-
Color is the most important factor for consumers to evaluate the sensory quality of meat products. The freshness of meat products is usually first judged by the color. In addition, the inherent normal color of meat products can increase consumers' appetite, and stimulate their desire to buy[1]. However, the color of meat products easily deteriorate during processing and storage, although it does not affect the safety of them, but it affects consumers' desire to buy to a certain extent. In order to eliminate the influence of bad color on meat products, the food industry usually adds food pigments during food processing to improve the color of meat[2].
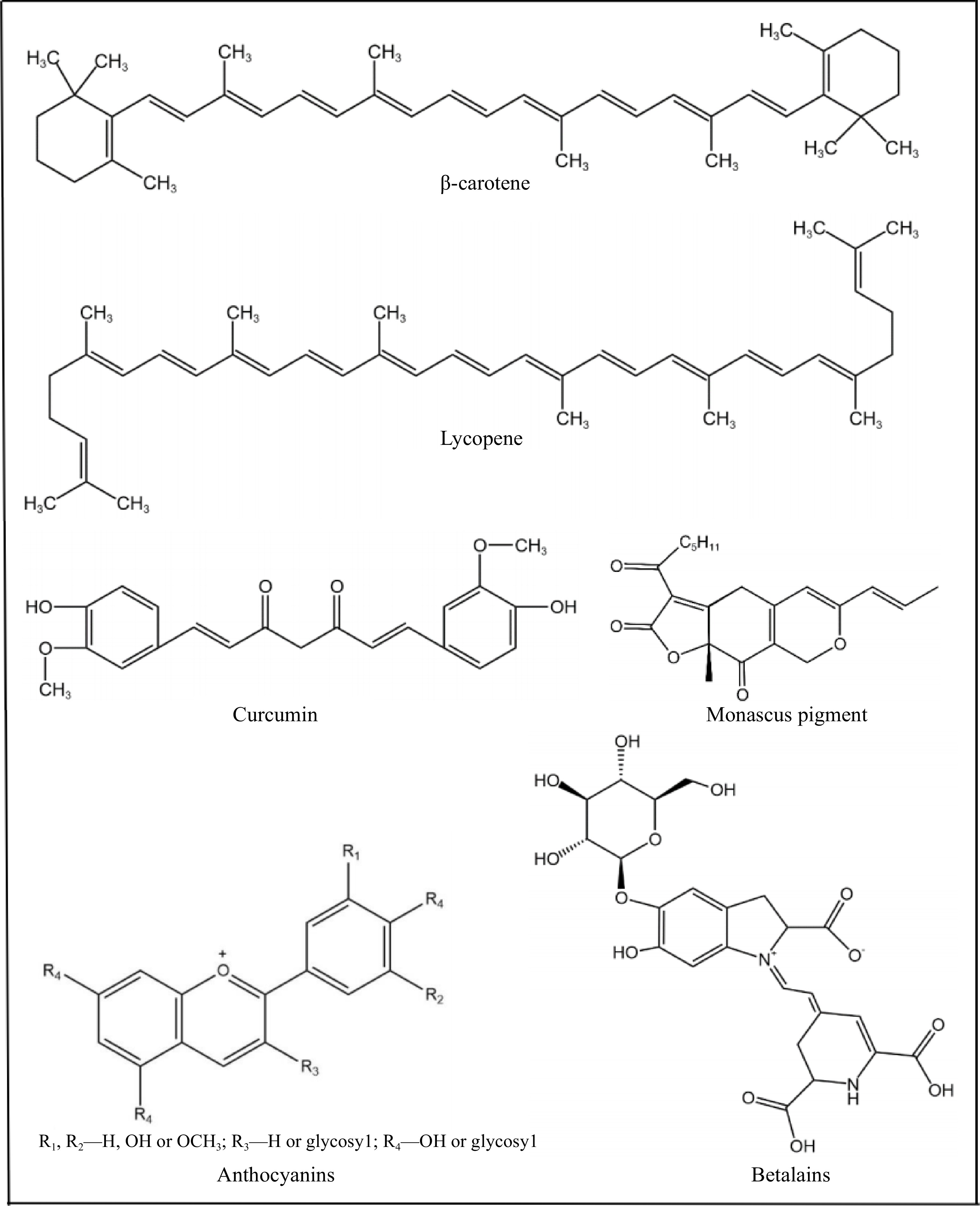
Pigments could be synthetic or natural. Synthetic pigments are chemically synthesized, while natural pigments are obtained from plants, animals and microorganisms. Synthetic pigments have the advantages of bright color, strong tinting strength, good stability and low cost. They have been widely used in various processed foods as food additives and have an integral position in the market[3]. However, most synthetic pigments are unhealthy, which may cause certain allergies, diarrhea, carcinogenicity, mutagenicity[4, 5] and attention deficit hyperactivity disorder (ADHD) in children[6]. Therefore, some synthetic food pigments, such as E128, E156, had been banned. Natural pigments are more popular because they are considered as safe. In addition to being environmentally friendly and safe, natural pigments also have health-promoting effects[2], and they are classified as bioactive substances. Natural pigments commonly used in the food industry are shown in Fig. 1, they mainly include β-carotene, lycopene, monascus pigment, betalains, anthocyanins, curcumin, etc. Studies have shown that these natural pigments are associated with low risk of many diseases, such as cancers[7], diabetes[8] and cardiovascular diseases[9]. In addition, natural pigments also have physiological functions such as antioxidant, antibacterial, and anti-inflammatory effects[10].
Although natural pigments have many advantages, they have low stability and are easily degraded by environmental factors such as pH, temperature, light, and metal ions[11], which lead to fading and loss of benefits. Secondly, the water solubility of some natural pigments is poor, these include β-carotene and curcumin, making them difficult to dissolve and spread evenly in the food matrix, and their low bioavailability makes them difficult to be effectively absorbed and utilized by the body. These defects greatly limit the development and application of natural pigments in the food industry[12]. Therefore, it is necessary to protect natural pigments, improve their stability, tinting strength, solubility and bioavailability, expand their application scope, and improve economic benefits.
This paper mainly reviews several methods currently used to improve the stability of natural pigments, such as adding antioxidants, modifying the molecular structure of the pigments and microencapsulation technologies. The advantages and disadvantages of each method are summarized, with the aim to provide theoretical reference and guidance for improving the stability of natural pigments. We also outline the use of stabilized and non-stabilized pigments in meat industry with the aim of providing guidance to the use of natural pigments in meat products.
-
Most natural pigments are prone to degradation in extreme pH (pH < 3 or pH > 7) environments, because acids and alkalis cause cis and trans isomerization of some double bonds, rearrangement, and de-esterification. Qin et al.[13] had shown that betalains had the best stability in the ranges of pH 3~7. When they are under highly acidic conditions, betalains are prone to deglycosylation. Betalains consist of red betacyanins and yellow betaxanthins, and when betacyanins are under highly alkaline conditions, they are degraded to the colorless cyclo-dopa-5-O-glucoside and the bright yellow betalamic acid. A few natural pigments have been documented to be resistant to extreme pH environments, such as anthocyanins that are stable in acidic conditions, and they degrade rapidly in alkaline conditions, showing changes in color[14].
Temperature is one of the most critical parameters for stability during food processing and storage. Heat treatment accelerates the degradation of natural pigments, because it will cause changes in their structure and color. In the study carried out by Rodriguez-Amaya et al.[15], betalains were exposed to high temperatures and found to be degraded by dehydrogenation leading to the formation of yellow neobetanin. During high temperatures, betacyanin is hydrolyzed to betalamic acid and cyclo-Dopa-5-O-β-glucoside. However, short-term heating can partially regenerate betacyanins.
In addition, many natural pigments are degraded when exposed to light. And light intensity and time will accelerate the degradation of pigments. Chen et al.[16] exposed anthocyanins from red cabbage to darkness, natural light and sunlight for 72 h. The results showed that light would cause the degradation of anthocyanins, and the degradation rate was proportional to the number of acylated groups. Therefore, natural pigments are generally stored away from light to avoid degradation.
Both metal ions and oxidants affect the stability of natural pigments. The former may have a protective effect on pigments and may also cause the degradation of pigments. Cortez et al.[17] had shown that some metal ions could stabilize anthocyanin extracts, because anthocyanin extracts can chelate with metal ions (potassium, aluminum, magnesium, etc.) to form stable complexes. But Li[18] added Fe3+ to the solution of anthocyanin, and the results showed that the preservation rate of anthocyanins was significantly decreased, and the preservation rate was inversely proportional to the concentration of Fe3+. At the same time, some studies had shown that curcumin was sensitive to Fe3+, betalains were sensitive to Cu2+ and Fe3+ [19]. Enaru et al. showed that the unsaturated chemical structure of natural pigments made them react with oxidants easily, which accelerate degradation[20].
As previously introduced, the main factors affecting the stability of natural pigments are pH, temperature, light, metal ions and oxidants. Therefore, a low temperature of 4°C and dark environment should be maintained during processing and storage. The effect of storage temperature and light on the preservation rate of beet red pigment has been previously studied[21]. The results showed that the preservation rate of pigment gradually decreased with the raise of storage temperature and the extension of time, and the degradation rate of the pigments stored in the light was significantly higher than that of the pigments stored in the dark. Oxidants (H2O2, O2, etc.) and metal ions (Cu2+ and Fe3+, etc.) will also affect the stability of pigments, so contact with these substances should be avoided as much as possible during processing and storage.
-
In order to improve the stability of natural pigments and prolong their shelf life, a certain amount of special antioxidants can be added during processing and storage, such as ascorbic acid and sodium erythorbate[22]. Studies have shown that these antioxidants play a significant role in enhancing the stability of natural pigments. They can effectively reduce the rate of oxidative degradation of natural pigments, and hydrophobic antioxidants can be concentrated in the oil-water interface area of emulsions as the main site of oil-phase or degradation of lipophilic natural pigments, thereby protecting natural pigments. In the study by Kim & Choi[23] , ascorbic acid was added to the aqueous phase in the experiment of preparing lycopene emulsion, and it was found that ascorbic acid could significantly inhibit the degradation of lycopene. It had been reported that addition of an appropriate amount of ascorbic acid could improve the stability of betalains[24], while sodium erythorbate showed greater advantages in stabilizing betalains. In addition, the synergy between different antioxidants is a hot spot. By adjusting the ratio of different antioxidants, the stability of natural pigments can be improved.
Modification of molecular structure
-
The stability and hydrophilicity of pigments can also be effectively enhanced by structural modification of the unstable functional group of the natural pigment molecule. Studies had shown that the chemical stability of anthocyanins can be improved by acylation. Acylation can increase anthocyanins acid-base stability, thermal stability and light stability[25]. Studies had shown that methylation may also increase the stability of pigments, while hydroxylation may reduce the stability[26]. The stability of pigments is determined by their structures, but the associations between the chemical structure and fading mechanism of many natural pigments need further studies. Although more stable anthocyanins can be obtained by acylation, they are not commercially available, so this method is difficult to apply in industry to improve the stability of natural pigments.
In addition, pigments can also form pigment-metal complexes by chelating metal ions to maintain their stability[27]. The ortho-dihydroxyl group of anthocyanins can quickly chelate metal ions, thereby inhibiting the generation of hydroxyl groups and slowing down the degradation of anthocyanins[28]. O-dihydroxy can also promote the formation of anthocyanin-metal-ascorbic acid complexes[29]. However, the use of metal ions alone will cause aggregation, the complexes will dissociate easily, leading to the degradation of pigments. The interaction of the chelate with alginate, pectin and other polysaccharides can further enhance the stability of pigments and avoid their aggregation[30]. It has been shown that the complexes formed of cyanidin-3-glucoside (C3G) and Fe3+ improved the thermal stability of C3G, but aggregation occurred, and further alginate addition to the complexes improved the thermal stability of C3G, significantly inhibiting the aggregation of C3G-Fe3+[31]. The complexes formed from anthocyanins and Zn2+ were easily degraded to colorless compounds, but the anthocyanins treated with sodium alginate and Zn2+ were quite stable, the treatment greatly extended the shelf life of anthocyanins[32]. Thus, the chelation of pigments with metal ions alone is prone to aggregation, addition of macromolecular substances such as polysaccharides or proteins is a good solution.
Furthermore, pigments can be stabilized by adding chelating agents such as citric acid and ethylenediaminetetraacetic acid (EDTA)[33]. Transition metals induce the degradation of β-carotene, while EDTA can chelate metal ions and improve the stability of β-carotene significantly[34]. Herbach et al.[35] had shown that chelating agents could effectively improve the stability of beet red pigment and prolong their shelf life, which worked by neutralizing the positively charged N in the molecular structure of this pigment. In order to obtain optimal flavor and smell, the selection of chelating agents should be further studied.
Microencapsulation
-
Microencapsulation is a technology that encapsulates bioactives into microcapsules by using encapsulant agents. The encapsulant agents are equivalent to the wall materials, and the encapsulated compounds are equivalent to the core materials. The core materials include solid, liquid or gas. Encapsulant agents can protect bioactives from external factors and improve the stability of bioactives, they play a role in extending the shelf life of bioactives[36]. In addition, microencapsulation technology can improve the water solubility of bioactives and increase their bioavailability[37]. For water-soluble substances, microencapsulation technology can increase their permeability and cause them to diffuse evenly in the food matrix[38]. For bioactives with strong taste and aroma, such as propolis and astaxanthin, microencapsulation technology can mask their odors and expand their applications as food additives in the food industry[39]. In addition, microencapsulation technology can convert gas and liquid that were not easy to process and store into a stable solid, thereby greatly preventing or delaying deterioration[40]. Microencapsulation technology can also control the release of bioactives, either slowly or at the target site[41], so as to improve the bioavailability of bioactives.
Proteins and polysaccharides are common materials used as wall materials. The proteins include gelatin, soy protein, and casein, and the polysaccharides include maltodextrin, sodium alginate, carboxymethyl cellulose, gum arabic, pectin, chitosan, and starch. The choice of wall materials should be based on the properties of the core materials. Generally speaking, lipophilic core materials, such as carotenoids, curcumin and chlorophyll, should choose hydrophilic wall materials, and vice versa. Core materials, such as betalains, polyphenols, and anthocyanins, should choose lipophilic wall materials[42]. The methods used to encapsulate bioactives can be physical, physicochemical and chemical. The commonly used physical methods include spray drying, freeze drying, supercritical anti-solvent method, complex coacervation, ionic gelation, and liposome entrapment. Chemical methods include in situ polymerization, interfacial polymerization, and molecular inclusion[43], Table 1 visually lists the advantages and disadvantages of the different encapsulation methods. The choice of the method of encapsulating the bioactives is also first made according to the properties of the core materials. Generally speaking, to avoid unnecessary release of the hydrophilic core materials in the aqueous phase, the core materials are required to have a certain degree of chemical interaction with the wall materials, and the physicochemical/chemical methods should be selected. If the core materials are lipophilic, only physical encapsulation can achieve the required effect[43]. Additionally, the size and water solubility of the required particles, matrix of the encapsulated bioactives, the feasibility and cost of methods should also be considered. The studies using different encapsulation methods and wall materials to encapsulate different core materials are summarized in Table 2.
Table 1. Advantages and disadvantages of different encapsulation methods.
Methods Advantages Disadvantages Spray drying Simple method, low cost, high flexibility, high encapsulation efficiency. High temperatures, the broad size distribution of the particles. Freeze drying Low temperatures, good rehydration properties. Time-consuming, energy-consuming. Supercritical anti-solvent Low cost, mild operation temperatures and simpler steps. The particles are of high purity, small size and uniformity. Use of organic solvents, organic solvent residual. Complex coacervation High encapsulation efficiency (up to 99%), scalability and reproducibility. Tedious, time-consuming, high cost, sensitivity to pH and ionic strength. Ionic gelation Good stability, good hydrogel sustained release, high encapsulation efficiency, low temperatures, no use of organic solvents, low cost. The larger size and lower stability of particles. Easy diffusion and fast release of core material through the ionic gel network. Ultrasound assisted High yield, being rapid and relatively simple without any purification steps. The narrow size distribution of the particles. High temperatures, high pressure. Liposome Good biocompatibility, sustained-releasing potential and targeting properties, biodegradable, non-immunogenic and non-toxic. It is easy to occur in aggregation, fusion, phospholipids hydrolysis and oxidation during storage. Table 2. Encapsulation of bioactives with different techniques and wall materials.
Stabilization technique Core material Wall material Results Reference Spray drying Beetroot extract Maltodextrin, inulin, whey protein isolate The values of retention was 88.45%~95.69%. The use of whey protein isolate together with inulin achieved high stability and antioxidant activity of beetroot pigments. [51] Spray drying Betacyanine Maltodextrin,
sweet potato starchEncapsulation efficiency reached 90%. The prepared microcapsules had a high degree of functional properties, defined morphology, and modal size distribution. [52] Spray drying Monascus pigments NaAlg, CaCO3 Spray-dried Monascus red pigments microcapsules had lower degradation constant and longest half-life under the treatments of heating, light and in vitro simulated gastrointestinal digestion [48] Spray drying Betalains Maltodextrin,
cladode mucilage from O. ficus-indicaThe addition of cladode mucilage from O. ficus-indica in the formulation increased the encapsulation efficiency, diminished the moisture content, and allowed to obtain more uniform size and spherical particles, with high dietary fiber content. [47] Spray drying Lutein Maltodextrin, gum arabic, modified starch Encapsulation efficiency ranged from 64.79% to 98.82%. The stability of Lutein was improved. [49] Spray drying Curcumin Porous starch,
gelatinThe microencapsules had good encapsulation efficiency. The stability of microencapsulation curcumin against light, heat, and pH was effectively improved and its solubility was increased greatly. [53] Freeze drying Anthocyanins Black glutinous rice maltodextrins Freeze dried anthocyanin powders showed the good properties in terms of bulk density, angle of repose, process yield and anthocyanin retention. [54] Freeze drying Betalains Guar gum, gum arabic, pectin, xanthan gum Encapsulation showed a higher recovery of betalains during freeze drying by 1.3 times than during spray drying. Freeze dried samples has a* (redness) higher than the spray dried samples. [55] Freeze drying Anthocyanins Soy protein isolate, gum arabic The microencapsulation rate reached 93.05%~98.87%, and the thermal stability of all treatment groups was improved in the range of 80~114 °C. [56] Freeze drying Thyme essential oil Whey protein The microcapsules demonstrated strong antibacterial activity against Salmonella ser. Enteritidis and Staphylococcus aureus. The thermal stability of encapsulated oil was effectively enhanced. [57] Ionic gelation Betalains Calcium alginate,
bovine serum albuminThe stability and antioxidant activity of the encapsulated betalains during processing and storage were improved in a lower humidity environment. [58] Ionic gelation Anthocyanins Curdlan, pectin and sodium alginate Encapsulation efficiency ranged from 80.3% to 96.7%. The release curves followed first order kinetics, with a strong burst effect,
80% to 100% of the anthocyanins released in solution at
pH 1 after 20 min.[59] Ionic gelation Epigallocatechin gallate (EGCG) Debranched starch, carboxymethyl debranched starch The highest encapsulation efficiency was 84.4%. The nanoparticles provided a controlled release of EGCG. [60] Complex coacervation Astaxanthin Gelatin, gum arabic Encapsulation efficiency reached 93.5%, Encapsulation reduced the characteristic odor, improved the coloring capacity effectively. [61] Complex coacervation Anthocyanins Gelatin, gum arabic The selected method significantly increased the stability of anthocyanins up to 23.66% after 2 months of storage at 37 ± 2 °C. The selected optimal microcapsules revealed intense red color over the time of storage. [62] Complex coacervation Carotenoids Whey proteins isolate, gum acacia Encapsulation efficiency reached 56%, the water solubility of the carotenoid was improved, and the encapsulated carotenoid showed good stability and microbiological properties during accelerated storage. [63] Complex coacervation Betacyanin Maltodextrin, gum arabic, xanthan gum, and gelatin were mixed with sodium alginate Encapsulation efficiency ranged from 80.53% to 92.27%. All of the wall material compositions provided protection against high temperatures and pH variations. [64] Complex coacervation Betanin Amaranth protein isolate, carboxymethylcellulose (CMC) The encapsulation efficiency of betanin was high, ranging from
61% to 87%. The microcapsules protected betanin from thermal degradation (50 °C), increasing its half-life by approximately
2.92-fold. After gastrointestinal digestion, the bioaccessibility of encapsulated betanin was approximately 84%.[65] Supercritical
anti-solventAstaxanthin Polylactic acid (PLLA) Encapsulation efficiency reached 91.5%. Astaxanthin/PLLA microspheres greatly enhanced the stability of astaxanthin during storage, and the levels of residual solvents were far lower than the ICH limits. [66] Supercritical
anti-solventAstaxanthin Polyhydroxy-butyrate-co-valerate Encapsulation efficiency reached 48.25%. Encapsulation enhanced the stability of astaxanthin during storage. [67] Supercritical
anti-solventLutein Hydrogenated phosphatidylcholine Encapsulation efficiency reached 90.0%. Encapsulation improved solubility, stability and bioavailability of lutein. [68] Ultrasonic Curcumin Zein, sodium caseinate With an encapsulation efficiency of 90.19 ± 0.33%, the sensitivity of curcumin to temperature was reduced, and the treatment improved the stability of storage. [69] Ultrasonic Betalains Maltodextrin The encapsulation efficiency of the betalains were above 79%. Therefore, modest ultrasound treatment can be used for microcapsulation to improve the stability of betalains. [70] Ultrasonic Anthocyanins Soy protein isolate (SPI), gum arabic (GA) Encapsulation efficiency ranged from 93.05% to 98.87% for all microcapsules. All microcapsules enhanced the thermal stability of anthocyanin in the temperature range 80~114 °C. In addition, SPI and GA combination presented good release behavior under simulated gastrointestinal conditions compared with unencapsulated anthocyanin. [56, 71] Self-assembly Lycopene β-lactoglobulin The results suggested that nanomicelles effectively improved the stability and bioavailability of hydrophobic bioactives lycopene. [72] Self-assembly Curcumin Hydroxypropylated debranched starch (HPDS) After encapsulation, the water solubility and physical stability of curcumin could be increased up to 226-fold and 6-fold, respectively. The HPDS nanospheres also exhibited good safety (including hemolysis and cytotoxicity) and sustainable release of curcumin. [73] Self-assembly Curcumin Lactoferrin peptides The encapsulation efficiency was 93.44%. Nano-micelles have been shown to improve thermal stability, dilution stability, storage stability, the transformation rate and bioaccessibility of curcumin. [74] Self-assembly Curcumin Acylated ovalbumin (AOVA) Curcumin encapsulated in AOVA nanogels displayed higher encapsulation efficiency (93.64%) and slower sustained release under simulated gastrointestinal conditions. [75] Self-assembly β-carotene Rapeseed peptides Encapsulation efficiency > 80%, encapsulation improved solubility, stability, flavor and bioavailability of β-carotene. [76] Liposome Vitamin C,
β-caroteneYolk lecithin, cholesterol The co-encapsulation of Vitamin C and β-carotene could significantly improve the storage stability of β-carotene. Liposomes could protect bioactives from damage in the stomach and release them in the small intestine, where they can be absorbed. [77] Liposome β-carotene Hydrogenated phosphatidylcholine and sucrose The liposome was highly solubility, and capable of preserving more than 90% of the incorporated beta-carotene for 60 d of refrigerated storage under vacuum. After 60 d, the color of the dispersions was preserved. [78] Liposome Epigallocatechin gallate (EGCG),
quercetinLecithin, cholesterol,
Tween 80The liposomes were homogeneous with a narrow size distribution and both the encapsulation efficiency for EGCG and quercetin were higher than 60%. High stability of the system was exhibited during the 30 d storage. [79] Liposome Betalains Lecithin The results showed that the color and stability of betalains increased to 76%. [80] Spray drying
-
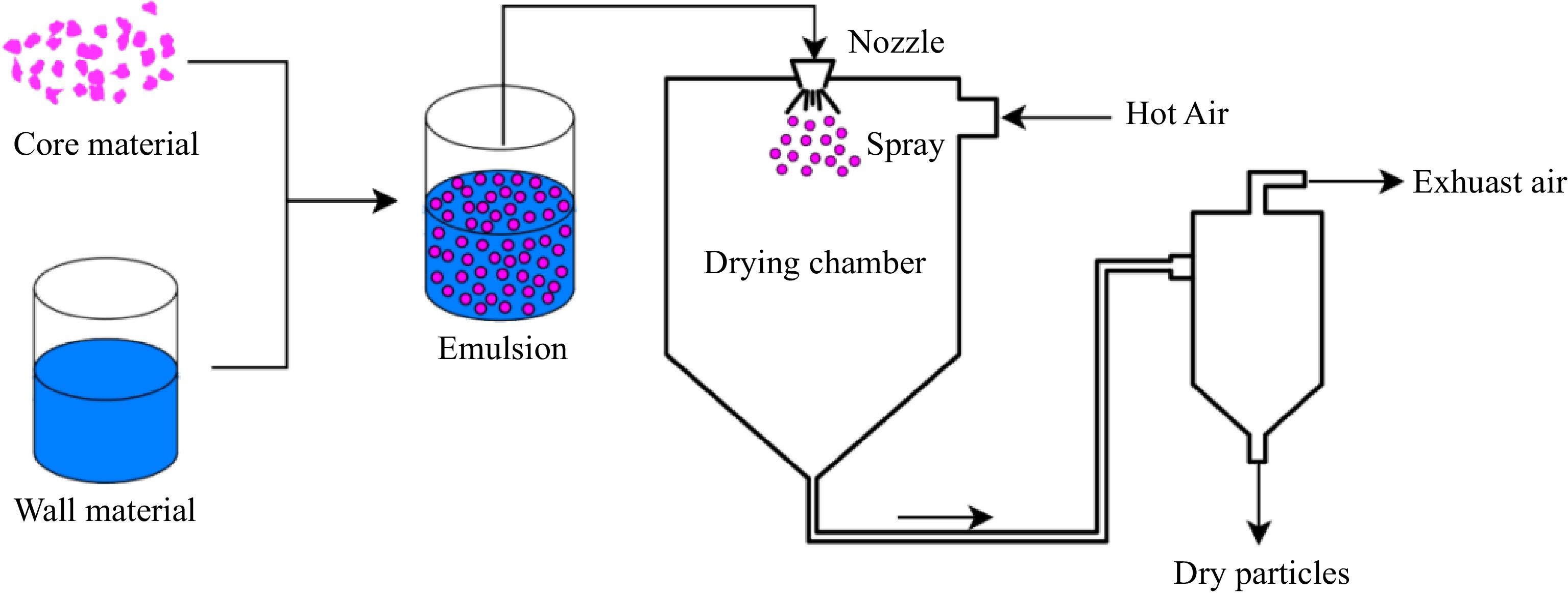
As shown in Fig. 2, spray drying, which converts liquids into solid particles with a high performance, is one of the most common methods for drying microencapsulated bioactives[44]. Spray drying is easy to operate and suitable for large-scale continuous production, it has the advantages of highly flexible, low cost, high encapsulation rate. The resulting particles have good stability and a long shelf life[45].
Baldin et al.[46] used spray-dried maltodextrin to microencapsulate jaboticaba extract, replacing carmine dye, and added to sausages. Inclusion of 2% jaboticaba extract positively affected sensory color, texture and overall acceptance of sausages. It can be considered as a replacement for carmine dye in fresh sausages to satisfy demand for new low-cost natural pigments. Maltodextrin and cladode mucilage from O. ficus-indica had been used as the wall materials and encapsulated betalains, the treatment increased the encapsulation efficiency and the thermal stability, diminished the moisture content, and allowed to obtain more uniform size and spherical particles[47]. In order to improve the stability of Monascus red pigments, Zhang et al.[48] used NaAlg and CaCO3 as wall materials, and prepared Monascus red pigments microcapsules by emulsification/internal gelation with spray/freeze-drying. The results showed that spray-dried Monascus red pigments microcapsules had lower degradation constant and longer half-life under the treatment of heating, light and vitro simulated gastrointestinal digestion. Alvarez-Henao et al.[49] microencapsulated lutein using spray-dried maltodextrin, gum arabic and modified starch, the encapsulation efficiency was as high as 91.94%. When the encapsulant agents were replaced by gum arabic with modified starch, the degradation rate of lutein after spray drying decreased from 97.62% to 8.06%. The combination of these three encapsulant agents provided better protection to lutein than gum arabic alone after 20 d of storage at 40 °C, probably because the presence of starch increased the film-forming capacity of the carrier. Maltodextrin may have enhanced antioxidant activity, thus improving the stability of lutein and maximizing its protection from the storage environment. Pal et al.[50] encapsulated lutein by spray dried maltodextrin and gum arabic, and improved the stability of lutein. The degradation rate decreased from 58.41% to 21.68%. It can be seen that the encapsulation efficiency is an important indicator in the spray drying process as it directly determines the protection and preservation of the encapsulated bioactives.
However, the disadvantages of this method are that heating will lead to the degradation of heat-sensitive substances. The temperatures required during the atomization of the dispersion is as high as 140−180 °C. Such a high temperature will lead to the loss of heat-sensitive bioactives such as anthocyanins and betalains[54]. Ersus & Yurdagel[81] observed that the process parameters during spray drying had a great influence on the stability of anthocyanins extracted from black carrots, in which a higher inlet temperature (160−180 °C) caused the loss of anthocyanins. Studies have also shown that when maltodextrin and natural/modified starch are used to encapsulate betalains, a higher inlet temperature (> 180 °C) will lead to a decrease in the preservation rate of pigments by at least 4%. However, compared with betalains before encapsulation, the encapsulated betalains had better stability[82]. These two examples underscore the importance of temperature during spray drying. Drying air flow, feed flow, and pressure will affect the yield and activity of bioactives[45, 50]. Therefore, it is of great significance to rationally optimize the process parameters of spray drying.
Freeze drying method
-
The same as spray drying, the preparation method of microcapsules is generally to first use high-pressure homogenization, microjet homogenization and other methods to mix the core materials and wall materials to prepare emulsion, and then use drying technologies to remove water to form microcapsules. Different from spray drying, freeze drying is carried out at low temperature (< 0 °C) and in vacuum conditions, and its lower temperature can effectively maintain the activity of bioactives[83]. Compared with spray drying, it can resist oxidation and effectively stabilize heat-sensitive compounds. Freeze drying is the best choice for drying heat-sensitive bioactives[55].
Laokuldilok & Kanha[54] used freeze drying and spray drying to encapsulate anthocyanins respectively. The antioxidant activity and stability of anthocyanin particles obtained by freeze drying were higher than spray drying. It had been reported that the recovery of betaine obtained by freeze drying encapsulation was higher than spray drying[55], because the conditions of freeze drying do not affect the color and nutritional values of bioactives. Microencapsulation of anthocyanins extracted from red raspberries using freeze-dried soy protein isolate (SPI), gum arabic (GA) and their combination, the microencapsulation rate reached 93.05%−98.87%, and the thermal stability of all treatment groups was improved in the range of 80−114 °C, the treatment groups were stored at 37 °C for 60 d, and the preservation rate reached 48%[56].
However, freeze drying is time-consuming and energy-intensive, causing a cost 30~50 times higher than spray drying[84]. Therefore, spray drying is still a better choice when drying substances with better thermal stability.
Ionic gelation method
-
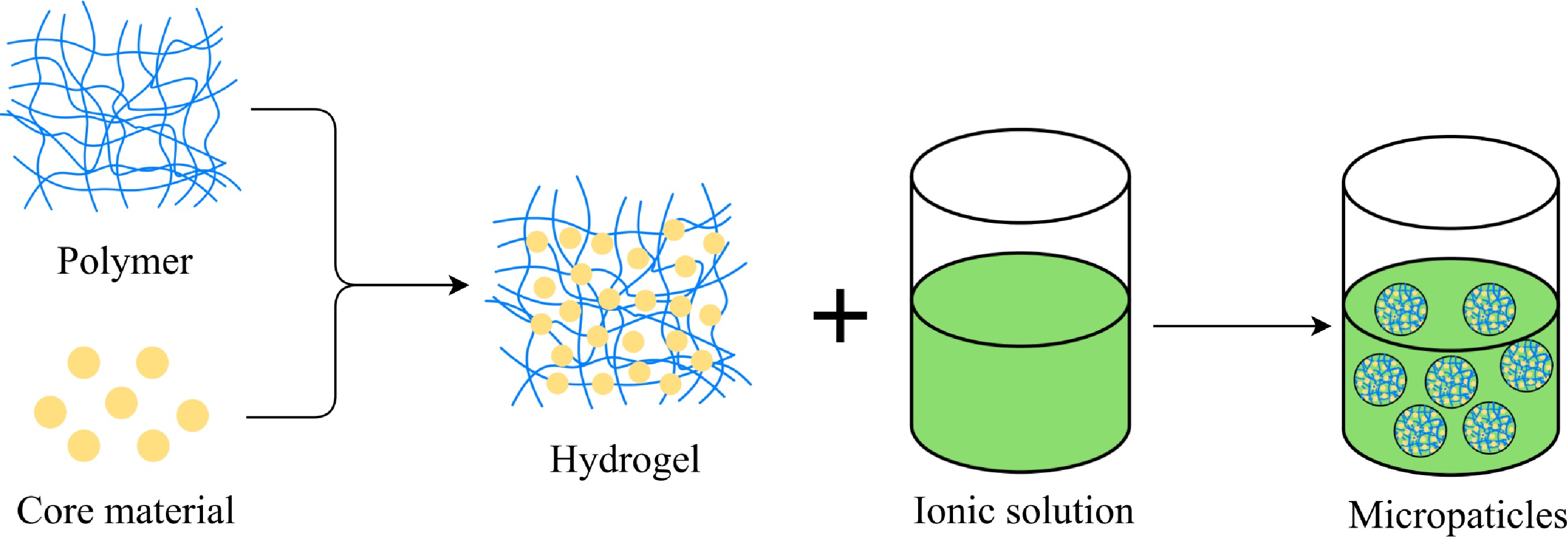
Ionic gelation is one of the most commonly used encapsulation methods. As shown in Fig. 3, it refers to the process of polyelectrolyte (usually alginate) forming a gel through ionic crosslinking with multivalent cations[58]. The ionic gelation method usually uses biodegradable hydrophilic polymers (sodium alginate, gelatin, chitosan, etc.) as raw materials. In order to avoid the miscibility of core materials and wall materials, ionic gelation method is generally used for the encapsulation of hydrophobic substances. In the encapsulation process, it does not need to use organic solvents and perform at high temperatures, it has the advantages of good stability, low cost, good hydrogel sustained release, and the encapsulation efficiency is up to 99%[42]. Some studies had used calcium alginate and bovine serum albumin to cross-link to form a gel to encapsulate betalains. The results showed that the stability and antioxidant activity of the encapsulated betalains during processing and storage were improved in a lower humidity environment[58]. Ferreira et al.[59] used ionic gelation method to encapsulate anthocyanins. In the presence of ions, sodium alginate and pectin form a gel, curd polysaccharide plays a role in inducing hypercoagulation. The results showed that the encapsulation rate of anthocyanins reached 80.3% − 96.7%, and the controlled release of anthocyanins could be achieved.
However, the ionic gelation method has limitations in particle size control, and the obtained capsules need to be stored in a closed environment, because in an open environment, the aqueous solution inside the hydrogel will gradually volatilize, and then the gel can not be stored for a long time. Due to the limitations of equipment and technology, the method is still at the small-scale or laboratory stage, it is difficult to realize large-scale production at an acceptable cost[42].
Complex coacervation method
-
As shown in Fig. 4a, complex coacervation is based on the complex interactions between two oppositely charged polymers in aqueous solution that are used as wall materials, polymers are incompatible with each other in the state of ordinary aqueous solution. Then, two oppositely charged polymers interact and wrap around the core material by changing the pH, temperature of the aqueous solution, the preparation of microcapsules of bioactives is completed[85]. Complex coacervation has been widely used for the encapsulation of bioactives, mainly for encapsulating lipophilic materials such as carotenoids and curcumin. The complex coacervation method does not require the use of organic solvents and high temperatures, and it has large loading capacity, high encapsulation efficiency and good stability[86], but it is time-consuming and costly, not suitable for large-scale industrial production[87].
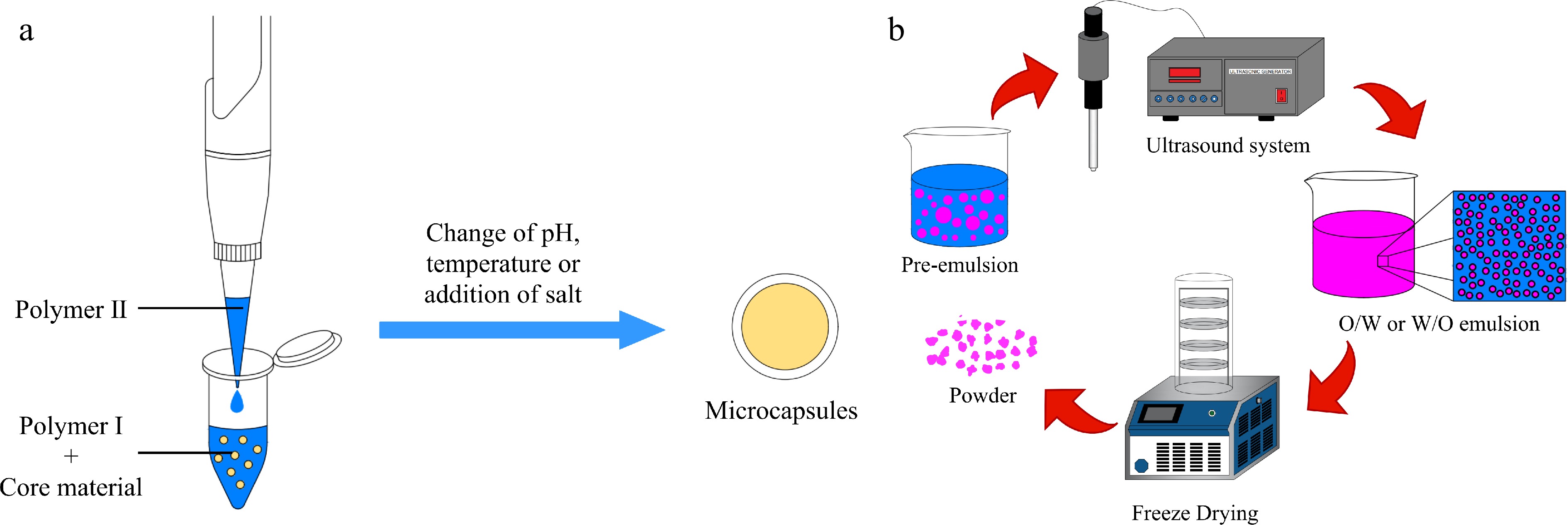
Figure 4.
Schematic representation of the microencapsulation process by (a) complex coacervation and (b) ultrasound.
Commonly used polymer materials can be divided into two categories: proteins and polysaccharides. As amphiphilic molecules, proteins can interact with anionic polysaccharides to condense and encapsulate bioactives[45]. Among them, gelatin and gum arabic can be used in combination. At low pH values, positively charged gelatin interacts with negatively charged gum arabic to form an insoluble complex, which can be used to encapsulate liquid or solid[88]. Gomez-Estaca et al.[61] used the interaction between gelatin and gum arabic to microencapsulate astaxanthin extracted from shrimp fat, and the encapsulation efficiency was as high as 93.5%. The results showed that the stability and tinting strength of the microencapsulated astaxanthin were greatly improved. Whey protein and gum arabic are also commonly used as encapsulant agents to encapsulate carotenoids. Ursache et al.[63] used whey protein isolate and gum arabic to form coagulation and freeze-drying technology to encapsulate carotenoids. The encapsulation efficiency was 56%. Microencapsulation improved the hydrophilic of carotenoids, and the encapsulated carotenoids showed good stability and microbiological properties during accelerated storage. Furthermore, chitosan and sodium alginate are often used as encapsulant agents for bioactives. Sodium alginate refers to an anionic polysaccharide extracted from brown algae, while chitosan is a positively charged polymer. Interactions at specific sites to form microencapsulated wall materials[89]. Deladino et al.[90] used calcium alginate and chitosan to microencapsulate yerba mate lyophilized extract, and the results showed that calcium alginate combined with chitosan to encapsulate yerba mate extract is a promising natural antioxidant food supplement technology. In addition, some studies had used ovalbumin and κ-carrageenan complexes to encapsulate curcumin, which not only had high encapsulation efficiency, but also improved the water solubility of curcumin after encapsulation, and encapsulated curcumin had high antioxidant activity[91]. More importantly, the complex coacervation method can not only protect the bioactives, but also reduce the unpleasant odor and flavor of the bioactives. Studies had shown that it could reduce the astringency of the cinnamon extract[92].
Ultrasound-assisted method
-
Ultrasound is an acoustic wave that vibrates at frequencies greater than 20 kHz and exceeds the upper limit of human hearing, and it has been widely used in food industry. Ultrasound does not work directly with substances, it increases the mass transfer and contact area between two phases mainly through the cavitation of liquids[93]. As shown in Fig. 4b, magnetic stirrer and high-speed homogenizer are usually used to pre-emulsify the two phases, and then put it in the ultrasound system to emulsify, the final microcapsule can be obtained. In order to obtain microcapsule powder, the emulsion can be spray dried or freeze dried. Ultrasound technology has shown good performance in microencapsulation studies, in addition to being an emerging technology for bioactives extraction. It was found that ultrasound can facilitate the interaction between compounds and assist the synthesis of shell-core structures with specific functions, and the applications of ultrasound to encapsulate bioactives are increasingly being studied[70, 71].
The ultrasound-assisted encapsulation process is relatively simple and rapid, and the particles prepared have a narrow and uniform size distribution and high encapsulation efficiency. Chen et al.[69] used the multi-frequency ultrasound-assisted dialysis method to regulate the self-assembly encapsulation of zein-sodium caseinate to encapsulate curcumin. With an encapsulation rate of 90.19 ± 0.33%, the sensitivity of curcumin to temperature was reduced, and the treatment improved the stability during storage.
Some studies had used ultrasonic technology and soybean isolate protein, gum arabic and their mixture to encapsulate anthocyanins separately to improve their stability, and the results showed that the encapsulation rate of microencapsulation was 93.05%−98.87%. The thermal stability of anthocyanins was all enhanced under the effect of high temperatures, and the anthocyanin microcapsules showed good release behavior under simulated gastrointestinal conditions[56]. Microencapsulation of betalains using ultrasound technology and maltodextrin can improve the environmental stability of betalains, especially the high temperature resistance, with an encapsulation efficiency of not less than 79%[70].
It is thus evident that ultrasound is one of the promising techniques to overcome the difficulty of preparing stable core-shell materials with controllable physical and biological functions by conventional methods. However, the use of ultrasound-assisted encapsulation is more demanding for the selection of core and wall materials, because the cavitation of the liquid is accompanied by high temperature and pressure, which may cause the destruction of the structure of bioactives that are extremely sensitive to temperature. At present, it can be improved by ice bath treatment or adjusting the ultrasonic time and frequency during the preparation process, but the specific parameters of ultrasound-assisted encapsulation of different bioactives need to be further explored.
Supercritical anti-solvent method
-
Compared with the traditional encapsulation technologies, supercritical anti-solvent method is suitable for heat-sensitive substances, and it has simpler steps and lower cost. The prepared microcapsules are not only small but also uniform. In the past decade, the study of supercritical fluids for encapsulation of bioactives has attracted increasing interest because of its advantages.
The supercritical anti-solvent method refers to dissolving the bioactives to be encapsulated in an organic solvent, and selecting supercritical CO2 as the anti-fluid, and then mixing the supercritical CO2 with the above solution, which makes the volume of the solution swell quickly and makes the solution soluble so the encapsulated compound decreases quickly and crystallizes[94]. The particles produced by this method are of high purity, small size and uniformity. Supercritical CO2 is not only safe, low-cost, but also the temperature at which the supercritical state occurs is close to the ambient temperature (Tc = 304.2 K, Pc = 7.38 MPa), which can protect heat-sensitive substances from degradation[95]. Machado et al.[67] used supercritical CO2 as the anti-solvent, and dissolved astaxanthin from Haematococcus pluvialis in dichloromethane and encapsulated it in polyhydroxy-butyrate-co-valerate (PHBV), which improved the stability of astaxanthin from Haematococcus pluvialis to environmental factors expanded its applications in the food and pharmaceutical industry. In order to protect the color and activity of β-carotene, Priamo et al.[96] also used supercritical CO2 as the anti-solvent and dichloromethane as the organic solvent to encapsulate β-carotene in PHBV.
Supercritical CO2 can be easily removed by decompression operations. Although supercritical CO2 is able to take away most of the organic solvent, there is still a small amount of organic solvent in the final particles[97]. At present, there are also studies that propose solutions to this problem. However, there are relatively few studies on this aspect, further work should be conducted.
Others
-
In addition to the techniques introduced above, techniques such as self-assembly and liposomes have also been used to encapsulate bioactives. Self-assembly is a process in which amphiphilic polymers spontaneously form thermodynamically stable aggregates based on the interaction of non-covalent bonds, aggregates have certain regular geometric appearance structures, which are very favorable for encapsulating lipid-soluble components. Hu et al.[75] prepared acylated ovalbumin nanogels for curcumin encapsulation by heat-induced self-assembly, which significantly improved the stability of curcumin to pH changes and metal ions, prolonged storage time, and improved the controlled release of curcumin. Liposomes are tiny vesicles composed of phospholipid bilayers that contain both hydrophilic and hydrophobic domains, so they can encapsulate both hydrophilic and hydrophobic components. In recent years, liposome technology has been widely used to encapsulate high-value hydrophilic and hydrophobic ingredients, with high encapsulation efficiency, and significant effects in improving the bioavailability of bioactives, however, due to the high fluidity of the phospholipid bilayers, it is easy to cause vesicle aggregation and membrane fusion, which can reduce the storage stability[98]. Sravan Kumar et al.[80] improved the stability of betalains by encapsulating it in lecithin nanoliposomes, and the results showed that the color and stability of betalains increased to 76%. The color of betalains was observed only at 121 °C (20 min) while the control group had already faded at 100 °C (20 min).
-
In recent years, there has been a growing trend of natural pigments as substitutes for synthetic pigments in food due to the increasing health concerns of consumers. The protection of natural pigments with microencapsulation has been a research hotspot in recent years, but the applications of encapsulated natural pigments as colorants are mainly focused on yogurt, ice cream, drinks, candy, etc. Accessible data on the use of encapsulated natural pigments as coloring agents in meat products is still rare. According to the research on natural pigments, sausages and pork/beef patties are the most studied products, because their special processing is suitable for addition of natural pigments. Therefore, this section mainly summarizes the applications of non-stabilized and stabilized pigments in meat products, and summarizes the results of these studies, with the aim to provide a reference for their application.
Betanin is one of the pigments approved for use in food products as a natural red colorant. It is widely used in frozen or refrigerated foods, such as pork/beef products and sausages. It was added to meat not only as an antioxidant to inhibit lipid oxidation, but also as a natural pigment to confer meat an attractive color[99]. Red pitaya is rich in polyphenol and betacyanin. Red pitaya extract is commonly used as a food colorant in the food industry. Bellucci et al.[100] added it to pork patties, when its concentration was greater than or equal to 0.1%, the redness (a*, belongs to the CIE Lab color space) of the patties was the best, the acceptance of consumer in pork patties was improved. Sucu & Turp[101] mixed a certain amount of beetroot powder with other ingredients to prepare fermented beef sausages, then sausages were stored at 4 °C for 84 d. The results showed that beetroot powder could increase the a* values of sausages, and the a* values of the sausages did not change significantly during storage. Aykın-Dinçer et al.[102] diluted carmine and beetroot extracts in the water used in the formulation and mixed into the batter to prepare fermented sausages and heat-processed sausages, the a* values, sensory appearance, flavor and overall acceptance of fermented sausages and heat-processed sausages were all improved. Due to the presence of betalains and phenolic compounds in beetroot extract, the degree of lipid oxidation was significantly lower than for the control and the carmine group. However, the a* values of heat-processed sausages was lower than that of fermented sausages, the reason is that a portion of beetroot extracts in the sausages are degraded by heating. The results showed that the natural beetroot extract can effectively take the place of carmine in sausages. Lycopene is widely found in tomatoes, tomato products and watermelon and other fruits. García et al.[103] added 0~6.0% (w/w) dry tomato peel (DTP) to the ground meat, mixed well and made the beef patties of hamburger. The addition of DTP increased the a* values of the beef patties, and the overall acceptability of the beef patties is the highest when the addition of DTP to 4.5%. The content of lycopene was 4.9 mg/100 g of cooked hamburger. Botella-Martinez et al.[104] used fresh beetroot juice rich in betalains as a colorant ingredient and then added it to the plant-based burgers, it was found that the color of the prepared plant-based burgers was similar to traditional meat burgers, and the overall acceptability of the plant-based burgers was high. However, in the plant-based burgers, more intense color changes would occur than in the traditional meat burgers during cooking, which was caused by the sensitivity of betalains to temperature, so it would affect the desire of consumers to buy and reduce the commercial value of products. Black mulberry water extract (BMWE) has remarkable potential as a natural colorant due to its high phenolic and anthocyanin content. It has been used to maintain the color stability of beef patties[105]. BMWE reduced the lightness (L*) and yellowness (b*) and increased the redness (a*) of beef patties. As the storage time progressed, the a* values of the BMWE containing groups decreased slowly.
Red raspberries are rich in phenolic compounds, especially anthocyanins and ellagitannins. Raspberry juice and maltodextrin were used as core material and wall material, they were mixed (2:3, w/v) for high-speed homogenization, and then the mixture entered the spray dryer to prepare spray-dried raspberry powder (SDRP). SDRP, 1.0%. 2.0% and 3.0% were dissolved in an equal volume of distilled water and added to the ground beef and mixed manually with gloves. The results showed that SDRP treatment had higher a* values, and SDRP treatment could inhibit lipid oxidation and discoloration, prolong the shelf life of the ground beef. When the addition of SDRP to 2% or 3%, the ground beef could effectively overcome the problem of discoloration. The extracts of red radish and hibiscus are rich in anthocyanins, the extracts of red beetroot are rich in betalains, but the stability of natural pigments is low. Therefore, Dias et al.[106] used soybean lecithin liposomes and maltodextrin microcapsules to encapsulate those extracts, and then used freeze drying method to prepare powder, finally, the powder was added as ingredients to the meat to prepare cooked ham. The E120 from the cochineal insect was used for comparison. The results showed that the color of cooked ham prepared from red beetroot extracts (0.88 g/kg cooked ham) was closest to the expected effect, it proved that red beetroot extracts were very promising in processed meat products such as cooked ham. Consumers are increasingly demanding healthy meat products due to health problems associated with a high-fat diet. In order to reduce the content of fat in meat products, Zheng et al.[107] used Monascus pigments as the internal aqueous phase, then prepared Monascus pigments W/O/W emulsions by a two-step emulsification procedure. The purpose of this research was to prepare meat products using Monascus pigments W/O/W emulsions instead of pork fat. The addition of Monascus pigments W/O/W emulsions reduced the content of lipids, improved the level of protein, and did not affect the hardness and color of the meat system, even after heating, the emulsions could still maintain a substantial amount of their red color. It can be seen from the above literature that the a* values of non-stabilized pigments decrease after heating, the stabilized pigments can still maintain an excellent red color after heating, which indicates that encapsulation can effectively protect and stabilize pigments.
-
Natural pigments have received attention due to their high safety and wide sources. However, the development and applications of natural pigments are restricted. Compared with synthetic pigments, natural pigments have higher cost, lower tinting strength, and poor stability. Environmental factors (such as pH, temperature, light, metal ions, etc.) may cause degradation of natural pigments. In addition, some natural pigments have poor water solubility, and are difficult to use in low-fat foods.
At present, the methods for improving the stability, hydrophilicity and bioavailability of natural pigments are still relatively limited, including adding antioxidants, modifying the molecular structure of pigments and microencapsulation. Among them, microencapsulation has been a potential method for the protection of bioactives, and it has significant advantages in protecting bioactives. Spray drying is the most commonly used encapsulation method, but heating will cause the degradation of heat-sensitive substances, while freeze drying, ionic gelation, and complex coacervation can effectively protect heat-sensitive substances, but they are expensive and not suitable for large-scale production. Therefore, it is necessary to consider many factors when choosing encapsulation technologies and encapsulation materials, such as chemical stability, matrix compatibility and economic feasibility. Appropriate encapsulation technologies and materials can not only improve the performance of bioactives but also has a health-promoting effect. However, the wise selection of encapsulation technologies and encapsulant agents is still a big problem. Future work will focus how to improve the encapsulation efficiency and reduce the cost, the bioavailability of the capsules in the body. In addition, it is necessary to evaluate the impact of various methods on the sensory quality of products.
By using different encapsulation technologies to protect natural pigments, the stability of natural pigments is improved and the application range of them in meat products is expanded. Therefore, it is necessary to analyze the physical and chemical properties of the encapsulated pigments and investigate the behavior of them in meat products, so as to better solve the possible problems of the encapsulated pigments and alleviate consumers' concerns about such processed foods.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Su L, Nian Y, Li C. 2023. Microencapsulation to improve the stability of natural pigments and their applications for meat products. Food Materials Research 3:10 doi: 10.48130/FMR-2023-0010
Microencapsulation to improve the stability of natural pigments and their applications for meat products
- Received: 29 October 2022
- Accepted: 04 May 2023
- Published online: 27 June 2023
Abstract: In recent years, natural pigments have been widely used in meat products (pork/beef patties, sausages, etc.) to maintain color, and as an antioxidant and antibacterial to extend the shelf life of meat products. To date, the food pigment industry has developed rapidly. Natural pigments are more popular among consumers because of their color and physiological functions such as bacteriostatic and antioxidant activity. However, compared with synthetic pigments, some natural pigments have poor stability and water solubility, which limit their application in the food industry. Therefore, taking certain methods to improve their stability and water solubility can expand the scope of use of the pigments and achieve favourable economic benefits. In this paper, we briefly analyze the main factors affecting the stability of natural pigments and summarize the methods commonly used to improve them. The methods mainly include the use of antioxidants and encapsulation of the pigments through spray/freeze drying, complex coacervation, ionic gelation, supercritical anti-solvent, etc. Finally, this paper also outlines the use of stabilized and non-stabilized pigments in meat products so as to serve as a baseline for use in meat and meat products.
-
Key words:
- Meat products /
- Natural pigments /
- Stability /
- Encapsulation.