-
Postmortem glycolysis is a crucial physiological and biochemical reaction during the transformation from muscle to meat, which could affect the degree and rate of pH decline, and ultimately influence the meat quality[1]. The process of glycolysis is catalyzed by a series of enzymes. Previous research has shown that glycolytic enzymes, including glycogen phosphorylase (GP), pyruvate kinase (PK), phosphofructokinase (PFK) and lactate dehydrogenase (LDH), may affect meat quality[2]. Protein S-nitrosylation might modulate structural and functional properties of the glycolytic enzymes including catalytic activity, protein stability, oligomeric state, binding of allosteric activators and conditional protein interaction. However, the effect of protein S-nitrosylation on glycolysis in postmortem muscle has not been explicitly understood.
Nitric oxide (NO) is a signaling molecule that could mediate cellular metabolism processes through reactions with metal ions including iron and copper, or thiol in proteins[3]. Specifically, protein S-nitrosylation is mediated by NO and cysteine (Cys) redox center response. The sites of S-nitrosylation have been identified in many proteins, including receptors, ion channels, small G proteins, and transcription factors, especially in enzymes[4]. Our previous work has demonstrated that NO could regulate glycolysis in postmortem pork through the treatments with NO synthase inhibitor of Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) and NO donor of S-nitrosoglutathione[5]. Besides, Zhu et al. reported that the S-nitrosylation levels of energy metabolism enzymes were up-regulated in high ultimate pH beef compared to intermediate ultimate pH[6]. Wang et al. demonstrated that protein S-nitrosylation could influence glycolysis by changing activities of glycolytic rate-limiting enzymes, ultimately causing the development of pale, soft and exudative (PSE) meat[2]. However, NO donors might alter confounding factors including calcium release and apoptosis, leading to the change of glycolysis rate in postmortem muscle in the in vivo studies[7−9]. In order to reduce disturbances of these in vivo complex factors, an in vitro muscle glycolysis model was used to simulate the postmortem glycolysis process[10, 11].
The purpose of this research was to study the role of protein S-nitrosylation in postmortem energy metabolism by establishing the in vitro model. To simulate the effect of pH on protein S-nitrosylation, different pH conditions were designed in the in vitro glycolysis model to determine metabolite content, protein S-nitrosylation levels and pH value.
-
Six castrated crossbred pigs with an age of 6 months (Landrace × Duroc × Yorkshire; 100 ± 10 kg) were harvested in the slaughterhouse (Sushi Company, Huai'an, China) based on national standard slaughtering practices (GB/T 19479-2019). The LT muscle samples were removed from the carcass at 0.5 h postmortem and 500 g samples were immediately frozen in liquid nitrogen. Based on the criteria proposed by Warner et al., the pH and the color of pork samples were measured at 1 h and 24 h and all parameters were within the normal ranges[12]. The samples were stored −80 °C for further biochemical analysis.
In vitro buffer system
-
A total of 0.5 g pork samples (n = 6) were ground into powder in liquid nitrogen and then homogenized in the phosphate buffer (0.1 M K2HPO4) at a ratio of 1:2 (wt/vol). The samples were then incubated at 37 °C for 1 h with three treatments, respectively. The treatments were as follows: control (0.1 M K2HPO4), 0.4 mM and 1 mM NO donor (NOR-3, (±)-(E)-4-Ethyl-2-(E)-hydroxyimino-5-nitro-3-hexenamide, E2895; Sigma-Aldrich). After the incubation, the reaction buffers (10 mM Na2HPO4, 0.5 mM adenosine diphosphate, 0.5 mM nicotinamide adenine dinucleotide, 5 mM adenosine triphosphate, 40 mM glycogen, 60 mM KCl, 30 mM creatine, 10 mM sodium acetate, 5 mM MgCl2 and 25 mM carnosine) were added to the mixtures at a ratio of 1:5 (mixtures volume/reaction buffers volume) and incubated at 25 °C[13]. The pH values of the reaction buffers were adjusted to 6.5, 6.0 and 5.5 respectively by 0.1 M HCl and 0.1 M NaOH. At the following time points: 0, 0.5, 2, 4, 6 and 24 h, 5 mL samples were taken from the reaction buffer for pH and metabolite analysis.
pH measurement
-
A pH meter (Mettler Toledo, Shanghai, China) was used to measure the pH value of the reaction buffer at 0, 2, 4, 6 and 24 h, respectively. The pH meter was calibrated with pH 4.0 and 7.0 standard buffers at 25 °C before measurement. All samples were measured three times and the values were averaged for statistical analysis.
Metabolite analysis
-
Samples (1 mL) were taken from the reaction buffer for metabolite analysis[2]. Hydrochloric acid (2.5 M, 1 mL) was added to the samples (1 mL) and then the mixture was heated in boiling water for 20 min and centrifuged at 10,000 g at 4 °C for 20 min. The pH of the supernatant was adjusted to 7 with 1.25 M KOH and then the glycogen content of the sample was determined using the glycogen assay kit (A043-1-1; Jiancheng Bioengineering, Nanjing, China) and microplate readers (Luminescence, Tecan Austria Gmbh, Austria) at 620 nm.
Samples (1 mL) were taken from the reaction buffer for glucose-6-phosphate (G6P) and lactate analysis. Perchloric acid (1 M, 1 mL) was added to the samples (1 mL) and heated in boiling water for 20 min. The samples were then centrifuged at 10,000 g at 4 °C for 20 min. The pH of the supernatant was adjusted to 7 with 2 M KOH. G6P and lactate content were determined using the G6P kit (6PG-1-J; Comin, Suzhou, China) and lactate analysis kit (A019-2-1; Jiancheng, Nanjing, China), respectively.
Enzyme activity assay
-
A sample (1 mL) was centrifuged at 10,000 g at 4 °C for 20 min. The supernatant was then collected to determine the protein concentration using the bicinchoninic acid protein assay kit (PC0020; Beijing Solarbio Science & Technology Co., Ltd., China). The GP, PFK, PK and LDH activities of samples were detected using corresponding activity assay kits (BC3345, BC0535, BC0545, BC0685; Beijing Solarbio Science & Technology Co., Ltd., China).
S-nitrosylation protein extraction
-
Referring to the method of Wang et al., the S-nitrosylation protein was purified by iodo tandem mass tags (Iodo TMT) zero label reagent (90100; ThermoFisher Scientific, USA) and anti-TMT resin (90076; ThermoFisher Scientific, USA)[2]. Firstly, 1 mL samples were taken at 0.5 h, 1 M methyl methanethiosulfonate (20 μL) was then added into the sample. The mixtures were incubated at room temperature for 0.5 h to block free Cys thiols. Next, 1 mL sample was added to 6 mL pre-chilled acetone and frozen at −20 °C for 1 h to remove methyl methanethiosulfonate. The samples were centrifuged at 10,000 g at 4 °C for 10 min. The tubes were inverted to decant the acetone and the resulting pellet was redissolved with 200 μL HENS buffer (0.1 M 2-[4-(2-hydroxyethyl) piperazin-1-yl] ethanesulfonic acid, 1% sodium dodecyl sulfate, 0.1 mM neocuproine, 1 mM ethylene diamine tetraacetic acid, pH 7.8). To irreversibly bind with Cys thiol, the dissolved iodoTMT reagent (20 μL) and 1 M sodium ascorbate (40 μL) were added to 200 μL samples. The reaction samples were the incubated for 1 h at 37 °C under dark conditions. Following the above processes, 260 μL samples and 400 μL anti-TMT resin were added to the pierce spin column (69705; ThermoFisher Scientific, USA) and shaken overnight at 4 °C. Finally, the samples were eluted with 600 μL TMT elution buffer (90104; ThermoFisher Scientific, USA) and lyophilized with a vacuum concentrator. The protein S-nitrosylation level was calculated depending on the ratio of enriched S-nitrosylation protein and total protein.
Gel electrophoresis and western blotting
-
A 1 mL sample was taken at 0.5 h and centrifuged at 10,000 g at 4 °C for 20 min. The protein was then dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer (CW0027; Cowin Bio., China) at a ratio of 1:4 (sample volume/loading buffer volume), and then separated by 10% gels running at 120 V for 1 h. Following this, the gels were transferred to nitrocellulose transfer membranes (STM2008; Invitrogen, USA) running at 90 V for 1.5 h. The membranes were then blocked with 5% non-fat milk (RP0004, Ryon Biological Technology, China) at room temperature for 1 h. After that, the membranes were immunoblotted with a primary antibody of GP (ab81901; Abcam, UK; 1:1,000), LDH (ab101562; Abcam, UK; 1:1,000), PFK (ab154804; Abcam, UK; 1:1,000), PK (ab150377; Abcam, UK; 1:1,000) and β-actin (4970; Cell Signaling Technology, USA; 1:1,000) at 4 °C for 12 h. The membranes were then washed six times (6 × 5 min) with tris-buffered saline with tween buffer (TBST, 20 mM tris hydroxymethyl methyl aminomethane, 5 mM KCl, 0.1% tween 20 and 150 mM NaCl). The mouse anti-rabbit immunoglobulin antibody (horseradish peroxidase) (A01827; GenScript, China; 1:5,000) was then used to treat the membranes for 1.5 h. The membranes were washed with TBST six times. The membranes were then reacted with the peroxide solution and ECL substrate (34580; ThermoFisher Scientific, USA) for 6 min. Finally, the relative amounts of the transferred proteins were quantified by scanning the Image Quant LAS4000 (GE, CT, USA). The β-actin was used as a reference protein and the total protein was normalized to the corresponding β-actin level[2].
Statistical analysis
-
Data were presented as mean ± standard error (SE). Statistical analysis system (SAS Institute Inc., Cary, NC, USA) software was employed to perform the least significant difference test. Duncan's multiple range tests were used to compare differences in the individual groups (p < 0.05).
-
The rate and the extent of pH decline are known to largely influence meat quality. Hence, the effect of NOR-3 on glycolysis was studied under different pH (6.5, 6.0 and 5.5) in vitro glycolysis buffer systems. NOR-3 was chosen at concentrations of 0.4 mM and 1 mM based on our previous studies in which the concentrations were effective to generate protein S-nitrosylation in porcine LT muscle[14]. Reaction vessels with NOR-3 had significantly lower pH value in comparison with the control group at pH 6.5, 6.0 and 5.5 (Fig. 1; p < 0.05). The results revealed that NOR-3 treatment resulted in a faster glycolysis rate. At pH 6.0 and 6.5, the addition of 0.4 mM NOR-3 also accelerated the decline of pH value, though at a significantly lesser extent compared to the treatment of 1 mM NOR-3 (p < 0.05). However, there was no significant difference in pH value among different reaction times at pH 5.5 (p > 0.05). A similar result was previously reported by England et al., which indicated that PFK activity significantly decreased at pH 5.5 and inhibited postmortem glycolysis[10]. Therefore, this phenomenon might be due to the inhibition of glycolytic enzyme activity by low pH value.
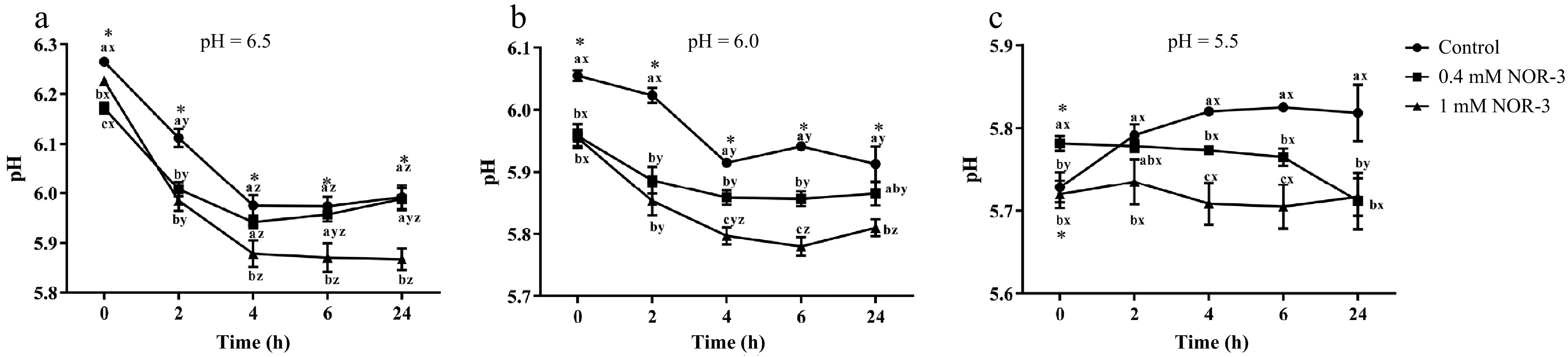
Figure 1.
pH of in vitro reactions. All measurements were expressed as the mean ± SE. a–c: Different letters at the same time points are significantly different between treatments (p < 0.05). x–z: Different letters are significantly different at different incubation times within the same treatment (p < 0.05). (n = 6).
The rate and degree of pH decline could significantly affect protein structure and activity in postmortem muscle and thus influence pork quality[15]. Our results are consistent with previous studies which proved that NO decreased the pH value. Bolaños et al. found that NO could increase the consumption rate of glucose and the accumulation of lactate in cultured astrocytes, indicating the occurrence of glycolysis activation[16]. According to Young et al., NO could regulate the glycolysis and increase the rate of glucose transport in skeletal muscle leading to the accelerated energy metabolism through the stimulation of guanylate cyclase[17]. However, Zhang et al. reported that L-NAME treatment led to a lower pH value at 24 h compared to the control group in postmortem pork[5]. It may contribute to the changes of enzyme activity and the occurrence of calcium release and apoptosis in vivo, which influences the rate of glycolysis in postmortem muscle[5,7,8].
Metabolite analysis
-
Lactic acid production and glycogen degradation can reflect the degree of energy metabolism. As shown in Fig. 2, NOR-3 treatments significantly influenced lactate accumulation over time. At pH 6.5, 6.0 and 5.5, NOR-3 significantly increased lactate formation at 0.5, 2 and 24 h compared to control group (p < 0.05). At 24 h, more lactic acid was significantly accumulated in the 1 mM NOR-3 treatment group indicating that NOR-3 could regulate postmortem glycolysis in the in vitro model even at low pH conditions (p < 0.05). The current results are similar to the previous study that NO promoted glucose utilization and lactic acid accumulation in vitro[17]. Jin et al. also found that NO could enhance glycolysis in osteoblasts by upregulating the transcription of multiple glycolytic genes[18].
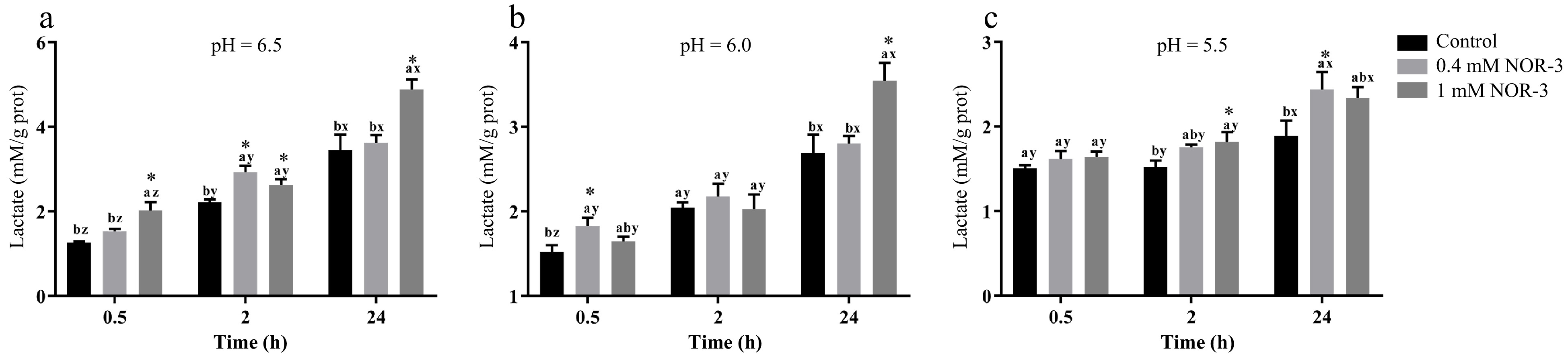
Figure 2.
The content of lactate in the in vitro reactions. All measurements were expressed as the mean ± SE. a–c: Different letters at the same time points are significantly different between treatments (p < 0.05). x–z: Different letters are significantly different at different incubation times within the same treatments (p < 0.05). (n = 6).
Glycogen can be degraded into glucose-6-phosphate by metabolic enzymes, which provide precursors for the metabolic pathway of glycolysis in muscle[13]. To investigate the effect of NOR-3 treatment on glycolysis, the glycogen and G6P concentrations were also measured in the homogenate. The content of glycogen decreased over time while there were no significant differences in glycogen degradation between different treatment groups at pH 6.0 and 5.5 (Fig. 3; p > 0.05). However, glycogen content significantly decreased at 0.5 h and 24 h when NOR-3 was added to reactions (Fig. 3; p < 0.05). The results suggest that glycogen degradation was enhanced compared to the samples without NOR-3 at pH 6.5. In addition, less glycogen was degraded and the declining trend was more gentle at pH 5.5 compared to pH 6.0 and 6.5 possibly due to the low pH induced-inhibition of enzyme activity (p > 0.05). This finding also indicates that low pH conditions weaken the effect of NOR-3, which is consistent with the pH result. At 0.5, 2 and 24 h, samples with NOR-3 at pH 6.0 and 5.5 showed significantly greater G6P content compared to the control group (Fig. 4; p < 0.05). However, samples with 1 mM NOR-3 (at pH 6.5) had significantly lower G6P content compared to the 0.4 mM NOR-3 and control groups (Fig. 4; p < 0.05). These results suggest that G6P was utilized faster than its accumulation at pH 6.5. The content of G6P increased initially and then decreased with time extension, which is consistent with the study of postmortem metabolism in vitro model[19]. Previous studies have shown that the rate and the extent of energy metabolism were controlled by regulating the balance of G6P consumption in the pentose phosphate and the glycolysis pathway when treating astrocytes with NO[16].
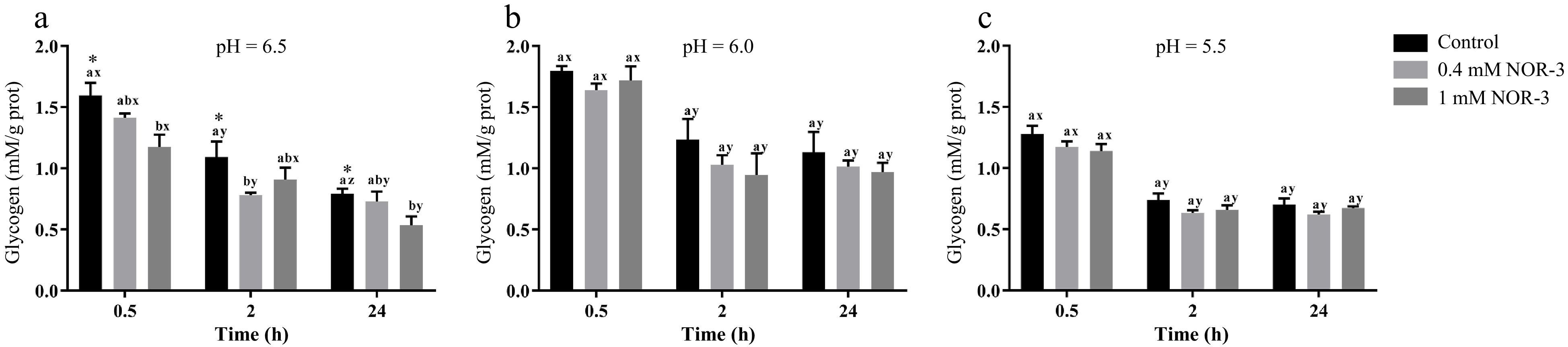
Figure 3.
The content of glycogen in the in vitro reactions. All measurements were expressed as the mean ± SE. a–c: Different letters at the same time points are significantly different between treatments (p < 0.05). x–z: Different letters are significantly different at different incubation times within the same treatments (p < 0.05). (n = 6).
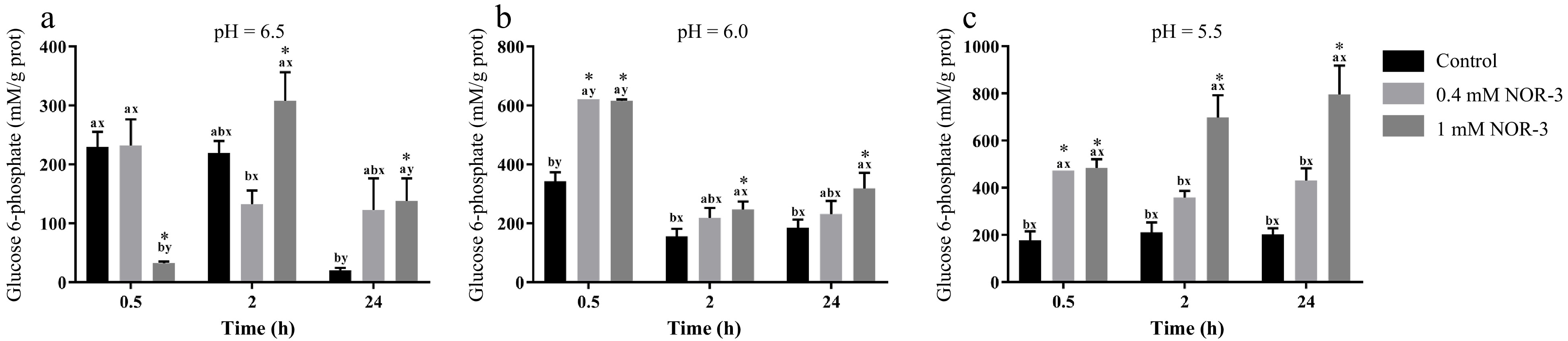
Figure 4.
The content of glucose-6-phosphate in the in vitro model. All measurements were expressed as the mean ± SE. a–c: Different letters at the same time points are significantly different between treatments (p < 0.05). x–z: Different letters are significantly different at different incubation times within the same treatments (p < 0.05). (n = 6).
Cidad et al. found that NO could improve glucose uptake by facilitating the migration of glucose transporter 1 and glucose transporter 3[20]. In addition, Merry et al. demonstrated that NO might be involved in regulating glucose consumption in muscle cells[21]. Our study confirms that NOR-3 stimulated glycolysis progress and resulted in greater lactate accumulation and higher glycogen degradation, which are consistent with the previous findings. This result may be attributed to the activation of cyclic guanosine-3',5'-monophosphate-dependent protein kinase, leading to a higher glycolysis rate[18]. However, Zhang et al. reported that the glycogen and lactate content at 24 h had no significant difference between the S-nitrosoglutathione and control groups in postmortem pork[5]. In addition, the GAPDH and GP activity of S-nitrosoglutathione treatment group had no significant difference with the control group. This may be due to the fact that the S-nitrosylation sites of GP and GAPDH were less sensitive in changing the activity.
Activities of PK, GP, LDH and PFK
-
The activities of the energy metabolism enzymes could affect the degradation of muscle glycogen and the decrease of pH value. In this study, the NOR-3 group showed significantly higher LDH activity than the control group at pH 6.5 and 5.5 (Fig. 5a; p < 0.05). Furthermore, reaction vessels with 1 mM NOR-3 had significantly lower PK activity compared to the 0.4 mM NOR-3 and control groups at pH 6.5 and 6.0 (Fig. 5b; p < 0.05). At pH 6.0 and 5.5, reaction vessels with NOR-3 had significantly lower activity of PFK compared to the control group (p < 0.05) while no significant differences were found among treatment groups at pH 6.5 (p > 0.05). The glycolytic enzyme activities are similar to those previously reported. Werner et al. showed that the level of PFK activity remained high at 0.6 h postmortem but decreased at 12 h[22]. This phenomenon might be due to the partial denaturation and the inactivation of PFK in postmortem muscles when pH decreased rapidly. Similarly, NOR-3 treatments significantly influenced GP activity (Fig. 5d; p < 0.05). At pH 6.5, reaction vessels with NOR-3 had significantly augmented activity of GP compared to the control group (p < 0.05). These data suggest that NOR-3 was responsible for the different effects on energy metabolism enzymes and accelerated the rate of glycolysis in postmortem muscle.
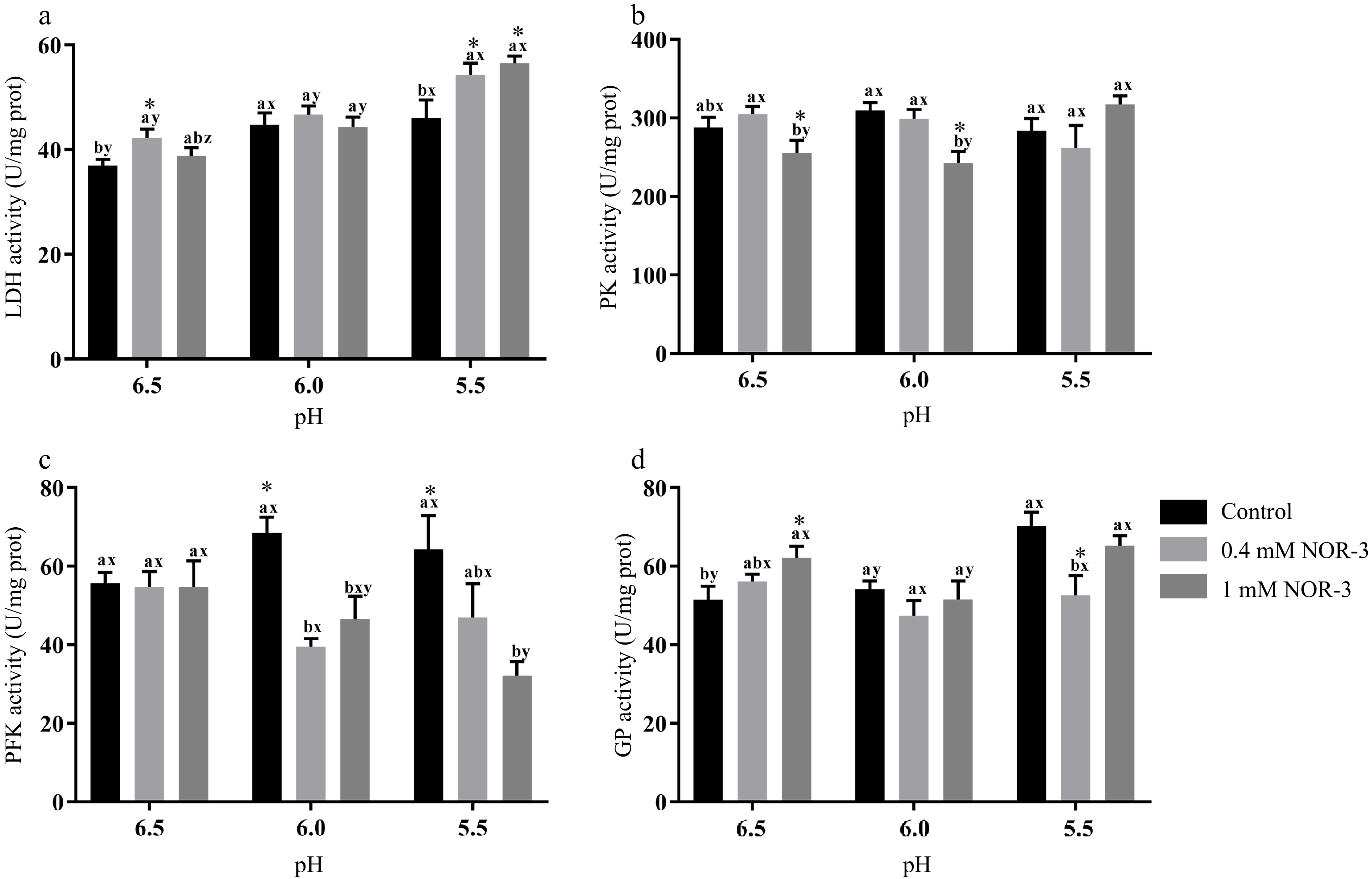
Figure 5.
The activities of glycolytic enzymes in the in vitro model at 0.5 h. All measurements were expressed as the mean ± SE. a–c: Different letters at the same pH conditions are significantly different between treatments (p < 0.05). x–z: Different letters are significantly different at different incubation pH within the same treatments (p < 0.05). (n = 6).
Similarly, Almeida et al. demonstrated that NO accelerated the rate of glycolysis by the phosphorylation of adenosine 5'-monophosphate activated protein kinase, resulting in the activation of phosphofructokinase muscle type[7]. Wehling-Henricks et al. demonstrated that the specific activity of PFK and glycogen utilization were increased by neuronal NO synthase in muscle, which was due to the positive allosteric interactions between neuronal NO synthase and PFK[23]. However, some reports showed NO could reversibly inhibit the activities of creatine kinase and aldolase by S-nitrosylation in vitro and rat skeletal muscle, respectively[24,25]. Zhang et al. reported that L-NAME treatment led to a higher level of GAPDH, GP and PK activities at 24 h compared to the control group in postmortem pork, possibly ascribed to the effect of protein S-nitrosylation[5]. Zhou et al. reported that protein S-nitrosylation inhibited the formation of tetrameric wild-type PKM2 (Cys 152/Cys 358) but not tetrameric PKM2 (Cys 423/Cys 424A) using purified proteins[26]. This result may be explained by the fact that different modification sites could produce different effects and that S-nitrosylation reactions may require negative electrostatic potential for amino acid residues adjacent to Cys residues within the protein or on the protein surface[27].
Expression and S-nitrosylation levels of LDH, GP, PFK and PK
-
Glycolytic enzymes are considered as good markers for meat tenderness including PK, GAPDH, enolase and LDH[15]. Many published studies have suggested that most of the widespread influences of NO on cellular function was attributed to the S-nitrosylation of Cys residues leading to the changed function and activity of protein[4]. In the present study, no significant differences were observed for the expressions of LDH, GP, PK and PFK in different treatments groups (Fig. 6; p > 0.05). As shown in Fig. 7, the S-nitrosylation levels of LDH, GP, PK and PFK in all treatment groups were significantly increased compared to the control group (p < 0.05). Su et al. found that GP, LDH, PFK and PK had a wide range of S-nitrosylated Cys sites identified by the biotin switch method[28]. Liu et al. also found that the sites of S-nitrosylation were identified in GP (Cys 496, Cys 260 and Cys 335), PFK (Cys 351 and Cys 709), PK (Cys 49) and LDH (Cys 163) during postmortem aging of porcine LT muscles[29]. For the enzymes containing critical thiols at their active sites, the covalent attachment with NO group led to functional changes[3]. In addition, these S-nitrosylated glycolysis rate-limiting enzymes have intense regulatory effects on physiological activities, which explain the changes of energy metabolism rate.
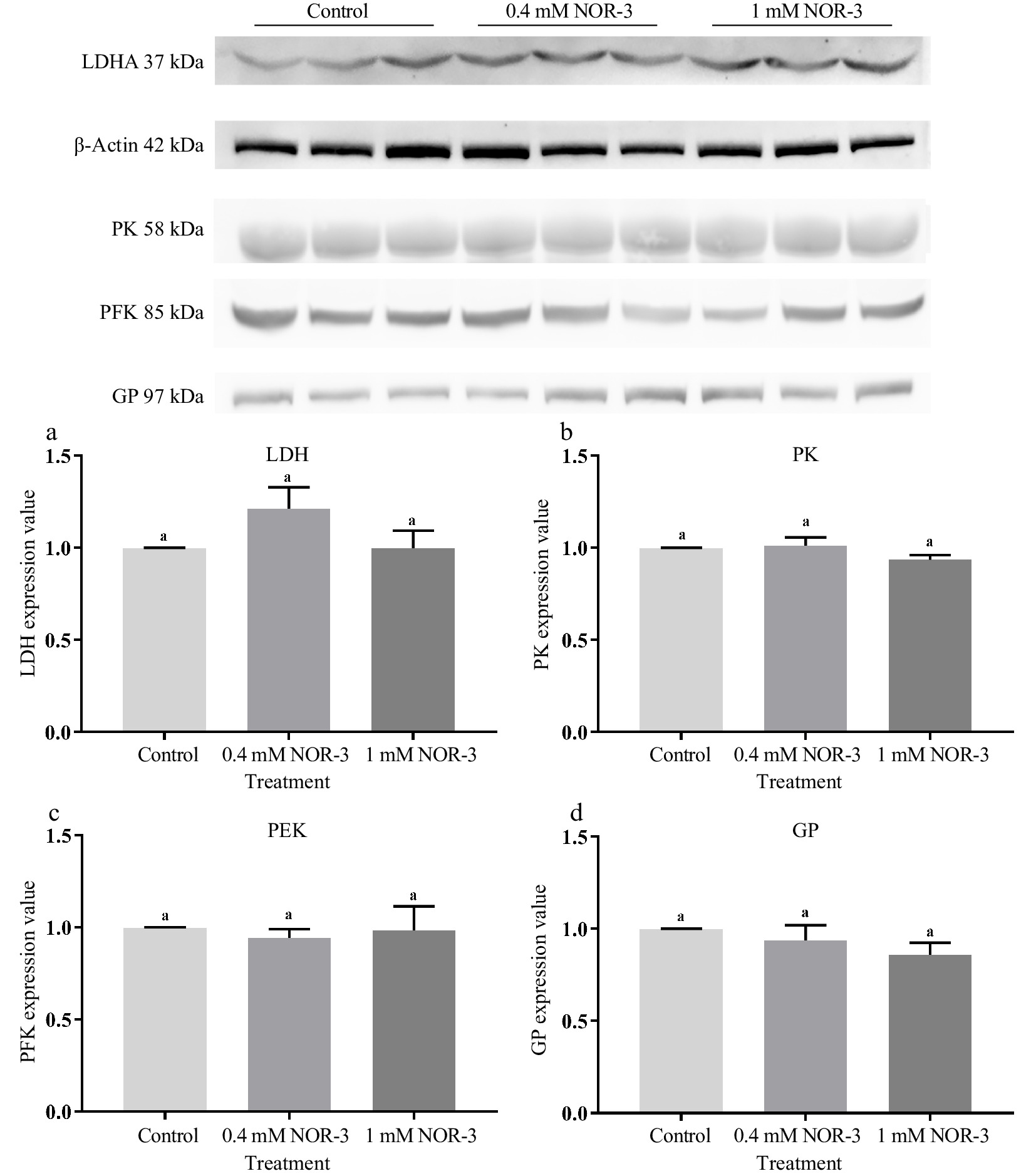
Figure 6.
The expression value of glycolytic enzymes in the in vitro model at 0.5 h. All measurements were expressed as the mean ± SE. a–c: Different letters are significantly different between treatments (p < 0.05). (n = 6).
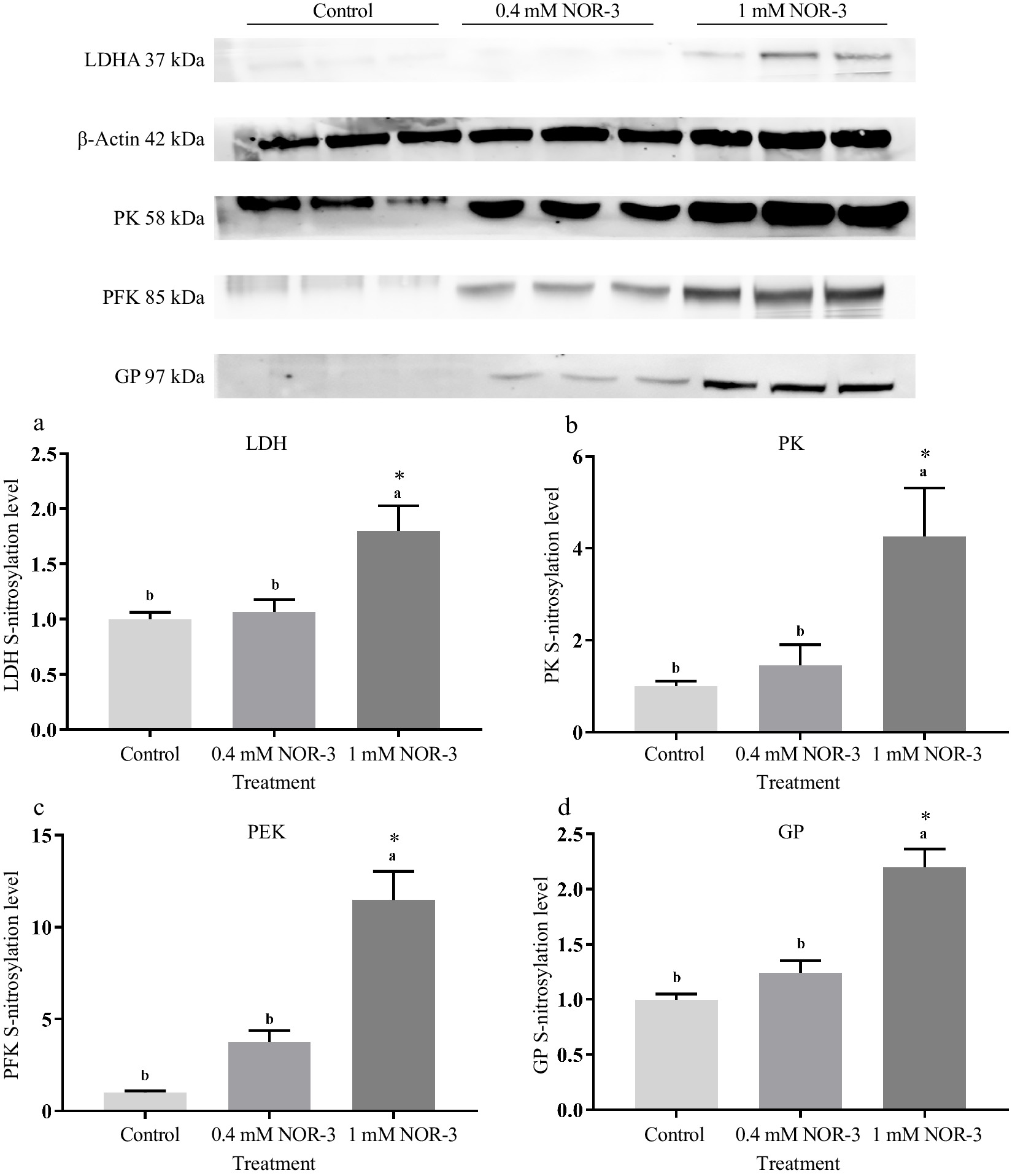
Figure 7.
The S-nitrosylation levels of glycolytic enzymes in the in vitro reactions at 0.5 h. All measurements were expressed as the mean ± SE. a–c: Different letters are significantly different between treatments (p < 0.05). (n = 6).
Mohr et al. observed that S-nitrosylation could protect GAPDH from inactivation in adverse environments and regulate glycolysis[30]. Previous studies reported that several Cys residues of sarcoendoplasmic reticulum Ca2+-ATPase (muscle type) and ryanodine receptor channel (muscle type) in skeletal muscle were S-nitrosylated, which influenced their activities, further resulting in the disturbance of calcium metabolism in sarcoplasm[31,32]. However, Zhang et al. suggested that inhibiting S-nitrosylation levels of GAPDH, PK and GP could increase enzyme activities and strengthen glycolysis rate[5]. This suggests that the modification of Cys may cause a conformational variation in the enzyme, leading to changed catalytic efficacy[25]. The inconsistent results may be due to the different regulatory effects on S-nitrosylation of the reactive Cys thiols at the enzyme active site, which is related to the distance between the modified site and the active site of the enzyme. In addition, some Cys residues are distributed within the protein and are not susceptible to S-nitrosylation[27]. Meanwhile, different types and concentration of NO donors may also produce inconsistent results, which might be attributed to the different NO release patterns and rates[33]. In summary, the results suggest that higher S-nitrosylation levels of PK, PFK, LDH and GP could influence the activities of glycolytic enzymes, leading to the elevated rate of energy metabolism.
-
This research provides evidence that NOR-3 (NO donor) increases the activities of GP and LDH by improving S-nitrosylation levels of GP and LDH, thereby enhancing postmortem glycolysis in the in vitro muscle glycolytic model. Additionally, low pH can weaken the effect of NOR-3 treatment and inhibit the rate of glycolysis. This work reveals the regulating mechanism of S-nitrosylation on the key rate-limiting enzymes, which is beneficial for future investigation on the relationship between protein S-nitrosylation and meat quality.
This work was financially supported by National Natural Science Foundation of China (Grant No: 31871827), China Agriculture Research System of MOF and MARA (CARS-35) and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS (2020) 425).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lu W, Hou Q, Zhang W. 2022. Protein S-nitrosylation regulates the energy metabolism of early postmortem pork using the in vitro model. Food Materials Research 2:4 doi: 10.48130/FMR-2022-0004
Protein S-nitrosylation regulates the energy metabolism of early postmortem pork using the in vitro model
- Received: 19 January 2022
- Accepted: 02 March 2022
- Published online: 30 March 2022
Abstract: This research aimed to investigate the regulation of energy metabolism by protein S-nitrosylation utilizing the in vitro muscle glycolysis model. Longissimus thoracis (LT) muscles homogenates were treated with nitric oxide donor NOR-3 ((±)-(E)-4-Ethyl-2-(E)-hydroxyimino-5-nitro-3-hexenamide) and control (0.1 M K2HPO4) under different pH conditions (6.5, 6.0 and 5.5) in vitro buffer system for 24 h, respectively. Results indicated that the NOR-3 treatment group had a significantly higher pH decline rate than the control group and resulted in a higher lactate accumulation and glycogen degradation at 24 h compared with the control group (p < 0.05). Moreover, NOR-3 treatment significantly increased the activities along with S-nitrosylation levels of lactate dehydrogenase and glycogen phosphorylase at pH 6.5 in a concentration-dependent manner (p < 0.05). In addition, low pH could weaken the NOR-3 treatment effect and inhibit glycolysis rate. Thus, protein S-nitrosylation could play a role in regulating postmortem glycolysis in vitro model even at low pH conditions.
-
Key words:
- Protein S-nitrosylation /
- Postmortem glycolysis /
- Enzymes /
- Meat quality












