis of T2D[101]. Besides, increased fatty acids within the pancreas or limited incretin action also leads to β-cell dysfunction[83].
-
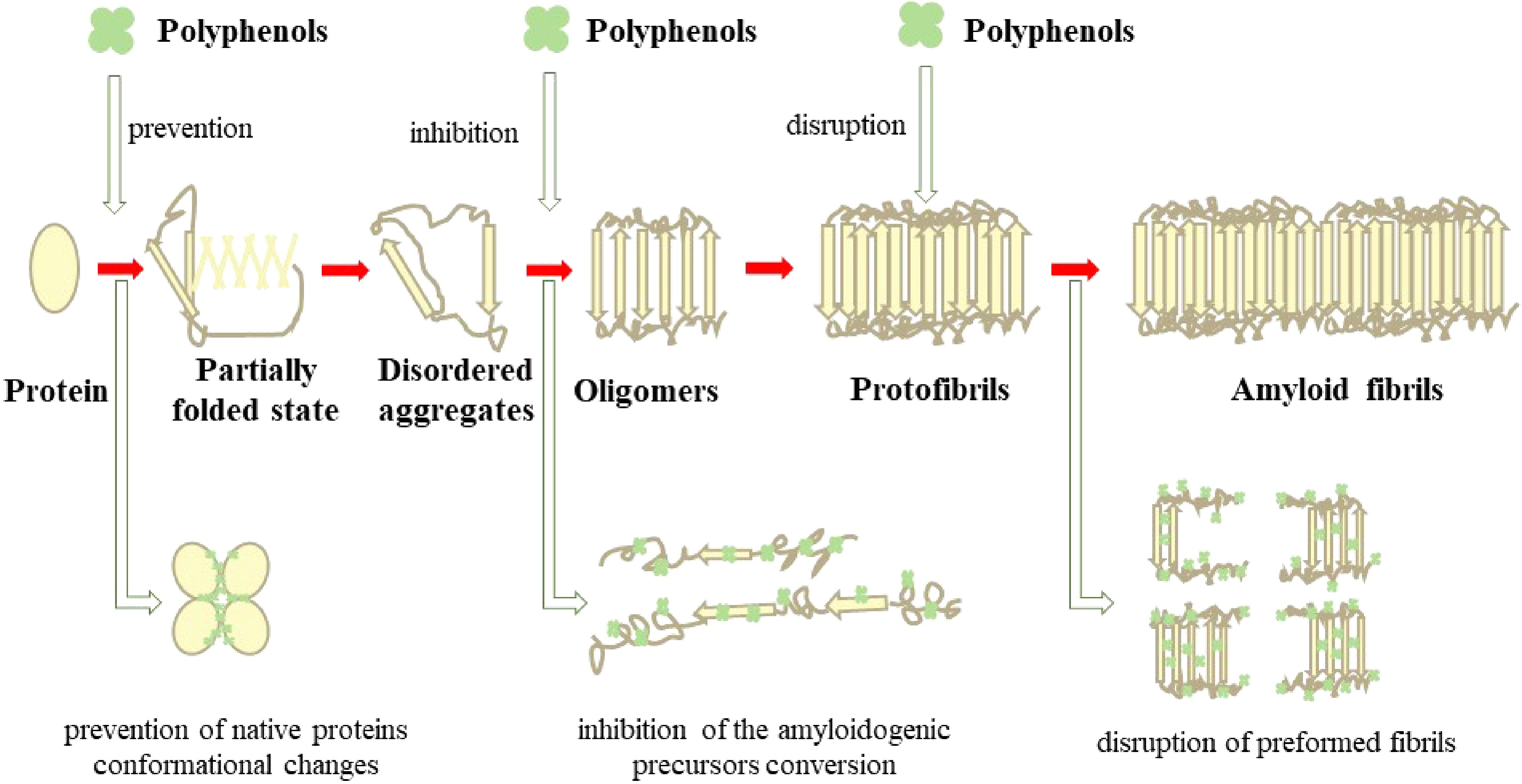
Some studies (Supplemental Table S1) have shown that polyphenols could interact with self-assembling proteins to inhibit the formation of amyloid fibrils in three major ways, as shown in Fig. 6: (1) prevention of conformational changes of precursor native proteins into amyloidogenic forms[17], (2) inhibition of the conversion of amyloidogenic precursors to amyloid fibrils[92], or (3) disruption of preformed fibrils with destruction of the aggregation conditions[102]. Different types of polyphenols have been demonstrated to interact with different protein conformations in the process of amyloid formation, such as oligomeric or fibrillary forms, which could account for the inhibition mechanism of amyloid fibrils (Supplemental Table S1). Some polyphenols inhibit the formation of oligomers to prevent the formation of amyloid fibrils while some polyphenols inhibit the formation of protofibrils and fibrils, not oligomers. Furthermore, some polyphenols inhibit not only the formation of oligomers, but also protofibrils and fibrils. For example, EGCG could redirect amyloid fibril formation from fibrillogenic forms to non-fibrillogenic oligomers[16] or change the property of oligomers upon interaction[17]. Overall, specific interaction between the molecular structure of polyphenols and amyloidogenic protein is an effective mechanism for inhibiting fibrillation. As is well known, the characteristics of polyphenols are the presence of aromatic rings and one or more phenolic rings[19]. When polyphenols bind with amyloidogenic proteins, aromatic rings of polyphenols could form non-covalent interactions with the fibril core where hydrophobic residues are dominant, and as a result, interfere with the fibril growth[85]. In addition, the hydroxyl groups on the phenol rings of polyphenol could form hydrogen bonds with amyloidogenic proteins, thus suppressing the self-assembly process conducting to amyloid fibril formation[16]. According to some studies, the mechanism of polyphenols inhibiting amyloid fibers is mainly to prevent the π–π interaction disturbing π-stacking between protein units within the β-structure and block the self-assembly[16], reduce the hydrophobic effect which is a major driving force that stabilizes the fibril structure, and change the conformation[2].
Polyphenols as therapeutic molecules in amyloidosis or amyloid disease
-
Recently, the role of polyphenols is receiving particular and extensive interest worldwide, given that they have been shown to interfere with the amyloid assembly pathway to inhibit the formation of amyloid fibrils or aggregates. Most polyphenols decrease amyloids, including tau, α-synuclein deposits, by inhibition of their formation or by disaggregation of them[83]. The attractiveness of polyphenols is due to them being natural biomolecules extracted from food or plants, and thereby they have many advantages, including high availability, bioactivity, and low side effects[19]. The antioxidant property of polyphenols endows their ability to inhibit the oxidation of hydrophobic residues in the peptide sequence[92]. Although polyphenols have limited absorption and low bioavailability in the brain, they are able to participate in neurogenesis and neuronal regeneration[92]. Most polyphenols showed permeability through the brain blood barrier (BBB), for instance, EGCG, daidzein, genistein and nobiletin with high BBB permeability. Whereas apigenin, luteolin, quercetin, and kaempferol showed medium permeability[103]. Thus, polyphenols could be considered neuroprotective and able to potentiate cognitive function, which is also likely due to their antioxidant properties[104]. Polyphenols could modulate amyloid precursor protein pathways to treat neurodegenerative diseases, such as activating α-secretase and inhibiting β-secretase or γ-secretase[2]. These secretases can catabolize amyloid precursor proteins to form non-amyloidal and/or amyloid-derived products. Polyphenols have been proven to increase neurogenesis and microvasculature in animals, reduce astrocyte hypertrophy, inhibit hippocampal microglial activation, and ultimately improve learning, memory, and emotional function to prevent neurological diseases[37]. When people ingest dietary polyphenols, polyphenols can reach the brain parenchyma through the BBB at a very low concentration and may induce neurite outgrowth, and the metabolites of polyphenols can also promote neurite outgrowth after the disappearance of polyphenols, thus preventing cognitive dysfunction and neurological diseases[54]. In addition, polyphenols and their metabolites which reached the brain may reduce oxidative damage due to their ability to suppress lipid peroxidation[37].
Epigallocatechin-3-gallate (EGCG) is the principal polyphenol constituent in green tea polyphenols, which has been shown to have many functional effects on human health, such as antioxidant[105], antimicrobial[106], anti-tumor, anti-viral, anti-inflammatory, anti-arteriosclerosis, anti-thrombosis and anti-angiogenesis activities[28,107]. It has been reported that EGCG is a potent inhibitor of amyloid formation and has promising effects on the reduction of aggregation and toxicity of a large number of proteins that are involved in PMDs[92,108]. EGCG was reported to inhibit the formation of amyloid fibrils by changing the oligomer's property through hydrophobic interaction[17], which might be due to the high affinity to oligomeric aggregation intermediates and a lower affinity to the monomeric peptide[109]. Some studies have also suggested that the amyloid fibrils remodeled by EGCG in vitro depends on the autooxidation of EGCG[110]. EGCG was proven to be the only active constituent inhibiting insulin amyloid aggregation among (−)-epigallocatechin gallate, (−)-epicatechin, gallic acid, and caffeine, the four compounds naturally present in green tea[111]. Researchers have studied the effects of the four compounds and their equimolar mixtures on the inhibition of the formation of insulin amyloid fibrils. The results showed that the fibrillization process of insulin amyloid fibrils was inhibited only in the presence of EGCG, either individually tested compounds or their equimolar mixtures. Zeng et al. also compared the effects of EGCG and oxidized EGCG on the inhibition of lysozyme fibrillization process. The results indicated that the oxidized EGCG had a stronger disruptive effect on preformed fibrils than the native form because EGCG was converted to quinones or quinonoid compounds by means of the autoxidation pathway, which covalently bound to the polypeptide chains, finally leading to the inhibition of amyloid growth[112]. Studies have showed that EGCG could inhibit the aggregation of various amyloidogenic proteins, for instance, Aβ, α-synuclein, insulin, prion protein, and lysozyme[16]. EGCG binds to the native monomers to prevent their conversion into stable, β-sheet-rich structures, which are a prerequisite for nucleation-dependent amyloid fibril assembly, thus interfering with the early stages in the amyloid formation pathway[83]. EGCG can also modulate the formation of toxic oligomers by binding and stabilizing species of α-synuclein and Aβ, reducing fibrillation and redirecting the aggregation pathway to form off-pathway, amorphous non-toxic aggregates, interdicting seeding and further conformational changes that may result in aggregation and cytotoxicity[104]. Besides, EGCG interacts with tau in a different way that binds with partially misfolded intermediates, preventing seeding and rescuing cells from tau-induced toxicity[113]. Kan et al. investigated the role of amyloid precursor protein cleavage, glial cell activation, neuroinflammation, and synaptic alterations in the protective effects of green tea against the amyloid β (Aβ) accumulation and cognitive impairment[114]. They treated 5XFAD mice with green tea extract (GTE) for 8 or 16 weeks. Their results demonstrated that spatial learning and memory ability were markedly improved by GTE treatment while GTE significantly alleviated the formation of Aβ. Their findings indicated GTE suppressed Aβ levels and alleviated cognitive impairment in 5XFAD mice, suggesting a beneficial effect of green tea on AD.
Curcumin is a symmetric molecule with chemical formula C21H20O6, which is the polyphenolic extract from turmeric, a spice derived from the Curcuma longa plant[115]. Due to its distinct structure and the ability to bind with other biomolecules through noncovalent interactions, curcumin has strong pharmacological advantages in antioxidant, anti-inflammatory, anticancer, anti-diabetes, cardioprotective and neuroprotective effects[116]. Curcumin has been shown to inhibit the formation of fibrils and oligomers, amyloid aggregation of various amyloidogenic proteins, such as Aβ, α-synuclein, prion protein, lysozyme and transthyretin (TTR), thereby curcumin has been extensively investigated in vitro and in vivo over the last decade[116,117]. The mechanism of curcumin inhibiting the formation of amyloid fibrils in different conditions is different, which can be divided into the following three types: (1) inhibiting the formation of oligomers while not protofibrils and fibrils, (2) inhibiting the formation of both oligomers and fibrils, and (3) inhibiting the formation of protofibrils and fibrils while not oligomers[115]. In general, curcumin affects not only the process of amyloid primary nucleation, but also the elongation of amyloid fibrils. In simple terms, curcumin could bind to amyloidogenic proteins tightly through hydrogen bonds and hydrophobic interactions, leading to the amyloid structure less flexible and ordered[116]. Moreover, curcumin could not only interact with the hexamer model of amyloid fibrils to effectively prevent the elongation of amyloid fibrils, but also decrease the number of β-strands, thereby inhibiting the formation of amyloid fibrils[116,118]. There was a study on the effect of curcumin on the formation of amyloid fibrils in the well-characterized model protein human lysozyme (HuL). The results revealed that curcumin played an important role in inhibiting the formation of HuL amyloid fibrils and disaggregated performed fibrils of HuL through interacting with the prefibrillar and fibrillar intermediates[119]. In addition to curcumin, curcumin derivatives also have an inhibitory effect on amyloid fibrils. Cui et al. investigated the inhibitory effect of two novel curcumin derivatives on the formation of hens egg white lysozyme (HEWL) amyloid fibrils. In the study, the water solubility of two novel Boc-L-isoleucine-functionalized curcumin derivatives is higher than that of the natural curcumin. Their results suggested the monosubstituted curcumin derivatives showed stronger inhibitory effect on the formation of HEWL amyloid fibrils than disubstituted curcumin derivatives. The inhibitory effect of the two curcumin derivatives on the formation of HEWL amyloid fibrils might be attributed to the interactions between the curcumin derivatives and HEWL to form the new ground state complexes before HEWL self-assembles into amyloid fibrils[120]. Curcumin efficiently destabilizes preformed α-synuclein and avoids fibril formation in a dose-dependent way[92,117]. In vitro studies suggested that curcumin could diminish aggregate formation and protect cells against amyloid-induced toxicity in the case of IAPP[121]. This might be attributed to curcumin retarding IAPP aggregations by modifying its conformation[118]. However, curcumin is protective against exogenous IAPP cytotoxicity within a narrow concentration range (10 – 25 µM); it is cytotoxic when the concentration increased above 25 µM[121]. Therefore, curcumin is limited in clinical treatment of PMDs.
-
Nowadays, PMDs prevalence continues to rise as a result of over-nutrition and lifestyle changes. Misfolding and aggregation of amyloidogenic proteins are the main reasons for the pathogenesis of a large group of diverse diseases. However, there is not an efficient therapy for curing or slowing the progression of the majority of PMDs. A large body of evidence indicate that polyphenols could play an important role in the inhibition of amyloidogenesis or PMDs. Accordingly, polyphenols are emerging as new active agents for slowing down the progression and preventing PMDs. The superior properties of polyphenols make them ideal candidates for treating PMDs, including antioxidant and anti-inflammatory effects. Some polyphenols could interact directly in the process of protein misfolding and aggregation, while others could prevent the toxicity of the accumulation of misfolded proteins. However, it is regrettable that despite numerous studies in vivo and in vitro, polyphenols have not yet been used for the clinical treatment of PMDs. This may be caused in part by polyphenols' poor metabolic stability and low bioavailability at the needed pharmacological concentrations. Especially, more efforts should be made to investigate the transportation of polyphenols through the blood brain barrier. In addition, after digestion, absorption, and metabolism, whether the interaction between the polyphenol metabolites and proteins is still the same as the in vitro interaction mechanism deserves further investigation. Therefore, more research is needed in the future to take full advantage of the prospects opened up by the use of polyphenols for the treatment and prevention of PMD.
This work was funded by the National Natural Science Foundation of China (No. 31901760, No. 31871843), the Natural Science Foundation of Jiangsu Province — Distinguished Youth Foundation (BK20200022), the Natural Science Foundation of Jiangsu Province — Youth Foundation (BK20190530), Jiangsu Agricultural Science and Technology Innovation Fund (CX(21)3040), Laboratory of Lingnan Modern Agriculture Project (NZ2021034), the Fundamental Research Funds for the Central Universities, China (KJJQ202102, KYDZ201903), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors thank X. Q. He at Nanjing Agricultural University for assistance in preparation of the graphics.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Inhibition of Amyloids by Polyphenols.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ruan C, Kong J, He X, Hu B, Zeng X. 2022. Interaction between polyphenols and amyloids: from the view of prevention of protein misfolding disorders related diseases. Food Materials Research 2:2 doi: 10.48130/FMR-2022-0002
Interaction between polyphenols and amyloids: from the view of prevention of protein misfolding disorders related diseases
- Received: 25 November 2021
- Accepted: 30 December 2021
- Published online: 14 January 2022
Abstract: Amyloid is the term usually used to describe a particular type of elongated, unbranched protein fibril with cross-β-sheet characteristics, formed through the ordered aggregation of peptides or denatured proteins. Amyloids show high association with many severe neurodegenerative diseases, such as Alzheimer's, Parkinson's, and Huntington's diseases. Unfortunately, there are still no effective medical treatments to cure these neurodegenerative diseases, including the failure of pharmaceutical approaches with amyloids as the targets. Polyphenols, the major phytochemicals in fruits, vegetables, tea and coffee have many beneficial functional properties, such as antioxidant, antimicrobial and anticancer properties. Polyphenols can interact with proteins through non-covalent interactions, including hydrophobic interaction, hydrogen bonding and electrostatic interaction. Polyphenols can also covalently interact with proteins mainly via the interaction between quinones, the oxidized products of polyphenols, and the nucleophilic residues (thiol, amino, guanidine, or imidazole) in protein side chains or peptides. Evidence from previous studies have indicated that polyphenols could reduce amyloid-formation via inhibiting fibril aggregation or steering oligomer formation into unstructured, nontoxic pathways. This effect was realized mainly through: (1) preventing conformational changes of precursor native proteins into amyloidogenic forms, (2) inhibiting the conversion of amyloidogenic precursors to amyloid fibrils, or (3) disrupting preformed fibrils meanwhile destructing the aggregation conditions. These findings imply that polyphenols could play a vital role in inhibiting protein misfolding, which might be useful for the prevention of various neurodegenerative diseases.
-
Key words:
- Polyphenols /
- Proteins /
- Amyloid /
- Interaction /
- Protein misfolding disorders