-
The escalating global population, coupled with the pervasive effects of climate change, highlights the imperative of augmenting and perpetuating agricultural productivity. In order to adequately address the escalating needs of the rapidly expanding global populace, which is estimated to exceed 3 billion individuals, it is projected that agricultural production will need to undergo a substantial augmentation of 70% by the midcentury.[1, 2]. Furthermore, the yield of horticultural crops is substantially impacted by changes in climate conditions. These plants are vulnerable to a wide variety of abiotic and biotic stressors, which play a major role in restricting production and productivity in the agricultural sector[3−5]. In the domains of agricultural science and plant biology, the quest for a comprehensive understanding of the intricate physiological and molecular mechanisms underlying plant responses to multifaceted environmental stimuli presents a formidable and pivotal scientific challenge. Heat stress, salt stress, and drought stress are considered some of the most prominent and prevalent environmental stressors that plants encounter during their growth and development[3,6,7].
The inherent limitations of conventional breeding methods in effectively addressing the multifaceted traits associated with stress tolerance underscore the pressing need for scientific innovation. This innovation is crucial to bridge the existing disparity between global food supply and demand on a scale that is commensurate with the challenges at hand. In this field, the formulation of novel and efficient approaches is an absolute necessity. Phytohormones have become a viable and practical option for cultivating highly productive crops that are resistant to the impacts of climate change. They have recently emerged as an environment-friendly alternative method that improves the ability of horticulture plants to withstand abiotic stress. Plant hormones (phytohormones) are chemical regulators produced by plants that govern how plants respond to various environmental stresses, including growth and development[7−10].
Phytohormones promote the coordinated activation of multiple signal transduction pathways, which is a key aspect of a plant's ability to respond to abiotic challenges[11,12]. They also modulate the impacts of a wide variety of both internal and external stimuli, leading to significant differences in plant expansion and development fields[13]. Based on previous studies, the investigation of horticultural crops' ability to withstand abiotic stress has been extensively explored[13]. These studies have revealed that phytohormones, such as ethylene, abscisic acid, and cytokinin, serve as crucial signaling molecules in the plant stress response[14].
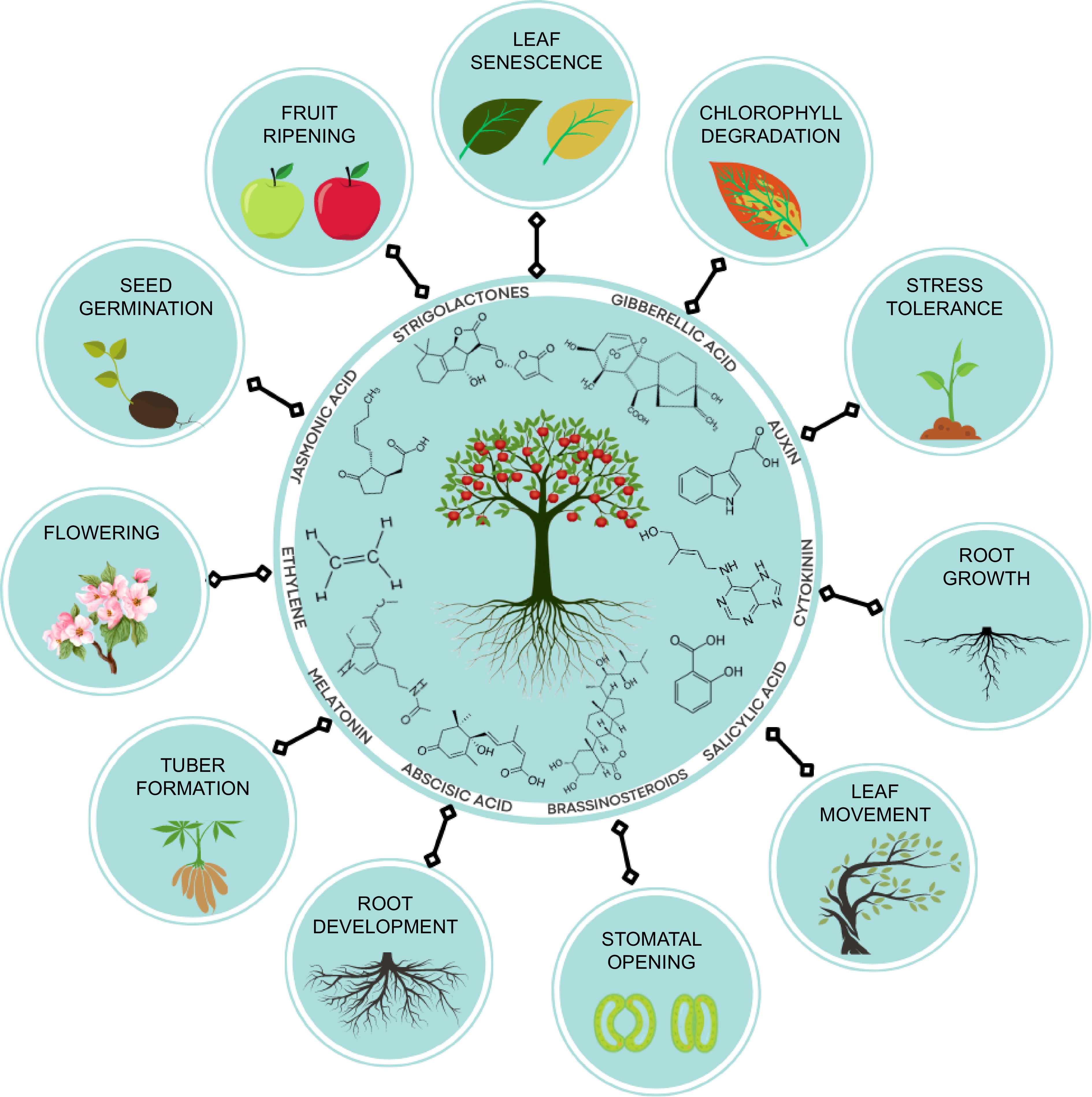
Recent scientific investigations into abiotic stress tolerance have highlighted the significant role of phytohormones as crucial regulators of physiological, molecular, and biochemical processes within plants. These findings contribute to our understanding of the intricate mechanisms underlying plant adaptation and response to adverse environmental conditions[15] and various phytohormones, including jasmonic acid, strigolactones, auxin, salicylic acid, cytokinin, brassinosteroids, ethylene, and others, are crucial in the process of cultivating crops that are resilient to abiotic stress and promote the growth and development and productivity of fruit crops as shown in Fig. 1.
-
The significance of investigating the interplay between horticultural crops and their environment is increasingly acknowledged, particularly considering the prevailing climate change scenario. These crops have significant nutritional value and have the potential to serve as a primary source of food for developed and developing countries alike. Global horticulture crop production is largely controlled by abiotic stress factors such as temperature extremes, salt, drought, metal pollution, lack of oxygen, UV radiation, inadequate nutrients, and pesticides, which have a negative impact on plant development and production. Abiotic stress-induced changes in a plant's physiological, anatomical, biochemical, morphological, and molecular composition have been linked to these negative effects[16]. There are various factors that affect how plants respond to abiotic stress, like the nature and intensity of the stress, environmental conditions, and growth phase of the plant, both living and non-living, under which it is cultivated[17]. The interplay of these factors can impact the plant's capacity to adapt and manage stress, which can ultimately influence its development and yield. Some of the ways in which plants may respond to abiotic stress include reducing their growth rate, losing photosynthetic pigments or membrane integrity, or even shutting down their photosynthetic machinery[6]. Therefore, it is imperative for the advancement of diverse horticultural methods to diligently investigate the intricate interplay between environmental stressors and the underlying morphological and physiological mechanisms that govern plant responses. Such scientific research endeavors are crucial to enhance our understanding of these complex relationships and developing effective strategies to mitigate the adverse effects of environmental pressures on horticultural crop productivity. (Table 1).
Table 1. Phytohormones and other associated compounds in the regulation of abiotic stress-impacts in horticultural crops.
Plant Phytohormone Effect Reference Melon SA The upregulation of the antioxidant defense system by salicylic acid helps to alleviate the inhibitory effects of cadmium on photosynthesis and plant growth [146] Pepper SA Exogenous salicylic acid plays a function in decreasing the impact of extreme temperature [71] Orange BRs Increased accumulation of secondary metabolites plays a significant response to low temperature stress [147] Orange SA To reduce heat stress conditions under SA [148] Banana AUX AUX regulate to salt stress [149] Grape SA Enhance cold stress tolerance under SA [150] Citrus ABA To negatively regulate water-stress under ABA treatment [151] Banana AUX Regulation of heat stress [149] Orange BRs Regulating antioxidant enzymes enhances cold stress tolerance [152] Apple ABA Upregulating expression of the mdsat1 gene enhance resistance to drought and salt stress [153] Grape BRs Elicitation of antioxidant enzymes, including peroxidase, superoxide dismutase, ascorbate peroxidase, catalase, and improves the self-life of fruit under cold storage
[154]Banana SA Decrease the photooxidative and heat stress [155] Grape wine ABA Upregulating of genes involved in chlorophyll synthesis, heat shock proteins, and defense-related genes improves drought tolerance [156] Mango ABA Water stress tolerance increase under ABA [157] Tomato Melatonin The mitigation of nickel toxicity in tomato seedlings is achieved by improving root architecture system, nutrient uptake fluxes, antioxidant potential and photosynthesis
[5]Tomato Ethylene The in vitro improvement of salinity-tolerant tomato is mediated by ethylene-induced physiological responses [16] Fragaria vesca ABA FveHDZs play a role in ABA-mediated processes. [153] Citrus ABA anoxia tolerance increased by ABA [158] Tomato Melatonin Melatonin can alleviate nickel phytotoxicity in tomato seedlings by improving secondary metabolism, oxidative stress tolerance and photosynthesis [67] Banana SA The activities of H2O2-metabolizing enzymes can be changed by salicylic acid, which can increase the chilling tolerance of banana seedlings [74] Grape wine BRs Regulating protein, sugar, and proline accumulation can enhance chilling stress tolerance [159] Citrus ABA Increasing ABA concentration in young leaves and Arbona can improve water stress tolerance. [160] Banana BRs Enhances the ability to tolerate high temperature at a concentration of 0.2 µm [161] Sweet orange ABA Under salt and water stress, an increase in the expression of Late Embryogenesis Abundant (LEA) proteins [162] Apple Ethylene The upregulation of the mdmyc2 gene enhances aluminum stress tolerance [163] Pepper SA Effect of ultraviolet radiation on inducing oxidative stress in leaves of pepper [76,164] Apple GA Enhance germination of seed under cold stress conditions [165] Grape ABA Exogenous application of ABA enhances resistance to different abiotic stresses [166] Apple Ethylene The upregulation of the mdnac047 gene enhances salt stress tolerance [163] Banana JA Polyethylene glycol (PEG) mediated water stress can be alleviated and oxidative stress can be reduced [167] Peach JA Hot air combined with MeJA vapor treatment can alleviate chilling injury in peach fruit [103] Almond ABA Regulate the movement of xylem sap under stress conditions of water [168] Apple JA ABA biosynthesis by the regulation of sunburn [169] Strawberry SA Mitigate salt stress by enhancing the leaf water holding capacity of plants, as reflected by the increase of water relative content in leaves [170] Strawberry ABA Maintaining fruit quality even under drought and salt stress conditions. [171] Apple AUX Plants can regulate photooxidative and heat stress by cross-talking with ethylene [169] Pear JA Phospholipid remodeling promotes alleviation of chilling injury [172] Apple GA Improved salt tolerance has been observed through the upregulation of the transcription factors mdbzr1 and mdbzr1-2like [173] Apple Ethylene The upregulation of mderf1b–mdcibhlh1 genes improves cold tolerance [173,174] Pea ABA Enhanced accumulation of abscisic acid in young leaves enhances water stress tolerance [175] Apple AUX Root development rate and architecture are maintained to minimize drought stress [176] Apple JA Regulating xylem sap movement improves water stress tolerance [177] Strawberry SA Combination with iron nanoparticles reduces the impact of drought stress under in vitro conditions [178] Apple BRs Regulating the photosynthesis mechanism can help minimize effects under water stress [179] In recent times, mounting concerns regarding the environment have gained prominence due to the rapid escalation of environmental contamination and substantial shifts in global climatic conditions. Moreover, prolonged exposure to higher temperatures in regions that receive rainfall may lead to an expansion in the severity of drought conditions worldwide. As per previous research, it has been projected that the average worldwide temperature will increase by 2.0−4.9 degrees Celsius by the start of the next century (2100 AD), aligning with the conclusion of the current century[18]. Soon, it is highly probable that there will be a decline in the quality and productivity of crops on a global scale, caused by a combination of elevated temperatures, drought, and salinity stress. High temperatures, salt stress, and drought, all put a strain on plants. Furthermore, at present, approximately 90% of agricultural land faces vulnerability to either a single stress scenario or the combined effect of multiple stressors. However, compelling evidence suggests that certain plant-derived hormones possess the capability to mitigate the detrimental effects of abiotic stresses on horticultural crops.
-
Endogenous phytohormones are often recognized as critical mediators of the responses that plants exhibit when exposed to stress. Endogenous phytohormones play an important part in a plants capacity to withstand abiotic stress. Plant hormones are minuscule molecules that serve as signaling agents and participate, to some extent, in all stages of a plant's growth and development (Fig. 2). Plant hormones may be found in all parts of a plant. It's possible that the underlying mechanism of many actions changes depending on which hormone is employed. Therefore, it is now evident that a single hormone may govern a broad variety of developmental and cellular processes, and that many hormones could perform the same function. BRs, MEL, SA, JA, GA, Aux, ABA, ET, BR, and SLs are all examples of hormones that are necessary for the growth and development of plants. These phytohormones offer support and management to plants in the face of biotic and abiotic stressors and are essential to the growth and development of plants. As a result, phytohormones are currently employed to enhance agricultural stress management.
Figure 2.
Phytohormones have been recognized as major regulators in facilitating the adaptive responses of plants to diverse abiotic stress conditions, thereby playing a crucial role not only in stress tolerance but also in promoting growth, development, and ultimately enhancing the overall productivity of horticultural crops. Through intricate signaling pathways and molecular mechanisms, these phytohormones such as brassinosteroids (BRs), gibberellin (GA), jasmonates (JAs), salicylic acid (SA), strigolactones (SLs), abscisic acid (ABA), and melatonin (MEL) orchestrate a complex network that modulates physiological, biochemical, and morphological changes, enabling plants to overcome the detrimental effects of abiotic stressors. Therefore, understanding the multifaceted roles and interactions of phytohormones in horticultural crop systems represents a vital avenue for devising innovative strategies to enhance adaptability, optimize growth, and maximize yield under challenging environmental conditions.
-
In the early 1960s, scientists discovered that abscisic acid was responsible for triggering the germination of dormant seeds[19]. Subsequent research revealed that it may also have a function in the development of plants and their adaptive responses to stress[20]. Abscisic acid (ABA) serves a pivotal role in regulating dormancy and seed growth, while also being involved in the modulation of water content through the regulation of stomatal pore opening and closing. Additionally, ABA plays a crucial role in modulating plant responses to external stresses. Under conditions of water scarcity, ABA synthesis primarily occurs in the roots and is subsequently transported to the leaves through the xylem, resulting in an increased concentration of ABA. The increase in ABA concentration acts as a pivotal signal to initiate a complex signaling cascade within guard cells. This intricate cascade ultimately governs the regulation of turgor pressure across the guard cell membrane.[21]. When the plant encounters abiotic stress, ABA synthesis and redistribution trigger the closure of stomata and a consequent decrease in transpiration rate. As a consequence, cell growth and development are suppressed[22]. ABA is renowned for its functions in regulating the water level of plants and promoting healthy plant-water relationships[23].
Abscisic acid (ABA) is accountable for activating hormone-responsive TFs. The phosphatases and kinases involved in ABA signaling are crucial for facilitating prompt responses to diverse abiotic stresses[24]. The precursors for the ABA production pathway are provided by the carotenoids[25]. The enzyme 9-cis-epoxy carotenoid di-oxygenase (NCED) is accountable for the biosynthesis of ABA, which is rapidly induced by stress stimuli through the oxidative cleavage of β-carotene. Additionally, the enzyme zeaxanthin epoxidase produces trans-violaxanthin, which undergoes a chemical transformation to become the 15-carbon molecule known as neoxanthin. Then, xanthoxin is generally converted into abscisic aldehyde, and the oxidation of that product results in the creation of ABA. Moreover, it has been found that ABA synthesis can occur via an alternative pathway involving ABA-GE (glucosidase homologs) in some plants when confronted with an abiotic stress[26,27]. In addition to mediating physiological responses by itself through signaling, ABA also regulates physiological changes by associating with other phytohormones in plants that are subjected to abiotic challenges. These interactions take place while the plants are under stress[28] and ABA is responsible for activating and mobilizing a broad range of metabolic defenses in plants. These defenses include producing proline, antioxidants, enzymes that eliminate ROS, unsaturated strengthening cuticular waxes, fatty acids, and heat shock proteins. Together, they help against the negative effect of abiotic stress[29−34].
-
Gibberellins (GAs) actively participate in a diverse array of plant growth and developmental processes, encompassing fruit development, seed germination, inter-nodal elongation, and promotion of blooming. These regulatory hormones are one of the earliest and most extensively researched groups of hormones[35]. Current research has employed genetic and biochemical methods to investigate the genetic foundation of gibberellic acids (GAs) and the genes encoding the enzymes responsible for their synthesis and inactivation[36,37]. The control of GAs on a cellular level is widely recognized to be highly complex. GAs is synthesized directly by enzymes belonging to a diverse array of multigene families, referred to as GA20ox (GA 20-oxidases) and GA3ox (GA 3-oxidases). Furthermore, these gene families aid in accelerating the process of bioactive GA production in its final stages[38]. Interaction between GA and GID1 enables the repressor DELLA protein to bind with the nuclear-localized receptor protein GID1. The F-Box protein binds with the protein-protein interaction domain of the DELLA protein, which then recruits the SLY1 complex for ubiquitination through SCF-E3 ligase. Additionally, the 26S proteasome degrades the interaction between the promoter region of GA-inducible genes and transcription factors. As a result, GA can regulate signaling by degrading repressor proteins and promoting a diverse range of physiological and biochemical responses.
The counteracting influence of gibberellins (GAs) in various plant tissues, whether endogenously synthesized or exogenously administered, serves as a pivotal mechanism for alleviating the adverse effects induced by stressors. This counteractive response facilitates the enhancement of plant resistance and fortification against the detrimental impacts of environmental stressors. For instance, the GAs regulatory function is illustrated by the increased seed yield and improved drought tolerance of rice under water-limited conditions, which resulted from a decrease in endogenous GA levels caused by the GA2ox6 ectopic expression[39]. Additionally, GA is known to modulate redox homeostasis by increasing electron mobilization in H. vulgare[40], enabling plants to adapt better to suboptimal development conditions. GRAS. TFs play an essential part in plant growth and signaling, particularly in pathways involving GA production and signal transduction. Exposure to abiotic stress treatment, such as NaCl, and H2O2 increased GRAS40 expression in tomato[41]. During the vegetative and reproductive stages of tomato development, SlGRAS40 is associated with the auxin and GA pathways. Overexpression of SlGRAS40 in plants resulted in increased resilience to both salt and drought stress, demonstrating its potential as a genetic tool for improving stress tolerance in various plants.
-
Auxins are endogenous plant hormones that function as growth regulators, mainly responsible for root and shoot formation, as well as relative growth[42]. Studies have shown that CKs and auxin collaborate to regulate a wide range of cellular and physiological processes such as cell elongation, apical dominance, leaf growth, cell cycle, and embryonic development that occurs throughout the seed generation process[43,44]. Clearly, auxins play a key role in the growth responses of plants to environmental stress. Though, future changes in auxin homeostasis caused by these dynamic changes could result in aberrant plant morphogenesis, which leads to normal morphogenesis. These morphogenic responses induced by stress exemplify an acclimatization strategy, which serves as a defensive mechanism enabling organisms to evade or alleviate the adverse impacts of environmental pressures[45,46]. Extensive genetic research and in vitro testing have elucidated the intricate nature of auxin biosynthesis pathways, revealing the existence of both tryptophan (Trp)-independent and tryptophan (Trp)-dependent processes. The four distinct tryptophan-dependent pathways, namely indole-3-acetaldoxime (IAOx), indole-3-acetamide (IAM), indole-3-pyruvate (IAP), and tryptamine (TAM) pathway, have been identified as integral components involved in the synthesis of auxins. These pathways collectively contribute to the intricate regulation of auxin production, highlighting the complex and multifaceted mechanisms underlying the biosynthesis of this essential plant hormone. Such a comprehensive understanding of auxin biosynthesis pathways provides valuable insights into the fundamental molecular processes that govern plant growth and development, paving the way for potential applications in agricultural practices and crop improvement strategies[47]. Among these, the IPA and TAM pathways are believed to have contributed to the expansion and maturation of the plant. The receptor protein for auxin is found in the nucleus. The fact that the auxin receptor protein is a repressor protein is an intriguing fact to take into consideration. An enzyme known as SCFTIR1-E3 ligase is brought into play whenever auxin forms a bond with either a receptor protein or a repressor protein. This enzyme adds ubiquitin to the repressor protein, which triggers the 26S proteasome to begin the process of breaking down the protein. Auxin response factors can effectively interact with the promoter region of the auxin-inducible gene because of the destruction of the repressor protein, thereby allowing the auxin response factors to moderate gene expression. The transcription factors that govern the effects are part of a family known as the auxin response factor family or ARF family. For example, certain ARFs have been found to play essential functions in lateral root formation.
A recent study has provided evidence of the involvement of the auxin-responsive regulatory network, which encompasses auxin-responsive factors, TAS3-derived trans-acting short interfering RNAs, and miR390. Collectively, these components play a crucial role in determining the extent of lateral root growth. The study demonstrated that the amount of lateral root growth is proportional to the number of microRNAs that are functioning together, indicating that the auxin-responsive factors and microRNAs create a regulatory network that is in response to auxin. The intricate nature of this regulatory network allows for the fine-tuning of lateral root growth to optimal levels, while simultaneously maintaining a high degree of flexibility. However, due to the limited research conducted thus far, the molecular mechanisms underlying changes in auxin dispersion in stressful environments are not yet fully understood. There are many distinct types of abiotic stresses, each of which has the potential to disrupt the homeostasis, distribution, and metabolic processes of auxins within the cellular environment. Alterations in the expression of the PIN gene, which is necessary for polar auxin transport, could be one potential explanation for these changes. The accumulation of phenolic compounds during stressful situations presents another potential factor that can disrupt polar auxin transport, thereby affecting various aspects of plant growth and development. Additionally, changes in auxin concentration could also play a role in the modification of auxin dispersion in stressful environments. The degradation of indole-3-acetic acid (IAA), facilitated by peroxidases, plays a pivotal role in regulating stress-induced auxin metabolism. Auxins consider stress hormones because they could directly or indirectly affect the profile of specific genes that respond to stress. One of the fundamental functions of auxin in plants is to facilitate the initiation and development of lateral roots. This process is vital for managing the structure of the plant's root system, as it helps to increase the plant's ability to absorb water and nutrients and enhances its overall efficiency. Therefore, the development of lateral roots is widely recognized as one of the most fundamental plant activities that auxins are responsible for, among their many other responsibilities.
-
Ethylene exists in the gaseous state and is an essential component in several different morpho-physiological processes. These mechanisms include the stimulation of plant responses to external stimuli and the triple response in the germination of seeds. Additionally, ET is responsible for regulating a wide range of stress-related biochemical reactions inside plants that are exposed to various abiotic stressors such as excessive heat, poor humidity, low temperature, water-logging, high salt, heavy metals, flooded, or submerged circumstances[48]. For example, a correlation between ET concentration and cold and freezing stress was identified in Arabidopsis[49] and Medicago truncatula[50]. In addition, modulation of ET homeostasis is required for adequate tolerance of suboptimal temperature stress, including chilling, and freezing. It has been demonstrated that having high ET levels is beneficial when it comes to salt stress resistance, as shown by salt-tolerant Arabidopsis plants. Under high-salt conditions, ethylene overproduction (ETO1) plays a crucial role in performing multiple beneficial functions, such as regulating the Na+/K+ balance and promoting the formation of reactive oxygen species (ROS)[51]. Furthermore, it is an essential factor in the process of modifying the way plants react to the assault of pathogens, external mechanical damage, ultraviolet radiation, and a deficiency of nutrients known as phylloprotection. In several instances, the production of ET, as well as its buildup, was seen as a response to mechanical traumas or other forms of physical injury[52]. As can be shown in Fig. 2, the production of ET has been quantified in a range of plant tissues, as well as in flowers that are wilting and mature fruits of plants that have been treated to abiotic stimuli[53]. S-adenosyl methionine also known as SAM, is the compound that initiates the fundamental process underlying the production of ethylthiourea (ET). SAM is frequently produced in high quantities in diverse range of plant and fruit trees and is the precursor of ethylthiourea (ET). The enzyme accountable for triggering the initial sequence of events that converts SAM into ACC and methylthioadenosine (MTA) is known as ACC synthase. The enzyme also plays a crucial function in the final conversion of SAM to L-methionine. In some circles, this enzyme is also referred to as an ACC synthase. Due to this recycling, the levels of L-methionine do not change even when there is a significant increase in the quantity of ethylene that is created because of biosynthesis. In addition, the very unstable ACC synthase enzyme has an impact on the ET biosynthesis route and the effect of making the system more unpredictable. This enzyme has a tendency to slow down the pace of biosynthesis, and the quantity of ethylene that is present in tissues, flowers, and fruits has been shown to have a direct correlation with the level of activity of the enzyme[54].
-
Brassinosteroids also known as BRs, are a class of non-toxic, multi-functional, and poly-hydroxylated steroidal plant compound[55]. The three most potent brassinosteroids (BRs) used to study physiological, cellular, and molecular processes in plants are Brassinolide (BL), 28-homobrassinolide (28-HBL), and 24-epibrassinolide (24-EBL). These compounds are commonly employed to investigate a wide range of plant-related topics. BRs can assist seed germination, root growth, seedling development, cell division, stomatal opening, vascular differentiation, and senescence[56]. BRs have been shown to exert efficient control over a wide range of physiological and morphological processes in horticulture crops[57]. Currently, research has demonstrated that BRs can provide significant tolerance in various plants against abiotic stimuli, for example, salt, waterlogging, drought, metals, and low and high temperatures[58]. The ability of BRs to defend against stress and regulate development is closely connected to the manufacture of proteins and nucleic acids and their impact on metabolic activities associated with photosynthesis[59].
Supplementation of brassinosteroids (BRs) has been demonstrated to significantly enhance growth and augment the pool of antioxidant enzymes in eggplant exposed to salt stress. Simultaneously, BRs supplementation leads to a reduction in the activity of sodium and chloride levels, as well as the levels of antioxidant enzymes and malondialdehyde (MDA)[60]. Furthermore, BRs have been found to significantly improve growth parameters, cellular membrane integrity, ion homeostasis, electrolyte leakage (EL), photosynthesis, and x electrolyte leakage (EL) when the strawberry plants were exposed to salt stress[61]. The 24-EBL treatment has also been found to improve peppers photosynthesis feature, even when the plant is subjected to drought[62]. Additionally, in a radish crop subjected to drought stress, treatment with 24-EBL and 28-HBL was found to increase the content of osmolytes and antioxidant enzymes while simultaneously lowering levels of MDA. It was also discovered that the 24-EBL treatment increased the amount of radish photosynthetic pigments[63]. Upon exposure to salt stress, the growth parameters of strawberry plants exhibited significant improvements when treated with brassinosteroids (BRs). These improvements were observed in various aspects, including boosting photosynthetic activity, enhanced cellular membrane integrity, reduced electrolyte leakage, improved ion homeostasis, and decreased x-electrolyte leakage[64].
-
SA shows a critical function in regulating numerous physiologic processes in plants, including secondary metabolite production, fruit development, leaf photosynthetic activity, ion homeostasis, root development, seedling growth, and system of antioxidant enzymes[65]. The application of SA greatly enhances the photosynthetic capability, root architectural system, and growth characteristics of salt-stressed Cucumis sativus[66]. The application of SA in tomato (Solanum lycopersicum) resulted in a decrease in ROS-induced damage and a significant increase in the antioxidant enzyme system, metabolites, leaf water potential, and leaf gaseous exchange.[67]. In the existence of SA, there is a considerable improvement in the growth status of potatoes but also in their antioxidant enzyme activity, proline contents, reduced oxidative damage, chlorophyll contents, and lowered cadmium deposition in the situation of cadmium toxicity[68]. The application of salicylic acid (SA) as a seed primer enhances the probability of seed germination, as well as promoting seedling growth and ultimately improving crop productivity in cucumber (Cucumis sativus)[69]. Under conditions of water scarcity, the application of salicylic acid (SA) proves advantageous for Solanum lycopersicum plants. It positively impacts various aspects, including leaf water potential, antioxidant enzyme system, photosynthetic apparatus, mitigation of cell damage, fresh weight, and anatomical responses[70]. The application of SA to Piper nigrum plants subjected to heat stress resulted in increased thermotolerance, leaf moisture content, antioxidant enzyme activity, and chlorophyll content. These positive effects on plant physiology were attributed to the presence of SA[71]. It has been shown that SA may improve the rate of photosynthesis in Piper nigrum[72], Prunus persica[73], and Cucumis sativus. Eggplant seedlings treated with SA may be protected from the oxidative damage affected by cold stress. Providing banana plants with additional SA under high-temperature circumstances resulted in a reduced concentration of H2O2 and greater management of the system of antioxidant enzymes[74]. After undergoing SA treatment, plants subjected to ozone stress exhibited a significant improvement in nitrogen uptake, root features, and seed germination[75].
Previous research has demonstrated that the utilization of salicylic acid (SA) in pepper leaves, which were previously exposed to UV-B radiation, results in an augmentation of antioxidant enzyme activity[76]. Other research has also shown the moderating effects of exogenous SA in horticultural crops such as spinach (Spinacia oleracea)[77], tobacco (Nicotiana tabacum)[78], rosemary (Salvia rosmarinus)[79], pea (Pisum sativum)[80], and strawberry (Fragaria × ananassa), which are all subject to abiotic stress[81]. The typical plant responses regulated by SA in horticulture plants include an increase in chlorophyll contents, proline concentrations, antioxidant enzyme activity, and synthesis of secondary metabolites. However, some plants like strawberries, rosemary, and tobacco respond to abiotic challenges by showing a decrease in oxidative damage, better growth traits, and greater yield. These examples demonstrate the various antagonistic and synergistic interactions of salicylic acid (SA) with nutrients that occur under favorable and unfavorable environmental conditions, and these interactions can significantly affect plant growth and development. In the case of cucumber plants subjected to high salt stress, the exogenous application of salicylic acid (SA) proved to be effective. It resulted in a notable decrease in salt (Na+) absorption, while simultaneously enhancing the uptake of other essential mineral elements[82]. During cold stress, eggplant seedlings exhibit upregulating of antioxidant enzymes through the AsA-GSH pathway, while downregulating oxidative damage and expression of genes involved in these processes[83]. Likewise, even when exposed to cold stress, the application of salicylic acid (SA) to okra plants demonstrated effectiveness in mitigating oxidative damage and reinforcing the antioxidant defense system[84].
-
Studies have shown that exposing stressed plants to melatonin can promote the synthesis of antioxidative defense pathways and increase the scavenging of reactive oxygen species (ROS), as melatonin have the ability to scavenge ROS[85]. Interestingly, melatonin was first discovered in 1993 in tomato and morning glory plants[86]. Since then, numerous investigations have highlighted the positive effects of MEL on horticulture crops, including strawberries[87], tomatoes[88], watermelons[89], peaches[90], cucumbers[91], and peppers[92]. Additionally, it has been shown in horticultural crops that MEL may reduce the amount of photosynthetic damage caused by abiotic stress[93]. In Chinese crab apple (Malus hupehensis), MEL successfully reduced the inhibitory effect of UV-B radiation on photosynthetic abilities and minimized leaf membrane damage[94]. Moreover, in grape leaves subjected to ozone stress, the application of MEL resulted in enhanced growth, photosynthesis, antioxidant enzyme activity, and decreased ROS production[95]. MEL has also been shown to improve the nutrient absorption capacity of horticulture crops while decreasing metal deposition in the soil. For example, MEL treatment significantly reduced the amount of nickel deposition in tomato roots and leaves while increasing the absorption of micro- and macronutrients under conditions of nickel toxicity[67].
In leafy crops such as fenugreek, the application of melatonin (MEL) has been observed to reduce the formation of reactive oxygen species (ROS) and chlorophyll degradation under drought conditions. Furthermore, MEL supplementation resulted in increased proline concentration, enhanced antioxidant enzyme activity, and elevated levels of photosynthetic pigments.[96]. In summary, due to its antioxidant properties and beneficial effects of growth and development on plants under abiotic stress, MEL is becoming increasingly popular as a potential growth regulator for horticulture crops. However, further research is required to fully understand the mechanism and action of MEL and its potential applications in various horticulture crops.
-
CKs are a significant family of hormones that are responsible for regulating the maturation and growth/development of horticultural plants. CKs control a range of biological activities, such as morphogenesis, shoot differentiation, seed germination, root development, cell division, chloroplast biogenesis, and the shedding of fruit and leaves. Incorporating CKs into agriculture methods can result in a significant increase in crop yield. For example, adding CKs to grapes improve root-shoot development and root morphology, and increases the quality of chlorophyll, soluble sugar, and soluble protein found in lettuce[7,97]. CKs can alleviate the deleterious effects of environmental stressors, resulting in higher growth and production. CKs mitigate the negative impact of low-temperature stress on the tobacco[98], salinity stress in the tomatoes[99], and drought stress in the radish[100].
Moreover, the application of cytokinins (CK) has been found to significantly reduce the levels of H2O2, O2, and malondialdehyde (MDA) in salt-stressed eggplant. Simultaneously, CK treatment increases the activities of ascorbic acid (AsA), glutathione (GSH), superoxide dismutase (SOD), and peroxidase (POD), as well as enhancing proline content. Increasing the concentration of CKs in horticulture crops can increase their tolerance to the toxicity caused by elements such as zinc. For example, modifying an IPT gene responsible for CK synthesis led to an improved CK concentration in tobacco plants, which strengthened their tolerance to zinc toxicity. Transformed plants demonstrated higher levels of photosynthesis and transpiration than their wild-type counterparts. Thus, CKs have an important function in mitigating the effects of abiotic stress in fruit crops.
-
Jasmonates (JA) is a group of plant hormones that play a crucial role in regulating various aspects of plant growth, development, and defense responses. They are derived from the amino acid, linolenic acid, through a series of enzymatic reactions. Jasmonic acid (JA) and its derivatives, such as methyl jasmonate (MeJA), are the most well-known and studied forms of jasmonates[56]. These compounds play a vital signaling role in plant development[101] and control diverse processes such as photosynthesis, leaf degeneration, stomatal formation, root growth, chlorophyll degradation, and nutrient homeostasis[102].
Numerous studies have provided substantial evidence demonstrating the significant impact of JA in enhancing plants' ability to withstand stress and adapt to new environmental challenges. For instance, the application of exogenous methyl jasmonate (MeJA) has been found to enhance cold stress resistance in peach plants. This effect is achieved by effectively mitigating ROS-mediated oxidative damage and reinforcing the antioxidant defense system[103]. The grape seedling antioxidant defense was boosted by JA treatment during heat stress[104]. JA also greatly improved sugar beet yield, drought tolerance, and antioxidant enzyme activity[105]. MeJA supplementation substantially boosted antioxidant enzyme activity, and secondary metabolite content, and reduced chilling damage in the pomegranate[106]. In a separate investigation, MeJA was used to cure cold-stressed loquat fruits. The treatment meaningfully alleviated the negative effects of cold damage and significantly improved the antioxidant enzyme system[107]. When applied to bitter melon under salt stress, Jasmonate significantly improved metabolite content, growth characteristics, and proline levels, while reducing oxidative damage[108].
Tabasco pepper plants exposed to cadmium toxicity exhibited notable improvements in various physiological aspects. Specifically, chlorophyll content, root growth, and the antioxidant enzyme defense system were all enhanced. Furthermore, the levels of malondialdehyde (MDA), a marker of oxidative stress, were significantly reduced[109]. MeJA pretreatment significantly increased the antioxidant activity in strawberry seedlings under a salt stress[110].
-
Plants are capable of producing various low molecular weight polyamines (PAs), including spermidine, putrescine, and spermine[111]. These PAs have a fundamental responsibility in regulating different physiological processes, including the division and differentiation of cells, root growth, embryogenesis, flowers, and fruit development, the transcription of genes, morphogenesis, organogenesis, organ maturation, and leaf aging[112]. PAs also have a role in mitigating a variety of abiotic stressors, such as salinity, high temperatures, and the accumulation of different types of heavy metals in the environment[113]. In a study, tomato was adversely affected by salt stress, leading to stunted growth. However, pretreatment with spermidine prior to the overall growth of the tomato plants. In addition, a higher dose of spermidine increased the levels of S-adenosylmethionine decarboxylase (SAMDC), ornithine decarboxylase (ODC), diamine oxidase activity (DAO), and polyamines oxidase (PAO) in the stems of tomato plants[114]. Diao et al.[115], conducted research that demonstrated the significant benefits of spermidine administration on growth, soluble sugar content, proline, and chlorophyll fluorescence components in tomato plants exposed to cold stress. In addition, ROS and MDA were decreased. Exogenous spermidine treatment in tomato plants also increased the enzymes antioxidant activity and concentrations such as PAO, DAO, ODC, and ADC. Moreover, PAs improved the antioxidant status of plants exposed to chromium toxicity, as evidenced by increased activity of enzymes such as SOD, POD, APX, and CAT. In the presence of saltwater, the application of polyamines (PAs) has demonstrated significant positive effects on plant growth parameters. Notably, in lettuce plants, PAs have been shown to lead to a substantial increase in both dry and fresh weight. Additionally, PAs have been observed to enhance root and stalk length, growth rate, and vigor indicators. These findings highlight the beneficial impact of PAs on the overall vigor of lettuce plants, particularly in the presence of saltwater conditions[116]. Polyamines have been shown to have a protective effect against various abiotic stresses, involving heavy metal toxicity. The use of PAs in Raphanus sativus plants exposed to chromium toxicity demonstrates their potential as a tool for mitigating the harmful effects of environmental stress on plants[117]. The application of spermidine and spermine to Rose damascena under water stress conditions resulted in successful improvements in the proline content, plant growth characteristics, CAT, gas exchange elements, and SOD enzymatic activities[113]. In conditions of water stress, exogenous supplementation with spermidine and spermine led to a decline in the endogenous level of putrescine and an increase in the concentrations of both spermidine and spermine. These results decrease the quantity of putrescine produced by organism itself. Supplementation with PAs led to higher levels of salt resilience in cucumber seedlings, as well as elevated levels of antioxidant enzymes[118]. Zapata et al. investigated the amounts of PAs present in several horticultural plants that had been exposed to seawater. Solanum lycopersicum, Capsicum annuum, Cucumis melo var. cantalupensis, Brassica oleracea var. botrytis, Lactuca sativa, and Spinacia oleracea[116]. The quantity of putrescine that was generated by each plant that had been grown in a saline environment resulted in a substantial decrease. Nevertheless, the addition of NaCl resulted in an increase in the total quantities of both spermine and spermidine across the board for all the species. Recent studies have used exogenous (PAs), regulators of PAs production, and recombinant approaches to examine the action mechanism of PAs. Research has shown the importance of PAs by providing distinctive insights into the function of PAs by providing unique insights into the function of PAs in plant growth and demonstrating the significance of these chemicals. According to past research that was conducted and published in this field, it was discovered that PAs had a connection with plant development, membrane structure and stability in nucleic acids, and tolerance to stress[119].
Numerous studies have consistently demonstrated that exogenous supplementation of polyamines (PAs) can induce various beneficial effects in plants. These effects include: 1) Regulating the expression of osmotically responsive genes in an appropriate manner. 2) Reversing or significantly alleviating growth restrictions induced by stress conditions. 3) Preserving the structural integrity of plant cell membranes. Reducing the levels of reactive oxygen species (ROS) such as H2O2 and O2. 4) Stimulating the production of antioxidant enzymes. These findings highlight the multifaceted positive impact of exogenous PAs supplementation on plant physiology, particularly in terms of stress tolerance, cellular integrity, and antioxidant defense mechanisms[120].
-
Strigolactones, also known as SLs, are multifaceted compounds recently discovered that are derived from carotenoids. They belong to the family of plant hormones known as stigolactones[121]. Previous research carried out on plants revealed that their primary function is to facilitate seed germination, rhizosphere structure, and root architecture[122]. In addition, they control the aging of the leaves, the elongation of the stems, the closing of the stomata, the spreading of the shoots, and the absorption of nutrients[123]. The application of SLs resulted in a considerable improvement in growth, gas exchange variables, relative water content, chlorophyll fluorescence elements photosynthetic pigments, and the activity of antioxidant enzymes in grape (Vitis vinifera) that was subjected to drought stress.
In addition, it has the effect of significantly lowering oxidative damage, the stomatal opening, and the EL level. The use of SLs also encourages the transcription of genes that are accountable for the creation of SLs, which is a further benefit[124]. Furthermore, when tomato plants were exposed to low light conditions, the application of synthetic strigolactones (SLs) to the vegetation resulted in notable improvements in various growth characteristics. Specifically, SLs enhanced the development of pigment molecules, photosynthetic metabolism, and chlorophyll fluorescence parameters in plants. These findings highlight the beneficial effects of SLs on the overall growth and physiological performance of tomato plants under low light exposure. In a different study, the results revealed that SLs sped up the deterioration of MdD14 in pepper plant[125]. The implementation of SLs led to a significant reduction in shoot branching and hypocotyl elongation that occurred. When apple seedlings were exposed to the harmful effects of potassium chloride, the administration of exogenous SL resulted in a considerable increase in both the net chlorophyll substance and the photosynthetic rate of the apple seedlings. This was the case even though the apple seedlings had been exposed to the toxicity of potassium chloride (KCL). Conversely, the addition of exogenous SL resulted in an increase in the accumulation of proline, maintained the osmotic equilibrium, and maintained the absorption of mineral nutrients. These findings were attained by increasing the activity of the antioxidant enzymes SOD and POD, while at the same time lowering the level of oxidative stress that was brought on by the detrimental effects of KCL[2]. Application of exogenous SL to peas resulted in a significant increase in the levels of photosynthetic pigments. This increase led to a higher number of stalk branches in response to the freezing stress[126]. The application of the SL treatment to tomatoes causes considerable upregulation of the transcript of numerous genes that are believed to play a role in light-harvesting and photosynthetic processes[127]. The presence of endogenous strigolactones (SLs) as growth regulators has been found to impart enhanced tolerance to abiotic stress in several horticultural crops, such as grapevine, tomato, lettuce, and apple. These crops have demonstrated improved resilience and adaptability in the face of adverse environmental conditions, highlighting the pivotal role of endogenous SLs in modulating stress responses and promoting the overall performance and productivity of these important horticultural species. These SLs play a crucial role in mitigating the effects of adverse environmental conditions on crops. Particularly when cultivated under stressful circumstances, these crops face significant challenges. However, the presence of SLs enables them to better withstand and adapt to these adverse conditions, ultimately improving their overall stress tolerance[128]. Priming lupine seeds with SL le to improve the growth of the seedlings and germination rate, as well as an elevated level of proline and decreased MDA content. The SLs application led to improved activity of antioxidant enzymes and glyoxalase system in lupine seedlings[129]. The addition of SL to tomato plants under dehydration stress resulted in a significant increase in stomatal sensitivity[130]. Strigolactones (SLs) exert their effects on various hormone response networks in diverse plant species. This modulation not only enhances the plants' resilience to challenging environmental conditions but also mitigates the detrimental effects of stress on agricultural productivity. By influencing hormone signaling pathways, SLs contribute to the optimization of plant responses to stress, allowing them to better cope with adverse factors. This multifaceted regulation of hormone networks by SLs underscores their potential as valuable tools for improving agricultural production under stress-prone environments[131]. Overall, the role of SL in the abiotic stress metabolism of agricultural plants is substantial, as it helps them cope with a variety of environmental stressors.
Plant hormones are chemical compounds that regulate diverse aspects of plant growth. They are responsible for controlling processes such as seed germination, cell division, differentiation, organ growth, and responses to environmental stimuli. Plant hormones include cytokinins, auxins, gibberellins, brassinosteroids, ethylene, and abscisic acid. The complex network of interactions between plant hormones and their signaling pathways is still not fully understood. However, researchers have discovered that by manipulating the biosynthesis, transport, metabolism, and signal transduction of plant hormones, they can influence plant growth and development as shown in Fig. 3. Chemical compounds that can achieve this are called plant growth regulators (PGRs). PGRs are commonly used in horticulture and agriculture to increase crop yield, improve plant quality, and control plant growth. For example, the application of auxins can promote root growth, while gibberellins can stimulate stem elongation. Similarly, abscisic acid can enhance drought tolerance in plants. Overall, the study of plant hormones and PGRs is a rapidly growing field that has significant implications for agriculture and environmental sustainability. By gaining a deeper understanding of the complex plant hormone network and developing targeted PGRs, researchers hope to improve food security, and plant productivity, and mitigate the effects of climate change.
-
The root system of plants plays a critical role in hormone-stress interactions, serving as an anchoring mechanism and facilitating nutrient uptake from the soil while regulating various physiological processes. An essential aspect of root functioning involves its participation in the plants response to diverse stressors, including hormonal responses. When confronted with stressors such as nutrient deficiency, salinity, or drought, plants initiate a series of intricate physiological responses. These responses enable plants to adapt and enhance their chances of survival. Plant hormones play a key function in mediating these responses, and the root system acts as an interface between the plant and its external environment, sensing and transmitting signals related to stress and hormones. Roots produce and release various signaling molecules, including hormones such as abscisic acid, ethylene, and cytokinin, which regulate the plants response related to stress. For example, during drought conditions, the root system detects the decrease in soil moisture and releases ABA, which then signals the stomata on the leaves to close, reducing water loss through transpiration[132]. This hormone-stress interaction helps the plant conserve water and prevent dehydration. Additionally, the root system also modulates the distribution and transport of hormones throughout the plant and provides a pathway for the movement of hormones from the roots to other plant parts, including the above-ground canopy. Through this hormone transport, the root system influences the growth, development, and physiological responses of the entire plant in response to stress[133].
-
The interaction between the root system and the canopy is essential for coordinating the plant's response to stress through hormone signaling. The canopy encompasses the above-ground portion of the plant, including the leaves, flowers, stems, and fruits. During stress situations, the root system communicates with the canopy through hormone signaling pathways. In response to stress, the root system of plants releases stress-related hormones that undergo transport towards the canopy. These hormones function as signaling molecules, conveying stress signals, and instigating precise responses within the canopy. For instance, the root system may release cytokinin during nutrient deficiency, which subsequently travels to the canopy region, thereby playing a role in regulating plant growth and development under challenging nutritional conditions. Cytokinin promotes shoot growth and development, stimulating the production of new leaves or lateral shoots. By doing so, the plant aims to increase its photosynthetic capacity and enhance nutrient uptake to compensate for the deficiency[134]. Additionally, the interaction between the root system and canopy is bidirectional. The canopy also produces hormones that can influence root growth and function. Notably, when the foliage undergoes damage caused by herbivory or pathogen attack, the injured leaves release jasmonic acid, a hormone that exerts inhibitory effects on primary root growth while simultaneously stimulating the formation of lateral roots. This response helps the plant allocate resources toward repairing and regenerating the canopy[135]. Overall, the root system and canopy interact through hormone signaling pathways to coordinate the plants response to stress. The root system detects and responds to stress by releasing hormones, which are then transported to the canopy, influencing growth and physiological processes. Conversely, the canopy can produce hormones that affect root growth and function. This intricate interplay ensures a coordinated response throughout the plant to optimize survival and adaptation under stress conditions.
-
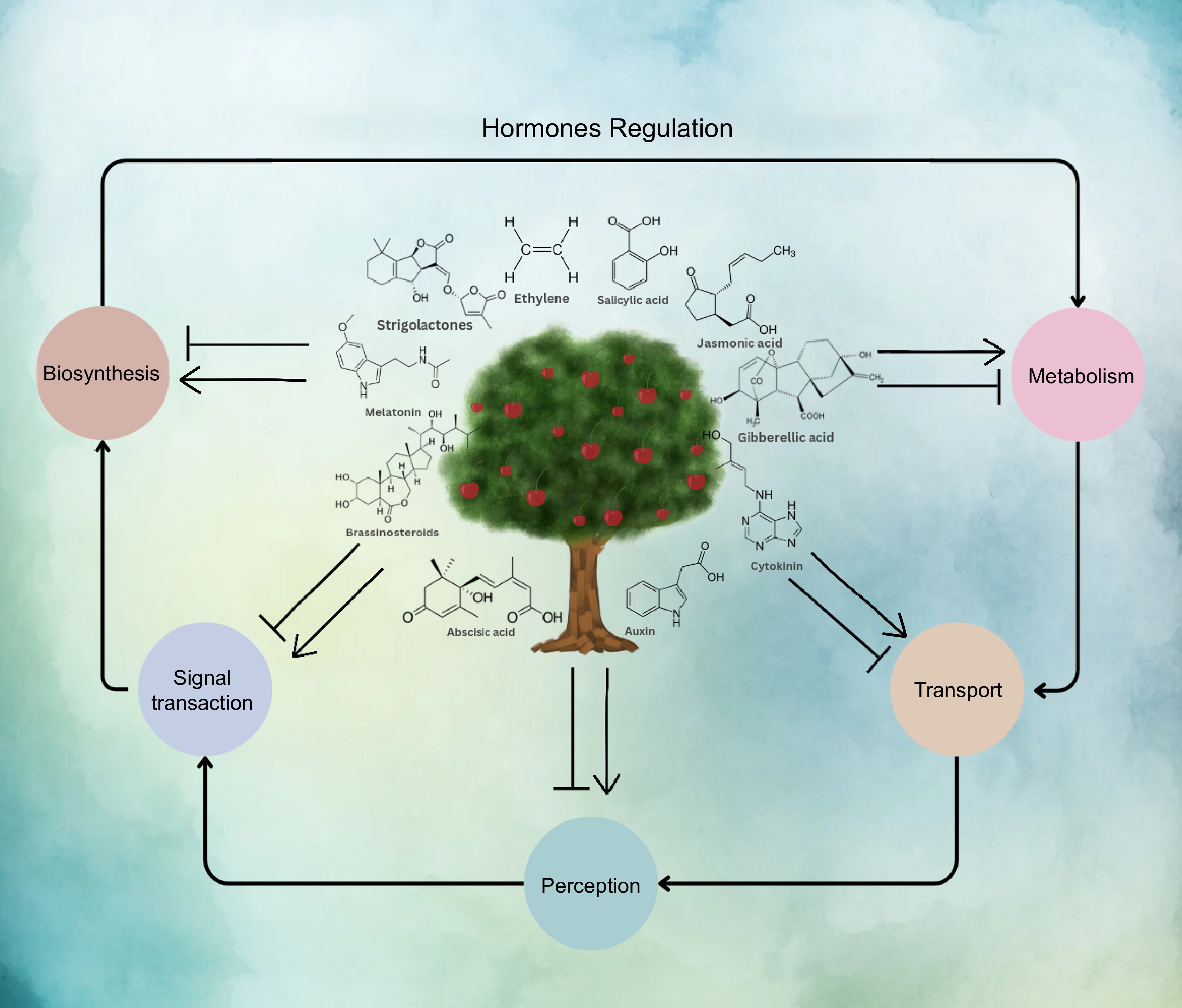
Plants have various receptors that can recognize specific environmental signals, such as abiotic stress, and trigger a signaling pathway to respond to these signals. The signaling pathway involves a complex network of biochemical processes that ultimately lead to changes in gene profile and the activation of various stress response mechanisms to help the plant cope with the stress[136]. At the crosstalk point in the cell, phytohormones interact with each other, including controllers located upstream or downstream, that can be stimulated by the hormones and secondary messengers involved in the phosphorylation cascade[137]. The signaling and transduction cascades initiated by the recognition of abiotic stress can ultimately lead to changes in gene expression, which in turn can affect the production of other phytohormones within the cell[9]. The exchange of phytohormones and other regulators for plant growth has the potential to cause notable alterations in the phenotypic manifestation of horticultural crops. In plant defense response, signaling pathways of JA, AUX, BR, SA, ABA, CK, ET, and GA interact with each other at different points, including hormone-responsive transcription factors (Fig. 4).
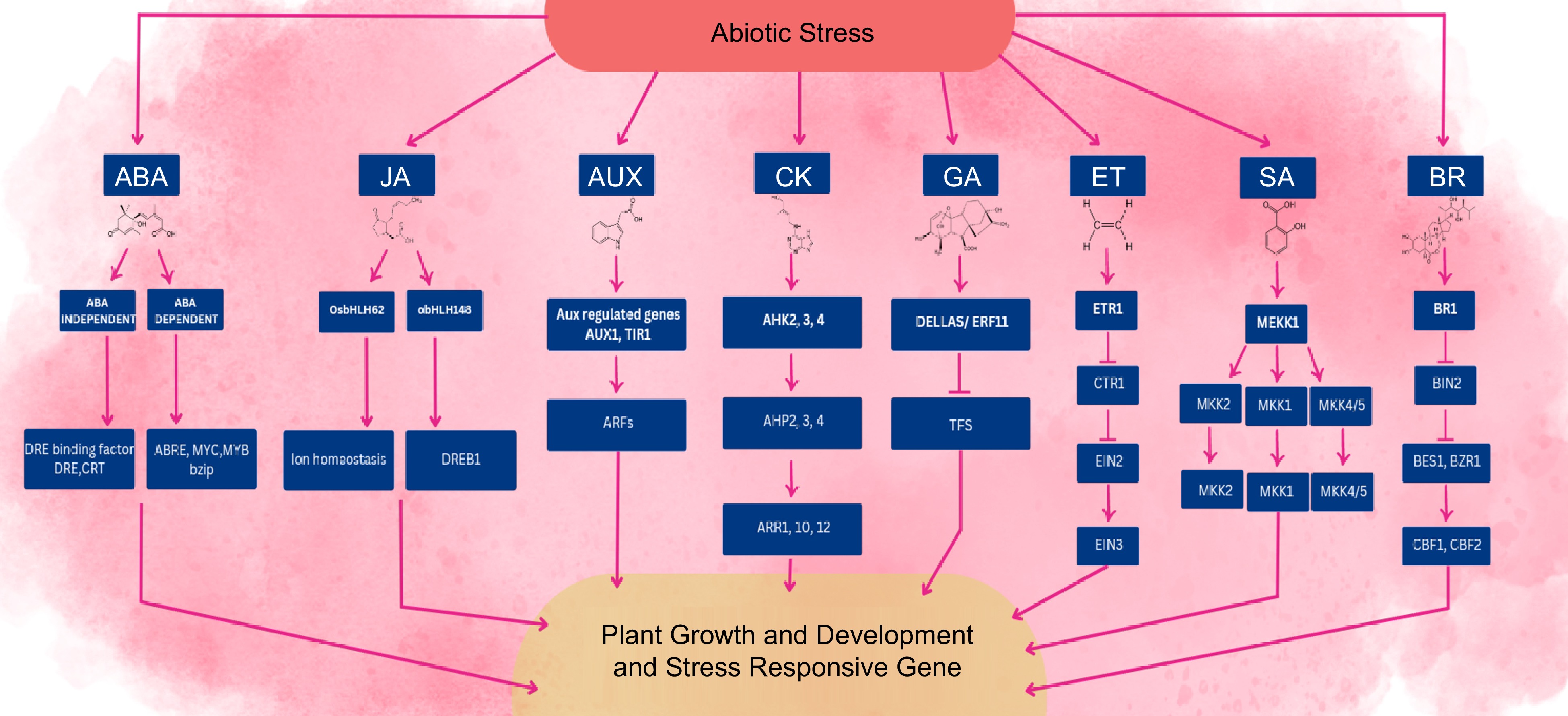
Figure 4.
An overview of phytohormone crosstalk and their signaling networks in abiotic stress responses. Under various conditions of abiotic stress, plant growth, and development are impacted by changes in phytohormonal signaling. Biosynthesis and signaling pathways of hormones are altered, which can affect specific genes within this network that are responsible for improving plant growth and defensive mechanisms to confer abiotic stress tolerance. The arrows bar end and simple arrow suggest repression effect and activation, respectively.
Nevertheless, it is crucial to emphasize that an effective defense response in plants encompasses not only stress adaptation but also sustained growth even under challenging conditions. The intricate interplay between defense hormones and growth-promoting phytohormones, such as gibberellins (GAs), cytokinins (CKs), and auxins, plays a pivotal role in mediating the stress response. This crosstalk between different hormone pathways enables plants to strike a balance between defense mechanisms and growth processes, ensuring their ability to withstand stress while maintaining optimal growth and development. Understanding and manipulating this hormone crosstalk is of utmost importance in enhancing plant resilience and productivity in the face of environmental stresses. The induction of defense responses in plants under diverse stress situations is determined by the interplay of hormone signaling pathways, rather than the individual contributions of specific hormones. Moreover, this section delves into the interplay between GA and ABA, which is mediated by DELLAs, in regulating the equilibrium between seed dormancy and germination. This balance is a critical mechanism for avoiding early abiotic stress conditions.
The objective of this discourse is to delve deeper into the complex interplay of hormonal communication that mediates stress responses in plants. Interactions among multiple phytohormones in governing diverse growth and developmental processes are prevalent. Plants possess the ability to regulate hormone actions through various mechanisms, including the control of hormone biosynthesis, modification of the hormone pool, and intricate regulation of signaling processes. The pleiotropic nature of phytohormones, wherein a single hormone influences multiple developmental events, has long been acknowledged by researchers in the field of plant biology. By understanding these intricate hormonal dynamics, we can gain valuable insights into the regulatory mechanisms underlying plant responses to stress and their broader implications for plant growth and development. The successful progression of plant development across various stages necessitates the coordination and fine-tuning of molecular pathways in response to diverse environmental conditions. This intricate process is achieved through the complex interplay and cooperative interactions of multiple hormonal regulations. The dynamic networking of these hormonal signals enables plants to adapt and respond effectively to changing environmental cues throughout their developmental journey. The concerted efforts of these hormonal regulations contribute to the precise timing and synchronization of key developmental events, ultimately ensuring optimal growth and successful reproduction in plants.
Phytohormones can bring about these modifications by interacting with several plant development regulators[138,139]. Recently discovered phytohormones, such as jasmonates and brassinosteroids, are essential in regulating both biotic and abiotic stresses in plants[140]. They achieve this primarily by increasing the concentration of Ca2+ as a secondary messenger intracellularly[140]. Under abiotic stresses such as salinity, cold, heat, and drought, inositol phosphate, and ROS are produced, leading to an increase in endogenous ABA levels[141]. The progress of a plant's life cycle is dependent on the coordination and adjustment of the molecular mechanisms of plant growth. This is achieved through complex networking and coordination of various hormonal regulations in response to diverse environmental conditions[142].
The crosstalk between hormones, such as ethylene, abscisic acid, jasmonic acid, auxin, and along with their interactions with signaling molecules like calcium ions (Ca2+), mitogen-activated protein kinases (MAPKs), and ROS (reactive oxygen species), is vital in harmonizing plant growth in response to abiotic stresses (Fig. 5)[12]. Plant defensive phytohormones like jasmonic acid (JA) salicylic acid (SA), and ethylene (ET) have an essential function in responding to biotic stress, and their levels considerably increase during pathogen infections. The content of these hormones is directly proportional to the severity of pathogen infection and abiotic stress[143,144]. Abscisic acid has a role in the long-term and short-term stress responses of a plant. In the short term, it controls stomatal conductance to control the water balance, while in the long term, it affects the profile of stress-responsive genes. The closure of the stomata is controlled by the regulation of phytohormones[145].
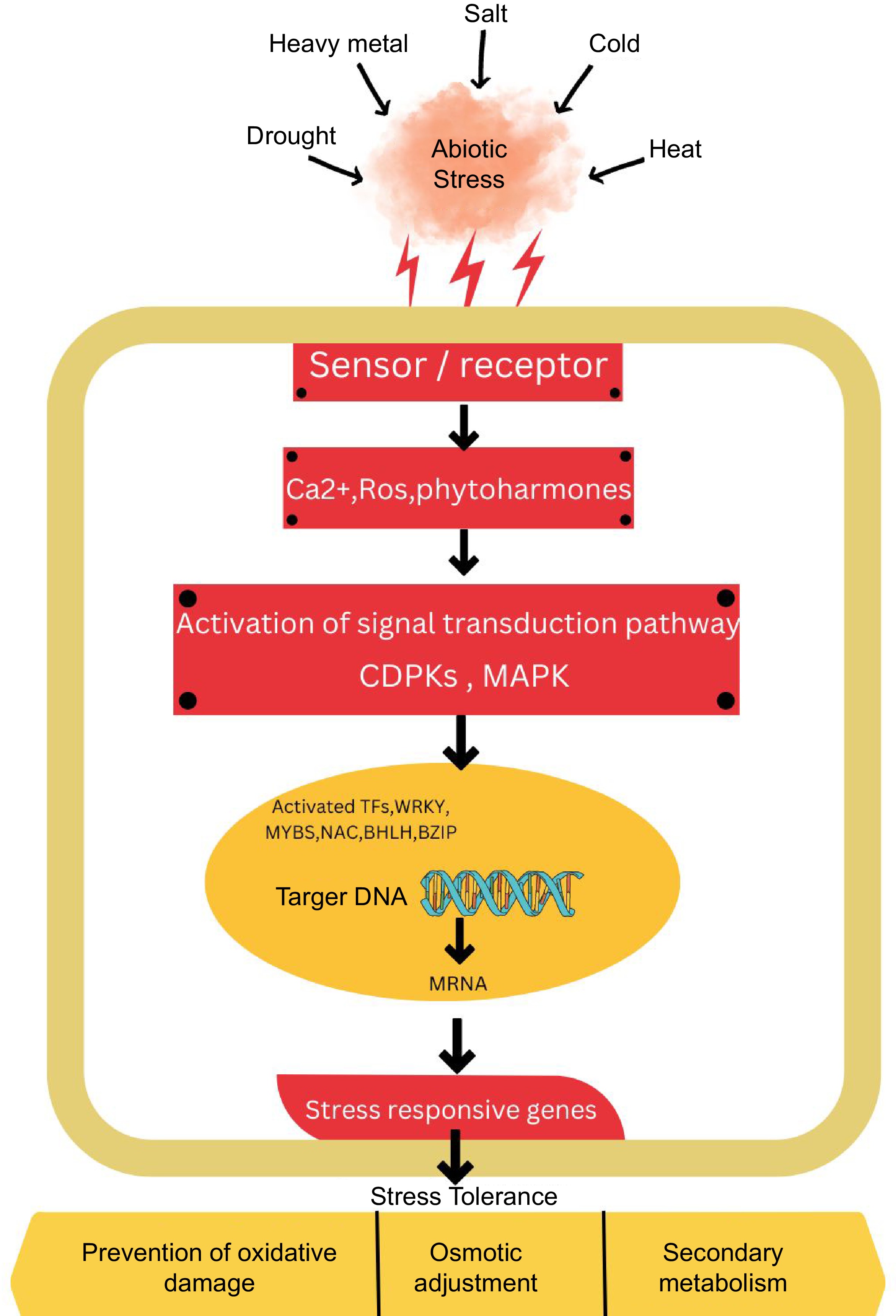
Figure 5.
The diagram illustrates the generic signaling pathway that plants use to respond to abiotic stress. The pathway begins with the perception of signal and extends towards stress responses. Initially, receptors and sensors perceive stress and cascading downstream stress responses via reactive oxygen (ROS), Ca2+, and phytohormones. Secondary messengers facilitate the transmission and amplification of signals in plants. This process involves the modulation of transcription factors and stress-responsive genes, which in turn triggers various molecular, biochemical, and physiological responses that contribute to enhancing stress tolerance in plants. The extension and transduction of signals are facilitated by secondary messengers. These secondary messengers result in the different regulation of stress-responsive genes and transcription factors. The regulation of TFs and genes leads to the adjustment of biochemical, physiological, and molecular responses that ultimately enhance stress tolerance in plants.
-
In summary, the integration of multiple signaling pathways inside plant cells is essential for their proper development and response to diverse environmental conditions. Horticultural crops are particularly sensitive to the disruption of environmental signals, which renders them vulnerable to an extensive range of abiotic stresses. Phytohormones are versatile compounds that have a substantial impact on the growth and development of horticulture plants, and they also play a key role in enabling them to adapt to different types of stress. The use of application of phytohormones such as melatonin and strigolactones may benefit the treatment of abiotic stress, and these strategies are becoming more popular. Extensive research has demonstrated that these compounds possess the ability to mitigate oxidative damage, enhance antioxidant enzyme activity, and promote plant growth, thereby enhancing the overall tolerance of horticultural crops to abiotic stress. However, to gain a comprehensive understanding of the precise mechanisms by which hormones alleviate abiotic stress, including their intricate interactions with other signaling molecules and transcription factors, further investigation is imperative. Future research endeavors should focus on unraveling the intricate molecular pathways and elucidating the regulatory networks involved in hormone-mediated stress responses in horticultural crops. Such studies will provide valuable insights into harnessing the potential of hormones for improving stress tolerance in crops and optimizing agricultural productivity in challenging environments.
Recent years have witnessed significant advancements in the identification and characterization of metabolizing enzymes associated with phytohormones, as well as the elucidation of phytohormone regulatory components and receptors involved in diverse signaling pathways. These two interconnected areas of research have garnered considerable attention and have been the subject of intensive investigations. The exploration of metabolizing enzymes has provided insights into the biosynthesis, catabolism, and interconversion of phytohormones, shedding light on their regulatory roles in plant growth and development. Concurrently, studies on phytohormone regulatory components and receptors have unraveled the intricate signaling networks that govern hormone perception and response. The synergistic progress in both these areas of study has significantly deepened our understanding of phytohormone biology and its implications for plant physiology. Continued research in these domains will undoubtedly contribute to further unraveling the complexities of phytohormone-mediated signaling and its impact on plant growth, development, and stress responses. Manipulating phytohormone levels and their subsequent action at the appropriate growth and developmental stage in the relevant tissue or organ is necessary to modernize agricultural production and engineer crops that are resistant to abiotic stress. As we look to the future, continuous research on the intricate interplay between phytohormones and other signaling molecules holds great promise for the development of novel cultivars with enhanced resilience to abiotic stress. In-depth investigations into the functional roles of phytohormones, such as strigolactones and melatonin, in mediating the alleviation of abiotic stress will shed light on the involvement of secondary metabolites and essential signaling molecules in these processes. These research endeavors are poised to pave the way for the creation of sustainable agricultural production systems that prioritize environmental friendliness while ensuring an ample food supply for future generations. By unraveling the complex mechanisms underlying phytohormone-mediated stress responses, we can harness their potential to develop resilient crop varieties capable of thriving in challenging environmental conditions, thus bolstering global food security and sustainability.
This study was funded by Shanghai Agriculture Applied Technology Development Program, China (Grant No. 2022-02-08-00-12-F01120), Natural Science Foundation of Shanghai (Grant No. 23ZR1430600), Shanghai Agriculture Applied Technology Development Program, China (Grant No. 2022-02-08-00-12-F01111), China Agriculture Research System (Grant No. CARS-30-2-08), National Natural Science Foundation of China (Grant No. 32102347), Shanghai Sailing Program (Grant No. 21YF1422100), Startup Fund for Young Faculty at SJTU (Grant No. 21X010500643).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Manzoor MA, Xu Y, lv Z, Xu J, Wang Y, et al. 2023. Fruit crop abiotic stress management: a comprehensive review of plant hormones mediated responses. Fruit Research 3:30 doi: 10.48130/FruRes-2023-0030
Fruit crop abiotic stress management: a comprehensive review of plant hormones mediated responses
- Received: 19 June 2023
- Accepted: 28 August 2023
- Published online: 10 October 2023
Abstract: Horticultural crop production is severely threatened by the changing global climate, as plants are vulnerable to a range of abiotic and biotic stresses. Abiotic stressors, such as floods, UV radiation, heat, drought, salt, and cold, often lead to crop damage and loss. However, plants have evolved sophisticated mechanisms enabling them to respond effectively to stressful conditions, thereby enhancing their ability to overcome challenges. They have evolved complex systems that detect stress signals and enable optimum growth responses, thus enabling them to survive in harsh environments. Due to their potential to mitigate the adverse impacts of abiotic stress, phytohormones have garnered significant research attention in recent years. Current findings have highlighted the crucial role that diverse phytohormones play in strengthening horticulture plants resistant to abiotic stress. Furthermore, plant growth regulators that function similarly to phytohormones, such as melatonin, have also been proven to be effective methods for mitigating the adverse impacts of both biotic and abiotic stress on fruit crops. A comprehensive understanding of the complex hormonal interactions that occur in a range of horticultural crops when exposed to biotic stress is a crucial summary of the function of phytohormones like plant growth regulators in decreasing abiotic stress and their associated crosstalk in the growth and development of plants under repeated stressful situations. The primary objective of this investigation is to focus on the fundamental advancements in the abiotic stress tolerance of fruit crops through the utilization of a diverse array of hormones including gibberellin (GA), brassinosteroids (BRs), abscisic acid (ABA), salicylic acid (SA), strigolactones (SLs), jasmonates (JAs), and melatonin (MEL). The insights gained from this study have the potential to facilitate sustainable plant growth, as hormones play a critical role in enhancing the abiotic stress resilience of horticultural crops.
-
Key words:
- Abiotic stress adaptation /
- Fruit crops /
- Hormonal crosstalk /
- Multi-stress tolerance