-
Salt-induced swelling of meat, especially of pork meat in the preparation of products such as ham, is an important industrial process affecting the water-holding capacity (WHC) of the raw material. As reviewed by Puolanne[1], there are three main hypotheses of the mechanisms of water-holding in meat, all of which centre on the swelling or shrinkage of the myofilament lattice within the myofibrils. Hamm[2,3] proposed that electrostatic interactions between myofilaments, balanced by hydrophobic effects and elastic cross-bridges, were the main mechanisms stabilizing water in the myofibrillar protein lattice. Offer & Knight[4] consider electrostatic forces to be possible at very long sarcomere lengths, where the myofilaments are very close together, but proposed that, at normal sarcomere lengths, osmotic forces created by the net charges of the thick and thin filaments and the ions bound to them, balanced by the elasticity of the actin-myosin cross-bridges, was a dominant mechanism. Offer et al.[5] extended this model to include the entropic effects of myosin dissociation from the thick filament in the presence of salt or polyphosphate, but no formulation of this mechanism was presented. The third main proposal was that capillary forces in the very small channels between myofilaments stabilize water in the muscle cell structure[6,7].
The highly-cited studies of Offer & Trinick[8] and other studies on salt swelling[9−11] all conducted experiments on either myofibrillar preparations, where the intermediate filament lattice joining the Z-discs of myofibrils is broken, or single fibre preparations. In all of these studies, the preparation of the myofibrils or fibres involved washing or soaking the preparations in a homogenizing solution (often 0.15 M KCl) that effectively removed all the soluble sarcoplasmic proteins from the study material. As Puolanne[1] observed, these papers do not consider the role of water-soluble proteins in water-holding in raw meat, or in the mechanism of salt-swelling.
Depending on the post-mortem pH and temperature history of post-mortem muscle, there can be denaturation of proteins, such as myosin[12], but also of the sarcoplasmic proteins. Hughes et al.[13] presented evidence to suggest that some of the denatured sarcoplasmic proteins can be precipitated onto the surface of myofilaments at some pH/temperature combinations. Liu et al.[14] showed that, in PSE-like conditions, denatured sarcoplasmic proteins improved the WHC of denatured myofibrils. Van Laack[15] pointed out that the reduced WHC of RSE (red, soft, exudative) pork is not due to excessive myosin denaturation, but suggested it is due to sarcoplasmic protein denaturation.
In relation to the osmotic forces that the widely-cited hypothesis of Offer & Knight[4] is based on, one question concerns the contribution that the water-soluble sarcoplasmic proteins can make to these. The osmotic pressure of post-rigor beef sternomandibularis and psoas muscles (480−540 mOs) is considerably higher than for the same muscles in the pre-rigor state (300 mOs), which Winger & Pope[16] ascribe to an increase in low molecular weight metabolic products during post-mortem metabolism. Winger & Pope[16] estimate that only a small fraction of the osmotic pressure in post-rigor meat arises from the muscle proteins. Christensen et al.[17] showed that muscle juice pressed from 24-h post-mortem bovine semitendinosus muscle had an osmolality of approximately 550 mOs/kg water. In agreement with previous results[16,18], Christensen et al.[17] showed that the osmolality of extracted muscle juice did not change substantially on ageing the meat (which involves proteolysis, increasing the number of protein moieties) and only decreased slightly on cooking at temperatures up to 80 °C, where the majority of the muscle proteins (both water-soluble and structural) are denatured. Thus, the osmolality of the muscle juice did not depend substantially on the concentration of water-soluble proteins and peptides within it. As the osmolality of a solution is a colligative property (i.e., depending on the ratio of the number of solute particles to the number of solvent molecules, rather than their identity), this result supports the interpretation that it is the low molecular weight ions and metabolites in muscle that largely determines the osmotic pressure within meat, rather than the proteins. Thus, the evidence from osmolality studies on muscle juices suggests a diametrically opposed hypothesis to that from experiments on meat and myofibrils reported by van Laack[15] and Liu et al.[14]; the osmolality studies suppose that water-soluble proteins are not important in determining water-holding due to osmotic force mechanisms, whereas the myofibril experiments proposed that WHC is markedly influenced by these proteins. Zhang et al.[19] showed that freeze-thaw cycles reduce the WHC of minced pork due to some myofibrillar protein denaturation. Akköse[20] showed that freezing-thawing increased the salt diffusion coefficients in bovine LL muscle. There is therefore reason to suppose that freezing and thawing may affect salt-swelling of pork due to several mechanisms, which may include its effect on the water-soluble muscle proteins.
The purpose of the experiments described here was to test the hypothesis that water-soluble muscle proteins can affect the swelling of meat that occurs in salt solutions. The experimental material chosen is porcine longissimus muscle, because of the large amount of pork used in salted meat products and that the composition of water-soluble proteins in this muscle has been previously documented[21].
-
The principal experimental material used was porcine longissimus lumborum muscle, for reasons stated above. Sections approximately 10 cm long of the loin were obtained from two commercially-available pigs (dressed half-carcass weight 45−50 kg) at 60 h post-mortem. The longissimus lumborum (LL) muscle was dissected, cleaned of all surface fat and connective tissue, and half was cut into blocks of approximately 5 cm long and 1-2 cm in width and depth, with the long axis of the block parallel to the muscle axis (the muscle fibre direction was observed to be at 45−50 degrees to this axis). These blocks were used immediately. The remaining half of the muscle sample was double-wrapped in polythene and frozen at −20 °C. After 3 weeks, this material was thawed at room temperature and cut into similar blocks.
Effect of regular replacement of salt solution on rate of swelling
-
This first part of the experiment investigated the effect of a regular replenishment of fresh salt solution on meat swelling, as compared to swelling for the same period of time but in an original volume of saline. The hypothesis being tested is that, by changing the saline, the removal of molecules leaching out of the meat, together with keeping the concentration of sodium and chloride in the soaking solution nearly constant, will produce a different amount or rate of swelling compared to keeping the meat in one volume of the soaking solution throughout the time period studied. Eight blocks of porcine LL muscle were initially weighed and four blocks placed in each of two baths containing 500 ml of 3% NaCl (0.513 M), pre-chilled at 4 °C. The baths were provided with tightly fitting lids and stored at 4 °C for the following 7 d. After each day of storage, the blocks were lightly blotted and weighed, and 2 sub-samples of 1 mL each taken from each bath. One bath was then returned to 4 °C without changing the saline, but for the other bath, the saline was removed each day and replaced with 500 mL of fresh pre-chilled 3% NaCl solution, before being returned to 4 °C.
Effect of restricting protein outflow on the rate of swelling
-
The purpose of this second part of the experiment was to test the hypothesis that leeching of soluble proteins out into the soaking solution altered the swelling capacity of the meat. One inch-diameter cellulose dialysis tubing (Sigma Aldrich, molecular weight cut-off 14 kDa) was used to restrict the removal of soluble proteins from the meat. Eight blocks of porcine LL muscle were initially weighed and four blocks placed in each of two baths containing 1 L of 3% NaCl (0.513 M), pre-chilled at 4 °C. In one bath, the four meat blocks were allowed to float freely within the salt solution, as in the experimental part one in above. Before being placed in the other bath, four meat blocks were individually placed within lengths of dialysis tubing, with the ends secured by clips. The baths were provided with tightly fitting lids and stored at 4 °C for the following 7 d. At 24-h intervals, the blocks were lightly blotted and weighed, and two sub-samples of 1 mL each taken from each bath. When weighing the blocks in the dialysis bags, one end of the bag was unclipped and the meat sample gently extracted without spilling any of the liquid that had accumulated inside each bag. After gentle blotting and weighing, the meat blocks were returned to their original dialysis bags, which were resealed with the clips. No changes of salt solution were performed in each of the baths during the 7-d soaking period.
Testing the possible restriction to swelling provided by dialysis tubing
-
Although the meat blocks were a loose fit inside the dialysis tubing, it was hypothesized that the tubing could affect the swelling behaviour of the meat samples by providing a physical restraint to lateral swelling. To test this, the second part of experiment described above was repeated with 16 slightly smaller blocks of porcine LL muscle (initial weight range 8−13 g) divided equally between two baths containing 1 L of 3% NaCl. All muscle samples in this thord part of the experiment were contained within dialysis tubing, but in one bath the dialysis tubes were all closed by clips, as above, whereas in the other bath, the dialysis tubes had been given pin-prick punctures every 2 cm along their length and the ends were left open (unclipped). All other procedures were as described in the second experimental part above.
Effect of one freeze-thaw cycle on swelling behaviour
-
Fourthly, to test the effect of freezing, the pork LL samples that had been frozen and thawed were used to repeat the third experimental part described previously.
In all parts of the experiment, the 3% NaCl solution used was unbuffered and maintained at 4 °C. Daily checks of the pH in the solutions showed that the buffering capacity of the raw meat maintained the pH of the salines in the range 5.5−5.6 in all cases.
Protein concentrations in the salines and protein identification
-
The concentration of protein in the small (1 mL) samples of saline taken at each time point in each experiment were measured by the ratio of UV absorbance at 260 : 280 nm, using serum albumin as a standard[22]. At the end of the experiment, samples of the salines in the soaking baths for each treatment were taken and analysed by SDS-Polyacrylamide gel electrophoresis (SDS-PAGE), using 12% polyacrylamide slab separating gels with a 5% polyacrylamide stacking gel (BioRad), with 20 μg of protein in each lane (except for the second treatment above (saline changed daily)), where 10 μg of protein was analysed. Molecular weight markers (Kaleidoscope Prestained Standard; BioRad) were run in an adjacent lane. Gels were run at room temperature (approximately 20 °C) for 10 min at 100 V for protein introduction into the gel, followed by 1.2 h at 150 V (Mini protean III machine; Biorad). Following staining with Coomassie blue, images of the gel were obtained using an Amersham ImageQuantTM camera (Global Life Sciences Solutions USA LLC, Cranbury, NJ, USA).
-
Table 1 shows the meat:brine ratios and measured protein content in salines at the end of each experimental part, compared to the theoretical maximum concentration of sarcoplasmic proteins, assuming that 5.5% of the initial wet weight of the meat pieces is sarcoplasmic protein and that all of this is extracted into the saline. The treatments where there was no restriction to the outflow of solubilized protein into the saline (i.e., no dialysis tubing in experiment part 2, and open-ended/punctured dialysis tubing in experiment parts 3 and 4) the protein concentration in the saline at the end of the experiment was higher than could be expected if only the sarcoplasmic proteins were extracted, indicating that there was some partial solubilization of myofibrillar proteins (e.g., myosin or actomyosin) in these treatments.
Table 1. Meat:brine ratios and protein content in salines at end of experiment (after 7 d).
Experiment part Treatment Total meat mass
(g)Saline volume
(ml)Meat : Brine ratio Measured final protein
(mg/ml)Maximum sarc prots
(mg/ml)1 Change 89.1 500 1:5.6 6.91* 9.80 1 No change 82.2 500 1:6.1 7.02 9.04 2 Dialysis 61.1 1,000 1:16.4 2.24** 3.36 2 No dialysis 69.9 1,000 1:14.3 5.25** 3.84 3 Open 90.6 1,000 1:11.4 7.91 4.98 3 Closed 82.5 1,000 1:12.1 3.83 4.54 4 Frozen open 78.7 1,000 1:12.7 6.99 4.33 4 Frozen closed 86.3 1,000 1:11.6 2.82 4.75 Maximum sarc prots (mg/ml): This is the maximum predicted values of protein concentration if sarcoplasmic proteins comprise 5.5% of wet muscle weight and all of this is extracted into the volume of saline used. Values of the measured final protein concentration that exceed the predicted maximum concentration of sarcoplasmic proteins are marked in bold font. * Value shown is for the summed concentration of protein extracted in the daily change of saline. ** These values were from day 6 of the study (values on day 7 not available). Regular replacement of bathing saline increases meat swelling
-
Figure 1a shows the percentage increase in weight of meat blocks immersed in 500 ml of 3% salt solution over a period of 7 d, compared to the percentage increase in weight of similar meat blocks when the salt solution was exchanged for an equivalent volume of fresh saline on each day of the studies. Figure 1b shows the protein concentration in the saline on each day of the study, for both treatments. The replacement of saline solution daily increases the weight gain in the meat blocks. This could be due to the egress of either soluble protein or small molecular weight metabolites, or conceivably, a more constant concentration of sodium and chloride ions in the solution. Protein concentration in the bathing solution that was not changed daily (no change in Fig. 1b) initially increased rapidly, but at a diminished rate on subsequent days. Because of the daily change of saline (change in Fig. 1b), the measured protein concentration on each day diminished. The cumulative extraction of protein with daily changes of saline is also shown in Fig. 1b (summed change). The magnitude and rate of protein extraction shown by this curve is comparable to the 'no change' curve.
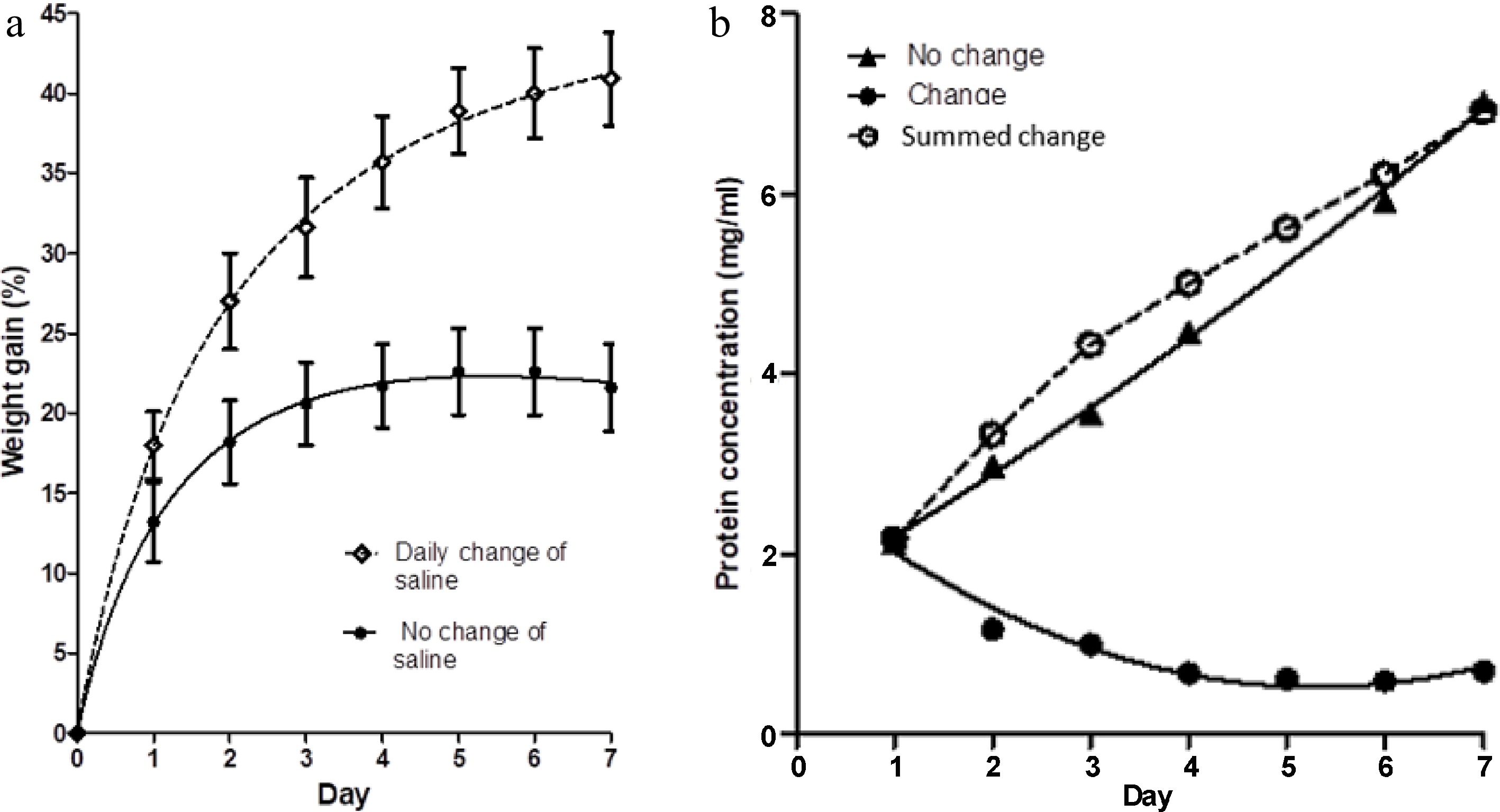
Figure 1.
(a) The percentage weight change in muscle blocks measured each day over a 7-d period of immersion in 3% NaCl solution, with two treatments; maintenance of the same saline solution throughout (no change of saline), and replacement of the saline each day with fresh, pre-chilled, 3% NaCl solution. Values shown are means ± standard errors. (b) Concentration of protein in the two saline baths (change and no change) on each day, measured just before replacement of the saline in the 'change' case. Each point is the mean of two determinations. Summed change is the cumulative concentration of protein in the salines of the treatment with daily changes of solution.
Dialysis membrane reduces protein egress and reduces meat swelling
-
Figure 2a shows a comparison of the percentage increase in weight of meat blocks immersed in one litre of 3% salt solution compared to the percentage weight gain of similar meat blocks in closed dialysis bags immersed in a second bath of 1 litre of 3% salt solution. The saline in both baths was not changed during the 7 d of observations. Figure 2b shows the protein concentration within both baths on each day. For the meat blocks within the dialysis bags, their weight gain and the concentration of protein extracted into their saline bath were reduced in comparison with the blocks in free solution. A small volume of pink-coloured viscous liquid (presumably concentrated solubilised protein) developed with time inside the dialysis bags.
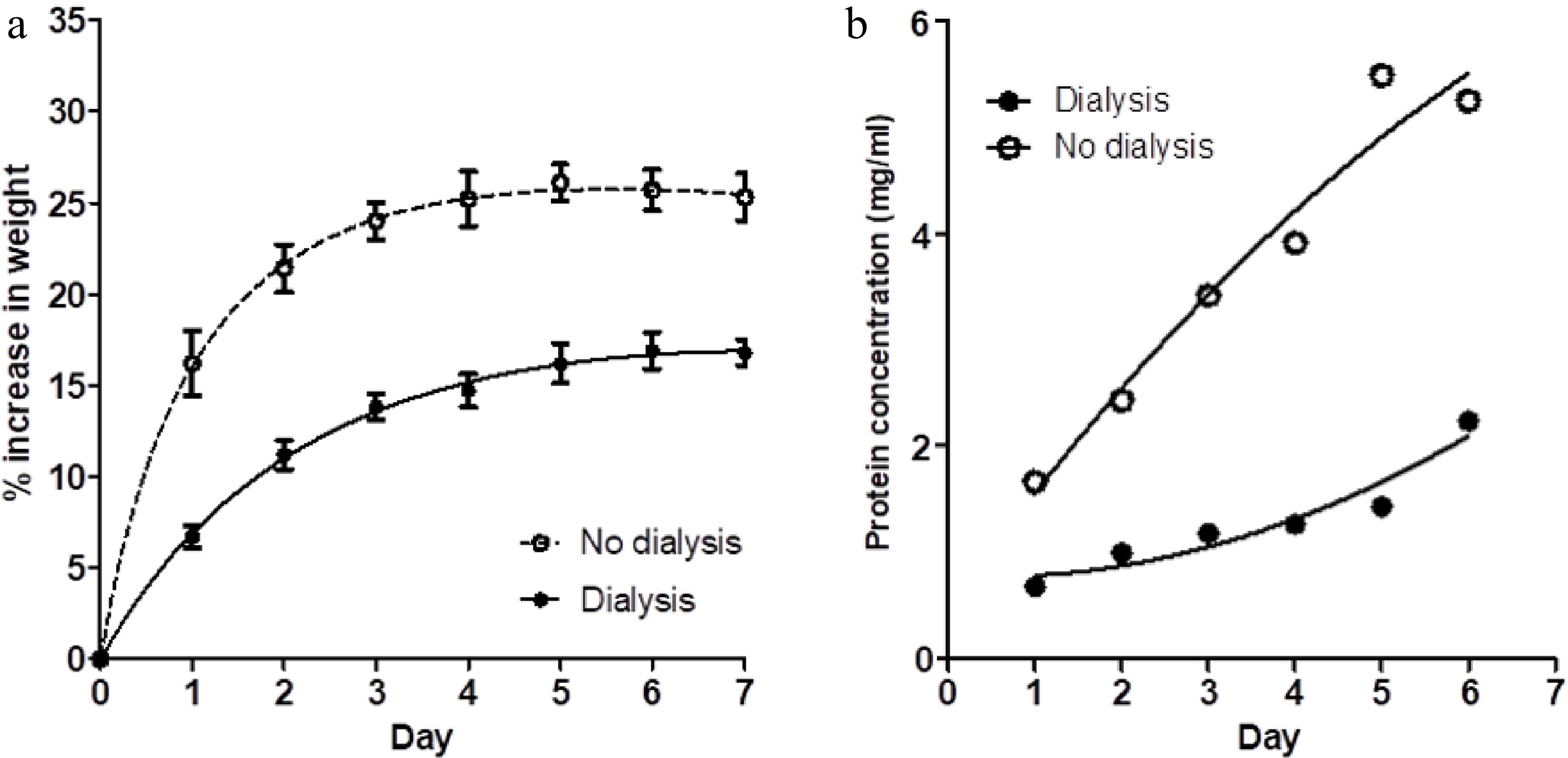
Figure 2.
(a) The percentage weight change in muscle blocks measured each day over a 7-d period of immersion in 3% NaCl solution, without changes of saline, for two treatments; muscle blocks free-floating in saline (no dialysis) and blocks enclosed in dialysis bags (dialysis). Values shown are means ± standard errors. (b) Concentration of protein in the two saline baths (dialysis and no dialysis) on each day. Each point is the mean of two determinations.
Although the brine:meat ratios in this experiment were roughly double those reported above (as documented in Table 1), the final protein concentration in the saline bath containing meat blocks without dialysis bags was only a little lower, showing that protein extraction was incomplete in the initial experiment reported above. However, the percentage weight gains for the meat blocks without daily change of saline (no change, Fig. 1a) and those shown in Fig. 2a are comparable.
Dialysis membrane provides no constraint to swelling in either fresh or frozen/thawed meat
-
Figure 3a shows the percentage weight gain of muscle blocks in four treatments (each treatment in their own one-litre bath of 3% salt solution); (i) blocks of fresh meat in dialysis tubing, but with open ends of the tubes (open dialysis bags), (ii) blocks of fresh meat in dialysis tubing, with the ends of the tubing sealed (closed dialysis bags), (iii) blocks of frozen and thawed meat in dialysis tubing, but with open ends of the tubes (freeze thaw open), and (iv) blocks of frozen and thawed meat in dialysis tubing, with the ends of the tubing sealed (freeze thaw closed).
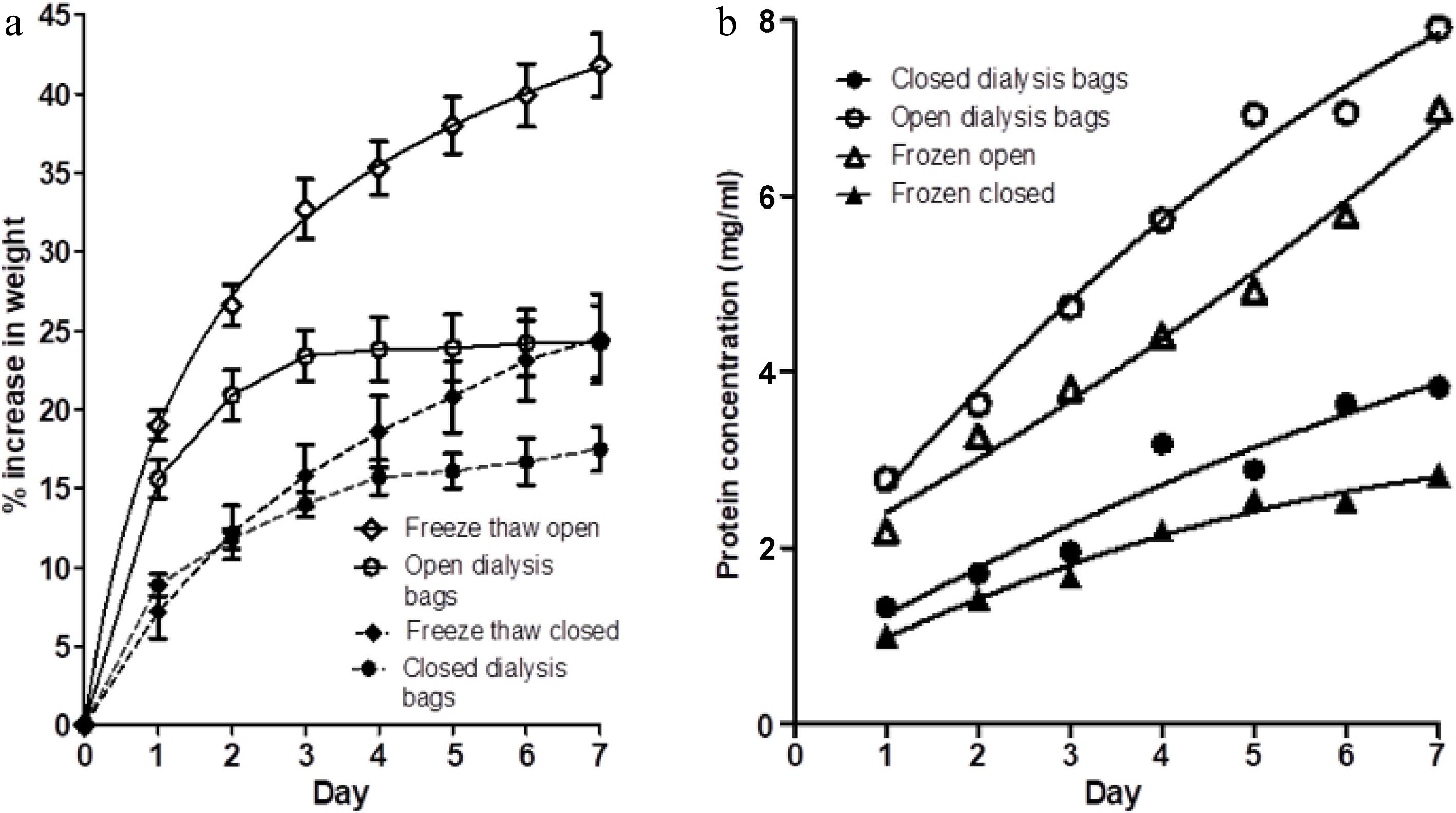
Figure 3.
(a) The percentage weight change in muscle blocks measured each day over a 7-d period of immersion in 3% NaCl solution, without changes of saline, for four treatments; fresh muscle in either (i) closed dialysis bags or in (ii) open-ended dialysis tubing, and frozen-thawed muscle blocks in either (iii) closed dialysis bags (freeze thaw closed) or (iv) open-ended dialysis tubing (freeze thaw open). Values shown are means ± standard errors. (b) Protein concentration on each day in each of the four treatments (i)−(iv) . Each point is the mean of two determinations.
Comparison of (i) and (ii) with the results shown in Fig. 2a shows that the dialysis tubing does not provide any constraint to the lateral swelling of the muscle blocks. The magnitude of the swelling of fresh meat in the open dialysis bags on each day shown in Fig. 3a is comparable to the swelling of the free-floating (no-dialysis) samples in Fig. 2a. The swelling of the fresh meat blocks in closed dialysis bags is consistent with that reported inFig. 2a. This demonstrates that the difference in percentage weight gain between the two treatments shown in Fig. 2a is solely due to the presence of a complete semipermeable membrane, and that the dialysis tubing did not in itself provide a physical (elastic) constraint to swelling. The changes in protein concentration in the saline baths for treatments (i) and (ii) shown in Fig. 3b are also consistent with those reported in Fig. 2b.
Meat blocks that had been frozen and thawed showed higher percentage weight gains than their fresh counterparts, in both the open and closed dialysis bags (Fig. 3a). However, freezing and thawing slightly reduced the concentration of protein extracted into the saline baths in both the open and closed dialysis bags (Fig. 3b). This may be due to some loss of protein on thawing (thaw loss) or possibly some denaturation of proteins. The frozen and thawed samples again showed a large difference in weight gain between open dialysis bags and closed dialysis bags.
SDS-PAGE characterization of extracted soluble proteins
-
Figure 4 shows the SDS-PAGE analysis of the proteins in the salines from each treatment at the end of the experiment. With the exception of the first lane in the gel (daily change of saline) which was loaded with 10 μg of protein, all other lanes were given an equal protein loading (20 μg). Thus, the differences between lanes are largely not due to difference in protein concentration. Actin (42 kDa) and myosin (220 kDa) can be seen in the samples from salines where there was no complete semipermeable barrier present, together with a substantial range of individual bands for the various soluble (sarcoplasmic) proteins. By comparison with the molecular weights of the major proteins identified in drip from porcine longissimus muscle, bands in the no dialysis and open dialysis bag treatments can be tentatively assigned to pyruvate kinase (237 kDa), GAPDH (homotrimer 144 kDa and subunits 36 kDa)[21], endolase and creatine kinase (81−85 kDa), aldolase (160 kDa), phosphoglycerate mutase, triosephosphate isomerase and phosphoglucose isomerase (54−55 kDa). The samples from salines where the dialysis bags were closed shows a great reduction (but not complete elimination) of proteins above the nominal molecular weight cut-off of the dialysis membrane used (14 kDa).
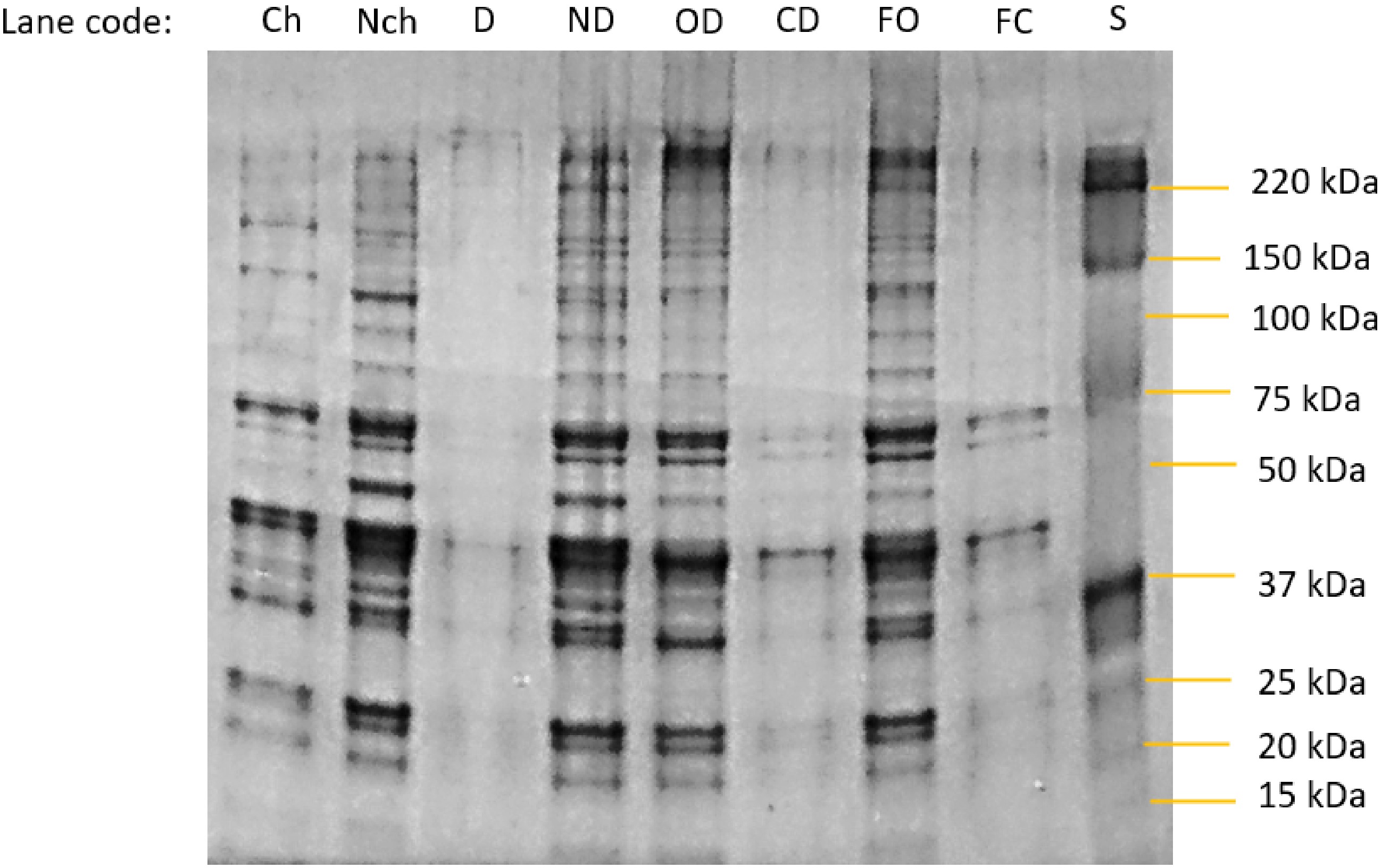
Figure 4.
Photograph of an SDS-Page gel separation of proteins in the bathing salines at the end point of each experimental treatment. Specimen codes: Ch and Nch are the change and no-change treatments from day 7, Fig. 1b. D and ND are the dialysis and no dialysis treatments from the endpoint of Fig 2b. OD = open dialysis bag and CD = closed dialysis bag treatments from the end of the experiment using fresh meat samples in Fig. 3b. FO = open dialysis bag and and F = closed dialysis bag treatments from the end of the experiment using fresh meat samples in Fig. 3b. S = molecular weight markers.
-
The experiments described here are basic but nevertheless reveal important aspects of the mechanisms contributing to the swelling of meat by sodium chloride solutions. The initial experiment demonstrated that swelling of meat pieces in 3% (0.513 M) NaCl during 7 d was greater if the saline solution was replaced with fresh solution daily. This could be due to greater efflux of small molecular weight molecules that dominate the osmotic pressure within meat[16], a greater efflux of high molecular weight soluble proteins, or an increased supply of sodium and chloride ions in the fresh bathing medium. To minimize the contribution of this last possibility, subsequent experiments were performed with a much higher brine:meat ratio (Table 1) and without daily changes of the saline.
The comparison of percentage weight gain in 3% NaCl between meat pieces with and without dialysis bags demonstrated that restriction of the efflux of higher molecular weight soluble proteins reduced the swelling. Subsequent experiments showed that the dialysis membrane did not provide a physical constraint to lateral swelling of the meat pieces.
For swelling to come to equilibrium, forces acting to laterally expand muscle fibres must be balanced by resisting lateral forces. Clearly, the efflux of soluble proteins from meat alters this balance, and begs the question of what mechanism is responsible for this. From measurements of osmotic compression by large molecular weight polymer solutions of mechanically-skinned muscle fibres in relaxing buffer (actomyosin cross-bridges not attached) , Godt & Maughan[23] concluded that lateral swelling of fibres with increasing ionic strength of the bathing medium could be due to two mechanisms; (i) repulsive forces between similarly charged myofilaments, and (ii) a Gibbs-Donnan osmotic force due to water entering the sarcomere to counterbalance the excess concentration of counterions attracted by the charged filaments. The fact that myofilament lattice spacing in the A-band region decreases with decreasing pH between pH 5.5−7 in relaxed fibres shows that filament charge repulsion is a potent mechanism. However, Godt & Maughan[23] point out that increasing ionic strength would be expected to decrease, not increase filament repulsion (and hence spacing) due to more effective ion screening. Although Gibbs-Donnan osmotic forces may also be expected to decrease with increased ionic strength, they considered that Gibbs-Donnan osmotic forces were more likely to be the driving mechanism behind increased fibre width with increasing ionic strength in the range studied. Offer & Knight[4] reviewed evidence to suggest that myofilaments can bind Cl- ions as well as Na+ ions, and that decreases in the isoelectric point of myosin with increasing salt concentrations suggest that anions such as Cl− bind to myosin in excess to Na+ ions. They also noted that, while increasing ionic concentration would be expected to increase the charge density of the myofilaments, and hence increase repulsive force, this effect is likely to be more than compensated for by a large increase in charge screening. Because of this, and because any repulsive force between filaments would be expected to diminish dramatically with only small increased in myofilament lattice spacing, Offer & Knight[4] discounted repulsive forces as being a major mechanism in the lateral expansion of muscle fibres with increasing salt concentration.
The Gibbs-Donnan osmotic effect describes the unequal distribution of diffusible ions between two compartments due to the presence of non-diffusible charged molecules[24,25]. This effect was originally formulated in consideration of solutions separated by a semipermeable membrane (such as dialysis tubing), but is also relevant if there is no intact membrane as long as one compartment holds non-diffusible charged molecules (e.g., insoluble myofilament proteins). At equilibrium, the solution in each compartment will be electrically neutral, and the product of diffusible ions in one compartment will be equal to the product of diffusible ions in the other compartment. However, the presence of non-diffusible charged molecules in one compartment creates an osmotic gradient that pulls water into that compartment.
The original theory developed by Donnan[24,25] was developed for one (anionic) charged species of non-diffusible molecule and explains why the osmotic force drawing water in increases with increasing ratios of NaCl concentration to non-diffusible molecule concentration. However, muscle fibres contain a large number of proteins, with different isoelectric points and hence different charge distributions at a given pH. As the sarcolemma becomes permeable during rigor and post-rigor, some of these proteins are free to leave the muscle fibres (as demonstrated by the high concentration of soluble protein in drip), whereas insoluble proteins are not.
The isoelectric point of a protein (pI) is the pH at which the net charge on it is zero. At pH's above the pI, the protein has a net negative charge (and so is termed anionic) and attracts positive counter-ions (cations) such as Na+ or K+. At pH's below the pI, the protein has a net positive charge (and so is termed cationic) and attracts positive counter-ions (anions) such as Cl− or OH−.
The isoelectric points of myosin and actin are in the region of 5.4[26−28]. The fact that these two proteins make up the majority of the mass fraction of muscle proteins explains why the minimum water-holding capacity of meat is seen at pH's just above 5. In the presence of KCl, the isoelectric point of myosin increases at low concentrations and then decreases, returning to 5.4 in 0.2 M KCl[4], indicating that K+ ions are preferentially bound at low concentrations, while Cl− ions are preferentially bound at higher concentrations. In the presence of 2% NaCl, the minimum water-holding capacity of meat occurs at pH 4[2], indicating that NaCl decreases the isoelectric point of muscle proteins at this concentration. It should also be borne in mind that chloride ions are chaotropic (disorder-making) whereas sodium ions are kosmotropic (order-making). Puolanne & Halonen[29] posited that, as chaotropic ions are more hydrophobic, they will bind to the non-polar core cavity surface of the myosin filament shaft, thus making the shaft to get looser and swell. Hamm noted the substantial concentration of divalent ion species (Mg2+, Ca2+, Zn2+ and Fe2+ ) naturally occurring in meat and showed that a protein-free ultrafiltrate containing these ions depressed the isoelectric point of raw meat[2].
The composition of water-soluble proteins extracted both by homogenization immediately post-mortem and in drip from 72-hr post-mortem pork longissimus lumborum muscle is essentially the same, and is dominated by enzymes involved in energy metabolism[21]. Table 2 shows the relative proportions of the 13 proteins identified by Savage et al.[21] in the extractable water-soluble protein phase, together with their approximate molecular weights. Apart from myoglobin and myokinase (present in only small amount), they are all reasonably large molecules, with molecular weights of above 50 kDa. Although they may suffer proteolysis during post-mortem ageing of meat, none of the intact enzymes should pass through standard dialysis tubing.
Table 2. Individual components of the water-soluble protein extracted from porcine longissimus muscle, showing the relative proportions of each (data from Savage, Warriss & Jolley, 1990) and their molecular weight (MW). The thirteen major components listed comprise 90.6% of the total amount of sarcoplasmic proteins.
Protein MW (kDa) % (w/w) of total
protein (sarcomeric)(Glyogen) Phosphorylase 188 3.4 Phosphoglucomutase 62 1.9 Pyruvate kinase 237 6.7 Phosphoglucose isomerase 55 2.2 Enolase 82−100 11.8 Creatine kinase (phosphoglycerate kinase) 81 23.2 Aldolase 160 13.6 Glyceraldehyde phosphate dehydrogenase 144 18.0 Lactate dehydrogenase 140 6.0 Phosphoglycerate mutase 54 6.7 Triosephosphate isomerase 54 2.7 Myokinase 25 trace Myoglobin 16.7 1.1 The largest mass fractions of the sarcoplasmic proteins are represented by aldolase, creatine kinase, endolase and glyceraldehyde phosphate dehydrogenase (GAPDH). As shown in Table 2, these four proteins together make up two-thirds of the total mass of sarcoplasmic proteins. Literature values for the isoelectric points of these enzymes give a range of values, but all are higher than pH 8.0. (Optimum pH for glycolysis is reported as 7.3) and so it is reasonable to assume that these enzymes are cationic (positively charged) at rigor pHs of 5.6−5.8, while actin and myosin are above their isoelectric point and so slightly anionic (but present in much higher concentrations in the muscle fibres). It is therefore reasonable to suggest that Gibbs-Donnan osmotic swelling effects due to the cationic sarcoplasmic proteins and anionic myofibrillar proteins are counter-effective at rigor pH in the region of 5.5−5.8. Based upon this idea, Fig. 5 schematically depicts the distribution of anionic myofilament proteins and cationic sarcoplasmic proteins at initial, intermediate and final times of immersion of muscle tissue in a 3% salt solution, both with and without dialysis membrane around the meat section.
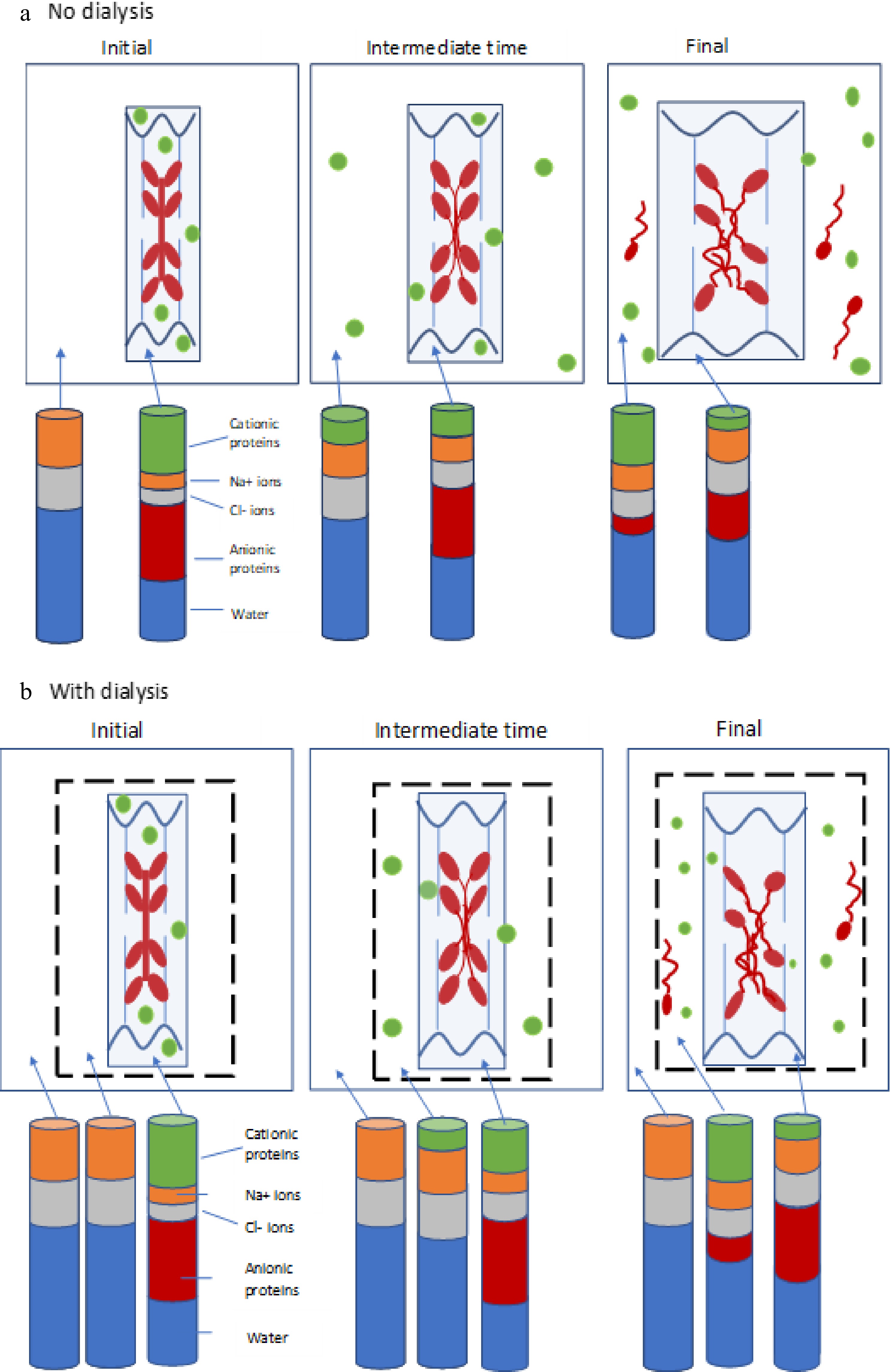
Figure 5.
(a) Schematic diagram depicting negatively-charged thick filaments (myosin, red) and thin filaments (actin, blue) attached to wavy z-discs (wavy dark blue lines) within a sarcomere (light blue box), together with positively-charged soluble sarcoplasmic proteins (green). The system is surrounded by surrounded by a solution of NaCl (white space). The distribution of these proteins is shown at the initial, intermediate and final time points of the experiment. The stacked columns represent the how the relative amounts of cationic proteins (green), anionic proteins (red), sodium ions (orange), chloride ions (grey) and water (blue) in the bathing solution and within the meat piece changes with time (not to scale). (b) Depiction of the same system, but with the addition of a dialysis membrane (dotted black line). Stacked bars now represent the relative amounts of the components within the meat piece, within the dialysis bag, and in the saline external to the dialysis bag. In (a), cationic soluble proteins are extracted from the meat with time, and eventually some myosin is also solubilised and extracted into the bathing medium. In (b), soluble proteins and any extracted myosin are contained within the dialysis bag, providing a smaller osmotic gradient driving water into the meat, and hence reducing swelling.
The stacked bars represent the expected changes in relative amounts of cationic protein, anionic protein, chloride ions, sodium ions and water between the compartments (not to scale). The main concept here (as emphasized in Fig. 6) is that cationic (positively charged) sarcoplasmic proteins provide some counterbalance to the Donnan effect of the anionic myofibrillar proteins, and the extraction of cationic sarcoplasmic proteins out into the saline reduces this counterbalancing effect, allowing the myofilbrillar lattice to develop its full Donnan osmotic force potential. Eventually, some myosin is also solubilised (especially from the H-band of the sarcomere). Micrographs of highly-swollen myofibrils[8] have shown that the M-line does not provide any constraint to the swelling of the entire A-band region due to sodium chloride. It is known that some myomesin is also solubilised in the presence of NaCl in highly-homogenized meat batters[30]. As depicted in Fig. 5b, swelling is less than in Fig. 5a because the soluble proteins are not transported out into the bulk of the saline solution, but remain within the dialysis tubing, so reducing the water gradient between the meat piece and the bulk solution. It is well-documented that pre-rigor single muscle fibres swell in relaxing solution on the order of 100%−200% in the radial direction after mechanical skinning[23, 31,32], while retaining contractile functionality. Watanabe et al.[32] ascribed this phenomenon to water moving into intra-myofibrillar spaces. The plasmalemma membrane on these fibres prior to skinning is still intact and functioning as an impermeable barrier to soluble proteins. This immediate radial swelling of muscle fibres after mechanical skinning (removal of the sarcolemma) supports the idea that release of sarcoplasmic proteins out into the bathing medium allows the myofilament lattice to expand.
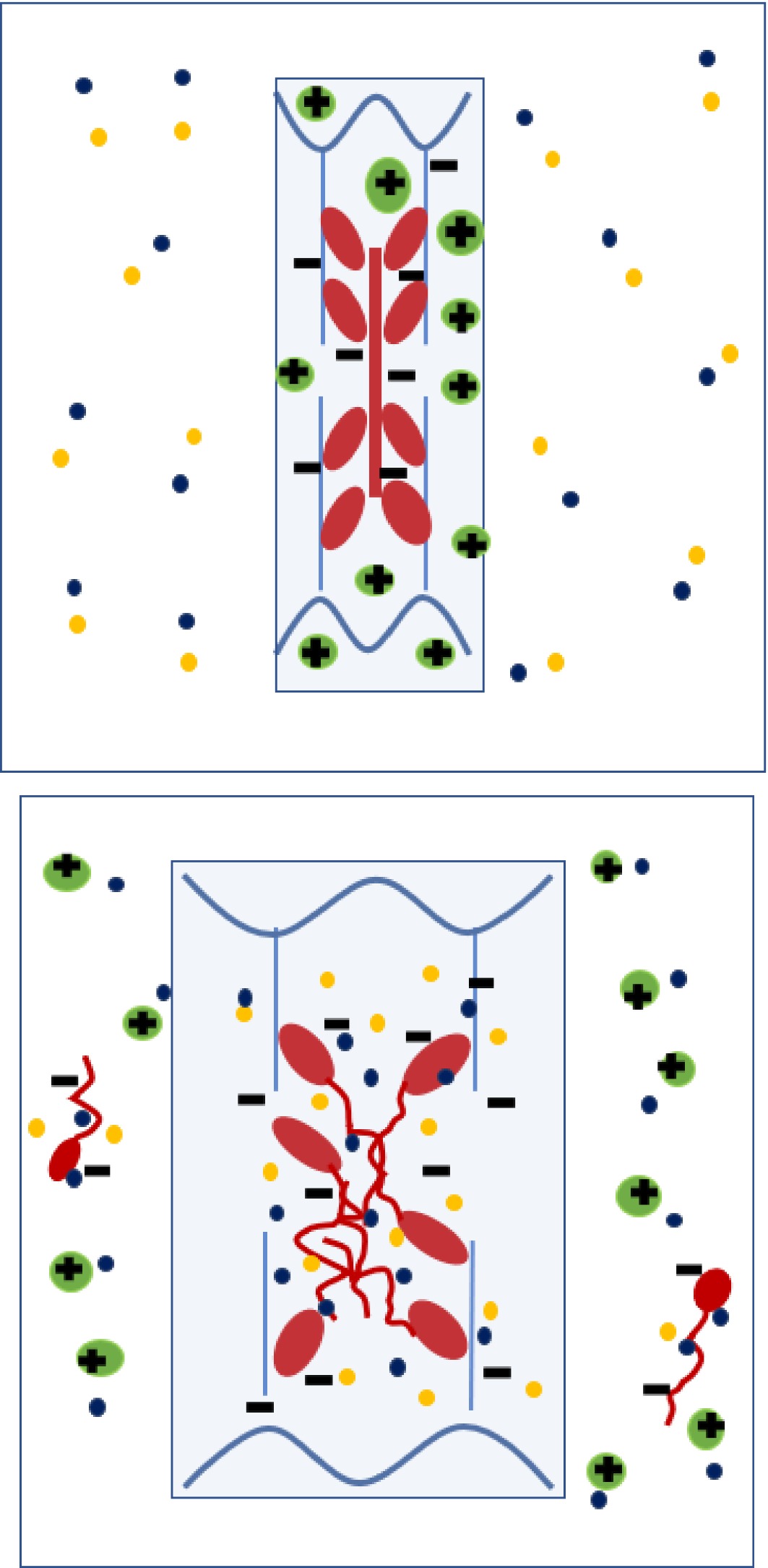
Figure 6.
Diagram depicting anionic (negatively-charged) thick filaments (myosin, red) and thin filaments (actin, blue) within a sarcomere at post-rigor pH, together with cationic (positively -charged) soluble sarcoplasmic proteins (green). Following initial immersion (left) in a solution containing Na+ and Cl- ions, the Cl- preferentially binds to myosin, making the myofilaments more anionic. Sodium counterions are attacted to the anionic myofibrillar proteins and Cl- counterions are attracted to the positively charged, cationic sarcoplasmic proteins. Swelling of the myofilament lattice (mainly anionic proteins) is initially counteracted by the presence of cationic sarcoplasmic protein, but as the soluble sarcoplasmic proteins diffuse out with time (right), their counteracting effect is extracted, leaving the thick filament to disassemble and the sarcomere to expand laterally due to Gibbs-Donnan osmotic forces. At medium to high NaCl concentrations, some of the anionic myosin is also extracted (right).
Without the presence of a lateral resistive force, the Gibbs-Donnan effect would cause constantly increasing separation of charged proteins in the myofilament lattice. The limits to swelling are provided by these resistive (elastic) forces[4]. Extracellular forces from intramuscular connective tissue will be not be considered here. Internal to the muscle cell, there are two main sources of elastic resistance to lateral expansion; (i) the elasticity of the Z-disc and M-line and intermediate filament structures, and (ii) the elastic stiffness of the actomyosin cross-bridges in the rigor state. It has been shown that the actomyosin cross-bridges are relatively stiff in both axial and radial directions with respect to the longitudinal axis of the sarcomere[33]. If the action of salt-swelling is to depolymerise, to some extent, the myosin tail regions from the thick filament backbone[4], then the flexibility of the alpha-helical coiled-coil tail region is an important consideration. However, measurements[34] have shown that this alpha-helical region is elastic, with a high stiffness. The radial stiffness of mechanically-skinned rigor fibres (with cross-bridges attached) is reported[31] as 2−4 times higher than relaxed fibres (where only Z-disc M-line and intermediate filament elasticity contribute). Whereas the radial stiffness of relaxed fibres did not change with sarcomere length, the radial stiffness of rigor fibres varied with the degree of thin-thick filament overlap. These findings[31] showed that the radial stiffness of the cross-bridges is substantially higher than the other transverse cytoskeletal structures. The depolymerization of myosin tails in the thick filament due to chloride ion binding suggested by Offer et al.[5] and the progressive solubilization of some myosin in 0.2-1.0 M NaCl will diminish the lateral restraint. This provides an explanation of why Offer & Trinick[8] observed an ever-increasing expansion of the width of myofibrils with increasing extraction of the A-band material.
Figure 3 suggests that freezing and thawing increases the swelling capacity of the meat substantially. This effect is most seen in the open dialysis bags. For frozen-thawed samples in the closed dialysis bags, there was a small increase in swelling at days 3−7 compared to unfrozen samples from the same muscle in closed dialysis bags. Although increased swelling in frozen-thawed muscle may be due to faster diffusion of sodium chloride into the myofibrils[20], the observation that a restriction of outflow of water-soluble proteins substantially reduces this effect in frozen-thawed meat suggests that soluble (and therefore undenatured) proteins are playing a substantial role in modifying the swelling capacity of the myofibrillar lattice in frozen-thawed meat as well as in fresh meat.
The model suggested in Fig. 5 is intentionally simplified in order to describe the major hypothesis clearly. It should be acknowledged that other factors affecting WHC and salt swelling are either known or have been suggested. These include the chaotropic/kosmotrophic properties of different ions[29], and differences in the degree of hydration of ions[35]. Both of these effects contribute to an explanation of the different effects of specific anions and cations. Puolanne & Halonen[29] also pointed out that two-thirds of the total water-accessible surface area available on the actomyosin complex is provided by the S1 + S2 regions of myosin, so that the role of these regions of the molecule should be highlighted in further study. Finally, it should be noted that the different isoforms of myosin present in different muscle fibre types can be expected to have an effect on the isoelectric point of the protein, and that deposition of sarcoplasmic proteins onto the myofilament in some post-mortem conditions[13] will also complicate the picture.
Figure 6 is presented as a summary diagram, depicting the proposed changes in the distribution of proteins and ions from initial to final states during salt-swelling.
-
This study demonstrates that the retention of soluble proteins within meat reduces the ability of the structural proteins in the myofibrillar lattice to drive swelling of meat in the presence of sodium chloride. This observation is relevant to ongoing studies of variations in meat quality by proteomics and metabolomics, and may provide another reason why variations in the amounts of several of the soluble enzymes associated with energy metabolism can be associated with variations in water-holding capacity. Post-mortem factors causing the denaturation of sarcoplasmic proteins (e.g., low rigor pH and/or high temperature) can also be expected to impact the salt-swelling behaviour of meat if the denaturation leads to precipitation of the proteins (so reducing their diffusion as well as possible changes in charge distribution).
-
We thank Dr. Matías Molino of ABM Laboratorios, Tandil for his help in protein concentration measurements. Professor T Eero J. Puolanne provided extensive and highly insightful discussion of the results and his comments on the manuscript were very much appreciated.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Purslow PP, Pouzo LB, Palacio MI. 2023. The extraction of soluble proteins aids salt swelling of pork meat. Food Materials Research 3:11 doi: 10.48130/FMR-2023-0011
The extraction of soluble proteins aids salt swelling of pork meat
- Received: 14 March 2023
- Accepted: 27 April 2023
- Published online: 27 June 2023
Abstract: The salt-induced swelling of meat is an important phenomenon in meat processing. Existing theories of the mechanism of fluid uptake by meat in NaCl solutions focus on myofibrillar proteins, especially myosin, but the role of sarcoplasmic proteins in salt swelling is unclear. This study clarifies the role of these soluble proteins. Experiments on the weight gain of blocks of porcine longissimus muscle show that multiple changes of fresh saline result in greater swelling and that enclosing meat blocks in dialysis tubing to reduce egress of soluble proteins decreases swelling over a 7-day period, compared to muscle blocks simply suspended in an excess of 3% NaCl. A hypothesis is presented that explains these findings, by proposing that sarcoplasmic proteins, which carry a net positive charge at rigor pH, balance out the Donnan osmotic effect of the myofibrillar proteins actin and myosin, which are reported to carry a net negative charge at the rigor pH of pork meat in the presence of salt. This hypothesis also explains why sarcoplasmic protein denaturation/precipitation may affect the weight uptake in salt-treated meat.
-
Key words:
- Myofibrillar proteins /
- Saarcoplasmic proteins /
- Water-holding /
- Mechanism of swelling












