-
Commercial apple production is still heavily reliant on monoculture of clonally propagated cultivars. Within a monoculture there is genetic homogeneity within a single block [except pollinator genotypes], because the commercial cultivars are inbred, clonal or F1 hybrids. Monoculture is popular in modern mechanised agriculture as they are easy to manage and harvest, however, they can also pose several issues. The crops in the same field all have the same resistance/susceptibility to specific pathogens, which can pose strong directional selection on pathogen populations to overcome plant resistance/tolerance. If host resistance/tolerance is broken down within a monoculture, founder populations capable of infection can emerge, which can cause epidemics and severe crop losses.
The arms race between plant R genes and pathogen Avr genes in monoculture can lead to a phenomenon known as a boom-and-bust cycle[1], whereby the widespread introduction of a resistant cultivar can increase selection pressure within the pathogen population. This causes the emergence and subsequent rapid spread of a resistance-breaking strain. Monocultures generally require higher inputs of pesticides, which in part is to prevent resistance-breaking strains from emerging, hence increasing costs and environmental impact[2, 3]. Increased fungicide application can promote the development of fungicide insensitive pathogen strains, leading to further problems when trying to achieve sustainable disease management[4].
Disease management can be partially achieved by the use of mixtures of several agronomically compatible cultivars that differ in their resistance against specific pathogens of interest. Mixtures introduce genetic diversity, specifically in disease resistance, into a single field to slow down epidemic development. It is particularly effective against airborne diseases because mixture can successfully disrupt inoculum dispersal - namely the inoculum landing onto compatible genotypes enabling infection to take place[2]. The emergence of pathogen strains which can infect several cultivars (genotypes) of differing resistance is expected to take a longer time in mixtures compared to monocultures, where there is only a single resistant genotype to overcome[2, 3]. Thus, although mixtures could impose selection on pathogens for acquiring virulence against multiple resistance genes present in the mixture, it is unlikely that many strains develop that can overcome the majority of resistance genes present within a mixed culture (super races) during the lifetime of the crop, especially for annual and short-lived perennial crops[2, 3].
The success of cultivar mixtures in reducing disease development can vary not only with the characteristics of specific diseases, such as reproduction and dispersal modes, but also with the exact mixture spatial layout[2, 5]. Proportion of individual cultivars present in the mixture, the size of a single plant (or Genotype Unit Area [GUA] - the area occupied by an independent unit of host tissue of the same genotype[6]), the dispersal gradient, and the number of pathogen generations (and their reproductive mode) per season can all influence the efficacy of mixed cultures for disease management[2, 5]. The relative frequency of autoinfection (donor host and the recipient host of the disease are the same genotype) or alloinfection (donor host and the recipient host of the disease are of different genotypes) are primarily determined by the dispersal gradient and the size of GUA[2, 7]. A high frequency of autoinfection will reduce the effectiveness of cultivar mixtures because disease development in much of the crops is essentially similar to monoculture. Autoinfection is higher if a pathogen has a steep dispersal gradient, meaning the inoculum dispersal is concentrated closer to the source, which is more common in rain splash-dispersed pathogens compared to wind-dispersed ones[2, 8]. The size of the GUA, relative to the pathogen dispersal gradient, is crucially important as it determines the extent of autoinfection. Larger crops, such as trees, generally have higher levels of autoinfection compared to smaller crops, such as cereals, because they have a larger surface area for infection and are likely to be spaced further apart[2, 6, 8]. Finally, the inoculum dilution factor also plays an important role when considering mixed cultivar cultures. Within a mixture, inoculum may need to travel a longer distance to reach a host which is susceptible to that particular strain; this can lead to a reduction in inoculum load due to the nature of dispersal and the likelihood of inoculum landing on the compatible genotype. Effectively, genotypes which are resistant to a specific pathogen strain (race) offer up a buffering effect, cutting off the inoculum from spreading between susceptible hosts[9, 10].
Cultivar mixtures have been well researched in the management of cereal crop diseases, and their implementation is becoming a common practice, particularly for non-mechanised crop production[2, 11, 12]. Mixing barley cultivars reduced the severity of powdery mildew (Erysiphe graminisf. sp.hordei) by between 33% and 71%[13]. Similar results were obtained for stripe rust/yellow rust (Puccinia striiformis) of wheat, with an average disease reduction of 28% in mixtures compared with monoculture[14]. Rice blast (Magnaporthe grisea) severity was reduced by 94% when rice varieties were planted in mixtures, leading to effective disease control without the need for fungicides[15]. When 25% of wheat plants in a field were resistant, there was a 50% reduction in the severity of Septoria tritici blotch, a splash-dispersed pathogen[16]. The impact of mixtures on disease development in non-cereal crops has also been investigated, including up to 20% reduction of late blight (Phytophthora infestans) in potato mixtures[17]. Mixtures of perennial crops as a means of disease management, have also been studied. For example, mixing coffee varieties has increased protection against coffee rust (Hemileia vastatrix) in Colombia[2, 11]. The overall consensus is that mixtures can suppress disease development, but need to be integrated with other crop protection measures in order to achieve satisfactory disease control.
On the other hand, there are also several issues for using mixtures in commercial agriculture, including the likelihood of pathogen super races emerging that can render the mixtures ineffective for disease management. Super races are pathogens that can infect multiple genotypes in a given mixture with different resistance characteristics because they accumulate necessary virulence factors through mutation, external inoculum and/or recombination over time[18, 19]. Thus, one key consideration for adopting mixtures in commercial agriculture is whether the likelihood of super races emerging can be predicted, and hence management intervention can be implemented to slow down the emergence of super races. Emergence of super races depends on several factors, including the nature of host resistance, pathogen reproductive mode, mutation rate, and pathogen sporulation intensity[20−22].
Something else to consider is that cultivar mixtures can introduce additional labour and costs pre-harvest if the cultivars are incompatible. Many arable crops/Cereal crops are sown and grown together in the same field, so it's essential to plant cultivars which have phenotypic similarities in their agronomic traits (such as plant height or vigour), as well as similar timelines for management (pesticide application, fertilisation, irrigation) and harvesting[23]. You also need to be certain that the blends are appropriate for their desired purpose; for example, mixed wheat flours need to have good bread making properties, or certain barley genotype combinations need to be compatible for brewing beer[23].
This review aims to assess the prospect of implementing orchards with mixed cultivars for managing apple diseases, particularly apple scab. Firstly, we briefly introduced the biology and epidemiology of apple scab, caused by Venturia inaequalis[24, 25], and its current control methods. Then, we reviewed recent findings on the population genetics of V. inaequalis within orchards of mixed cultivar cultures to infer the likelihood of V. inaequalis super races emerging. Finally, we reviewed the prospect of using mixed orchards as a management strategy for the future of commercial apple production and any problems which may be encountered with their implementation.
-
Apple scab is one of the most damaging diseases of cultivated apples (Malus spp.) worldwide, particularly in temperate growing regions. It causes significant economic losses to apple growers and allied industries, including the cider and juice industries[26, 27]. Infection by V. inaequalis and subsequent scab development are most commonly observed on the fruit and leaves, but it has also been reported on shoots, twigs and the wood where is it generally referred to as wood scab (Fig. 1a, b)[24, 28]. Usually around 10−15 fungicide sprays per season are required to control scab, but this can be much higher depending primarily on climatic conditions within the particular region[29, 30]. More than 70% of the crop can be lost as a result of severe scab outbreaks in the UK, causing destruction to both the trees and fruit[31]. Severe scab development may reduce tree growth and vigour the following season[26, 32].
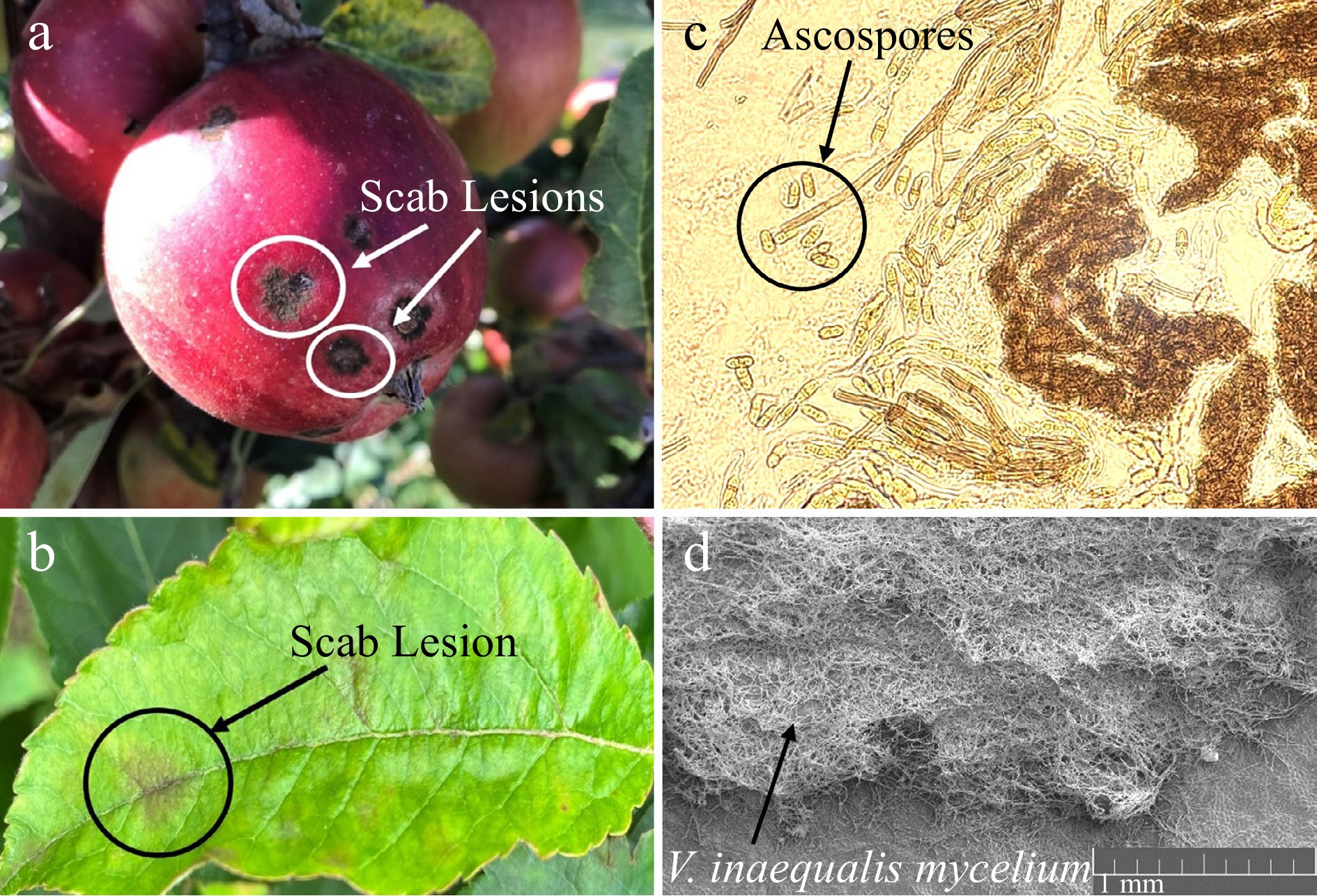
Figure 1.
Images showing the different aspects of apple scab infection: Distinctive scab lesions on the apple (a) fruit and (b) leaves. (c) V. inaequalis ascospores following ejection from pseudothecia, imaged under a stereomicroscope. (d) Leaf scab lesion made up of fungal mycelium, imaged using a scanning electron microscope (VEGA3 TESCAN). All images taken by Katherine Stewart, 2022/2023.
The disease cycle of V. inaequalis can be divided into two phases (Fig. 2); a primary infection phase where inoculum (ascospores) results predominantly from sexual reproduction over the winter on leaf litter, and a secondary infection phase initiated by conidia from asexual reproduction[24].
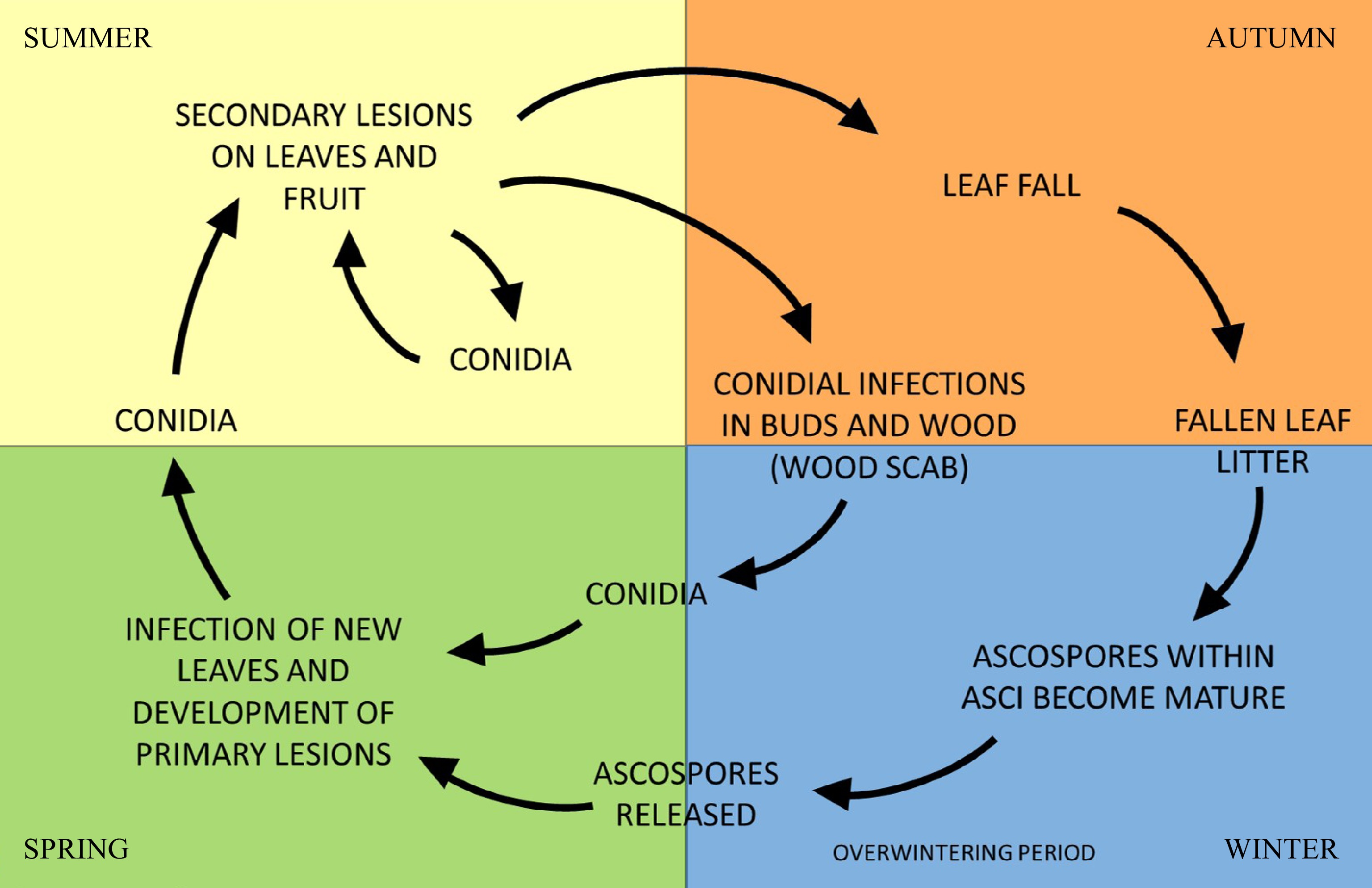
Figure 2.
Simplified diagram outlining the reproductive cycle of Venturia inaequalis throughout the year. The life cycle comprises a sexual and asexual phase of reproduction, where ascospores and conidiospores are formed, respectively. Ascospores develop and mature over winter, infecting new leaves upon release in spring, subsequently forming lesions which release conidia. Conidial infection can occur throughout the growing season and can develop from buds, wood and secondary lesions of leaves and fruit.
V. inaequalis primarily overwinters as sexual fruiting bodies (pseudothecia) on fallen leaf litter, where abscised leaves are used for nutrition during a saprobic phase of growth[24, 32]. Primary inoculum can also arise from conidia overwintering in buds, on the shoots of the trees and on diseased wood (wood scab)[33−35]. In spring, mature ascospores are released from pseudothecia (Fig. 1c) into the air following sufficient rainfall and dispersed by wind. Once landed on susceptible tissue, they can initiate infections under suitable conditions (temperature and presence of surface water or high humidity) through germination and penetration of the leaf cuticle[26, 32, 36].
Secondary infection is initiated by conidia arising from lesions from primary infections. Conidiophores develop on dense mycelium (Fig. 1d), situated between the leaf cuticle and epidermal tissue, and rupture the leaf cuticle as they push out[24, 32, 37]. Conidia and conidiophores form the distinct velvety appearance found in young scabby lesions (Fig. 1a, b). Conidium production is dependent on temperature and moisture, with optimal conditions at temperatures between 15 and 20oC and relative humidity ≥ 90%[24, 26, 35]. Once formed, the conidia are dispersed, primarily by rain splash, germinate and penetrate the cuticle, establishing new infections. Splash dispersal of conidia generally leads to a steeper dispersal gradient than aerial dispersal of ascospores; thus, more conidia are deposited within the canopy of the tree near their origin[24, 32]. There can be multiple cycles of conidial infection during the growing season with young leaves and fruitlets being the most susceptible[26, 32, 38]. In addition to this, late infection of old fruit may lead to pin-point scab lesions, which can develop into storage scab post-harvest[39].
-
Presently, apple scab is managed using integrated strategies that can broadly be divided into three categories: chemical, cultural, and biological[26]. Fungicide application is still the most common method of scab management, and usually achieves satisfactory control[24, 40]. Fungicides for scab control are classified into types based upon their modes of action. Protectants are applied to the trees before infection as a preventive measure. Examples of these fungicides include sulphur, copper fungicides, captan and mancozeb[41]. Curative products aim to kill young developing infections to prevent scab lesions from becoming visible; these products usually need to applied within 24−72 h of infection[29, 41]. Dodine and DMI (triazoles) are examples of curative fungicides[42]. A protectant and curative treatment are often both recommended, and their timings may partially be determined by a scab forecasting model[41−43]. The earliest and simplest model is the Mill's infection model that combines the criteria of temperature and hours of leaf wetness required to predict infection[38, 44]. Whilst effective, chemical fungicides can be undesirable as frequent application is required, with growers often using >10 sprays per season, making them expensive and unsustainable in terms of negative environmental impacts. These may include water and soil pollution, as well as affecting non-target organisms[30, 45, 46]. Excessive use of fungicides, particularly copper, lime sulfur and triazoles, can cause phytotoxicity or reduced fruit quality in certain apple cultivars, and using high doses can cause symptoms such as leaf spotting[29, 30, 47]. Furthermore, reliance on fungicides may increase the selection pressure on the fungal population for reduced fungicide-sensitive strains, making the disease more difficult to manage[29, 48, 49].
The application of biological control agents (BCAs) has been suggested as an alternative to fungicides for controlling plant diseases. Formulated biocontrol products containing fungal antagonists such as bacteria, fungi or yeasts can be applied to the trees where they have the potential to either compete with V. inaequalis for resources or kill the fungus through antibiosis and/or direct mycoparasitism[50, 51]. Whilst preliminary laboratory and field work has been carried out to screen and test potential BCAs against V. inaequalis[50−52], there are currently no commercial BCAs available for scab-specific control in the UK[53].
Cultural control involves ensuring good sanitation within the orchard. Leaf shredding, burning, burying and urea application to fallen leaf litter for accelerated decomposition can all reduce the level of overwintering inoculum and hence primary infection[26, 54]. Good soil conditions encourage earthworms which can degrade leaf litter in the orchard, thus reducing primary inoculum levels[55, 56]. Pruning trees can promote sunlight penetration and air movement, which can lower humidity in the canopy, reducing V. inaequalis infection and sporulation[57]. Furthermore, shoots with woody scab need to pruned off in order to reduce inoculum potential[28, 58]. Orchard layout and, more specifically, the spacing between trees is important to ensure each tree has sufficient resources and space to grow effectively. More space between the trees may also reduce infection because the inoculum will be more dispersed, reducing the volume and infection potential[56, 59]. Furthermore, the application of plant growth regulators (PGR's) can improve fruit set whilst reducing shoot production (increasing air movement in the canopy). They can also encourage the production of biocidal compounds which can reduce apple scab infection[60].
Resistance breeding is a popular method of plant disease management. This entails breeding resistance (R) genes into apple cultivars to improve disease resistance to V. inaequalis. R genes in Malus spp. against V. inaequalis are called Rvi genes, and there have been 20 R genes discovered to date[61−63], numbered Rvi1–Rvi20 based on the nomenclature proposed by Bus et al[64]. The first scab resistance gene to be discovered was the Rvi6 (Vf) gene from the wild relative of apple; Malus floribunda 821[62, 63], and this gene was bred into several cultivars for resistance to scab[62, 64, 65].
An ongoing study, VINQUEST[66], has been characterising the performance of indicator genotypes with different Rvi genes in several regions worldwide. The results so far have shown that the strongest host resistance arose from genes Rvi5, Rvi11, Rvi12, Rvi14 and Rvi15[66]. These genes produce different responses to the pathogen and were extensively reviewed by Bus et al in 2011[64]. Rvi5 (Vm) induces a hypersensitive response which acts as an effective defence mechanism[62−64]. Rvi11 (Vbj) and Rvi12 (Vb) can exhibit different reactions to the fungus dependent on the presence of other resistance genes[62−64]. Rvi14 (vdr1) produces a chlorotic-type reaction similar to that of Rvi6, the most commonly used R gene[64, 67]. Rvi15 (vr2) initiates necrosis of infected plant tissues[62, 64, 68]. Most R genes are still useful for breeding but would have to be combined with other R genes via gene stacking (gene pyramiding) to increase their durability and long-term success within a mixed cultivar orchard[66]. Many commercial varieties show high susceptibility to scab, either because they contain no known Rvi genes such as 'Royal Gala' or 'Cox's Orange Pippin', or because the Rvi gene they do contain has been broken down by specific scab races. An example being 'Golden Delicious' which contains the Rvi1 gene but remains an incredibly susceptible variety. Rvi6 has also been eroded in most varieties, including M. Floribunda from which the gene originates. Many of the more effective Rvi genes have not yet been introduced into commercial varieties[66, 69].
Whilst the VINQUEST study has shown promising results for the future of breeding durable resistance against V. inaequalis, breeding is still a long and difficult process for apple trees because of their long growth cycles. It usually takes between 10 and 20 years to develop new apple varieties through traditional breeding[70, 71]. New methods of breeding are slowly being introduced to the apple breeding industry, including speed breeding, marker assisted selection and gene editing (e.g. CRISPR/cas9)[70, 72−74], however there is still a lot of improvement required to produce cultivars which are resistant to scab, without compromising fruit quality traits or resistance to other diseases. There is also the additional issue of some techniques being banned in certain countries because the cultivars are classified as 'genetically modified organisms (GMO's)[71]. The UK has taken a step towards improving breeding by passing the Genetic Technology (Precision Breeding) act earlier this year (2023)[75].
-
Based on the information we know about cultivar mixtures in other crops[2], and differential resistance against apple scab, it seems logical to introduce mixed orchards as a means to manage it. In addition, implementation of mixed orchards should be theoretically simpler than cereal crops. Within an orchard, trees are individually planted, therefore a specific layout mixture design can hypothetically be adopted to maximise disease reduction. In contrast, cereal crops are usually broadcast sown and grow within the same plot, so it's essential that agronomic characteristics (height, vigour etc) are compatible[2, 11, 23]. For trees, similar rates of maturity and harvesting are desirable, however characteristics like tree height, width and vigour do not need to be compatible as they are harvested tree-by-tree[2]. In dessert apple production, the fruit are picked individually and generally marketed as their named varieties. Mixtures may introduce additional labour and costs as the varieties will need to be separately harvested. Applying a mixture in cider apple orchards may be more commercially viable compared to dessert apples, because disease control requirements in cider apple production are less strict.[76]. Also, a vast majority of ciders are blends of several apple varieties, thus cultivars with similar maturing dates may not need to be harvested separately[77].
A key requirement for using apple mixtures as a management strategy against scab is that there is differential resistance against apple scab strains present among the cultivars planted, whether this resistance be conferred by major R genes or through quantitative resistance[2, 9, 78]. Patocchi et al.[66] have shown that there are several R genes which are still effective, and even the ones showing breakdown could still be useful if stacked within a cultivar[66]. Resistance to apple scab can either be polygenic (quantitative), meaning there are several resistance quantitative trait loci (QTL's) contributing towards resistance, or monogenic (qualitative), where resistance is controlled by a single R gene[79, 80]. Race-specific resistance is often monogenic, utilising major R genes to confer resistance to a specific scab race[79], whereas polygenic often includes combining R genes of QTL's through techniques of gene stacking (pyramiding), which can provide a range of gene effects from partial resistance to immunity[64, 71]. Barbara et al.[81] demonstrated that scab isolates, taken from either a monoculture or a mixed culture, show adaptation to their host cultivar. The pathogen isolates can exhibit different levels of 'aggressiveness', defined by Pariaud et al.[82] as the 'quantitative variation of pathogenicity on susceptible hosts'. The level of aggressiveness within the isolate is likely to accelerate the adaptation to its cultivar, and therefore the evolution of the isolate into a more successful pathogen on that specific host genotype[82, 83].
Research into host resistance revealed that quantitative resistance is also vulnerable to erosion or even complete breakdown. In one case[84], quantitative resistance resulted in the emergence of a generalist population containing a wider range of pathogenicity, meaning it could infect both susceptible and resistant varieties. Another study[85] investigated the effect of selection pressure on a mixed scab inoculum taken from apple genotypes with quantitative resistance. Broad spectrum resistance (resistance against the majority of scab races) did not cause selection pressure on the mixed inoculum, however narrow spectrum resistance (specific resistance to particular races) produced fewer isolates on the susceptible genotypes, indicating selection pressure[85]. Pyramiding broad spectrum factors and introducing mixtures with genotypes that carry different narrow spectrum resistance are both methods of improving the durability of resistance within the hosts[85].
Further studies into the population genetics of V. inaequalis in mixed orchards have been carried out. Guerin & Le Cam[86] discovered that there was less genetic variation in populations of V. inaequalis found on cultivars containing Vf (Rvi6) resistance, which was causing a founder effect where small populations were becoming virulent against the Vf resistance gene. Leroy et al.[87] discovered that the presence of the Rvi6 resistance gene strongly affected the V. inaequalis population in a mixed culture of several Malus species. The population split into two subpopulations; one virulent subpopulation infecting Malus trees containing Rvi6, and a subpopulation only infecting trees without Rvi6. Both of these studies[86, 87] add to the argument that there is a separation of populations due to host resistance, and no sexual mating is occurring between isolates from different hosts.
Cultivars showing R gene breakdown can still be useful for the future. A mixture can still be implemented with susceptible cultivars, because they may contain narrow spectrum resistance genes, meaning they are susceptible to particular strains whilst showing resistance against others[76, 78, 81]. However, mixtures with fully susceptible cultivars (no known R genes present) may be significantly less effective for disease reduction. Avirulent isolates of V. inaequalis have the capacity to infect cultivars without R genes because the host cannot recognise the Avr genes of the fungus[64, 88].
In summary, there is sufficient genetic diversity in apple against V. inaequalis in commercial apple cultivars, as well as specific host-pathogen interactions for apple scab, which suggests a potential for using mixture to reduce scab development. The theoretical explanation for using mixtures to manage apple scab has been supported by several orchard studies[56, 89, 90]. Two types of mixtures can be used in an orchard; a within-row mixture, where the cultivars alternate within the row, and a between-row mixture where different cultivars are grown in parallel rows, but the rows themselves are monoculture[89]. The between row mixtures are easier for applying differential agronomic measures (e.g., irrigation and fertilisers) appropriate for individual cultivars, but disease suppression efficacy is expected to be reduced. Didelot et al.[89] investigated the efficacy of mixtures in reducing scab development, as well as the impact of fungicide application. Prior to fungicide application, mixtures led to a significant reduction in the scab incidence (~21.3%) and severity (~35.4%). There was little difference between within-row and between-row mixtures. Nevertheless, this study clearly demonstrated that using a mixed culture alone is insufficient for scab management, and other management strategies are needed as well, such as orchard sanitation, fungicide application and using cultivars with more complementary resistance backgrounds[56, 89, 90]. The extent of disease reduction through mixture varied greatly with season, which could have resulted from the relative importance of conidia and ascospores and differences in temporal climatic conditions[89].
-
As discussed above, the most important risk associated with using cultivar mixtures is the development of pathogen super-races. This is particularly true for perennial tree fruit crops, such as apple, where an orchard usually needs to last for at least 15 years in order to make it commercially viable. Thus, we need to be confident that super-races will not become dominant in mixed orchards within that time-span before cultivar mixtures can be recommended for commercial apple production.
It is also important to consider the pathogens natural evolution; the domestication of apple likely caused a shift in the scab pathogen population which made it more virulent, possibly due to higher levels of homogeneity within agro-ecosystems. It has been discovered that isolates from M. domestica can still infect wild relatives despite a lack of gene flow. This could cause a 'boomerang' effect, where isolates will infect wild relatives and increase their genetic diversity before re-infecting the commercial varieties[91−93].
To determine the risk of super race development in established orchards, a series of studies have been conducted at East Malling, Kent, UK on a long-term mixed orchard, as well as several monoculture orchards. First, it was demonstrated that scab isolates from a mixed orchard of three susceptible cultivars ('Bramley' (B), 'Cox's Orange Pippin' (COP) and 'Worcester Pearmain' (WP)) in West England differed in their virulence characteristics[81]. Isolates from 'COP' can often infect 'B' and vice versa; in contrast, isolates from 'WP' cannot infect 'B' and, to a lesser extent 'COP'. This result indicates that super-races have not yet dominated in the mixed orchards at the time of sampling (the orchard was about 25 years old). This cultivar-specific virulence characteristics agree with previous studies[94, 95] showing specific isolates from two different cultivars differed in virulence. None of the tested isolates from mixture or monoculture orchards could infect all three cultivars (B, WP, COP), although some ascospore progeny obtained from artificial crosses between the tested isolates could infect all three cultivars. This indicates that new combinations of virulence genes can occur in ascospores, which is important when considering the durability of resistance for the future[81]. Similarly, Sierotzki & Gessler[94, 95] demonstrated segregation of virulence when two isolates were mated from two specific cultivars, and confirmed that a gene-for-gene interaction[96] could be observed between V. inaequalis and susceptible M x domestica cultivars[95].
Based on SSR markers, there are significant differences between isolates from individual cultivars in the mixed orchard in West England and that isolates from the same cultivar in the mixed or monoculture were similar, as well as between groups of isolates from trees of the same cultivar[78] . In addition, fungal variability between trees of the same cultivar suggests that the role of conidia as a primary inoculum source is greater than previously suggested[48, 78], which was supported by another study[97]. If scab super-races were spreading in the mixed orchard, we would have expected the differentiation among pathogen populations among cultivars in the mixture to decline over time. However, over a 7-year period the decreased population differentiation was observed only between isolates from 'COP' and 'B', and in contrast the population differentiation between isolates from 'WP' and 'COP'/'B' increased significantly over the 7 years[76]. This suggests that 'B' and 'COP' share some common resistance features against scab, but differ largely from 'WP'. Furthermore, it shows that there is a lack of exchange of genetic materials between scab isolates from 'WP' and those from 'B' or 'COP'. The lack of recombination between isolates from 'WP' and 'B'/'COP' was further supported by genomic sequence data[98], where isolates of V. inaequalis, taken from 'COP', 'B' and 'WP' from the mixed orchard, were sequenced and compared on the basis of single nucleotide polymorphism (SNPs).
The data collected from the mixture and monoculture orchards in the UK suggested that scab super races are unlikely to develop rapidly within the studied mixed orchard of ~50 years, which is far longer than the life-span of any commercial orchard. The lack of apparent sexual recombination between isolates from 'WP' and 'B'/'COP' inferred from the molecular, as well as phenotypic virulence data, is unlikely due to fitness cost associated with progeny ascospores from such crosses, as no obvious fitness cost in virulence and in vitro colony development was observed in controlled crossing and inoculation studies[81]. We speculate that such lack of sexual recombination among isolates from specific cultivars in a mixed orchard is due to assortative mating (rather than random mating as commonly assumed). Sexual reproduction can only occur between isolates that infect the same leaf because physical proximity of the hyphae for a longer period of time that may be initiated before leaf fall in autumn is likely to be required for mating. Even if isolates on different leaves (thus potentially different cultivars) can mate post leaf-fall, the probability of success for such a mating is likely to be far less than among lesions on the same leaves because sufficient contact time is needed for mating. Thus, isolates from 'B' and 'COP' can mate as they can frequently cross-infect[81], whereas isolates from 'WP' almost can only infect 'WP' and hence remain in isolation from 'B'/'COP' isolates under the assortative mating hypothesis.
These studies suggested that scab super-races are unlikely to become dominant within a orchard's commercial lifetime, which is further supported by theoretical modelling[18]. Therefore, mixed orchards can be used as an alternative tool to manage apple scab (and other diseases as well[90]) and could be adopted in commercial apple production. Of course, considerations need to be given to other issues such as added cost to crop management and harvesting. Fruit picking will likely be solved in the near future with successful research into automated picking given each tree has a pre-defined spatial location[99, 100]. The additional cost associated with using mixtures needs to be balanced against the continuing decline in available fungicides and lack of effective biocontrol products.
-
This review highlights both the benefits and risks associated with the implementation of mixed orchards for the management of apple scab.
In any orchard design an integrated approach to disease management should be adopted. It is unlikely that any single measure can contribute to scab management effectively all the time. Therefore, we believe that mixtures should be considered seriously as one component of an integrated disease management, especially for cider apple production. With the combination of both sexual and asexual reproduction, and the influence of a changing climate, it is difficult to predict accurately temporal pathogen dynamics with regards to virulence characteristics. Whilst breeding cultivars with durable resistance will remain the favourite and long-term goal, it is sensible to consider other alternatives of exploiting genetic diversity in scab resistance, such as the introduction of cultivar mixtures. Future research into speed breeding of apples and the introgression of R gene pyramids within single cultivars will all improve breeding efficiency, contributing to disease management in the future.
With the research already carried out into cultivar mixtures, it is clear that mixed cultivar orchards should be considered as a management strategy to reduce the incidence and severity of apple scab. However, further studies into how sex may be initiated will be crucial for estimating the likelihood of super race development within a given mixed orchard in addition to understanding the genetics of host resistance and pathogen virulence. Understanding whether sex can only occur before leaf-fall, or between lesions on the same leaf, will improve our ability of estimating the risk of super races emerging, hence the potential risks associated with using mixtures to suppress disease development. Another key component in using mixtures is to understand the nature of host resistance/susceptibility, which will enable us to select cultivars with complementary resistance for use in mixtures. We are currently conducting research at East Malling on sexual reproduction in V. inaequalis and the nature of host-pathogen interaction.
This PhD project is funded by the BBSRC and the Collaborative Training Partnership for Fruit Crop Research (CTP-FCR).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Stewart K, Passey T, Verheecke-Vaessen C, Kevei Z, Xu X. 2023. Is it feasible to use mixed orchards to manage apple scab? Fruit Research 3:28 doi: 10.48130/FruRes-2023-0028
Is it feasible to use mixed orchards to manage apple scab?
- Received: 24 March 2023
- Accepted: 25 August 2023
- Published online: 10 October 2023
Abstract: Apple scab, caused by the fungus Venturia inaequalis, is one of the most damaging diseases of cultivated apples (Malus x domestica) worldwide. It results in huge losses as it diminishes fruit quality and impacts tree growth. Current management revolves around the application of fungicides, however the number of sprays required per season is unsustainable and expensive. Further to this, populations of V. inaequalis have developed fungicide resistance. Breeding new cultivars with higher levels of resistance to scab is a priority, however, this process is long, so introducing mixed cultivar orchards may be a faster solution. We reviewed the general principles of using mixtures to manage plant diseases, and then considered specifically using mixed cultivars to manage apple scab in commercial production. Limited field studies have demonstrated the potential of using mixture to suppress apple scab development; but scab super-races that could emerge from mixture can pose a significant risk in commercial production. However, recent research on population genetics of apple scab in monoculture and mixed orchards suggests that the risk of super-race emergence is probably over-stated, because assortative mating among lesions on the same leaves is likely to occur, rather than commonly assumed random mating. Thus, we conclude that cultivar mixtures can contribute towards sustainable scab management, particularly in commercial cider apple production.
-
Key words:
- Apple scab /
- Venturia inaequalis /
- Mixed cultivar culture /
- Disease management












