-
Pummelo (Citrus maxima), a cash crop from the Rutaceae family, has been known as a 'natural canned food' since ancient times and is an important fruit in the market. Pummelo fruits are widely consumed by people because of their health benefits and taste. Pummelo trees have been mainly grown in Southeast Asia for more than 3,000 years[1]. Pummelos are widely cultivated in southern China and annually produce 4.5 million tons of fruits, which accounted for 70% of global production[2]. 'Guanxi', 'Hongrou' and 'Sanhong' pummelo are the most popular commercial cultivars grown in the subtropical regions of China, followed by 'Hongzuan' and 'Guanxihuang'. 'Hongrou', 'Hongzuan' and 'Guanxihuang' are the bud mutants of 'Guanxi', and 'Sanhong' is the bud mutant of 'Hongrou'. The fruits of 'Guanxi', 'Hongrou' and 'Sanhong' mature from early to mid-October, while 'Hongzuan' and 'Guanxihuang' ripen in early to mid-November and mid to late November, respectively, in Pinghe, Fujian (China) (Supplemental Table S1).
Pummelo fruits are divided into three parts including flavedo, albedo and pulp (membrane and juice sacs). The flavedo of pummelo and other citrus is often used in foods, pharmaceuticals, and the cosmetics industry in the form of essential oil. Pummelo fruits contain unique and complex phytochemical profiles, including dietary fiber, vitamin C, carotenoids and flavonoids such as naringin, naringenin, hesperidin and neohesperidin[3]. Genetic variation plays a key role in driving the diversity of the primary and secondary metabolites produced by different pummelo genotypes[4,5], the fruits from different cultivars show large variances in nutrients and bioactives. Large differences were found in the fruit sugars, organic acids, bioactives and antioxidant activity among the red-, pink- and white-fleshed pummelos[6]. The number and content of volatile compounds are the main factors affecting the pummelo fruit aroma, and the quality and quantity of aroma released during fruit consumption can influence consumption behaviors. Pummelo flavedo contains a large-scale volatile production, especially rich in terpenes, alcohols, aldehydes and esters[3,7,8]. Among the flavedo volatiles, limonene, β-myrcene, β-pinene, α-pinene, germacrene D, linalool, cis-linalool oxide, sabinene and nootkatone are the most abundant compounds[5,9]. Interestingly, some volatile compounds, such as indole and 3-ethylphenol, were exclusively identified in pummelo flavedo[10,11]. Compared to flavedo, pulp generally contains fewer numbers and/or less content of volatile compounds. Most of the volatiles in pummelo pulp are alcohols, aldehydes and terpenes[4,12,13]. The number and content of volatile compounds in pummelo fruits could be affected by external factors, for example, the cultivation region[12], illumination time[14] and oxygen and heat[15].
Terpenes account for 85%−95% of volatile compounds in mandarin fruit, and generally present piney, pleasant green and citrus aromas[16]. Monoterpenes and sesquiterpenes are biosynthesized from the methylerythritol 4-phosphate (MEP) pathway in plastids and mevalonate (MVA) pathway in cytosol, respectively. Geranyl diphosphate (GPP, C10) and farnesyl pyrophosphate (FPP, C15) are the precursors for monoterpene and sesquiterpene synthesis, separately, both catalyzed by terpene synthases (TPSs). A total of 95 TPS loci have been detected in the sweet orange (C. sinensis) genome[17], and some of them have been functionally studied, including CsTPS1[18] and STPS[19] in sweet orange, CitMTSE1, CitMTS3, CitMTS61, CitMTS62, CitMTSE2 and CuSTS1 in Satsuma (C. unshiu)[20−22], CitMTSL1, CitMTSL4 and RlemTPS2 in Rough lemon (C. jambhiri)[23,24], and CmTPS1 in finger citron (C. medica)[25]. Few transcription factors were reported to regulate terpene synthesis, like CitAP2.10[26] and CitERF71[27] regulating valencene and (E)-geraniol in sweet orange, respectively. Aldehydes often generate green and citrus-like fruity aroma in orange juice[28], are produced from fatty acid oxidation, amino acid derivation and terpenoid pathways. (Z)-3-hexenal and hexanal, as the important aldehydes in citrus flavor, are formed via 13- and 9-hydroperoxides of linolenic acid catalyzed by lipoxygenase (LOX)[29]. The LOX genes (LOX1, LOX2, and LOX6) were identified in citrus elaioplasts and chromoplasts, which may be involved in generating LOX-derived volatiles in fruit[30], respectively. Terpene alcohols originate from the hydration of terpenes, like linalool, the important volatile possessing a distinctive fresh and floral note in orange juice[28]. Aliphatic alcohols are products of linoleate oxidation via LOX and hydroperoxide lyase, such as (Z)-3-hexenol and hexanol in citrus. Alcohol dehydrogenase (ADH) is one of the key enzymes in alcohol and ester biosynthesis via catalyzing the interconversion between aldehydes and subsequent alcohols.
The molecular mechanisms of volatiles biosynthesis in pummelo have not been fully investigated. The aims of this study are mainly to perform a comprehensive analysis of the nutrients (soluble solids, acidity and vitamin C) and bioactives (phenols, flavonoids, carotenoids, volatiles and antioxidant activity) in pummelo fruits of 'Guanxi' and its four bud mutants, to study how the genotypes would vary in the distribution of main nutritional components, and to identify the candidate genes associated with the volatile formation in pummelo fruits. Information from this study will benefit current pummelo breeding programs and the biotechnologies of fruit and fruit-derived products in pummelo.
-
Ascorbic acid (vitamin C), gallic acid, catechin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-hexanone (internal standard), n-alkanes standard (C8−C20) and sodium chloride (NaCl) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). For the determination of volatile compounds on GC-MS, 53 authentic standards were obtained from Yuanye Bio-Technology Co., Ltd. (Shanghai, China), Rhawn Co., Ltd. (Shanghai, China) and ZZBIO Co., Ltd. (Shanghai, China) (Supplemental Table S2).
Plant materials and sample preparation
-
Mature fruits of five pummelo genotypes were obtained from Pinghe, Fujian province in the 2018 harvest season, including 'Guanxi', 'Hongrou', 'Guanxihuang', 'Hongzuan' and 'Sanhong'. 'Guanxi', 'Hongrou' and 'Sanhong' were sampled in October and others were harvested in November (Supplemental Table S1). The fruits free of any peel defect were collected from nine trees for each cultivar. Then, a total of 15 fruits from three trees of each genotype were randomly collected and assigned into one group, and each group represented a biological replicate and three biological replicates were made. The fruits were washed with dH2O and anhydrous ethanol was used to wipe any other chemicals that adhered to the peel. The flavedo layer was sliced from the peel and collected. The fruits were manually peeled and membranes were removed, then juice sacs were collected as pulp samples. In addition, the juice samples were prepared from the collected juice sacs using a manual commercial citrus juicer. All samples of juice, pulp and flavedo were collected after harvest and then immediately stored at −80 °C until analyzed.
SSC, acidity and vitamin C
-
Soluble solid content (SSC) and acidity were determined using an ATAGO PAL-BX|ACID1 Pocket Brix-Acidity Meter (ATAGO, Tokyo, Japan). Four hundred μL of undiluted juice sample and diluted juice sample (1:50) were taken for SSC (g·100 mL−1) and acidity (g·100 mL−1, g citric acid per 100 mL of juice) measurement, respectively. Each measurement included three technical replications. Vitamin C levels in pummelo juice were evaluated by titration against 2,6-dichlorophenolindophenol[31]. The content of vitamin C (mg·100 mL−1) was determined by comparing the volume of titrant used for the pummelo juice sample with those for 0.1% vitamin C. Each measurement included three technical replications.
Total carotenoids
-
Total carotenoids were isolated as described by Lee & Castle[32]. Briefly, 0.5 mL of juice was mixed with 1 mL of carotenoid extracting solvent (hexane/acetone/ethanol, 2:1:1, v/v) for 0.5 min, followed by centrifugation at 6,500 rpm for 5 min at 5 °C. Then, 0.45 mL of the colored supernatant was collected, and the absorbance value at 450 nm was read in a spectrophotometer. Total carotenoids (mg·L−1) were calculated using an equation with E1% (extinction coefficient of β-carotene) = 2,400. Each measurement included three technical replications.
Phenols and flavonoids
-
Juice-methanol extracts were prepared by stirring 0.2 mL of pummelo juice with 1.8 mL of 80% methanol for 30 min, then centrifuged at 10,000 g for 10 min. Total phenols were quantified using the Folin-Ciocalteu method[33], the reaction solution contained 0.1 mL of juice-methanol extracts, 0.5 mL of ddH2O, 0.1 mL of Folin-Ciocalteu's reagent, and 1 mL of 7% Na2CO3. The reaction solution was incubated for 60 min, and the absorbance value at 750 nm was obtained by comparison with a control. Total phenols (mg·L−1) were reported as gallic acid equivalents, and each measurement included three technical replications.
Total flavonoids were quantified according to Shin et al.[34], the reaction solution contained 0.5 mL of juice-methanol extracts, 0.12 mL of 10% Al(NO3)3, and 0.12 mL of 5% NaNO2. The reaction solution was incubated for 6 min, then 1 mL of 1 mol·L−1 NaOH was added to end the reaction. The absorbance value at 510 nm was calculated by comparison with a control, and total flavonoids (mg·L−1) were expressed as catechin equivalents. Each measurement included three technical replications.
Antioxidant activity
-
Antioxidant activity was accessed using the DPPH free radical-scavenging method as reported by Sdiri et al.[35]. The reaction mixtures contained 0.1 mL of juice-methanol extracts, 0.8 mL of methanolic DPPH (0.2 mmoL·L−1 in methanol) and 0.5 mL of ddH2O. The mixture was placed for 30 min incubation, and the absorbance value at 517 nm was obtained by comparison with a control. The results (mg·100 mL−1) were reported as ascorbic acid equivalents per 100 mL of pummelo juice, and each measurement included three technical replications.
Volatile extraction
-
Briefly, 4 g fine powders of pulp or flavedo were thoroughly mixed with 4 mL saturated NaCl solution (0.36 g·mL−1) in equal proportion, then 6 g of the mixture and 6 μL 3-hexanone (401.20 g·mol−1, internal standard) were added into a 20 mL glass vial[36]. The glass vials were immediately stored at −20 °C. For analysis, the samples were thawed for 30 min at room temperature and then put on a tray holder for 30 min incubation at 40 °C. A 1 cm tri-phase (DVB/CAR/PDMS) solid phase microextraction (SPME) fiber (50/30 μm, Supelco, PA, USA) was used to absorb the volatile compounds for 60 min at 40 °C. Then the volatile compounds were released from the fiber for 5 min at 250 °C in the GC injector port (splitless mode).
Gas chromatography-mass spectrometry (GC-MS) analysis and volatile compound identification
-
Fruit samples were analyzed through an Agilent 7890B GC (Agilent, CA, USA) and a Pegasus HT time-of-flight MS (LECO, MI, USA) coupled with a DB-5MS capillary column (30 m × 0.25 μm × 0.25 mm, Rxi-5 Sil MS; Restek, PA, USA). The temperature settings were: 40 °C for 5 min, 40−230 °C at 3 °C·min−1, kept at 230 °C for 3 min, 230−260 °C at 15 °C·min−1, held at 260 °C for 5 min. The flow rate of carrier gas (helium) was 1 mL·min−1. The ionizing source was set at 250 °C and the electron energy was set to 70 eV with a scan range from 30 to 500 m/z, the scan time started from 200 s. The saturated NaCl solution as a blank sample and then n-alkane standards (C8−C20) were uploaded at the beginning of each day separately to calculate the RIs.
Volatile compound identification was completed by comparing the mass spectra with authentic standards and the mass spectral libraries including ADAMS.L[37] and NIST05.L (National Institute of Standards and Technology, Gaithersburg, MA, USA)[38], and comparing RIs with reported RIs from the same column. The volatile amount was expressed as relative content that was calculated by normalizing the compound peak area to the internal standard peak area.
Real-time quantitative PCR (RT-qPCR)
-
According to the transcriptomic analysis (unpublished), several candidate genes from the biosynthetic pathways of terpenes, alcohols and aldehydes were screened. Primer pairs for the four biosynthetic gene family members are listed in Supplemental Table S3, and their expression levels were determined through RT-qPCR. Total RNA was isolated from pummelo pulp or flavedo by the TRIzol Reagent (Thermo Fisher Scientific, CA, USA). The first strand cDNA was synthesized by the StarScript II First-strand cDNA Synthesis Mix (GenStar, Beijing, China). RT-qPCR was performed in the Bio-Rad CFX Connect System (Bio-Rad, CA, USA) using the 2× RealStar Green Fast Mixture (GenStar). Each sample was examined in three biological replicates and three technical replicates. The gene expression data were analyzed using the Bio-Rad CFX Manager Software with the 2−ΔΔCᴛ method, and the gene expression levels were normalized to the reference gene actin. The threshold cycle (CT) is the cycle at which the fluorescence level reaches the threshold. The first ΔCT is the CT difference between the target and reference genes, and the ΔΔCT is the ΔCT difference between the target and reference samples[39].
Statistical analysis
-
Three biological replicates were used for the quantification of volatile compounds and the determination of gene expression levels in pummelo fruits. The descriptive statistical analysis was mainly run in the JMP 13.0. Principal component analysis (PCA) was performed based on Pearson's correlations. A score plot was applied to separate the five pummelo genotypes and a loading plot was used to distinguish fruit volatile compounds. Hierarchical cluster analysis (HCA) with Ward's method was employed to investigate the relationships between the five pummelo genotypes. The mean content of three biological replicates for each volatile compound was used for HCA.
-
The colors of flavedo and pulp were different among the five pummelo genotypes (Fig. 1). 'Sanhong' formed a light red flavedo, whereas 'Guanxi' and other mutants had a light yellow flavedo. 'Guanxi' and 'Hongzuan' produced pale pulp, while 'Hongrou' and 'Sanhong' generated red pulp and 'Guanxihuang' produced yellow pulp. Carotenoids serve as the primary pigments for characteristic yellow to red colors, were highly accumulated in 'Hongrou' (1.94 mg·L−1) and 'Sanhong' (4.54 mg·L−1) pulp (Table 1). The 'Guanxi' and 'Hongzuan' pulp, was pale in color and contained low levels of carotenoids (0.28−0.34 mg L−1). Lycopene is the predominant carotenoid in pummelo flesh, especially in red-fleshed pummelos[3]. Liu et al.[11] reported that the amount of pulp carotenoids in red-flesh pummelo 'Chuhong' was 57 times as much as in pale green-flesh pummelo 'Feicui', mainly all-trans-lycopene and phytoene. The levels of sugars and acids in the juice sacs mainly determine the taste of citrus fruit. The remarkable differences were observed among pummelos in their juice SSC, ranging from 9.27 g·100 mL−1 in 'Hongzuan' to 11.93 g·100 mL−1 in 'Hongrou'. The overall juice acidity varied from 0.65 g·100 mL−1 in 'Guanxihuang' to 0.87 g·100 mL−1 in 'Hongrou'. SSC/acids ratio as a basic taste indicator is more reliable to judge fruits than the value of individual SSC or acidity, a higher ratio represents a better flavor[40]. Here the SSC/acids ratio ranged from 13.56 in 'Hongzuan' to 16.89 in 'Guanxihuang' among the five pummelo genotypes, suggesting that the bud mutation may have few effects on SSC and acids. This agrees with the previous report that little differences were found for juice SSC and acids between 'Valencia' orange (SSC: 11.03, acid: 1.04) and its mutant 'Rohde Red Valencia' (SSC: 11.47, acid: 1.20)[41]. The relatively high SSC/acids ratio in 'Guanxihuang' and 'Sanhong' mainly contributed to their rich sweet and moderately sour taste (unofficial taste test).

Figure 1.
Fruits of ‘Guanxi’ (GXB) pummelo and its bud mutants. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong.
Table 1. Fruit pulp quality traits of the five pummelo genotypes.
Traits Hongrou Guanxihuang Hongzuan Sanhong Guanxi Soluble solid content (g·100 mL−1) 11.93 ± 0.25 a 11.03 ± 0.38 b 9.27 ± 0.12 c 11.90 ± 0.10 a 11.63 ± 0.40 ab Acidity (g·100 mL−1) 0.87 ± 0.06 a 0.65 ± 0.05 b 0.68 ± 0.01 b 0.81 ± 0.04 a 0.85 ± 0.04 a Soluble solids/acids 13.76 ± 0.99 b 16.97 ± 1.71 a 13.56 ± 0.21 b 14.71 ± 0.67 ab 13.77 ± 1.06 b Vitamin C (mg·100 mL−1) 39.93 ± 1.20 bc 42.01 ± 3.01 ab 34.03 ± 0.60 c 48.26 ± 3.18 a 37.50 ± 4.54 bc Carotenoids (mg·L−1) 1.94 ± 0.36 b 0.50 ± 0.02 c 0.28 ± 0.04 c 4.54 ± 1.06 a 0.24 ± 0.02 c Phenols (mg·L−1) 368.92 ± 11.82 abc 396.42 ± 34.05 ab 310.92 ± 19.15 c 441.82 ± 33.72 a 336.19 ± 39.08 bc Flavonoids (mg·L−1) 46.69 ± 1.89 b 59.90 ± 6.57 a 53.14 ± 6.03 ab 47.28 ± 0.59 b 44.33 ± 0.94 b Antioxidant activity (mg·100 mL−1) 48.08 ± 4.16 a 46.71 ± 5.11 a 34.08 ± 2.72 b 45.89 ± 4.86 ab 34.61 ± 5.20 b All the trees were grafted on sour pummelo rootstock in 2009. All values are mean ± SD of three biological replicates (15 fruits each) per cultivar; different letters in the same row indicate significant differences according to Tukey’s honestly significant difference test at P < 0.05. Juice bioactives and antioxidant activity in different pummelo genotypes
-
The overall antioxidant activity of citrus fruit is governed by the primary nutritional constituents including vitamin C, phenols, flavonoids, and carotenoids. Significant variability was detected among pummelo genotypes in their juice antioxidant activity and bioactive contents (Table 1). Among the citrus varieties, vitamin C content is higher in pummelos and sweet oranges than in lemons, grapefruits and mandarins[3]. Here, the highest and lowest vitamin C levels were found in 'Sanhong' (48.26 mg·100 mL−1) and 'Hongzuan' (34.03 mg·100 mL−1) juice samples, respectively. Most pummelo juices had relatively high phenol levels, varying from 310.92 mg·L−1 in 'Hongzuan' up to 441.82 mg·L−1 in 'Sanhong'. Compared to sweet oranges, pummelo and grapefruit contain lower concentrations of total flavonoids in pulp[42]. Here, wide variability was found among pummelo juices in their flavonoid levels, ranging from 44.33 mg·L−1 in 'Guanxi' to 59.9 mg·L−1 in 'Guanxihuang'. The total antioxidant activity varied from 34.08 mg·100 mL−1 in 'Hongzuan' to 48.08 mg·100 mL−1 in 'Hongrou'. Overall, the two pale-fleshed pummelos ('Guanxi' and 'Hongzuan') contained relatively low levels of carotenoids, vitamin C, phenols and total antioxidant activity. In addition, we observed great correlations between the total antioxidant activity of pummelo juices and carotenoid (0.52), phenol (0.79) and vitamin C contents (0.75) (Fig. 2). It seems that phenols and vitamin C highly contributed to the total antioxidant activity of pummelo fruit. In mandarin juice, vitamin C provided the highest contribution to total antioxidant activity[43].
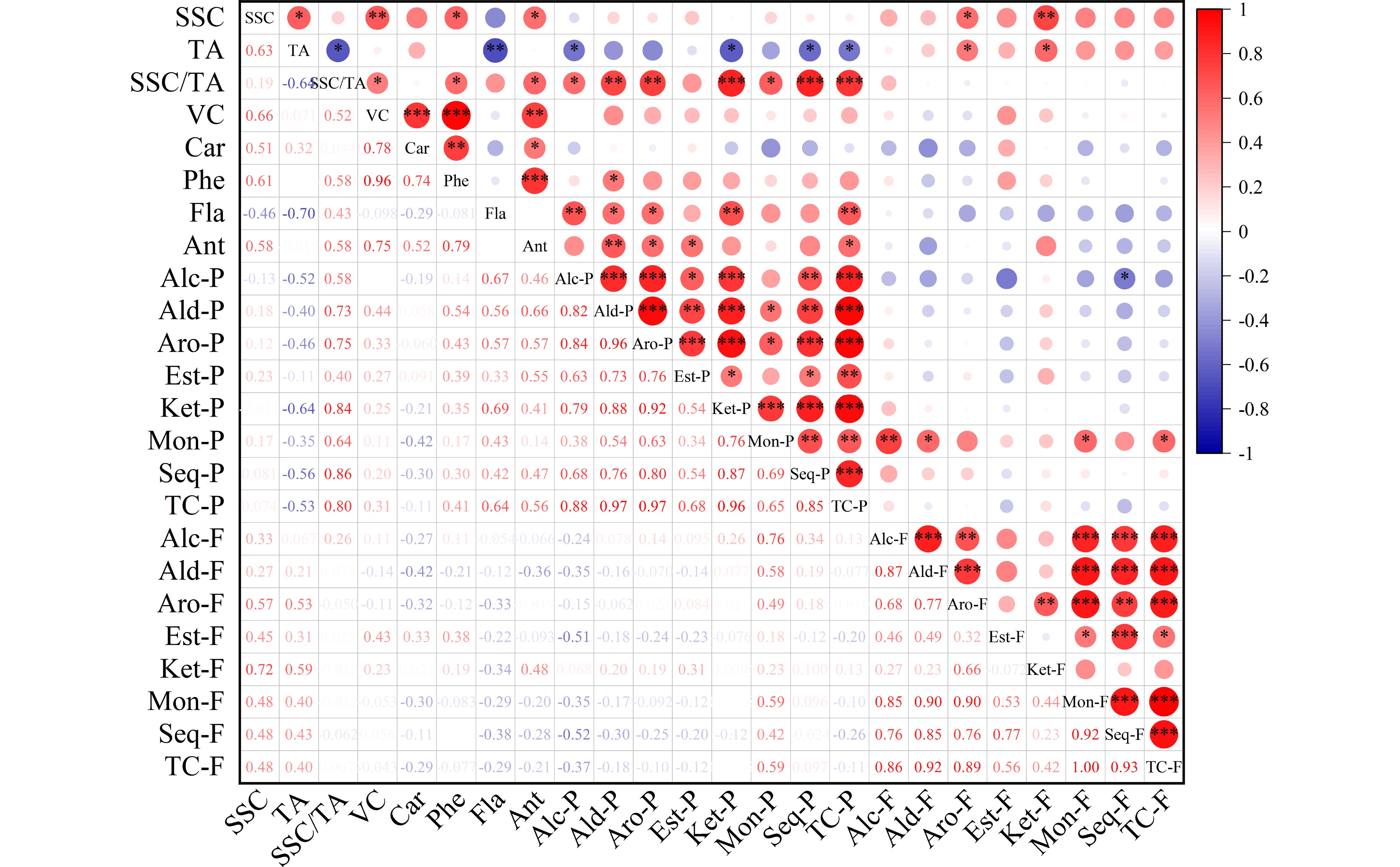
Figure 2.
Correlation analysis heatmap of fruit quality traits and volatiles in pummelo pulp and flavedo. The fruit quality traits included soluble solid content (SSC), acidity (TA), SSC/TA, vitamin C (VC), carotenoids (Car), phenols (Phe), flavonoids (Fla) and antioxidant activity (Ant). The volatile groups contained alcohol (Alc), aldehyde (Ald), aromatic hydrocarbon (Aro), ester (Est), ketone (Ket), monoterpene (Mon), sesquiterpene (Seq) and total compounds (TC) from the pulp (-P) and flavedo (-F) samples. * P < 0.05, ** P < 0.01, *** P < 0.001.
Pulp volatiles in different pummelo genotypes
-
A total of 92 aroma volatiles were detected in pulp samples from the five pummelo genotypes, including 18 alcohols, 22 aldehydes, eight ketones, two esters, five monoterpenes, 27 sesquiterpenes, four aromatic hydrocarbons, one furan, two epoxides, one phenol and one acid (Supplemental Tables S4 & S5). Of the 92 pulp volatiles, 66 consensus compounds were present in all pummelo genotypes, and no genotype-specific volatile was detected (Fig. 3c). Hexanal, (Z)-3-hexenol and hexanol were the most abundant volatile compounds in pulp samples, which is similar to the previous volatile study in pummelo juice[1]. Compared to 'Guanxi', 13, 11, 11 and 19 different volatiles were found in 'Hongzuan', 'Guanxihuang', 'Hongrou' and 'Sanhong', separately (Supplemental Fig. S1a & S1b). Hexanal, β-myrcene, (E)-2-nonenal, α-pinene, limonene, linalool and octanal were reported as the main contributors to the total aroma in pummelo pulp[4]. In the present study, hexanal (described as green and grassy odor) was abundant in all five pummelo genotypes, ranging from 2.28 in 'Guanxi' to 9.70 in 'Guanxihuang'. Limonene (described as citrus-like, fresh odor), the most abundant volatile compound in flavedo, was identified in a higher amount in 'Guanxi' (0.16) than that in its mutants (0.02−0.13) in pulp. The relative content of β-myrcene (described as musty and wet soil aroma) in 'Guanxihuang', 'Hongrou', 'Sanhong' and 'Hongzuan' were about 3.73, 0.92, 0.71 and 0.21 times as much as in 'Guanxi' pulp (0.05), separately. 'Guanxihuang' (0.24) contained a higher level of linalool (described as floral odor) than that in 'Guanxi' (0.008) and other mutants (0.003−0.005), while 'Sanhong' (0.06) produced a larger amount of octanal (described as citrus, fruity odor) than that in 'Guanxi' (0.008) and other pummelos (0.004−0.04). Octanal was one of the essential odorants for orange-like aroma, whereas linalool, octanal and limonene were the main contributors to mandarin-like aroma[44]. The pummelo samples contained very low levels of (E)-2-nonenal (described as cucumber, perfumey odor), varying from 0.0003 in 'Hongzuan' to 0.006 in 'Guanxihuang' in pulp. The common alcohols and aldehydes between pummelo and mandarin were hexanal, hexanol and 2-hexenal[11,36]. Compared to 'Guanxi' and the other three mutants, 'Guanxihuang' contained higher levels of 2-hexenal (1.54), hexanal and hexanol (2.81) in the pulp. It is noticed that the altered contents of multiple components rather than specific volatiles resulted in the distinctive flavor of each citrus variety[45].
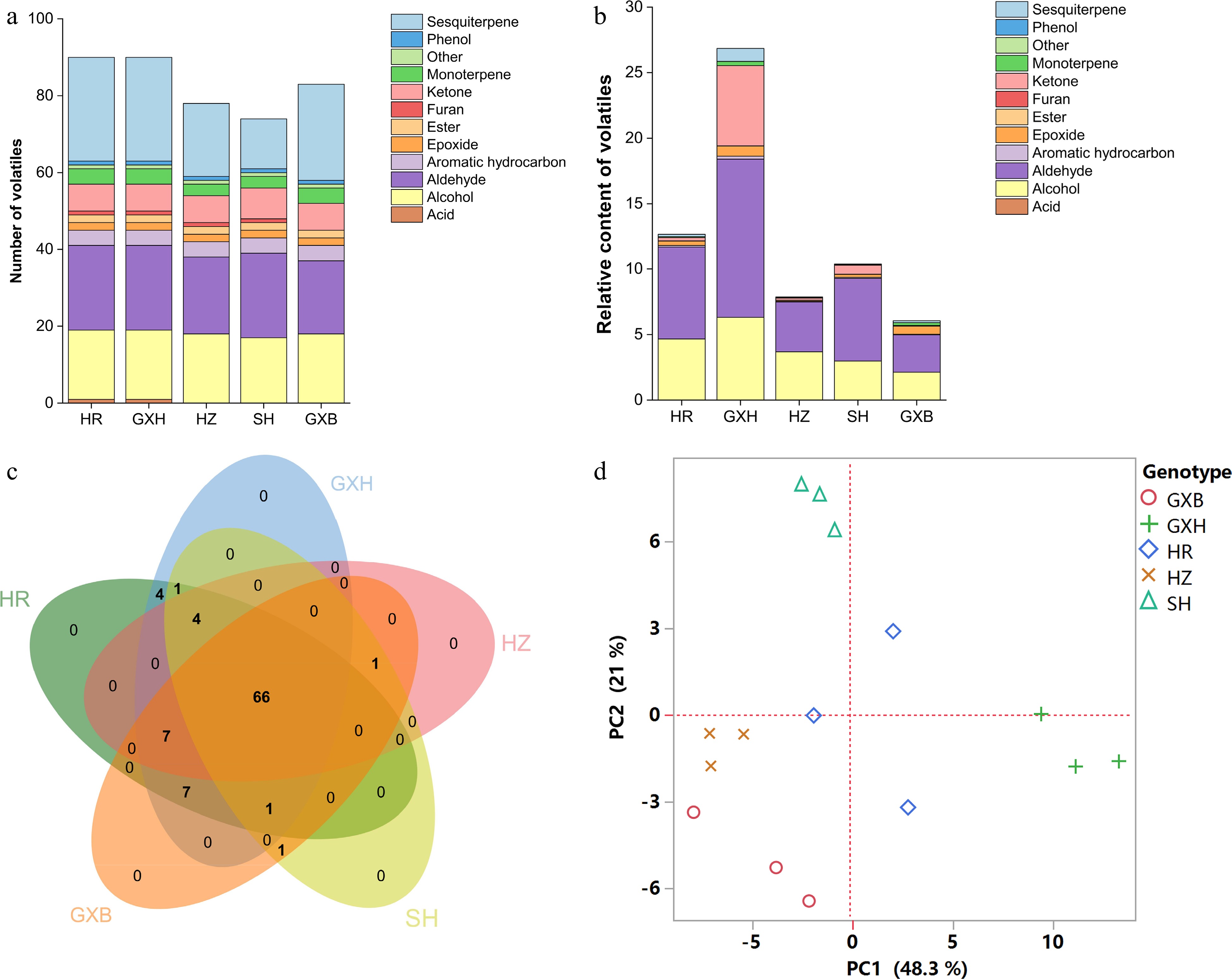
Figure 3.
Multivariate statistical analysis of pulp volatile profiles from the five pummelo genotypes. (a) Number and (b) relative content of volatile compounds in each chemical class. (c) Venn diagram analysis of volatile compounds. (d) PCA score plot of volatile compounds. The volatile relative content was calculated by normalizing the compound peak area to the internal standard peak area. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi.
A previous study suggested that the differences in juice volatile profiles were associated with carotenoid content differences between 'Valencia' orange and its mutant 'Rohde Red Valencia'[41]. Here, the yellow-fleshed cultivar 'Guanxihuang' contained the greatest number (90) and the largest amount (26.85) of pulp volatiles, followed by 'Hongrou' (red-fleshed) with the number and content being 90 and 12.67 (Fig. 3a & b, Supplemental Table S4 & S6). The white-fleshed pummelo contained lower content of alcohols, aldehydes and total juice volatiles but higher terpenoids compared to the pink-fleshed pummelo[46]. In this study, 'Guanxi' and 'Hongzuan', the two pale-fleshed cultivars, contained lower levels of aldehydes and total volatiles compared to the others. Interestingly, higher amounts of monoterpenes and sesquiterpenes were observed in 'Guanxi' (0.35) than the mutants 'Hongrou' (0.24) and 'Sanhong' (0.09), which was supported by the negative correlations between carotenoids and terpenes in the pulp (Fig. 2). This agrees with the previous report that the decreased carotenoids were accompanied by increased production of volatile terpenoids in pummelo fruit[11], suggesting a balance in the formation of various terpenoids. In mandarin juice, color space value a negatively correlated with monoterpene content over four harvests[47]. The pairwise correlation coefficient for each chemical group against every other group and against every fruit quality trait was calculated in the pulp samples (Fig. 2). Positive correlation coefficients were observed for most of the chemical groups (0.34−0.96), and total volatile content was positively correlated with fruit quality traits including SSC/acids ratio (0.8), vitamin C (0.31), phenols (0.41) and flavonoids (0.64). In agreement with a study in mandarin[47], most of the chemical groups of juice volatiles exhibited positive correlations.
In the PCA analysis based on the pulp volatile profiles, principal components (PC1 and PC2) represented 48.3% and 21% of the total variance, respectively, and 'Sanhong' and 'Guanxihuang' were clearly separated from each other and from other genotypes (Fig. 3d). 'Sanhong' presented a strong and typical pummelo aroma, while 'Guanxihuang' generated a strong and complicated aroma of citrus/pummelo note (unofficial taste test). The PC1 separated 'Guanxihuang' from the other four pummelos primarily based on the relative content of several aldehydes, ketones and sesquiterpenes, including geranial (150; code as shown in Supplemental Table S7), 2-hexenal (27), hexanal (17), 1-octen-3-one (64), geranylaceton (185), 6-methyl-5-hepten-2-one (68), elemene isomer (159), γ-gurjunene (189), (−)-β-bourbonene (170), aromandendrene (183), α-ylangene (165) and epizonarene (197). The alcohols, aldehydes and ketones, including (E)-2-nonenol (131), octanol (107), 4-methylhexanol (54), heptanol (63), heptanal (46), (E)-2-pentenal (6), 2-heptanone (41) and 2-nonanone (96) were crucial for the differentiation of 'Sanhong' from the other pummelos across PC2. Octanol (described as soapy odor) was reported as a main contributor to the fruity/floral attribute in citrus fruits[48]. α-terpineol and linalool oxide (furanoid) isomers were identified as the key contributors to the separation of different pummelo genotypes[5], but not in this study. Within most of the genotypes, the three biological replicates were closely clustered. However, the replicates of 'Hongrou' were relatively separated from each other, which may be caused by the individual differences among trees and fruits, as 15 fruits from three trees were randomly sampled, grouped and represented as a biological replicate.
HCA using the relative content of pulp volatiles divided the pummelo genotypes into two main clusters (Fig. 4). 'Hongrou' and 'Guanxihuang' were grouped into a cluster, whereas the second cluster contained 'Sanhong', 'Hongzuan' and 'Guanxi'. 'Guanxihuang' was greatly different from others in pulp volatile profiles, with being rich in aldehydes, ketones and sesquiterpenes. It was previously reported that 6-methyl-5-hepten-2-one and geranylaceton were produced from the digestion of phytoene and lycopene that were cleaved by CCD10a (carotenoid cleavage dioxygenase) in maize (Zea mays), respectively[49]. Here, 'Guanxi' pulp contained the smallest 6-methyl-5-hepten-2-one amount (0.03), which was 20.93%, 11.45%, 4.66% and 0.53% of the amount in 'Hongzuan', 'Hongrou', 'Sanhong' and 'Guanxihuang', respectively. The relative content of geranylaceton in 'Guanxi' pulp (0.005) was about 25.64%, 23.39%, 18.47% and 0.49% as much as in 'Hongrou', 'Sanhong', 'Hongzuan' and 'Guanxihuang', separately (Supplemental Table S5).
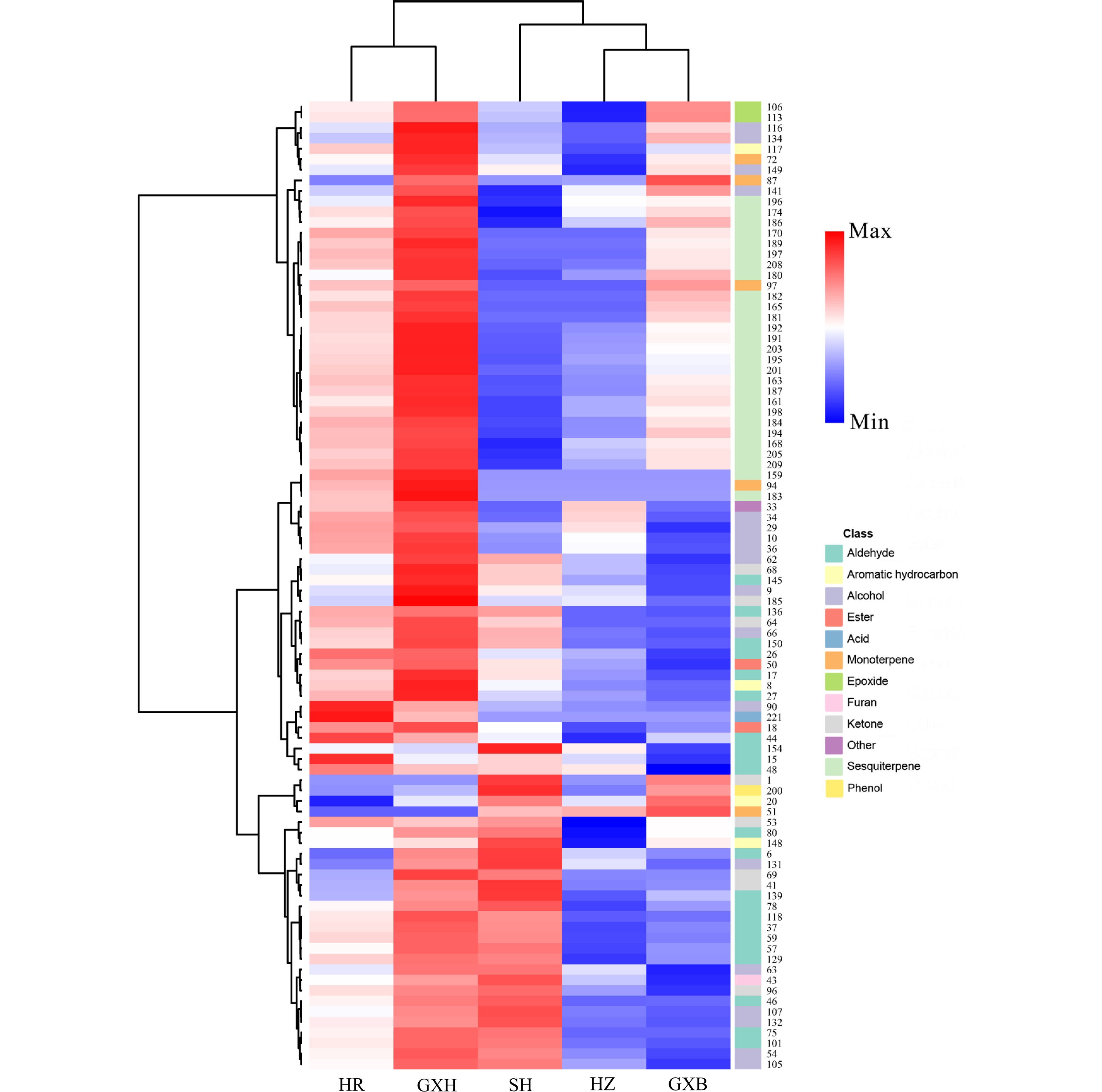
Figure 4.
HCA of pulp volatile profile from the five pummelo genotypes. The volatile codes can be found in the first column in Table S5. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi.
Flavedo volatiles in different pummelo genotypes
-
Pummelo peels are abundant in essential oil, pectin, polyphenols and dietary fiber, and can be directly used to produce food ingredients, pickles, jams and tea[8,50,51]. Citrus essential oils are abundant in aroma-active volatiles and include bioactive compounds with antimicrobial and anti-inflammatory effects. A total of 167 volatile compounds were detected in pummelo flavedo, including 35 alcohols, 23 aldehydes, 7 ketones, 14 esters, 17 monoterpenes, 59 sesquiterpenes, six aromatic hydrocarbons, one furan, three epoxides and one acid (Supplemental Tables S4 & S8). Out of the 167 flavedo compounds, 152 common volatiles were detected in all pummelo genotypes, and no genotype-specific volatile was found (Fig. 5c). Compared to 'Guanxi', 11, 7, 5 and 7 different volatiles were found in 'Hongzuan', 'Guanxihuang', 'Hongrou' and 'Sanhong', separately (Supplemental Fig. S1c & S1d). The pairwise correlation coefficient for each chemical group against every other group was calculated and strong positive correlations were observed for most of the chemical groups in the flavedo samples (0.23−0.92) (Fig. 2).
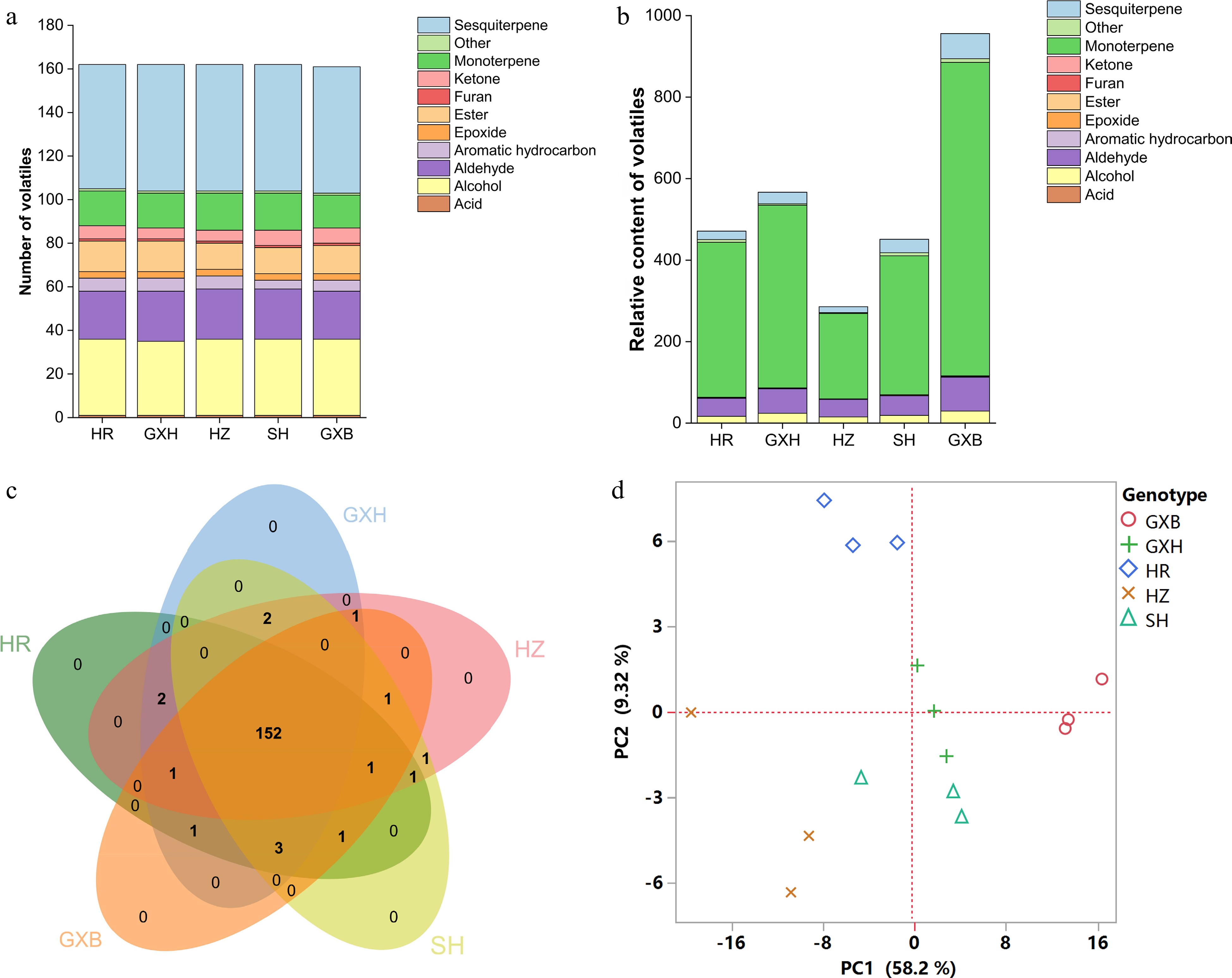
Figure 5.
Multivariate statistical analysis of flavedo volatile profiles from the five pummelo genotypes. (a) Number and (b) relative content of volatile compounds in each chemical class. (c) Venn diagram analysis of volatile compounds. (d) PCA score plot of volatile compounds. The volatile relative content was calculated by normalizing the compound peak area to the internal standard peak area. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi.
Large differences were found in the total amounts of flavedo volatiles among the five pummelo genotypes even though they contained similar numbers of volatiles (Fig. 5a & 5b, Supplemental Tables S2 & S6). 'Guanxi' flavedo contained the largest volatile production level (955.88), which was about 2.03, 1.69, 3.34 and 2.12 times as much as in 'Hongrou', 'Guanxihuang', 'Hongzuan' and 'Sanhong', separately (Fig. 5b). Limonene was the most abundant volatile in flavedo samples, ranging from 133.28 in 'Hongzuan' to 480.36 in 'Guanxi', in agreement with the reported observations in mandarin, grapefruit (C. paradisi), sweet orange, sour orange (C. aurantium) and pummelo[5,52−57]. Jing et al.[58] reported that limonene accounted for 31% to 70% of the total volatile content in pummelo flavedo, and even accounted for up to 96% of the orange essential oil. The lowest and highest levels of β-myrcene were observed in 'Hongzuan' (69.72) and 'Guanxi' (273.37), respectively. Germacrene D as the most abundant sesquiterpene ranged from 3.93 in 'Hongzuan' to 15.93 in 'Guanxi', which usually serves as an important precursor of many other sesquiterpenes, such as amorphene and cadinene[59]. Geranial (22.84−47.04) and neral (11.22−21.85) were identified as the most abundant terpene aldehydes in flavedo. Sun et al.[15] suggested β-myrcene, limonene, octanal, decanal and linalool as the major odorants to the aroma of fresh pummel essential oil. Linalool is the most prominent terpene alcohol in citrus fruits, with being rich in pummelo flavedo samples (4.23−7.91). The three aldehydes including hexanal (0.92−4.32) decanal (2.36−4.19) and octanal (0.65−1.30) were also abundant in flavedo.
PCA was performed to analyze the differences in flavedo volatile composition among the five pummelo genotypes. The PC1 and PC2 explained 58.2% and 9.32% of the total variance, respectively, and 'Guanxi' and 'Hongrou' could be separated from each other and from the other three genotypes (Fig. 5d). The aldehydes and sesquiterpenes including (E)-2-dodecenal (277, code as shown in Supplemental Table S9), undecanal (197), (2E, 4E)-decadienal (202), β-elemene (233), δ-elemene (210), γ-elemene (256), germacrene B (318), guaia-6,9-diene (260), were crucial for the differentiation of 'Guanxi' from its mutants across PC1. The PC2 separated 'Hongrou' from the other four pummelos primarily based on the relative content of several ketones and aromatic hydrocarbons, including trans-dihydrocarvone (154), 2-methyl-6-methylideneocta-1,7-dien-3-one (107), (3E)-4,8-dimethylnona-1,3,7-triene (94), octadecane (352) and heptadecane (348). The peel samples of pummelos from different countries were separated in PCA mainly by citronellol and octanol[5].
HCA divided the pummelo genotypes into two clusters, the first cluster contained 'Guanxi', and the second cluster included its four bud mutants. The four mutants were largely different from 'Guanxi' in flavedo volatile profiles, and most of the different volatile compounds were terpenes, alcohols and aldehydes, such as limonene, cis-β-ocimene, copaene, δ-elemene, linalool, octanol, hexanal and neral (Fig. 6). A small proportion of flavedo volatiles was detected in higher levels in 'Guanxihuang' than them in 'Guanxi'. For example, 'Guanxihuang' flavedo contained about 14.2 and 3.19 times of the α-pinene and 2-hexenal amounts in 'Guanxi' flavedo, and α-pinene was described as pine-like, resinous odor and was found to be enriched in bergamot sour orange[60].
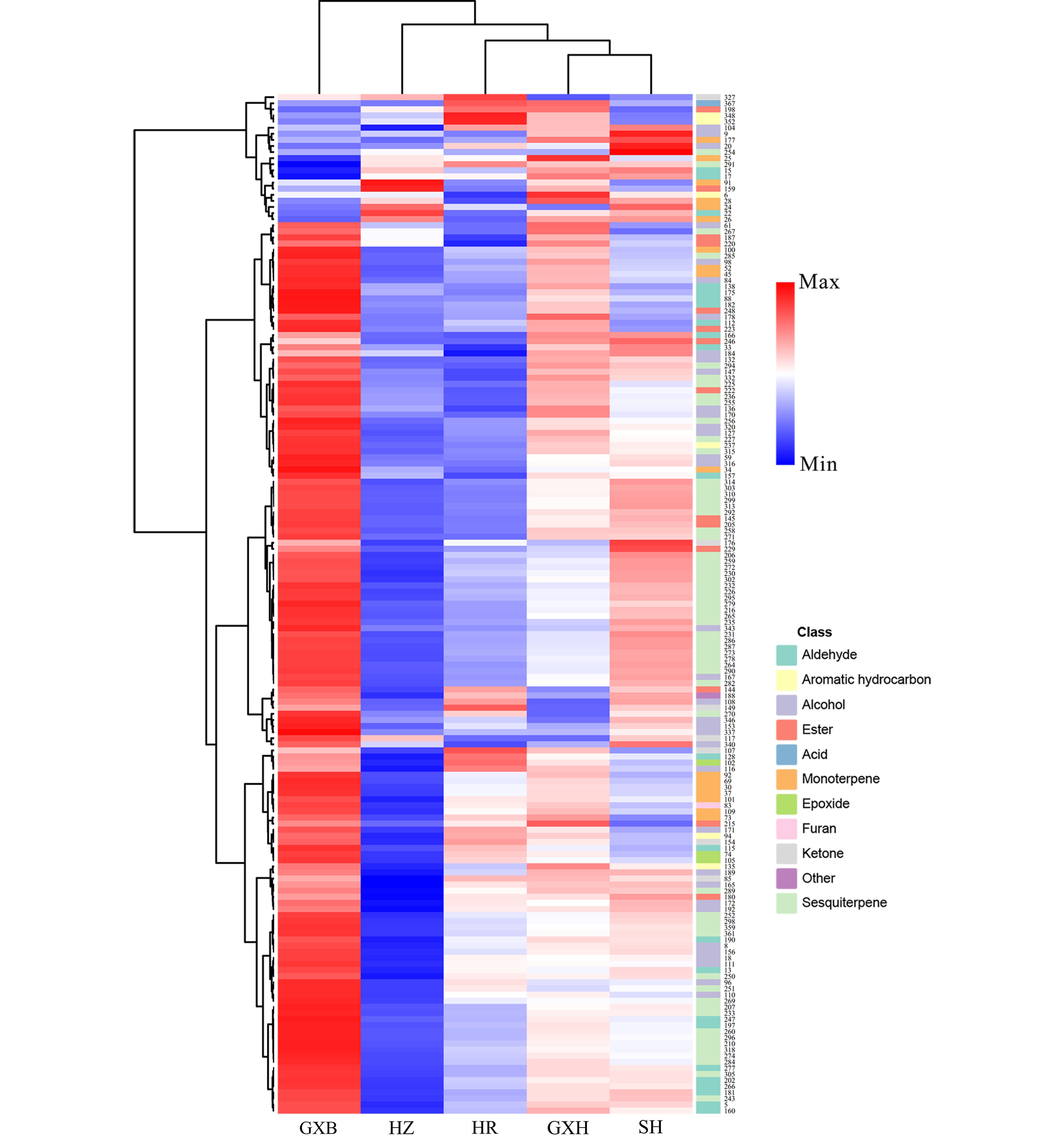
Figure 6.
HCA of flavedo volatile profile from the five pummelo genotypes. The volatile codes can be found in the first column in Table S8. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi.
Comparative analysis of the volatile compounds between pulp and flavedo
-
Citrus flavedo and albedo are the major byproducts of the citrus juice industry, containing high levels of soluble and insoluble fiber, lipids and organic acids, and phenolic compounds[8,61,62]. However, limited amounts of citrus flavedo have been applied in the production of confectionary, jams and cosmetics[63,64]. A total of 167 and 92 volatile compounds were detected in the flavedo and pulp of pummelo fruits, respectively (Supplemental Table S4). Totally 45 common volatiles were observed in both the two tissues, while 47 and 122 were exclusively detected in pulp and flavedo samples, respectively. The important tissue-specific aroma contributors included α-pinene and indole (described as floral odor) in flavedo, and 6-methyl-5-hepten-2-one (described as sweet, green odor) in pulp. Indole as a citrus flower characteristic compound was present in the flavedo samples of all five pummelos (2.04−9.28). Extremely large numbers of sesquiterpenes (59) and alcohols (35) were the main chemical groups in flavedo, whereas great numbers of sesquiterpenes (27) and aldehydes (22) were present in the pulp. The pairwise correlation coefficient for each chemical group between pulp and flavedo volatiles was calculated and no significant correlation was observed for most of the chemical groups between pulp and flavedo, except for the monoterpene group which showed a positive correlation of 0.59 (Fig. 2).
Pummelo flavedo (285.85−955.88, Supplemental Table S8) generally produced much higher levels of volatile compounds compared to the pulp (6.05−26.85, Supplemental Table S5). Aldehydes (2.85−12.1) and alcohols (2.21−6.31) were the most abundant chemical groups in pummelo pulp. Low amounts of monoterpenes (0.04−0.31) and sesquiterpenes (0.03−0.99) were present in pummelo pulp, and similar observations were reported in citrus juice made from peeled fruits[1,36]. However, large amounts of limonene and β-myrcene were detected in mandarin juice produced from unpeeled fruits[65]. Here, pummelo flavedo mainly yielded limonene (133.28−480.36, Supplemental Table S8), while pulp was relatively rich in hexanal (2.28−9.7, Supplemental Table S5). Limonene accounted for, on average 43.8% and 44.4% of total volatile compounds in juice and pomace samples, respectively, of mandarin, orange, lemon (C. limon) and bergamot fruits from Southern Italy[60].
Screening of candidate genes in pulp and flavedo samples
-
Several major genes that govern citrus taste characteristics including sweetness, bitterness and sourness have been reported[66]. Four candidate genes from the biosynthetic pathways of terpenes and aldehydes were screened via analyzing the pulp transcriptomic data of the five pummelo genotypes, and then were validated in the pulp samples with RT-qPCR (Supplemental Fig. S2a−S2d). In pulp samples, the expression levels of Cg5g022140 (TPS1), Cg5g022150 (TPS1), Cg2g002080 (LOX2.1) and Cg2g002010 (LOX2.1) were higher in 'Guanxihuang' than them in other genotypes. This was consistent with the higher amount of terpenes and aldehydes in 'Guanxihuang' than others, suggesting that the increase in volatile production may be caused by the up-regulation of related biosynthetic genes. Terpenoids are mainly formed by various TPS genes in plants, and a small number of them have been functionally characterized in citrus. The TPS1 (Cg5g022140 and Cg5g022150) expressions were positively correlated with β-myrcene and several sesquiterpenes (Supplemental Table S10), and their higher expression levels may contribute to the higher amounts of β-myrcene and several sesquiterpenes in 'Guanxihuang' pulp. In sweet orange, Cstps1 encodes valencene synthase that catalyzes the biosynthesis of valencene from FPP as the substrate[18]. Valencene positively correlates with the increased orange flavor quality and gives citrus juice flavor and fragrance, can be used as a marker of citrus fruit maturity[67]. The relative content of valencene in 'Guanxihuang' pulp (0.02) was about 4.56, 7.08, 14 and 26.86 times as much as in 'Hongrou', 'Guanxi', 'Hongzuan' and 'Sanhong', separately. The lipid-derived volatiles are generated by the LOX pathway. It was previously reported that the great accumulation of volatile alcohols resulted from the high expression of LOX and ADH genes in pear (Pyrus bretschneideri) fruits[68]. Here, the higher expression levels of LOX2.1 (Cg2g002080 and Cg2g002010) may lead to greater amounts of 2-hexenal and (E)-2-hexenal in 'Guanxihuang' pulp, while their lower expressions may induce the fewer amounts of 2-hexenal and (E)-2-hexenal in 'Guanxi' pulp. This was consistent with the positive correlations between LOX2.1 expressions with 2-hexenal and (E)-2-hexenal (Table S10).
In flavedo samples, the TPS1 (Cg5g022140 and Cg5g022150) expressions were positively correlated with several monoterpenes and sesquiterpenes (Supplemental Table S11), and their much lower expression levels were consistent with the fewer amounts of terpenes in 'Hongzuan' (Supplemental Fig. S2e & S2f). Correspondingly, the up-regulated TPS1 expressions (Cg5g022140 and Cg5g022150) may cause the higher contents of several monoterpenes and sesquiterpenes in 'Guanxi' flavedo, for example, limonene, β-myrcene, germacrene D, δ-elemene and (Z)-caryophyllene. Valencene, the major sesquiterpene in citrus peel oil, is largely used for flavoring citrus beverages and is considered as one of the most commercially valuable terpenes[67]. 'Guanxi', with the higher TPS1 expressions, contained about 1.37, 2.15, 2.69 and 3.71 times of the valencene amount in 'Sanhong', 'Guanxihuang', 'Hongrou' and 'Hongzuan', separately. The relatively strong correlations between the expressions of candidate genes (LOX2.1, TPS1) and relative content of volatiles (aldehydes, monoterpenes and sesquiterpenes) suggest that those gene expressions have the potential to predict the related volatile profiles but need validations in broad citrus/pummelo breeding germplasm and segregating populations.
-
In summary, the fruit sensory and nutritional compositions were investigated in 'Guanxi' pummelo and its four bud mutants. 'Guanxi' and 'Hongzuan' were pale in pulp color, and contained relatively low levels of carotenoids, vitamin C, phenols and total antioxidant activity. Phenols and vitamin C provided relatively high contributions to the total antioxidant activity of pummelo fruit. The SSC/acids ratios varied little among the five pummelo genotypes, however, the volatile profiles of the five pummelo genotypes exhibited large qualitative and quantitative variations within each tissue. The majority of the different pulp volatiles were ketones, terpenes, alcohols and aldehydes, and were highly accumulated in 'Guanxihuang'. Compared to the pulp samples, pummelo flavedo contained a greater number of volatile compounds and a larger volatile production level. In pulp, 'Guanxihuang' generated higher levels of ketones, alcohols, aldehydes and terpenes than 'Guanxi' and other mutants, which may partly be explained by the higher expression levels of related biosynthetic genes. The candidate genes including LOX2.1 and TPS1 were associated with the amounts of aldehydes, monoterpenes and sesquiterpenes in pummelo, and future studies are required to confirm their functions. 'Sanhong' and 'Guanxihuang' fruits, with strong aroma, rich sweet and moderately sour taste as well as high nutrients, were preferred by several tasters. The large variations in fruit volatile constitutes among pummelo genotypes and among fruit tissues provide important information for understanding citrus aroma and the potential utilization of pummelo fruits in processing. These results may help understand the molecular mechanisms underlying volatile metabolism in pummelo fruits and provide fundamental information for the genetic improvement of pummelo flavor.
This study was financially supported by the National Natural Science Foundation of China (Grant number 31972369 to Y.Y.) and the startup fund from Fujian Agriculture and Forestry University horticulture science plateau discipline construction project (Grant number 712018011).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Xinxin Zhang, Zhijun Fu
- Supplemental Table S1 Fruit sampling date of the five pummelo genotypes in Pinghe, Fujian, 2018.
- Supplemental Table S2 Fifty-three standards used in the volatile compound determination.
- Supplemental Table S3 Primer sequences for amplification by qRT-PCR.
- Supplemental Table S4 Number of volatiles in 11 chemical classes detected in pummelo flavedo and pulp.
- Supplemental Table S5 Relative content of pulp volatiles in the five pummelo genotypes.
- Supplemental Table S6 Number of volatiles in 11 chemical classes detected in pulp and flavedo among the five pummelo genotypes.
- Supplemental Table S7 Compounds with high contribution to the PC1 and PC2 in Figure 3.
- Supplemental Table S8 Relative content of flavedo volatiles in the five pummelo genotypes.
- Supplemental Table S9 Compounds with high contribution to the PC1 and PC2 in Figure 6.
- Supplemental Table S10 Correlation coefficients between candidate genes and volatiles in pummelo pulp.
- Supplemental Table S11 Correlation coefficients between candidate genes and volatiles in pummelo flavedo.
- Supplemental Fig. S1 Venn diagram analysis of pummelo volatile compounds. The pummelo samples included pulp (A and B) and flavedo (C and D) samples. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi.
- Supplemental Fig. S2 Expression profiles of candidate genes from the biosynthetic pathways of terpenes, alcohols and aldehydes in pummelo. The pummelo samples included pulp (A-D) and flavedo (E-F) samples. HR: Hongrou; GXH: Guanxihuang; HZ: Hongzuan; SH: Sanhong; GXB: Guanxi. TPS: terpene synthase; LOX: lipoxygenase. Data are mean ± SD of three biological replicates. Different letters indicate significant differences according to Tukey’s honestly significant difference test at p < 0.05.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang X, Fu Z, Kong L, Zhou J, Pan T, et al. 2023. Metabolomic analyses provides insight into the fruit nutritional and sensory attributes among 'Guanxi' pummelo and its bud mutants. Fruit Research 3:6 doi: 10.48130/FruRes-2023-0006
Metabolomic analyses provides insight into the fruit nutritional and sensory attributes among 'Guanxi' pummelo and its bud mutants
- Received: 18 December 2022
- Accepted: 01 March 2023
- Published online: 14 March 2023
Abstract: The biochemical compositions in pummelo (Citrus maxima) fruits are key determining factors for the formation of nutrients and flavor. In this study, sensory (soluble solids, acidity and volatiles) and nutritional (vitamin C, carotenoids, phenols, flavonoids and antioxidant activity) profiles of fruit samples from 'Guanxi' pummelo and its four bud mutants were investigated. 'Guanxi' and 'Hongzuan', two pale-fleshed pummelo cultivars, contained relatively lower levels of carotenoids, vitamin C, phenols and total antioxidant activity compared to the other pummelos with red or yellow flesh. A total of 92 and 167 volatile compounds were detected in the pulp and flavedo of pummelo fruits, respectively. The majority of the different pulp volatiles were ketones, terpenes, alcohols and aldehydes, and were highly accumulated in 'Guanxihuang' pummelo (yellow-fleshed). The contents of volatiles in flavedo (285.85−955.88) were much larger than those in pulp (6.05−26.85) samples. Limonene was the most abundant volatile in flavedo, ranging from 133.28 in 'Hongzuan' to 480.36 in 'Guanxi'. The candidate genes TPS1 and LOX2.1 were associated with the amounts of terpenes and aldehydes in pummelo fruits. 'Sanhong' and 'Guanxihuang' fruits were preferred by tasters for their strong aroma, rich sweet and moderately sour taste, and high nutritional constituents. These results may provide insight into understanding the flavor metabolism pathways in pummelo fruits and facilitate further genetic improvement of pummelo fruit quality.
-
Key words:
- Citrus maxima /
- Fruit quality /
- Nutritional quality /
- Flavor /
- Aroma /
- Citrus breeding













