-
H2S, a toxic gas with a rotten egg odor, has recently warranted inclusion as a messenger molecule. H2S diffuses readily through membranes as small lipophilic molecules, presenting a membrane receptor-independent signaling mechanism[1]. H2S signaling in mammalian systems occurs through reactions with metals and oxidants as well as protein persulfidation, which either activates or inhibits enzyme activities directly, or by forming protein adducts[2]. Moreover, H2S in plants induces defense systems against biotic and abiotic stress from seed germination to fruit ripening[3], protein persulfidation in postharvest has also obtained keen interest. After fruits and vegetables are picked, they lose their nutritional supply and gradually senescence during the storage process, so preservation has always been a relevant issue. Recent reports on H2S application show great potential for delaying senescence in cut flowers, vegetables, climacteric and non-climacteric fruit under shelf and low temperature conditions, including enhancing the antioxidant system, mitigating chilling injury (CI) symptoms, inhibiting fungal growth, regulating senescence-related genes and interacting with other molecules[4−6]. Nevertheless, the mechanisms of H2S on postharvest physiological metabolism require further research. The residual levels of H2S after exogenous treatment are lower than human plasma levels[6], and there is usually no disagreeable smell in the preparation of plant extracts[3]. Consequently, a stimulate concentration of H2S may have safe applications in postharvest storage and transportation. Intricate interactions between H2S and other secondary messengers like Ca2+, NO, abscisic acid (ABA) and hydrogen peroxide (H2O2) form a cross-adaptation signaling network[7]. The function of H2S to activate or inhibit target proteins via persulfidation deserves more attention, while possibly contending with other PTMs for the same residue positions, especially NO-mediated S-nitrosation and nitration[3,8]. Although there have been many reports, the effects of H2S on postharvest physiology of fruits and vegetables still need comprehensive evaluation. This review focuses on the intrinsic mechanisms of H2S signaling on senescence, CI and disease resistance, and also highlights the current progress of persulfidation with other modifications.
-
Transsulfuration is the major pathway for plants to produce endogenous H2S. Wilson et al.[9] first discovered that the emission rate of H2S in higher plants was light-dependent. It seemed that H2S was a crucial part of sulfur metabolism and helped plants to remove excessive inorganic sulfur anions[9]. Since then, researchers have discovered that there are at least five pathways to generate endogenous H2S in plants (Fig. 1). The five known pathways are as follows: (1) Plants convert the combined sulfate to the free state that is activated to form adenosine 50-phosphosulfate (APS) in the plastids. Then APS is reduced to H2S by APS reductase (APR) and sulfite reductase (SIR), with reduced glutathione (GSH) as the electron donor[10]. (2) Cysteine desulfhydrase is the main pathway to generate endogenous H2S, including L-cysteine desulfhydrase (L-DES), D-cysteine desulfhydrase (D-DES) and L-cysteine desulfhydrase 1 (DES1). L-DES and D-DES are not directly related, they have different substrates and subcellular localization[5,11]. (3) O-acetylserine thiol lyase (OAS-TL), also known as cysteine synthase (CS), catalyzes sulfide into O-acetylserine (OAS) to form cysteine, its reversible reactions can release H2S[12]. (4) Pyridoxal 5′-phosphate-dependent L-DES catalyzes L-cysteine to generate elemental sulfur, which is further reduced to H2S[13]. Two candidate genes AtNFS1 and AtNFS2 have been identified in Arabidopsis thaliana, encoding NifS-like plastidial cysteine desulphurase, which are addressed to plastids and chloroplasts, respectively[14]. (5) β-cyanoalanine synthase (β-CAS) converts L-cysteine into H2S through the detoxification of cyanide[15]. NaHS, a commonly used exogenous H2S donor, gives a rapid burst of H2S gas in a water solution and keeps a constant concentration, but actual physiological H2S concentration triggering the defense response is yet unclear[7].
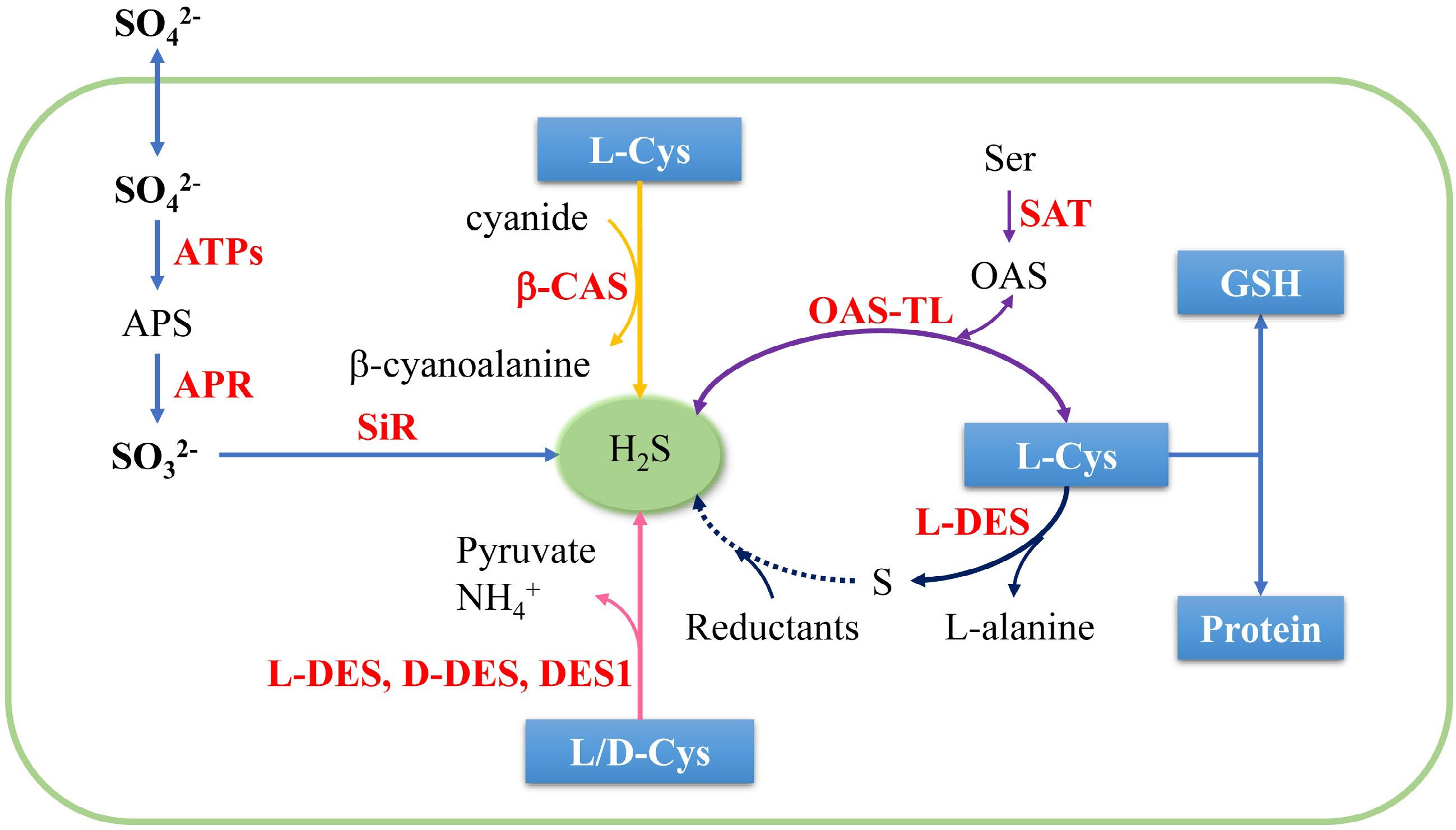
Figure 1.
Endogenous H2S biosynthesis in higher plants. SO42−: sulfate ion; SO32−: sulfite ion; ATPs: ATP sulfurylase; APS: adenosine 50-phosphosulfate; APR: APS reductase; SiR: sulfite reductase; β-CAS: β-cyanoalanine synthase; L-Cys: L-cysteine; D-Cys: D-cysteine; L-DES: L-cysteine desulfhydrase; D-DES: D-cysteine desulfhydrase; DES1: L-cysteine desulfhydrase 1; Ser: serine; SAT: serine acetyltransferase; OAS: Oacetylserine; OAS-TL: O-acetylserine thiol lyase; GSH: reduced glutathione.
-
The chemical properties of H2S allow it to diffuse readily through membranes without the necessity of membrane channels or specific transporters, and confers its biological signaling via protein persulfidation[2,3]. Protein cysteine residues undergo H2S-derived persulfidation to form persulfides (R-SSH), which is the mechanism by which H2S performs its biological function[2]. In plant physiobiochemistry, complex crosstalk between H2S and phytohormones such as salicylic acid (SA), gibberellins (GAs) and jasmonic acid (JA) have been reported. It also interacts with other messengers like NO, H2O2 and carbon monoxide (CO) to regulate plant growth and development[16]. This review focuses on the inter-connection of Ca2+, ABA and H2S. Recent studies show that the relationship between Ca2+ signaling and H2S is not a simple upstream-downstream relationship. Under stress, intracellular Ca2+ signaling responded to environmental stimuli to promote endogenous H2S accumulation, while H2S mediated Ca2+ signaling transduction by up-regulating the transcription of calmodulin (CaM), calcineurin B-like (CBL) and calcium dependent protein kinase (CDPKs) genes. Furthermore, H2S and Ca2+ triggered ascorbic acid-glutathione (AsA-GSH) cycle against heavy metals (HMs)[17,18] and low temperature stress[19]. Fang et al.[20] confirmed how Ca2+ promoted endogenous H2S production under chromium (Cr6+) stress in Arabidopsis. The interaction between Ca2+/CaM2 and bZIP transcription factor (TF) TGA3 activated LCD expression by enhancing binding ability of TGA3 to the motif 'TGACG' of LCD promoter, forming a CaM2-TGA3-pLCD complex, resulting in H2S accumulation[20]. Nevertheless, the correlative studies on Ca2+ and H2S signaling still remain in plant growth and development.
H2S seems to interplay with ABA in drought responses. Shen et al. found a negative feedback loop between reactive oxygen species (ROS) and ABA signaling. ABA-triggered overproduction of ROS which further produces negative feedback to regulate respiratory burst oxidase homolog protein D (RbohD) and DES1 activity, resulting in ABA de-sensitivity[21]. Then ABA-triggered DES1 function contributed to long hypocotyl 1 (HY1) retrograde signaling[22]. Chen et al. also reported that ABA-induced H2S persulfidatd the TF abscisic acid insensitive 4 (ABI4) at the residue Cys250, triggering the transactivation of mitogen-activated protein kinase kinase kinase 18 (MAPKKK18) involved in autophosphorylation or phosphorylation of the sucrose nonfermenting 1 (SNF1)-related protein kinase 2.6 (SnRK2.6)[23], while ABI4 trans-activated DES1 to form a DES1-ABI4 loop[24]. Moreover, HY1 can negatively regulate ABA-induced stomatal closure by activating ABI4 expression[22]. Accordingly, there appears to be a redox-based regulatory loop between H2S signaling and ABA-induced stomatal closure. Furthermore, H2S mediated ABA signaling by persulfidating SnRK2.6 at Cys131 and Cys137 residues. The interaction between SnRK2.6 and downstream ABA response factor 2 (ABF2) was enhanced by persulfidation, hence promoting ABA-induced stomatal closure[25]. Ca2+ signaling might harmonizes persulfidation and phosphorylation of SnRK2.6, and probably establishes a connection with ABA via ROS signaling[23]. However, more is pending on how this complex regulatory mechanism controls postharvest ripening and senescence.
-
It has been well-established that H2S modulates ripening and senescence processes by mediating ethylene biosynthesis and signal transduction, and H2S plays a positive role in both climacteric and non-climacteric fruit (Table 1). After fumigating three green leafy vegetables with exogenous H2S, then using 0.1 μL L−1 ethylene to ventilate, respiration rate and chlorophyll loss were decreased[26]. Compared with ethylene treatment, H2S reduced the accumulation of reactive oxygen species (ROS) by enhancing the antioxidant system, and obviously delayed the color change of tomato fruit[27]. Consequently, H2S antagonizes ethylene-induced fruit ripening, which was associated with the antioxidant system. At the transcriptional level, Li et al. found that exogenous H2S attenuated the expression of ethylene synthesis-related genes, such as AdSAM, AdACS1, AdACS2, AdACO2 and AdACO3[28]. In addition, H2S also affects the expression patterns of genes linked to ethylene synthesis and signaling, including ethylene receptor genes and ethylene response factors (ERFs). Apple slices treated with 0.4 mM NaHS showed lower expression of MdACO1, MdERS1 and MdETR1[29]. Similarly, H2S (1.0 mM NaHS) was combined with ethylene (1.0 g L−1 ethephon) to fumigate banana, the released H2S significantly down-regulated ethylene biosynthesis genes expression (MaACS1, MaACS2 and MaACO1) and up-regulated ethylene receptor genes expression (MaETR, MaERS1 and MaERS2)[30]. In this case, ethylene receptors may act as a negative regulator to inactivate by ethylene binding[31]. Lin et al. reported that H2S treatment reduced the expression of ethylene receptor 2 (ETR2) and ERF genes (ERF003, ERF5 and ERF016), but increased the expression of ERF4 and ERF113[32]. Generally, H2S counteracts the quality deterioration caused by ethylene via regulating ethylene-related genes, which is essential to postpone postharvest senescence.
Table 1. H2S counteracts the effects of ethylene in postharvest fruits and vegetables.
Materials Treatment Effect Reference Vegetables Pak choy (Shanghai) Green curly kale (Sabellica) Sweet Italian basil (Ocimum basilicum) NaHS fumigation for 4 h (0, 50,
100 and 250 μL L−1 H2S)Decreased ethylene production, chlorophyll loss and respiration rate [26] Non-climacteric fruit Strawberry (Fengxiang) 0.8 mM NaHS and 5 μM SNP immersion for 10 min Inhibited respiration rate and maintained crust color [33] Climacteric fruit Peach (Dahong) 15 μL L−1 NO gas and 20 μL L−1
H2S gas fumigation for 20 minReduced ACS and ACO activities and inhibited respiration rate [34] Kiwifruit (Qinmei) 1.0 mM NaHS fumigation for 8 d Down-regulated the expression of ethylene synthesis-related genes like AdSAM, AdACS1, AdACS2, AdACO2 and AdACO3 [28] Kiwifruit (Jinkui) 20 μL L−1 H2S gas fumigation for 0.5 h Down-regulated the expression of ethylene receptor 2 (ETR2), ERF003, ERF5 and ERF016; up-regulated the expression of ERF4 and ERF113 [32] Fresh-cut apple (Fuji) 0.4 mM NaHS fumigation for 5 d Up-regulated MdDHAR expression and down-regulated the expression of MdLOX2, MdPG1, MdPPO, MdACO1, MdERS1 and MdETR1 [29] Banana (Brazil) 1.0 mM NaHS and 1.0 g L−1 ethephon solution fumigation
for 6 dInhibited chlorophyll loss; up-regulated the expression of ethylene receptor genes MaETR, MaERS1 and MaERS2; down-regulated the expression of ethylene synthesis-related genes like MaACS1, MaACS2 and MaACO1 [30] Tomato (Micro Tom) 0.9 mM NaHS and 1.0 g L−1 ethephon solution fumigation
for 24 hMaintained better appearance and postharvest quality and down-regulated the expression of ERF003 and DOF22 [37] There appears to be a cooperative relationship between H2S and NO against ethylene. The combination of H2S and NO delayed ripening and decay of strawberry[33] and peach fruit[34]. Combinatorial treatment (20 μL L−1 H2S gas and 15 μL L−1 of NO gas) further attenuated ACC synthase (ACS) and oxidase (ACO) activities, and significantly decreased ACC and MACC content[34]. Munoz-Vargas et al. also found that H2S levels were increased during sweet pepper fruit ripening, while NO levels were reduced[35]. NO binding with ACO to form a ternary complex reduces ethylene generation during the fruit ripening process[36]. However, it remains uncertain how the intricate crosstalk between H2S and NO co-regulates ethylene-induced ripening and senescence.
Regulation of senescence-related genes
-
The genes associated with ethylene mentioned above are also senescence-related genes[5]. Co-treatment (0.9 mM NaHS and 1.0 g L−1 ethephon) decreased protease activity and sustained a high level of bioactive compounds which were essential for tomato postharvest quality by principal component analysis (PCA), including soluble protein, starch, titratable acids (TA) and ascorbic acid (AsA). Meanwhile, H2S reduced the transcription of ripening-related TFs (ERF003 and DOF22), and changed the expression pattern of genes encoding beta-amylase and UDP-glycosyltransferase including BAM3, UFGT73 and UFGT5[37]. Consistent results were obtained from studies on fresh-cut pears. Reducing sugar, soluble protein and total amino acids content of pear slices under H2S fumigation were higher[38]. After harvest, chlorophyll and some proteins are gradually degraded under the regulation of senescence-related genes. Extrapolating from recent studies, H2S affects the transformation of large molecules at the transcriptional level. During postharvest, Li et al. reported a decreased expression of cysteine protease genes AdCP1and AdCP3 in H2S-treated kiwifruit[28]. Moreover, H2S reduced the expression of cysteine protease genes BoCP1 and BoCP3 and aspartic protease gene BoLSC807, while genes involved in chlorophyll degradation were successively found to be down-regulated by H2S in postharvest broccoli, such as BoSGR, BoRCCR, BoCLH2, BoPaO, BoNYC, BoCLH1 and BoPPH[39,40]. In water spinach, exogenous H2S fumigation reduced the activities of chlorophyll degradation-related enzymes, including chlorophyllase (Chlase), Mg-dechelatase (MD) and Mg-dechelating substance (MDS), further sustaining chlorophyll levels and slowing the decomposition of chloroplasts[41]. Studies on banana[30], pak choy[26] and broccoli[42] were consistent with the aforementioned results, where H2S alleviated senescence-induced yellowing by mitigating chlorophyll loss. Ning et al. found that the TF IbERF71 formed a IbERF71-IbMYB340-IbbHLH2 complex to co-target the IbANS1 promoter, resulting in anthocyanin accumulation in purple-fleshed sweet potato[43]. Understanding the molecular mechanism by which H2S regulates pigment metabolism is conducive to comprehending ripening and senescence processes. In addition, Lipoxygenase (LOX) catalyzes the hydroperoxidation of polyunsaturated fatty acids, and according to studies, H2S treatment can inhibit its activity[28] and gene expression[39,40]. In summary, H2S regulates senescence-related genes to ameliorate ethylene-induced quality deterioration, as well as higher nutritional compounds.
Regulation of cell wall metabolism
-
Softening and textural changes are modified by several enzymes, and this process maybe different in various types of fruits[44]. It is well known that cell wall modifying enzymes taking part in fruit ripening are controlled by ethylene. Zhu et al.[34] found that H2S treatment (20 μL L−1 H2S gas) attenuated ethylene production and avoided cell wall loosening, while markedly decreasing the activities of cell wall modifying enzymes, such as polygalacturonase (PG), pectin methylesterase (PME) and endo-β-1,4-glucanase (EGase). H2S-NO co-treatment (15 μL L−1 NO gas and 20 μL L−1 H2S gas) further reduced the increases of water-soluble polysaccharides (WSP) and CDTA-soluble polysaccharides (CSP) in peach fruit, as well as the breakdown of Na2CO3-soluble polysaccharides (NSP)[34]. Similarly, in postharvest strawberry, synergistic interactions between H2S and NO (0.8 mM NaHS and 5 μM SNP) reduced cell wall modifying enzyme activity, and further improved softening and shelf life[33]. The genes linked to cell wall degradation (EGase, PME and β-galactosidase) were down-regulated under H2S treatment (20 μL L-1 H2S gas), while the expression pattern of ethylene responsive genes were also changed in kiwifruit, indicating that H2S modifies the integrity of the cell wall at the transcription level[32]. Besides, H2S participates in cell wall metabolism and provides tolerance for cold stress. Lower activities of PG, pectate lyase (PL) and β-galactosidase (β-gal) and lower yields of WSP and CSP under H2S treatment (1.0 or 2.0 mM NaHS) contributed positively to improve surface pitting symptoms in cold-stored sweet cherry[45]. Cumulative reports demonstrate that H2S inhibits the depolymerization and solubilization of cell wall polysaccharides. In addition, Forlani et al. proposed that ABA is also a key ripening-associated regulator, which can promote ethylene biosynthesis[44]. Whether the synergistic or antagonistic effects of H2S and other molecules in fruit softening and senescence regulate cell wall metabolism directly or indirectly needs to be explored.
-
The ROS scavenging system is divided into non-enzymatic and enzymatic. The enzymatic antioxidant system is composed of antioxidant enzymes like glutathione reductase (GR), catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR) and glutathione S-transferase (GST), and the non-enzymatic antioxidant system consists of antioxidants, such as GSH, AsA, polyphenols, and carotenoids[46]. Abiotic stresses can destroy the dynamic balance of reactive oxygen species and reactive nitrogen species (ROS/RNS), which may ultimately cause irreversible nitro-oxidative damage[3]. Accumulating evidence verifies that H2S counteracts ROS accumulation to reduce oxidative damage. Ni et al.[47] reported that high concentrations of H2S (NaHS as donor) had toxic effects on Kyoho grape, described as severe decay and threshing rate. The optimal concentration of H2S (1.0 mM NaHS) increased antioxidant content like AsA, flavonoid and total phenolics, as well as higher APX and CAT activity, while inhibiting H2O2, superoxide anion (O2−·) and malondialdehyde (MDA) accumulation due to its higher antioxidant capacity[47]. Furthermore, it was reported that H2S treatment (0.7 mM NaHS) showed higher relative expression of genes encoding SOD, CAT and GST, which ameliorated surface browning of fresh-cut apple by stimulating non-enzymatic and enzymatic antioxidant systems[48]. Similarly, Yao et al. found that additional H2S (0.9 mM NaHS) enhanced the activities of POD, APX, CAT and SOD, and also up-regulated the relative expression of SlAPX2, SlCAT1, SlPOD12 and SlCuZnSOD[27]. In a nutshell, this is a possible mechanism for H2S to delay postharvest senescence by sustaining the redox balance.
Like senescence, CI also induces ROS burst[46]. Application of H2S induced chilling tolerance by enhancing antioxidant capacity as reported in banana[49], hawthorn[50] and kiwifruit[51] during postharvest (Table 1). It is indicated that H2S alleviates CI-induced quality deterioration during postharvest, which is associated with the antioxidant system. Interestingly, water spinach treated with NaHS enhanced L-DES and D-DES activity that generated more endogenous H2S. However, it was recovered by using DL-propargylglycine (PAG, L-DES inhibitor), while the hypotaurine (HT, H2S-scavenger) group was unaffected. Hence, Hu et al. deduced that endogenous H2S was involved in the regulation of senescence[41]. During cold storage, NaHS application on hawthorn fruit[50] and mulberry fruit[52] also exhibited higher levels of endogenous H2S, as well as H2S-generating enzyme activity (L-DES and D-DES). Moreover, low temperature induced endogenous H2S accumulation and higher expression levels of H2S synthesis-related genes like CsaLCD (Csa2G034800.1), CsaDES1 (Csa1G574800.1) and CsaDES2 (Csa1G574810.1)[53]. As mentioned earlier, endogenous H2S regulates ethylene-induced ripening, which indicates that exogenous H2S triggers endogenous H2S production to further regulate ripening, senescence and chilling injury processes during storage.
-
If chilling stress occurs over time, it will induce membrane phase transitions and increase membrane permeability, resulting in a series of adverse effects such as intracellular ATP (iATP) shortages, ROS accumulation and lipid peroxidation[55]. Therefore, sufficient iATP plays a pivotal role in postharvest fruits and vegetables. During cold storage and ripening period, banana fruit treated with 0.5 mM NaHS showed higher ATP levels, and increased the activities of energy-related enzymes like H+-ATPase, Ca2+-ATPase, cytochrome C oxidase (CCO) and succinate dehydrogenase (SDH)[56]. Thus, H2S seemingly induced chilling tolerance of banana fruit by regulating energy metabolism. In water spinach, H2S treatment (2.4 mM NaHS) delayed dark-induced senescence resulting from maintenance of energy status, along with higher ATP and ADP levels and lower AMP levels[41]. Sugar is the substrate of energy metabolism. Li et al. reported that H2S treatment (0.8 mM NaHS) not only triggered energy metabolism, but also increased the activity of enzymes involved in embden-meyerhof-parnas (EMP) and pentose phosphate pathway (PPP), such as glucokinase (GK), fructokinase (FK), glucose-6-phosphate dehydrogenase (G6PDH) and 6-phospho-gluconate dehydrogenase (6PGDH), while the broccol treated with 0.5 mM PAG exhibited a negative role[42]. H2S participates in the inclusion of sugar and energy metabolism, resulting in adequate energy availability and better postharvest quality, but how H2S regulates these enzymes still requires further investigation.
GABA, a 'sensor' for intracellular energy status, indicates plants whether to need or institute processes for more energy[57]. GABA shunt metabolism reduces oxidative damage and ensures ATP supply by providing NADH and succinate to the tricarboxylic acid (TCA) cycle and electron transfer system (ETS)[55]. Moreover, recent studies proposed that GABA is not only a plant metabolite, but also an endogenous signaling molecule in response to stress[58]. NO has been reported to promote coordinated biosynthesis of polyamines (PAs), proline and GABA in banana fruit, thereby blocking the development of CI symptoms[59]. Nevertheless, whether H2S and GABA have a positive response to cold stress has not been reported.
Roles of H2S in membrane integrity and membrane lipid peroxidation
-
Chilling attenuates the fluidity of the cytomembrane, resulting in electrolyte leakage, MDA accumulation, eventually resulting in irreversible oxidative damage and CI symptoms[60]. Most chilling-induced changes are associated with the integrity of the cell membrane. Table 2 shows that exogenous H2S reduces ROS accumulation and membrane lipid peroxidation during shelf life and cold storage. Supplementation with 0.5 mM NaHS confered chilling tolerance of banana fruit by reducing electrolyte leakage, MDA and ROS, and because of higher CAT, POD, APX, SOD and GR activities. Meanwhile, H2S regulated Δ1-pyrroline-5-carboxylate synthetase (P5CS) and proline dehydrogenase (PDH) activity that synthesized more proline[49]. Proline, a major osmolyte, can sustain membrane permeability[61], and stabilize antioxidant enzymes or stimulate additional ROS scavenging pathways in response to abiotic and biotic stresses[62]. Thus, it is implied that H2S mediates the production of ROS and proline to mitigate CI. Soluble protein and soluble sugar are also important osmotic substances. As mentioned earlier, they can be improved by H2S. Besides, H2S up-regulated most differentially expressed unigenes (DEGs) associated with antioxidant and energy, while down-regulating the expression of genes encoding membrane-degrading enzymes like PLD and LOX[48]. Thus, H2S postpones membrane lipid metabolism to reduce the damage of lipid peroxidation at the transcriptional level. In combination with the above, the roles of H2S in CI may be achieved by regulating physiological metabolism including (i) ROS, (ii) energy, (iii) membrane lipid, (iv) proline and (v) cell wall metabolism, there are still vast areas to be studied.
Table 2. Effects of H2S on antioxidant system in postharvest.
Materials Treatment Results Reference Delay ripening and senescence Tomato (Micro Tom) 0.9 mM NaHS and 1.0 g L−1 ethephon fumigation for 24 h Enhanced CAT, POD, APX, SOD and PAL activities; reduced LOX and PPO activities; inhibited H2O2, O2−· and MDA accumulation [27] Fresh-cut pear (Dangshan) 2.0 mM NaHS fumigation for 6 d Enhanced CAT, APX and POD activities; reduced PAL, PPO and LOX activities; reduced H2O2, O2−· and MDA content [38] Water spinach (Ipomoea aquatica) 2.4 mM NaHS fumigation for 8 d Enhanced CAT, POD and SOD activities, as well as higher DPPH scavenging activity; reduced O2−· and MDA content; increased endogenous H2S content by higher LCD and DCD activities [41] Grape (Kyoho) 1.0 mM NaHS fumigation for 7 d Enhanced CAT and APX activities; reduced LOX activities; reduced H2O2, O2−· and MDA content [47] Apple (Red Fuji) 0.7 mM NaHS fumigation for 24 h Enhanced CAT, SOD, PAL and GR activities; increased antioxidants content like AsA and phenolic; reduced PPO, POD and APX activities, as well as H2O2 accumulation [48] Mulberry (Dianmian-1) 0.8 mM NaHS fumigation for 6 d Enhanced DPPH and O2−· scavenging activity by higher CAT, POD and SOD activities; reduced O2−· and MDA content; increased endogenous H2S levels by higher LCD and DCD activities [52] Fresh-cut lotus roots
(Taikong Lian III)15 μL L−1 H2S gas fumigation for
30 minEnhanced total phenols and antioxidant capacity; higher PAL and CAT activities; lower PPO and POD activities; reduced H2O2 and O2−· production [54] Alleviate chilling injury Banana (Brazil) 0.5 mM NaHS fumigation for 24 h Enhanced CAT, POD, APX, SOD and GR activities; reduced H2O2, O2−· and MDA content; alleviated electrolyte leakage and CI [49] Hawthorn (Crataegus monogyna) 1.5 mM NaHS fumigation for 48 h Alleviated CI; enhanced CAT, APX, SOD and PAL activities; reduced H2O2 and MDA content; enhanced LCD and DCD activities, while increased endogenous H2S levels [50] Kiwifruit (Jinkui) 45 μM H2S immersion for 30 min Enhanced CAT, POD and SOD activities; reduced H2O2 and O2−· content; inhibited electrolyte leakage [51] -
Fruits and vegetables often rot and deteriorate due to fungal infection during the process of transportation and storage. Recent studies have verified that H2S-induced reduction in postharvest decay primarily operates through inhibiting fungal growth (Table 3). For example, Tang et al. reported that H2S fumigation (1.0−2.5 mM NaHS) effectively inhibited the growth of fungal pathogens on medium and sweet potato slices in a dose-dependent manner, while reducing the incidence of black rot or soft rot[63]. Additionally, low doses of H2S also controlled the mycelium growth on medium and pears[38]. How does H2S rely on dosage to combat fungi? This study seems to explain, 0.5 mM NaHS treatment inhibited the growth of Aspergillus niger and Penicillium italicum on medium and inoculated fruits, and this facilitation may be attributed to lower CAT and SOD activities and down-regulation of relative genes in A. niger. H2S in high concentrations had a bactericidal effect on the growth of some foodborne pathogens, among which Staphylococcus aureus was more sensitive to H2S[64]. Taken together, the potential mechanism by which H2S, in low doses, exert antibacterial effects is to induce ROS overproduction in fungi by attenuating antioxidant capacity, and high concentrations of H2S principally act as a fungicide to inhibit spore germination, germ tube elongation and mycelial growth (Table 3).
Table 3. Effects of H2S on disease resistance in postharvest fruits and vegetables.
Materials Pathogens Treatment Effect Reference Pear (Dangshan) Aspergillus niger Penicillium expansum NaHS fumigation for 6 d (2.0 mM) Enhanced antioxidant capacity of fresh-cut pear and inhibited ROS accumulation; increased POD activity; reduced PAL and PPO activities; inhibited mycelium growth and lesion diameter on pears [38] Fresh-cut sweet potato (Xushu 18) Mucor rouxianus Rhizopus nigricans Geotrichum candidum NaHS fumigation for 4 d (2.0 mM) decreased PPO and LOX activities; lower POD activity; inhibited fugal growth and its pathogenicity [63] Apple (Malus domestica), mandarin (Citrus reticulata), sweet orange (Citrus sinensis), kiwifruits (Actinidia deliciosa), pear (Pyrus bretschneideri Rehd), tomato (Lycopersicon esculentum) Aspergillus niger Penicillium italicum NaHS fumigation for 9 d (0.5 mM) Inhibited spore germination, germ tube elongation, mycelial growth; promoted ROS accumulation by reducing SOD and CAT activities in A. niger, as well as higher expression of SOD and CAT [64] Chinese white pear (Pyrus bretschneideri) Aspergillus niger Deletion of the cbsA Gene in A. niger niger MA: induced higher resistance to cadmium stress and stronger infectivity to pears; increased Cys and GSH levels; decreased endogenous H2S content [67] infection with above mutant Pear fruit: increased the accumulation of H2O2, O2−· and MDA; induced larger lesion diameter Peach (Mengyin) Monilinia fructicola NaHS fumigation for 2 h (50 mM) M. fructicola: inhibited spore germination, mycelial growth and pathogenicity Peach fruit: enhanced CHT, GLU and PAL activities, while up-regulated the expression of related genes; reduced disease incidence and lesion diameter [70] In mammals and yeast, cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) are key enzymes involved in H2S synthesis from sulfur metabolism. As shown in Fig. 2a, CBS catalyzes the dehydration condensation of homocysteine (Hcy) with Ser or Cys to form H2O or H2S, respectively. Additionally, CBS also converts Cys to H2S through the β-replacement reaction of Cys with thiols, and CSE produces H2S via the γ-replacement reaction between Hcy molecules and the α,β-elimination reaction with cystine (Fig. 2b)[65,66]. Interesting results suggest that H2S in fungi is also produced through the transsulfuration pathway. Guo et al.[67] reported that the deletion of the An05g00160 gene (encoding CBS, named cbsA) in A. niger promoted Cys and GSH levels while decreasing endogenous H2S content, which is responsible for enhancing cadmium (Cd2+) tolerance and pathogenicity. Pear fruits infected with the cbsA mutant brought about an excessive production of ROS. In accordance with these results, it is suggested that CBS mainly decomposed Cys to produce H2S, and cbsA deletion harmonized redox homeostasis in A. niger to resist ROS burst of the host[67]. This maybe the reason why fungi can still grow on fruits, the detailed mechanisms require further research.
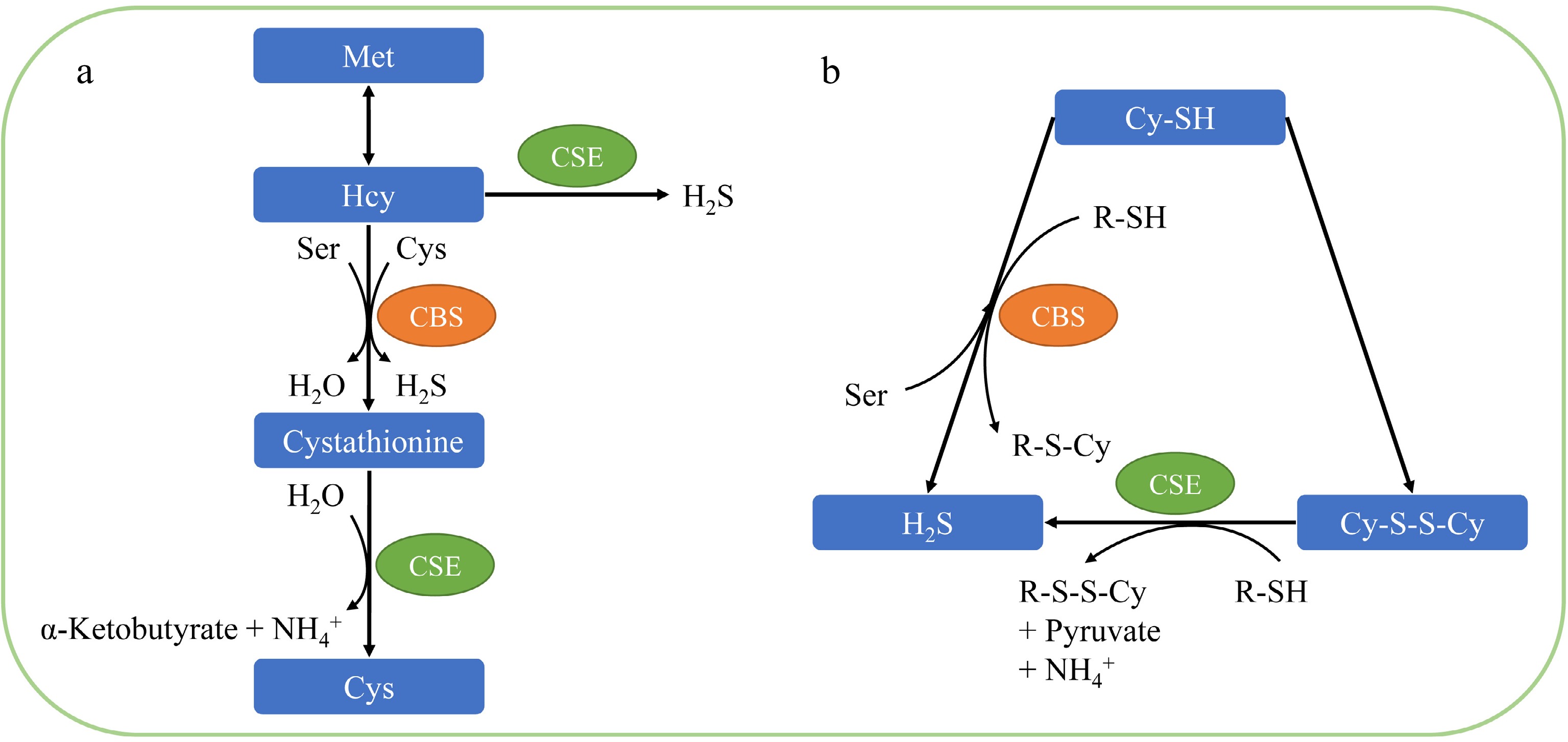
Figure 2.
Generation of H2S in mammals and yeast. (a) H2S is produced by sulfur metabolism, wherein CSE and CBS participate in each step. (b) Cys with thiols can be further interacted by CSE and CBS to synthesize H2S endogenously through triggering replacement reactions. Met, methionine; Hcy, homocysteine; CSE, cystathionine-γ-lyase; CBS, cystathionine-β-synthase; Ser, serine; Cys, cysteine; Cy-SH, cysteine; Cy-S-S-Cy, disulfide.
Regulation of defense-related proteins
-
Under abiotic and biotic stresses, pathogenesis-related proteins (PRs) are triggered through the signaling network like SA, JA, and ethylene. PRs are divided into 17 families according to their biological characteristics, among which PR-2 (β-1,3-glucanase, GLU) and PR-11 (chitinases, CHT) proteins have antifreeze[68] and fungicide activities[69]. GLU and CHT enzymes could decomposed the basic components of the fungal cell wall to deal with fungal infection. Zhang et al. observed that H2S and NO in association, caused an overall reduction in the decay incidence of strawberry, associated with an increase in GLU and CHT activity[33]. Analogous results were obtained on peach fruit, where H2S and HT application both effectively controlled lesion development diameter by enhancing GLU, CHT and PAL activities, with accompanying higher gene expression (U49454, AF20663 and KC757351). But HT treatment had no obvious impact on the incidence of brown rot compared with control[70]. HT function usually acts as a H2S scavenger, but the assumptions of how H2S and HT promote PRs responsible in disease resistance remains unclear. Related proteins triggering in the absence of fungal infection were considered as 'PR-like' proteins such as PPO, POD and PAL[69], also known as PRs. The metabolites lignin and phenols produced through phenylpropanoid metabolism are correlated with disease resistance, and the metabolic network composed of PAL, POD, PPO, 4-coumarate coenzyme A ligase (4CL), cinnamate-4-hydroxylase (C4H) and chalcone isomerase (CHI) co-regulate their synthesis[71,72]. POD and PPO activity are also major drivers of the fungicidal substance quinone. Vacuum infiltrated H2S (1.0 and 2.0 mM) increased total phenols, diminished quinone accumulation and further mitigated pericarp browning in cold-stored Litchi by altering the activity of PAL, POD and PPO. Interestingly, POD and PPO activity were higher than control at the later stages[73]. Likewise, in fresh-cut lotus root, 15 μL L−1 H2S showed positive effects on reducing browning degree, which corresponded to higher phenolic content and antioxidant capacity[54]. Moreover, Chen et al. found that H2S incompletely suppressed POD activity and relative expression in fresh-cut apple, and the reduction of enzymatic browning by H2S is not realized by reducing phenolic substrates[48]. It is worth noting that phenols are not only regulators of disease resistance, but also antioxidants to delay postharvest senescence. However, how H2S induces phenolic synthesis involved in the equilibrium between antioxidant and disease resistance is vague, less is known about whether H2S regulates other key enzymes of phenolic metabolism.
-
H2S triggers antioxidant system and controls ROS/RNS to harmonize the redox homeostasis, which may be pivotal to its functions[3]. It occurs primarily through mediating PTMs of proteins that convert cysteine (Cy-SH) into R-SSH[74]. This process was initially called S-sulfidation which can not signify hydration, but persulfidation is more suitable[3]. Cysteine residues are the 'switch' of redox and undergo other PTMs such as S-nitrosation (NO), S-glutathionylation (GSH), S-cyanylation (cyanide) and S-acylation (fatty acids) during different stress periods, which compete with H2S for thiol (-SH) groups[75]. Persulfidation modifies the structure, function and subcellular localization of target proteins. Proteomic analysis showed that the number of targets identified in Arabidopsis was in the following order: persulfidation > S-nitrosation > S-glutathionylation[8]. This implies that H2S-derived persulfidation plays a crucial role in biology. Two bHLH TFs, Csa5G156220 and Csa5G157230, regulated the expression of the gene cluster involved in cucurbitacin C (CuC) biosynthesis[76]. H2S-derived persulfidation enhanced binding ability of two TFs with bitter leaf (Bi) promoter, further resulting in CuC accumulation and helping cucumber to withstand biotic and cold stress[53]. Likewise, H2S persulfidated mitogen-activated protein kinase MPK4 to resist cold stress in Arabidopsis[77]. Jia et al. also proposed that ethylene-induced H2S feedback regulated ehtylene generation by reducing ACO activities via persulfidation under osmotic stress, and the residue Cys60 was a potential binding site in LeACO1[78].
As mentioned previously, H2S forms a regulatory loop with ABA and redox signaling based on protein persulfidation. There is growing evidence that the interconnection between H2S and ROS signaling operates through persulfidation. Rboh, also called NADPH oxidase (NOX), takes part in ROS/RNS metabolism. During sweet pepper fruit ripening, NOX activity is suppressed by S-nitrosation, nitration and S-glutathionylation[79]. NADPH is a key cofactor in ROS/RNS metabolism, where some enzymes like GR, NOX and NO synthase-like (NOS) are NADPH-dependent to perform their functions[80]. H2S and NO regulate NADPH-generating enzymes based on PTMs to harmonize cellular redox homeostasis[81]. G6PDH, 6PGDH, NADP-malic enzyme (NADP-ME) and NADP-dependent isocitrate dehydrogenase (NADP-ICDH) enzymes are responsible for NADPH supply. NADP-ICDH, a potential H2S target, was probably suppressed by S-nitrosylation at Cys133 and nitration at Tyr450 during the ripening phase[35]. Moreover, further studies showed that higher H2S and NO levels were designed to diminish NADP-ME activity to modulate pepper fruit ripening, while 6PGDH activity was unaffected[82]. Cumulative reports extrapolate that NADPH generation involved in fruit ripening is a good example of the association between H2S and NO via PTMs. Similarly, APX and CAT are targets of both persulfidation and S-nitrosylation at the same position, where H2S acts upstream or downstream of NO in response to different stimuli[83,84]. H2S and NO co-regulate root development, stomatal closure, and programmed cell death (PCD) in plants, where ABA and ROS signaling play an integral role[85]. These incorporating reports mean that synergistic or antagonistic properties of H2S and NO under various stresses are based on PTMs, which is in agreement with previous studies. Nonetheless, the precise molecular mechanism of how they work remains ambiguous.
-
There is growing proof that exogenous H2S promotes endogenous H2S directly or by synthetic enzymes to further oppose metabolic disorders in fruits and vegetables caused by abiotic stress, which appears to affect enzyme activity via persulfidation (Fig. 3a). However, large numbers of reports suggest that H2S can regulate various metabolic enzyme systems to improve postharvest quality (Fig. 3b), but fewer persulfidated target proteins have been identified. Many issues have emerged that require urgent attention. H2S-induced chilling tolerance remains an extensive area of research, and it is necessary to investigate the effects of H2S application on GABA and PAs catabolism. Whether H2S stimulates the expression of cold responsive proteins at low temperature is also a research interest. Multiple studies have corroborated that H2S can delay ripening and senescence and arrest ethylene-induced quality deterioration, but the accurate analysis of the cooperation between H2S and other molecules remains obscure (Fig. 3a), especially H2S and NO seem to provide mutual regulation. Moreover, future work should focus on the interaction network of H2S, ABA and Ca2+ signaling during postharvest. H2S inhibits fungal growth in a dose-dependent manner, the reasons why H2S has different impacts on the redox homeostasis of fruits and fungi needs further discussion. Some enzymes involved in ripening have been reported to be persulfidated by H2S, but it is essential to understand how H2S recognizes specific targets. Cysteine residues also undergo other PTMs, whose interactions benefit our understanding of how H2S responds to various stresses and explore its application value postharvest. Nevertheless, maximum residue limit (MRL) of H2S has not been reported[6], which limits its commercial applicability, but H2S has huge potential for postharvest commercialization.
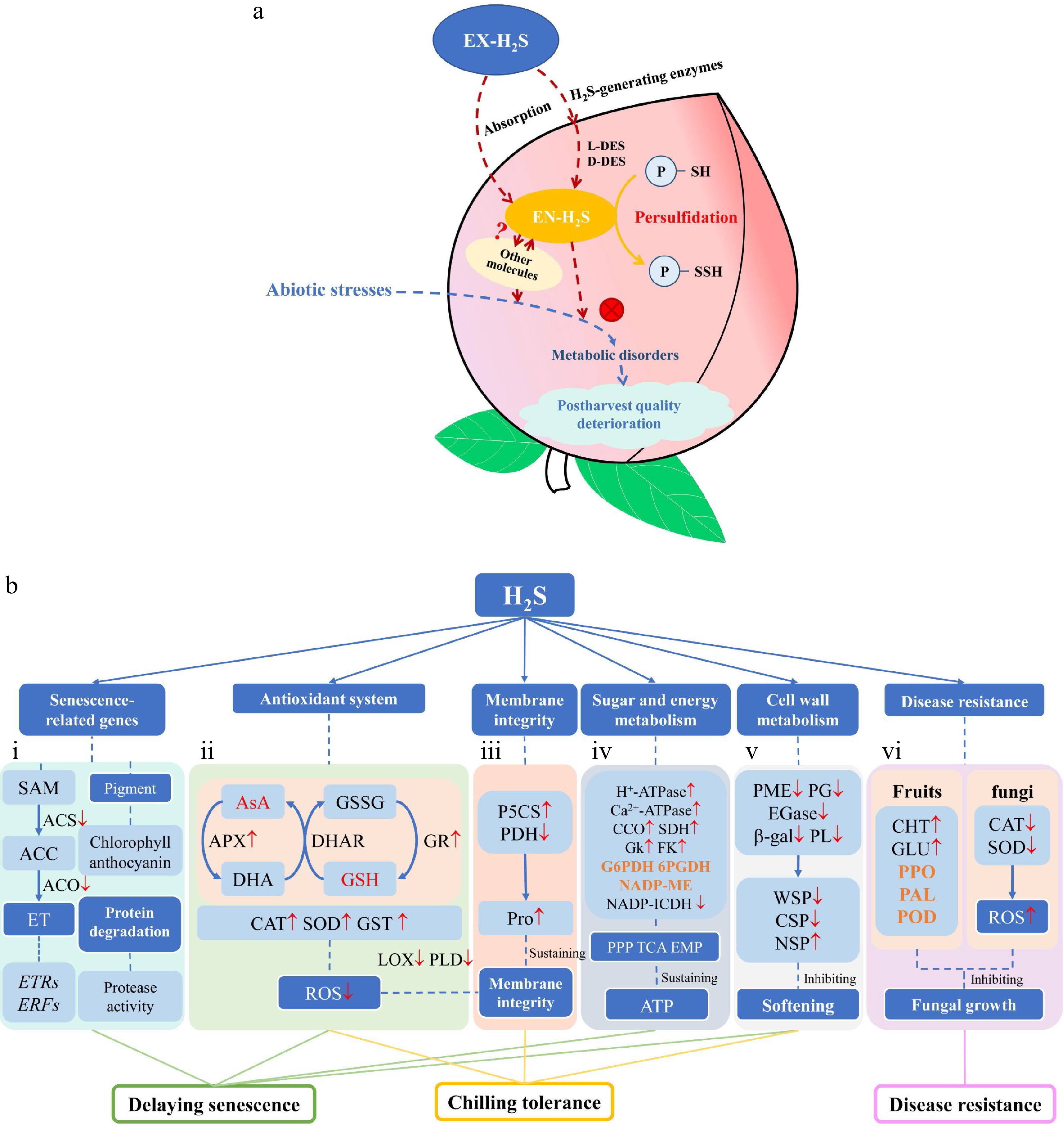
Figure 3.
Exogenous H2S regulates numerous physiological processes to improve postharvest quality of fruits and vegetables. (a) A simple model showing a cascade of events after exogenous H2S, which may stimulate various components of enzyme systems to maintain cellular homeostasis, but it is still not well understood how H2S works with other molecules like ABA, NO, Ca2+ and ROS, etc. Broken red arrows represent processes triggered by H2S, and broken blue arrows indicate abiotic stress-induced. (b) Various functions of H2S in metabolic enzyme systems. (b-i) H2S regulates related genes to delay senescence, especially ethylene (ET) biosynthesis and signaling transduction. (b-ii) The roles of H2S in the antioxidant system, which is essential for H2S-derived beneficial effects. (b-iii) H2S sustains the integrity of cell membrane. (b-iv) H2S facilitates ATP supply. (b-v) Inhibitory effect of H2S on cell wall loosening. (b-vi) H2S shows different mechanisms of redox homeostasis in hosts and fungi. Red arrows indicate that exogenous H2S enhanced the enzyme activity. The orange font implies that different results are obtained after H2S treatment, which correlates with whether the analyses are performed under cellular/subcellular or cell-free conditions (purified proteins)[81].
This work was supported by the National Natural Science Foundation of China (31972125,32172265)
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhao Y, Zhao L, Hu S, Hou Y, Zheng Y, et al. 2022. Hydrogen sulfide: a luminous future in the postharvest preservation of fruits and vegetables. Food Materials Research 2:3 doi: 10.48130/FMR-2022-0003
Hydrogen sulfide: a luminous future in the postharvest preservation of fruits and vegetables
- Received: 23 November 2021
- Accepted: 30 December 2021
- Published online: 14 February 2022
Abstract: Hydrogen sulfide (H2S) has emerged as a signaling molecule that plays a crucial role in the postharvest preservation of fruits and vegetables. This review summarizes the various functions of H2S such as delaying ripening and senescence, enhancing the resistance to cold and disease, and emphasizes the underlying mechanisms. Appropriate concentrations of H2S primarily operate through stimulating the antioxidant system, while showing positive effects on physiological metabolism relevant to storage quality and shelf life including energy, sugar, proline, phenolic, membrane lipid and cell wall metabolism. Moreover, H2S may reduce storage loss by modulating the expression patterns of senescence-related genes, like those linked to ethylene. The coordination of H2S and nitric oxide (NO) combats ethylene-derived negative effects during ripening and senescence. High concentrations of H2S not only act as a regulator to induce disease resistance, but also as a fungicide to inhibit the growth and pathogenicity of fungi. The intricate crosstalk between H2S and other molecules exists via synergistic and antagonistic roles based on protein persulfidation, which is the major signaling process of H2S and appears to compete with other post-translational modifications (PTMs) for the same cysteine residues. This review summarizes H2S synthesis pathways and also discusses the correlation between the signals of H2S, Ca2+ and ABA, while highlighting the intrinsic mechanisms of H2S performing its functions in postharvest preservation.
-
Key words:
- Hydrogen sulfide /
- Postharvest /
- Quality /
- Senescence /
- Chilling injury /
- Decay












