-
Sunburn in horticultural crops, including apples, is one of the major problems affecting fruit quality in regions with high levels of solar radiation and temperature [ 1] . In the literature, three types of sunburn damage have been identified in apple ( Malus domestica): sunburn browning (SB), sunburn necrosis and photooxidative sunburn [ 2] , with SB being the most prevalent. SB causes significant economic loss to apple production, which is expected to increase under the climate change as extreme heat events occur more frequently [ 3− 5] . Many studies have been conducted to determine the causes and effects of sunburn damage in apples [ 6] . It has been reported that SB is activated by a fruit peel temperature in the range of 45–49 °C in combination with high ultraviolet B radiation [ 1] . Further, sunburn damage is known as a defense mechanism against oxidative stress, as plants have developed a defense system based on antioxidant compounds including vitamin C (ascorbic acid), vitamin E (α-tocopherol), polyphenolics, and enzymes such as peroxidase and polyphenol oxidase [ 6] . Hao & Huang [ 7] studied the changes in the antioxidative system and cell ultrastructure in the fruit peels of apple during sunburn development and found that as the peel became brown, chlorogenic acid, quercetin, rutin and myricetin accumulated, and cells of the outer layers of the epidermis collapsed and cell walls became thicker. In addition, chloroplast degradation and thickening of cuticular layer were reported in 'Ambrosia' apple [ 5] . Felicetti & Schrader [ 8] reported that lower chlorophyll and anthocyanin concentrations observed in the SB 'Fuji' apples allowed the yellow color from the carotenoids and quercetin glycosides to be more prominent; as SB intensified, there was further decrease in chlorophyll and idaein, and further increase in quercetin glycosides and carotenoids. Similarly, Liu et al. reported that sunburn promoted the gene expression for carotenoid, phenolic and flavonoid synthesis, but inhibited the accumulation of chlorophyll and anthocyanins, which led to the development of light and yellow color in the apple peel after sunburn [ 9] .
Further, fruit with SB increases the likelihood of the fruit developing postharvest disorders during storage, such as browning or blackening of the sunburned tissues, lenticel breakdown, bitter pit, stain or watercore [ 1, 10, 11] . Due to the economic impact of apples developing postharvest quality issues coupled with the fact there is no effective method currently available to prevent development of these physiological disorders during cold storage, development of a technique capable of detecting fruit with initial sunburn symptoms would benefit the apple sector. However, an appropriate technique for early assessment of apple sunburn has not yet been developed. Meanwhile, the Delta Absorbance (DA) meter is a hand held tool developed from vis/NIR spectroscopy, to non-destructively measure the Difference of Absorbance ( δA, DA or I AD) between 670 and 720 nm, for rapid detection of skin chlorophyll breakdown and non-invasive determination of ripeness and optimum harvest timing for apples [ 12] . Our previous study on the bicolor 'Ambrosia' apple demonstrated a significantly elevated skin δA on the sunlit side of SB fruits [ 5] . It remains unknown whether there is any change in δA in dark red cultivars such as 'Buckeye Gala'. If so, can a DA meter detect the differences in δA of fruits with different SB severities? Is it feasible to use δA as an index to rule out fruits of severer SB? How does the change in δA correspond to the changes in stress response compounds as SB intensifies? To elucidate these questions, we examined the external and internal attributes of the SB apple with reference to damage-free apples, including skin cuticle and epidermal anatomy, skin δA 670–720 nm, fruit mass at harvest and postharvest mass retention, dry matter content, soluble solids content, acidity, firmness, ethylene emission, fruit flesh water potential, and phenolics contents in peel and flesh. The study aimed to understand the impacts of SB severities on fruit quality and the heat stress responses in the fruit, and to demonstrate the potential use of DA meter as a rapid, non-destructive tool in facilitating heat stress ecophysiology studies and assessing SB severity in red apple varieties.
-
The high-density planting of 'Buckeye Gala' affected by the 2021 heat waves was located in Summerland, British Columbia, Canada (49°33′53.0′′N, 119°38′57.2′′W, elevation 454 m; Summerland Research and Development Centre, Agriculture and Agri-Food, Canada). Since 2019, the trees were grown in Tall Spindle Axe training system, in 3' × 11' spacing (0.91 m × 3.35 m), in silt-loam farm soil and under the semi-arid Okanagan climate with hot, dry summers and cool winters. Irrigation was supplied through drip line from May to early October. In July 2021, five continuous days of daily maximum temperatures above 38 °C led to fruit sunburn necrosis and browning [ 13] , with the symptoms particularly manifesting in the south and southwest zones of the canopy. Defect-free and SB apples were harvested in mid-September (≥ 400 fruits for defect-free and SB each). Samples were categorized based on SB severities as such: 'Normal' for fruits without obvious browning or discolored skin (n = 45) ( Fig. 1a); 'Subtle SB' for discolored sunlit side and localized compression at harvest but no browning patch, and symptoms becoming less obvious after storage (n = 45) ( Fig. 1b); 'Mild SB' for browning patch size about 20%−30% of surface area, no russeting or microcracking (n = 45) ( Fig. 1c); 'Moderate SB' for apparent browning and patch size 30%−50%, with russeting and microcracking but no shrivel after storage (n = 45) ( Fig. 1d); 'Severe SB' for apparent browning, patch size about 40%−60%, with russeting and microcracking, and significant fruit shrivel after storage in air at 4 °C (n = 15; less replications due to fewer apples in this category) ( Fig. 1e). Additional fruits with mild and moderate SB symptoms were grouped together for general SB assessment ('Mild-Moderate SB', Fig. 1c, d).
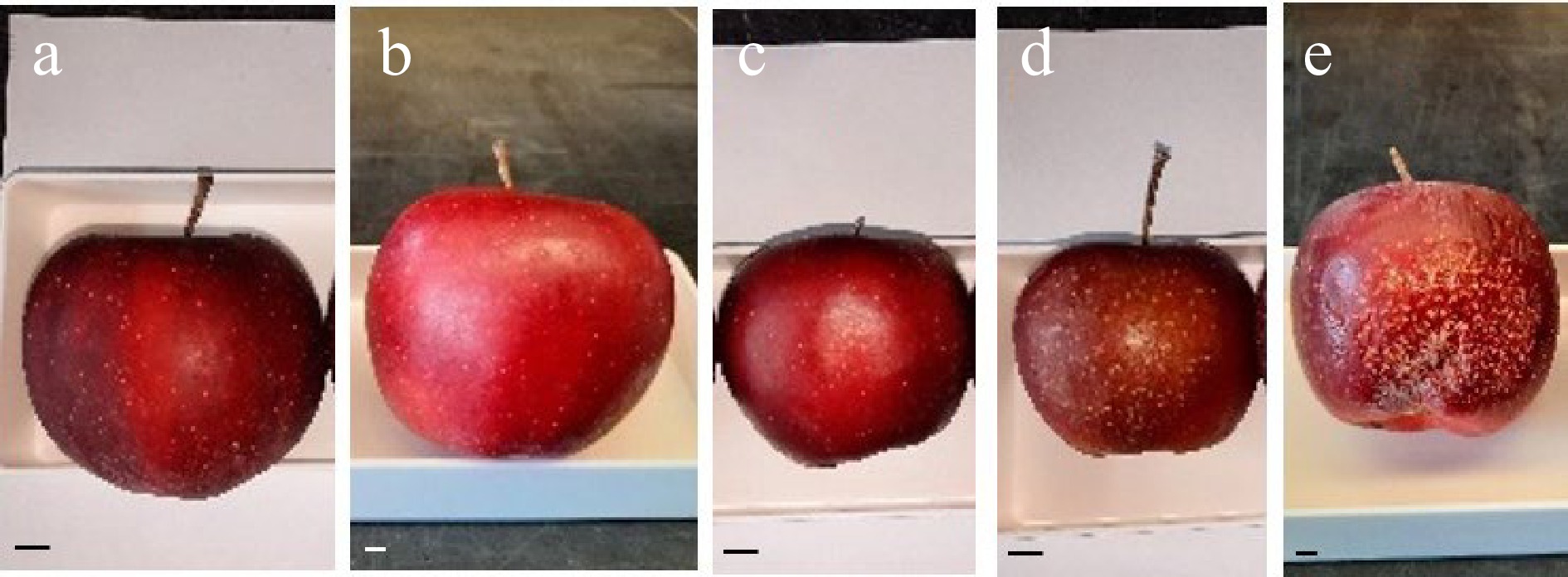
Figure 1.
'Buckeye Gala' apples with normal appearance and different sunburn browning (SB) severities. (a) Normal, (b) Subtle SB, (c) Mild SB, (d) Moderate SB, and (e) Severe SB. The photos were taken after 7 months of 4 °C storage. Scale bar in each subpanel is 10 mm.
Assessment of fruit quality attributes
-
To assess general SB impacts on fruit quality attributes, samples with mild-moderate symptoms ( Fig. 1c, d) were assessed at harvest, and after 2-month and 7-month air storage at 4 °C, in comparison with normal fruits. At harvest, fruit skin δA bsorbance ( δA 670–720 nm; Apple DA meter, Sinteleia, Bologna, Italy; n = 100), fruit dry matter content (DMC; Felix-750 Produce Quality Meter, Felix Instruments Inc., Camas, WA, USA; n = 100) and fruit flesh water potential ( Ψ; WP4C potentiometer, Meter Environment; n = 15) were measured on both sunlit and shaded sides of Normal and Mild-Moderate SB samples [ 5] ; the differentials were calculated as the value of sunlit side subtracted by that of shaded side (Eqns 1−3). The following additional fruit quality attributes were assessed on Normal and Mild-Moderate SB samples at harvest, and after 2-month and 7-month air storage at 4 °C, for the general evaluation of SB impacts on fruit quality retention: fruit mass (Ohaus R71MHD35 Ranger 7000, Parsippany, NJ, USA; n = 100), firmness (Fruit Texture Analyzer-G25, GÜSS Manufacturing Ltd., Strand, South Africa; n = 30), and soluble solids content (SSC%; 30P refractometer, Mettler Toledo, Columbus, OH, USA) and titratable acidity (TA, in gram of malic acid equivalents / L OrionStar T940 titrator, Thermo Scientific, Waltham, MA, USA) (n = 6 juice samples, each consisting of juice from five fruits, four slices per fruit).
To assess the effects of SB severities on fruits after 7-month storage, Normal, Subtle SB, Mild SB, Moderate SB and Severe SB samples were analyzed respectively for fruit mass and δA (Normal: n = 30; Subtle: n = 30; Mild: n = 28; Moderate: n = 27; Severe: n = 15), firmness (Normal: n = 9; Subtle: n = 12; Mild: n = 12; Moderate: n = 9; Severe: n = 9), and flesh Ψ (n = 6).
$ \rm d \delta A=\delta A_{sunlit}-\delta A_{shaded} $ (1) $ \rm dDMC=DMC_{sunlit}-DMC_{shaded} $ (2) $ \rm d\varPsi=\varPsi_{sunlit}-\varPsi_{shaded} $ (3) Ethylene emission
-
Internal ethylene concentrations (IECs) were measured in both Normal and Moderate SB groups of fruit (n = 15) according to the method of Jung & Watkins [ 14] . Average maturity based on δA 670−720nm at harvest was between 0.2 and 0.24. After harvest, fruits were stored at 20 °C in air storage during the evaluation. Specifically, one milliliter of gas from the core cavity of each fruit was taken through the calix end using a syringe and then injected into a gas chromatograph with a flame ionization detector (Model 8610 C, SRI Instruments, Torrance, CA, USA), fitted with a 3 m × 2.1 mm i.d. stainless steel column packed with > 0.254 mm / < 0.318 mm particle diameter Hayesep D (Supelco, Oakville, ON, Canada). Measurements were conducted periodically after harvest and during storage at 20 °C in air until the end of storage life. Storage life in current study was determined based on the severity of shriveling, where 100% severe shriveling within the sample group was considered as end of storage life.
Microscopy
-
Fresh skin peels from 7-month stored samples were hand sectioned from the sunlit side of Normal, Mild SB, Moderate SB, and Severe SB apples using a double edge razor blade and mounted onto a 35 mm diameter glass bottom petri dish (Electron Microscopy Sciences, Hatfield, PA, USA). Sections were immersed in distilled water and weighed down by an aluminum stub. Samples were immediately imaged on a Leica Microsystem CMS GmbH (Wetzlar, Germany) TCS SP8 white light laser confocal system with a 25 × 0.95NA water immersion objective. Chlorophyll auto-fluorescence was imaged using a 488 nm laser and 490−568 nm emission filter (green fluorescence channel) while anthocyanin auto-fluorescence was imaged using a 577 nm laser and 582−720 nm channel (red fluorescence channel). Cuticle thickness was measured from the outer edge of the cuticle to the closet edge of the first living cell layer, identified by the presence of plastids and/or vacuoles. Fifty measurements of cuticle thickness were made from 10 micrographs of each degree of SB damage. Because the δA values of Normal and Subtle SB apples were not significantly different, Subtle SB was omitted from microscopic analysis.
Characterization of phenolic compounds
Sample preparation
-
After 7-month storage, apple peel and flesh samples from the sunlit and shaded sides were freeze dried for Normal, Mild SB and Moderate SB fruits (n = 6). To ensure adequate extraction of phenolic compounds, the samples were subjected to grinding on a ball mill (Mixer Mill MM 301, Retsch GmbH, Haan, Germany) to reduce particle size. Sample material was placed in 2 mL capacity flat bottom microtubes along with one zirconium ball (0.4 mm) and two steel balls (5 mm) (Qiagen, Toronto, ON, Canada). The microtubes were placed on racks (Qiagen, Toronto, ON, Canada) in the aluminum plate holders attached to the ball mill. The racks were placed in the mill in one direction and the samples were ground at 20 Hz for 60 s. After the initial 60 s of grinding, the direction of the racks was changed and the samples were ground for another 60 s at 20 Hz. This procedure resulted in finely ground powder samples. The sunlit side of severe SB apples were damaged and shriveled severely, so this group was omitted from phenolics analysis.
Preparation of methanol extracts
-
Total phenolics content was assessed using both the Folin Ciocalteu and Glories methods. The use of the Glories method also allowed for the determination of levels of total tartaric esters, flavonols, and anthocyanins. Extracts used for testing in these methods were obtained using a procedure based on the work of Velioglu et al. [ 15] and Ross et al. [ 16] . An aliquot of 60mg sample (freeze dried apple peel or freeze dried apple flesh) was weighed into a micro-centrifuge tube and 1.2 mL of 70% MeOH was added to the tube (1 : 20 ratio). Tubes were vortexed for 30 min at speed 5 (Fisherbrand heavy duty vortex, Fisher Scientific, Toronto, ON, Canada). The tubes were then centrifuged (Eppendorf Minispin, Eppendorf, Mississauga, ON, Canada) for 5 min at 13,400 rpm. A pipettor was used to transfer the supernatant into a 3 mL syringe with 0.22 µm PVDF filter attached. Apple peel samples were further diluted with 70% methanol (1/5) to be within the standard curve for total phenolics analyses while the apple flesh samples did not require further dilution.
Phenolics content: total phenolics analyses
-
Measurement of total phenolics using the Folin-Ciocalteu colorimetric method was based on the procedure described by Singleton & Rossi [ 17] . Briefly, 50 µl of sample extract or chlorogenic acid standard was added to a test tube along with 150 µl MilliQ water. The system was vortexed to ensure adequate mixing and 1 mL of 1/30 Folin-Ciocalteu reagent was added. The system was vortexed and 0.8 mL of Na 2CO 3 solution (75 g/L) was added. The system was vortexed again to ensure adequate mixing and allowed to sit for 60 min before reading absorbance. Quantification was determined based on a standard curve for chlorogenic acid by measuring absorbance at 765 nm on a spectrophotometer (Cary 60, Agilent Technologies, Mississauga, ON, Canada). The total amount of phenolic content was expressed as mg chlorogenic acid equivalent per 100 gram sample on a fresh weight basis. Experiments were performed in duplicate.
Measurement of total phenolics, tartaric esters, flavonols, anthocyanins using the Glories Method was performed using a procedure based on the method provided by Ross et al. [ 16] and Harrison et al. [ 18] . An aliquot of 0.1 mL of sample extract or standard was added to a test tube. Chlorogenic acid, caffeic acid, quercetin and cyanidin-3-galactoside were used as the standards for quantification of total phenolics, tartaric esters, flavonols and anthocyanins, respectively. To the sample or standard, 0.1 ml of 0.1% HCl in 95% EtOH along with 1.82 ml of 2% HCl was added. The system was vortexed and allowed to sit for ~15 min before reading the absorbance. Quantification was determined based on a standard curve for each standard by measuring absorbance at 280, 320, 360, and 520 nm on a spectrophotometer (Cary 60, Agilent Technologies, Mississauga, ON, Canada) for determination of total phenolics, tartaric esters, flavonols, and anthocyanins, respectively. The total amount of phenolic content was expressed as mg standard equivalent per 100 gram sample on a fresh weight basis for total phenolics, tartaric esters and flavonols and on a dry weight basis for anthocyanins. Experiments were performed in duplicate.
Data analysis
-
Statistical significance analysis (One-Way or Two-Way ANOVA, Tukey Pairwise Comparisons) and graphing were conducted in OriginPro 8.0 (OriginLab, Northampton, MA, USA). Lines in box plots from bottom to top represent the minimum, lower quartile, median, upper quartile and maximum values; different letters in the same figure subpanel indicate significant differences at p ≤ 0.05. Principal Component Analysis was conducted to explore the correlation between fruit skin δA and the contents of stress response compounds.
-
Sunburn affected skin δA, DMC and Ψ on the sunlit side of the affected apples, and aggravated the inconsistencies between the sunlit side and the shaded side. Compared to Normal, Mild-Moderate SB led to the increase in δA and DMC, and the decrease in Ψ in the affected tissues at harvest, which resulted in significantly higher d δA (F(1,199) = 95.9, p < 0.001) and dDMC (F(1,199) = 201.3, p < 0.001), and significantly lower d Ψ in SB fruits (F(1,29) = 39.3, p < 0.001) ( Table 1). The altered spectral absorbance indicated the changes in skin pigments (such as chlorophylls and anthocyanins) and stress response compounds (such as phenolics and tartaric esters) in the superficial layer of the affected tissues. Compared to Normal, fruit mass was lower at harvest in Mild-Moderate SB fruits ( p < 0.001) but did not decrease significantly during the storage ( p = 0.82). This suggests that preharvest fruit development and mass accumulation was hindered by SB damage. Attributed to larger surface area (higher mass and larger fruit size) and higher water content (lower average DMC as 14.11% ± 0.20% versus 16.58% ± 0.38% (mean ± standard error), F(1,42) = 36.31, p < 0.001; higher average flesh water potential as −1.74 ± 0.05 MPa versus −2.15 ± 0.04 MPa, F(1,20) = 27.67, p < 0.001), Normal fruits lost mass over storage significantly ( p < 0.001) and at a higher rate than Mild-Moderate SB fruits. Firmness was higher in Mild-Moderate SB fruits at harvest, and after 2-month and 7-month storage, attributed to the high firmness of dehydrated and rigid tissues after SB damage. Both Normal and Mild-Moderate SB fruits lost firmness by 38% over 7-month storage, however firmness loss was significant only during the first two months of storage in both groups ( Table 1).
Table 1. Differential skin delta Absorbance d δA), differential dry matter content (dDMC) and differential flesh water potential (d Ψ) at harvest, and mass, firmness, soluble solids content (SSC), titratable acids concentration (TA) of Normal and Mild-Moderate sunburn browning (SB) 'Buckeye Gala' apple at harvest, and after 2-month (2 m) and 7-month (7 m) air storage at 4 °C.
Fruit condition Normal Mild-Moderate SB d δA At harvest 0.074 ± 0.01a 0.351 ± 0.02b dDMC (%) At harvest 0.74 ± 0.2a 6.00 ± 0.2b d Ψ (MPa) At harvest −0.09 ± 0.07a −0.65 ± 0.07b Fruit mass (g) At harvest 188.5 ± 4.1a, A 158.1 ± 2.5b, A 2 m 163.0 ± 3.2b, B 150.9 ± 2.6b, A 7 m 158.8 ± 2.9b, B 146.9 ± 2.9b, A Firmness (lbs) At harvest 18.26 ± 0.10a, A 21.33 ± 0.41b, A 2 m 11.78 ± 0.19a, B 12.30 ± 0.35b, B 7 m 11.34 ± 0.29a, B 13.26 ± 0.42b, B SSC (%) At harvest 12.89 ± 0.07a, A 14.18 ± 0.18b, B 2 m 13.46 ± 0.58a, A 15.73 ± 0.46b, A 7 m 12.4 ± 0.12a, C 14.05 ± 0.32b, B TA
(g malic acid/L)At harvest 4.69 ± 0.11a, A 4.66 ± 0.25a, A 2 m 4.05 ± 0.14a, B 3.37 ± 0.03b, B 7 m 1.95 ± 0.21a, C 2.93 ± 0.28b, C Values are mean ± standard error. For each parameter, lowercase letters in the same row represent significant difference of SB effect, and uppercase letters in the same column represent significant difference of time effect (one-way ANOVA, Tukey's test, p ≤ 0.05). SSC was higher in Mild-Moderate SB fruits at harvest, and after 2-month and 7-month storage ( p = 0.001) ( Table 1). Mild-Moderate SB fruits had lower abundance of soluble solids and dry matter per fruit despite the higher percentage of SSC and DMC, because of their lower fruit mass. Storage effect on SSC was significant in both groups; SSC after 2-month storage was higher than at harvest and after 7-month storage; the decrease in SSC after 7-month storage was significant in Normal fruits. TA significantly decreased over the storage in both groups ( p = 0.02); Compared to Normal, Mild-Moderate SB fruits had similar TA at harvest, lower TA after 2- month storage but higher TA after 7-month storage. Similar to 'Ambrosia' apple [ 5] , the increased SSC% and DMC%, and the lower Ψ, implied water deficit in Mild-Moderate SB apples at the preharvest stage. Mild-Moderate SB fruits appeared to have better SSC and TA retention over storage; less water content may have slowed down the postharvest metabolism.
The changes of ethylene emission during storage were investigated in both Normal and Moderate SB fruit, as shown in Fig. 2. Overall, there was no significant difference in ethylene emission observed between the groups ( p = 0.9). IECs in both groups of fruit were above 1 µL/L at the beginning of storage due to the advanced harvest maturity in 2021. Onset of climacteric increase occurred at 3 days after harvest (DAH) in both groups until it reached the peak at 11 DAH in Normal fruit and 16 DAH in Moderate SB fruit. Ethylene emission then gradually declined towards the end of storage period. It is worth noted that, when stored at 20 °C in air storage, Moderate SB fruit started shriveling at 15 DAH while Normal fruit developed the disorder of shrivel at 20 DAH (as marked with arrows). There was a significant difference in duration (days) from ethylene peak to the end of storage life between normal (M = 17.8, SD = 4.8) and Moderate SB fruit (M = 12.3, SD = 4; t(27) = 3.3788, p = 0.0022).
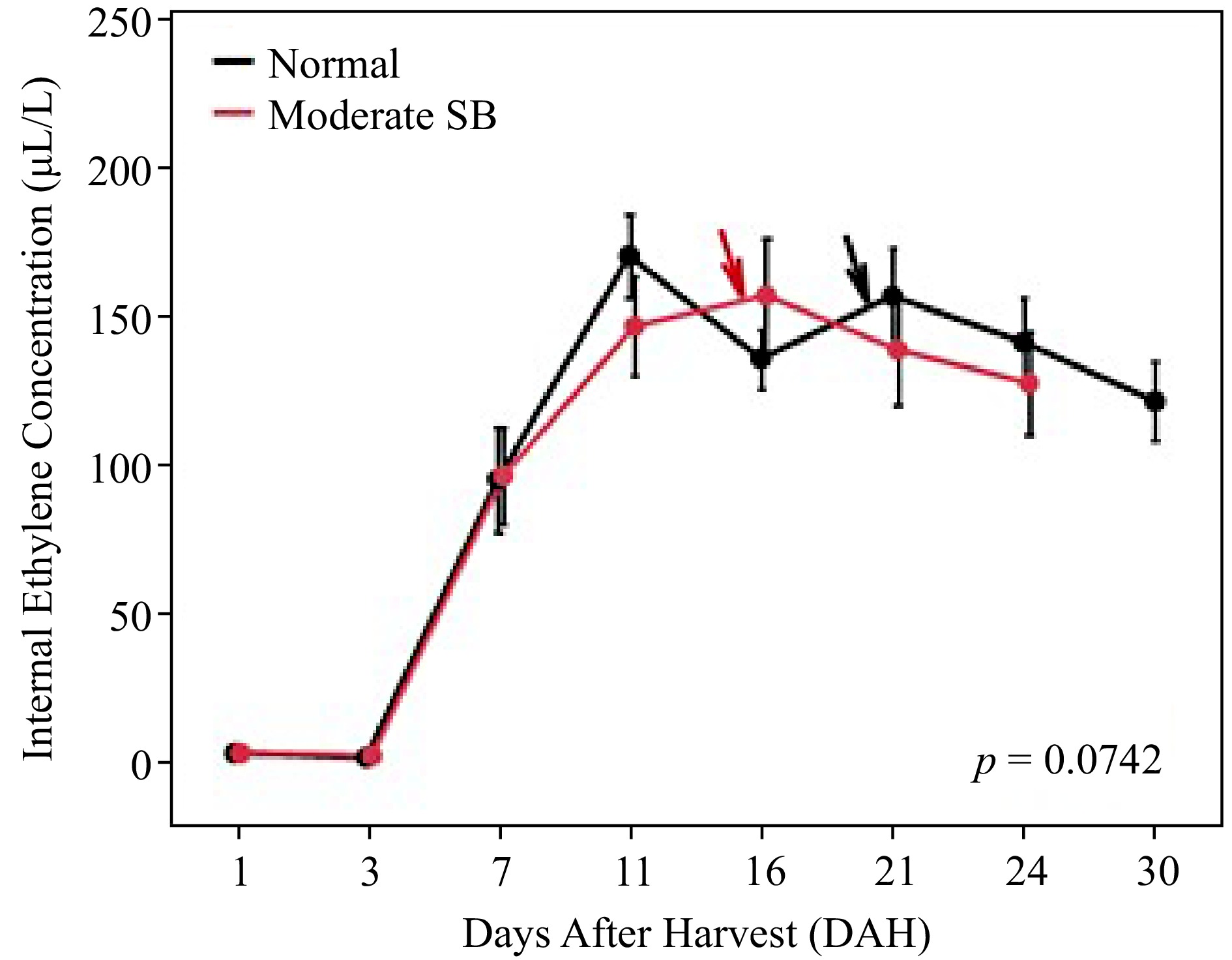
Figure 2.
Ethylene emission of 'Buckeye Gala' apples during air storage at 20 °C. Dots and error bars are mean ± standard error at each sampling point (n = 15). Lines indicate the changes over time. Arrows point out the dates when fruits started shriveling.
Both Normal and Moderate SB fruit exhibited typical ethylene climacteric pattern at postharvest stage. Sunburn damage slightly suppressed the overall ethylene production ( p = 0.0742) as well as delayed the ethylene climacteric peak in 'Buckeye Gala' apples. The result also showed that sunburn damage advanced the development of shriveling (as marked with arrows) and significantly shortened the storage life after the onset of ethylene climacteric peak ( p = 0.0022). In our previous report on sunburn damage in 'Ambrosia' apple, a cultivar that has significantly lower ethylene production during fruit ripening, significantly higher levels of ethylene emission was found in SB apple [ 5] . The important role of ethylene in response to abiotic and biotic stress has been recognized in many horticultural plants [ 19] . Both findings in 'Buckeye Gala' and 'Ambrosia' apples strongly suggested the involvement of ethylene in response to excessive sun exposure during fruit development, although further study is necessary to investigate the difference in stress responses between these cultivars.
After 7-month storage, fruits with Moderate SB ad Severe SB had significantly lower mass compared to Normal – Mild SB (F(4,126) = 7.79, p < 0.001) ( Fig. 3a). Severe SB led to significantly lower Ψ (F(4,26) = 5.54, p = 0.002) ( Fig. 3b). Firmness increased as SB severity intensified (F(4,47) = 26.47, p < 0.001), except that Subtle SB was not significantly different from Normal ( p = 0.99) ( Fig. 3c). This suggests that Subtle SB had negligible impact on fruit mass, Ψ and firmness after 7-month storage, whereas negative impacts were manifested in Moderate-Severe SB fruits. About 18.2% of moderate and severe SB fruits developed disorders of decay and shrivel during cold storage (data not graphed).
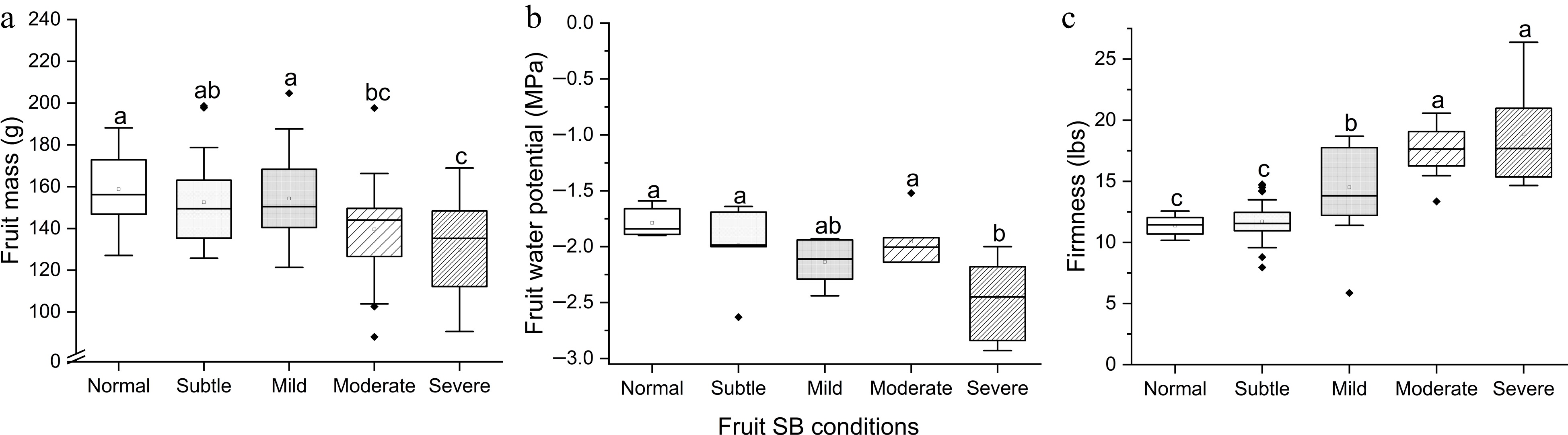
Figure 3.
(a) Fruit mass, (b) flesh water potential Ψ and (c) firmness of 'Buckeye Gala' apples with different severities of sunburn browning (SB) after 7-month air storage at 4 °C. Different letters in each subpanel represent significant difference of SB severity effect (one-way ANOVA, Tukey's test, p ≤ 0.05).
The after-storage fruit skin δA was higher on the sunlit side than the shaded side of all fruits (F(1, 259) = 294.26, p < 0.001), whereas δA on the shaded side was not significantly different between any groups ( p = 0.99) ( Fig. 4a). Normal and Subtle SB fruits had similar δA on both sides and similar d δA ( p = 0.99). The difference between the two sides (d δA) increased as the SB severity intensified (F(4, 126) = 26.52, p < 0.001) ( Fig. 4b). The δA on the sunlit side ( Fig. 4a) and d δA ( Fig. 4b) were significantly higher in Severe SB ( δA: 0.447 ± 0.044 (mean ± standard error); d δA: 0.332 ± 0.045) and Moderate SB ( δA: 0.456 ± 0.032; d δA: 0.303 ± 0.029) than in Mild SB ( δA: 0.352 ± 0.022; d δA: 0.215 ± 0.021), followed by Subtle SB ( δA: 0.230 ± 0.009; d δA: 0.090 ± 0.010) and Normal ( δA: 0.234 ± 0.008; d δA: 0.081 ± 0.012). Our limited data suggest that when d δA was set at 0.17, all Normal fruits and a majority of Subtle SB fruits could be separated from 3/4 of Mild-Moderate SB. As Subtle SB didn't significantly affect the retention of fruit mass, fruit water potential or firmness during storage, it would be practical to keep Subtle SB apples for storage meanwhile rule out the apples with severer symptoms which deteriorated fruit quality. Measured rapidly and non-destructively by DA meter, the d δA can be used to facilitate such preharvest apple sorting and early decision making. The preliminary threshold shown in Fig. 4b was based on δA measurements after 7-month storage of limited samples of 'Buckeye Gala' only. More reliable d δA threshold should be identified by multiple measurements before harvest with a larger sample pool, and should be tested for a variety of apple cultivars with different skin spectral characteristics in future studies.

Figure 4.
(a) Fruit skin δA bsorbance on the sunlit and shaded sides and (b) differential δA bsorbance between the two sides of 'Buckeye Gala' apples with different severities of sunburn browning (SB) after 7-month air storage at 4 °C. Different letters in each subpanel represent significant difference: (a), fruit side × SB severity interaction effect, two-way ANOVA; (b), SB severity effect, one-way ANOVA; Tukey's test, p ≤ 0.05.
Heat stress responses
Degradation of cellular structures of SB fruits
-
As fruit color is determined primarily by fluorescent compounds such as anthocyanins and chlorophyll within the epidermal layer of the fruit, we sought to determine their quantity and the integrity of the intracellular structures which contain these compounds by confocal microscopy. As reported previously in SB 'Ambrosia' apples [ 5] , both chlorophyll within chloroplasts ( Fig. 5a, Cl) and anthocyanins in vacuoles ( Fig. 5a, V) were clearly visible within undamaged cells ( Fig. 5a, Cl). These distinct structures however diminished in both fluorescence intensity and quantity in SB damaged areas and were entirely absent in severely damaged tissue. Similar to 'Ambrosia' apples [ 5] , damaged cells accumulated a highly autofluorescent compound that exhibited intense broad spectrum fluorescence. This compound was present in several cell layers of damaged tissue as shown in transverse sections ( Fig. 5b), resulting in an aberrant thickened cuticle layer made up of both true cuticle and dead cells. The resulting thickness of this layer appeared to follow the degree of SB damage ( Fig. 5c).
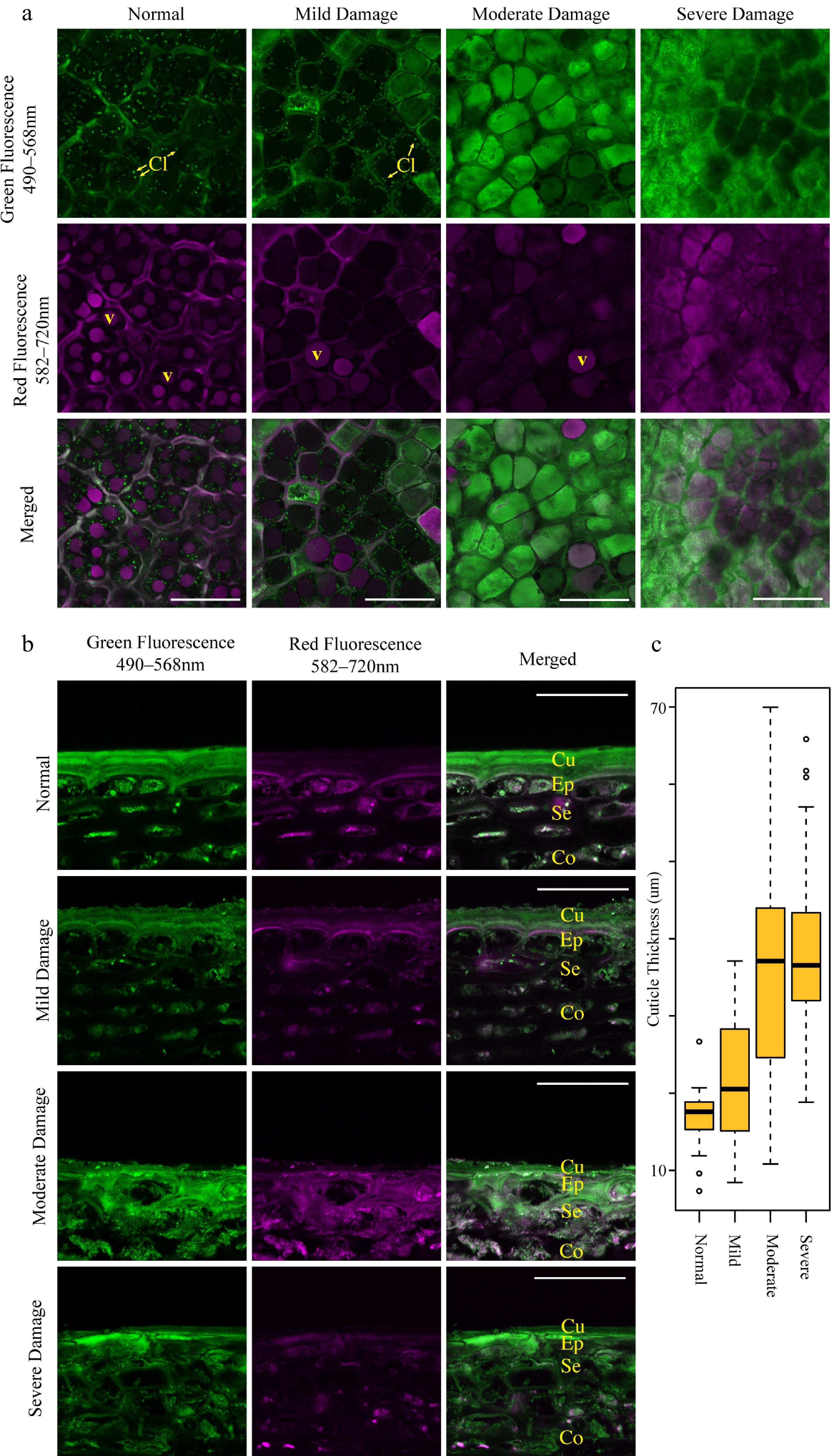
Figure 5.
Sunburn browning (SB) damage results in altered cellular structures including decreased chloroplast and anthocyanin autofluorescence and increased cuticle thickness. Confocal micrographs of (a) tangential sections and (b) transverse sections of 'Buckeye Gala' apple epidermis exhibit no, mild and moderate SB damage. Scale bars = 75 μm in (a) and 50 μm in (b). V, vacuole; Cl, chloroplasts; Cu, cuticle; Ep, epidermal tissue; Se, subepidermal tissue; Co, cortex. (c) Boxplot measurements of cuticular thickness in sun-exposed skin with various degrees of SB damage, as measured from the edge of the cuticle to the first living cell layer. (n = 50 measurements from 10 micrographs each; circles represent outliers).
Phenolic characteristics of SB fruits
-
The composition of these autofluorescent compound(s) cannot be determined via confocal microscopy, thus quantitative chemical analysis of candidates was carried out. This work provides data on levels of total phenolics, tartaric esters, flavonols and anthocyanins for flesh and peel for both the sunlit and shaded side of the apples ( Figs 6 & 7). In general, peel contained substantially higher levels of these compounds than flesh (shown in different scales on Y axis in Figs 6 & 7). For the flesh portion sampled from the sunlit side of the apple depending on sunburn (SB) damage severity (Normal, Mild SB or Moderate SB), average values of total phenolics content assessed with the Folin Ciocalteu method ranged from 80.9 to 136.1 mg chlorogenic acid equivalent (CGAE) /100g fresh weight basis (data not graphed); average values of total phenolics content assessed with the Glories method ranged from 47.7 to 91.7 mg CGAE /100 g fresh weight basis ( Fig. 6a); average values of flavonols ranged from 3.6 to 6.8 quercetin equivalents (QE)/100 g fresh weight basis ( Fig. 6b); average values of tartaric esters range from 9.9 to 20.5 mg caffeic acid equivalents (CAE)/100g fresh weigh basis ( Fig. 6c); and average values of anthocyanins ranged from ~5.0 to 6.3 mg cyanidin-3-galactoside equivalents (Cy-3-GalE)/100 g dry weight basis ( Fig. 6d). For the flesh portion sampled from the shaded side of the apple depending on SB damage severity (Normal, Mild SB or Moderate SB), total phenolics content assessed with the Folin Ciocalteu method ranged from 76.5 to 95.2 mg CGAE/100 g fresh weight basis (data not graphed); total phenolics content assessed with the Glories method ranged from 46.3 to 58.9 mg CGAE/100 g fresh weight basis ( Fig. 6a); flavonols ranged from 3.7 to 4.7 QE/100g fresh weight basis ( Fig. 6b); tartaric esters range from 10.0 to 12.4 mg CAE/100 g fresh weight basis ( Fig. 6c); and average values of anthocyanins ranged from ~5.3 to 7.8 mg Cy-3-GalE/100 g dry weight basis ( Fig. 6d). Yuri et al. [ 6] has also examined levels of phenolic compounds in apple fresh with sunburn damage compared to apples without sunburn damage. For various apple cultivars, they reported levels of total phenolics to range from 90 to 120 mg CGAE/100 g fresh weight in the flesh of healthy fruit and levels of total phenolics to range from 100 to 160 mg CGAE/100 g fresh weight in the flesh of sunburn damaged fruit, which are in agreement with levels and trends determined in the present work. Chen et al. reported levels of flavonols in healthy flesh of various apple cultivars to be up to ~1mg flavonols per 100g fresh weight [ 20] , which is in the same order of magnitude observed in the present work.
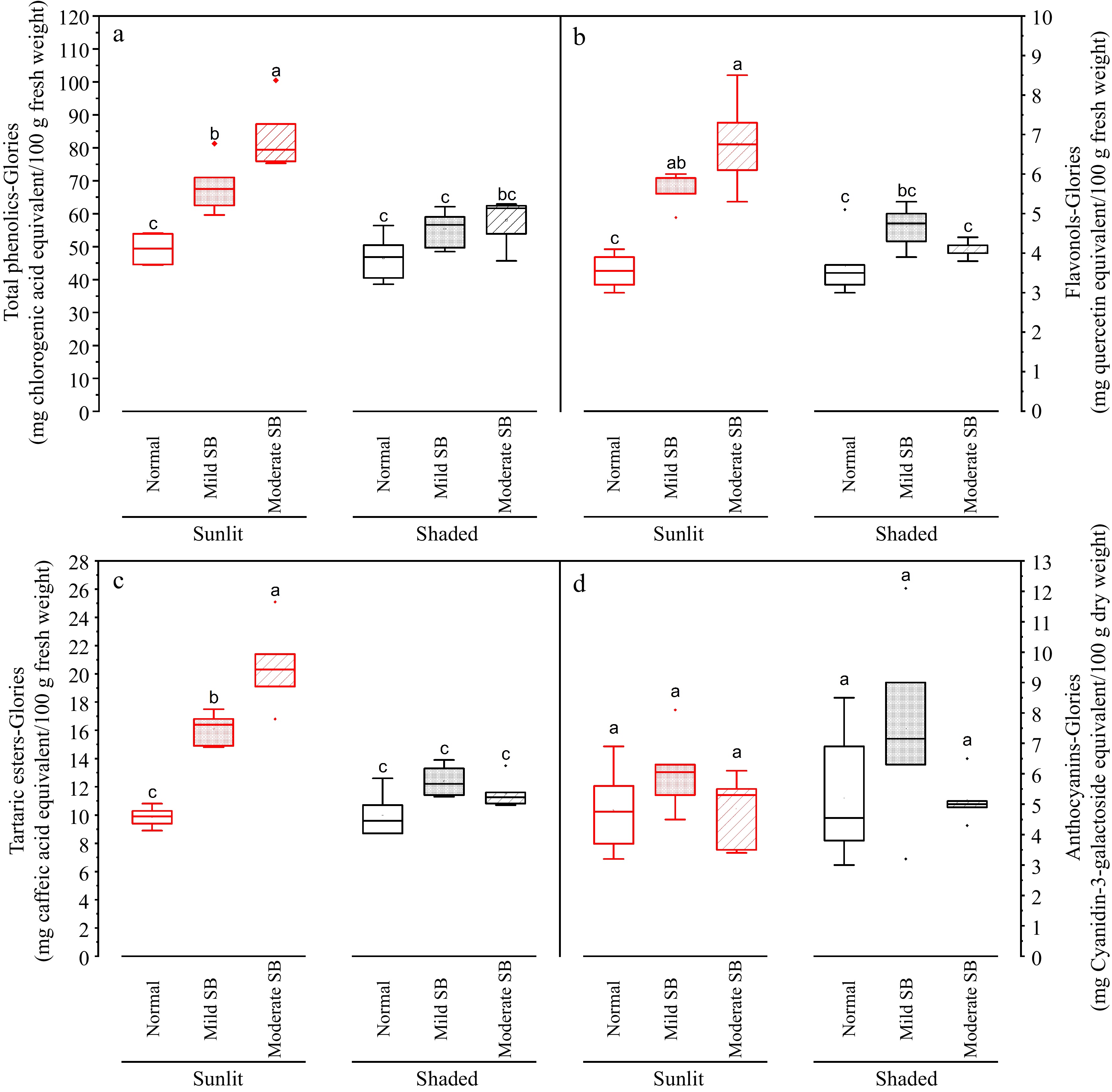
Figure 6.
Total phenolics, flavonols, tartaric esters and anthocyanin in the flesh on sunlit and shaded sides of normal, mild sunburn browning (SB) and moderate SB Buckeye Gala apples. Different letters in each subpanel represent significant difference of fruit side × SB severity interaction effect (two-way ANOVA, Tukey's test, p ≤ 0.05).
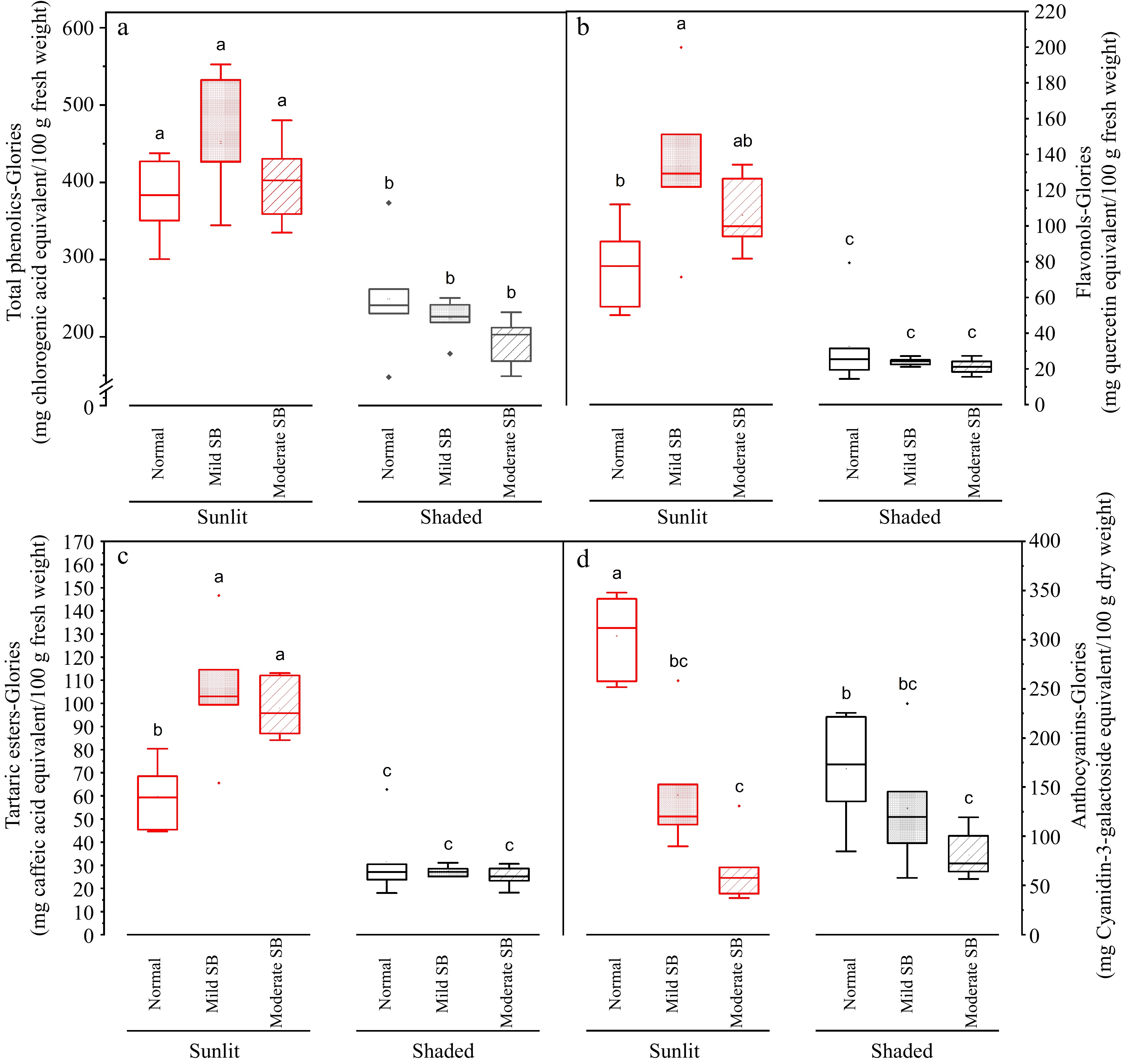
Figure 7.
Total phenolics, flavonols, tartaric esters and anthocyanin in the peel on sunlit and shaded sides of normal, mild sunburn browning (SB) and moderate SB Buckeye Gala apples. Different letters in each subpanel represent significant difference of fruit side × SB severity interaction effect (two-way ANOVA, Tukey's test, p ≤ 0.05).
For the peel portion sampled from the sunlit side of the apple depending on SB damage severity (Normal, Mild SB or Moderate SB), average values of total phenolics content assessed with the Folin Ciocalteu method ranged from 565.7 to 632.4 mg CGAE/100 g fresh weight basis (data not graphed); average values of total phenolics content assessed with the Glories method ranged from 380.5 to 451.7 mg CGAE/100 g fresh weight basis ( Fig. 7a); average values of flavonols ranged from 77.2 to 133.8 QE/100 g fresh weight basis ( Fig. 7b); average values of tartaric esters range from 59.6 to 105.4 mg CAE/100 g fresh weight basis ( Fig. 7c); and average values of anthocyanins ranged from ~68.2 to 316.0 mg Cy-3-GalE/100 g dry weight basis ( Fig. 7d). For the peel portion sampled from the shaded side of the apple depending on SB damage severity (Normal, Mild SB or Moderate SB), average values of total phenolics content assessed with the Folin Ciocalteu method ranged from 384.0 to 438.4 mg CGAE/100 g fresh weight basis (data not graphed); average values of total phenolics content assessed with the Glories method ranged from 194.6 to 249.2 mg CGAE /100 g fresh weight basis ( Fig. 7a); average values of flavonols ranged from 21.3 to 32.6 QE/100 g fresh weight basis ( Fig. 7b); average values of tartaric esters range from 25.2 to 31.5 mg CAE/100 g fresh weight basis ( Fig. 7c); and average values of anthocyanins ranged from ~84.1 to 175.7 mg Cy-3-GalE/100 g dry weight basis ( Fig. 7d).
In general, levels of total phenolics, tartaric esters, and flavonols in apple flesh sampled from the sunlit side and shaded side were not significantly different in fruit without SB damage ( Fig. 6). For the peel, levels of total phenolics, tartaric esters, flavonols, and anthocyanins sampled from the sunlit side were higher than levels measured in peels sampled from the shaded side in fruit with various degrees of SB damage (Normal, Mild SB or Moderate SB) ( Fig. 7). Yuri et al. reported levels of total phenolics to range from 430 to 780 mg CGAE/100 g fresh weight in the peel of healthy apple fruit and levels of total phenols to range from 630 to 1,440 mg CGAE/100 g fresh weight in the peel of sunburn damaged apple fruit [ 6] , which are in agreement with levels and trends determined in the present work. Chen et al. reported levels of flavonols in healthy peels of various apple cultivars to be up to ~840 mg flavonols per 100 g fresh weight [ 20] which is higher but in the same order of magnitude observed in the present work, indicating the validity of measurements. Levels of anthocyanins healthy apple peel was reported to be up to ~150 mg Cy-3-GalE/100 g fresh weight and therefore approximately 750 mg Cy-3-GalE/100 g dry weight. This value is higher but in the same order of magnitude presented in the current work, which again indicates validity of measurements. The phenolic content of fruits can be affected by different preharvest factors such as inherent cultivar differences, horticultural practices (e.g. pruning, fertilization, irrigation, canopy position) and growing environment [ 21] . With respect to environmental effects, Yang et al. examined the influence of weather conditions on phenolic compounds in different currant cultivars and found that high UV radiation levels and high temperatures were associated with low phenolic contents in all the currant cultivars studied [ 22] . High temperatures (30–40 °C) during ripening have been shown to result in lower levels of flavonol accumulation in grape berries [ 23] . Additionally research has shown high temperatures reduce anthocyanin accumulation in many plants, including in the skin of fruits such as grape berries and apples [ 24, 25] . Lin-Wang et al. showed in 'Mondial Gala' and 'Royal Gala' apples that heat caused a reduction of both peel anthocyanin concentration and transcripts of the genes of the anthocyanin biosynthetic pathway [ 24] . The work of Kim et al. demonstrated that high ambient temperatures repressed anthocyanin biosynthesis through degradation of elongated hypocotyl 5 (HY5 signaling module) as an increase in ambient temperature decreased expression of genes required in both the early and late stages of the anthocyanin biosynthesis pathway [ 25] . Therefore, based on the literature, the results of lower levels of anthocyanins and flavonols in the sunburned apple peels compared to levels reported in healthy apple peels by different researchers may be due to cultivar differences, differences in horticultural practices, growing location and the high temperatures experienced during growth.
With further respect to the effect of SB damage on levels of phenolic compounds, there were no significant differences between levels of anthocyanins for flesh samples taken from the sunlit side of the apple with varying levels of sunburn browning (SB, normal, mild and moderate) ( Fig. 6d). This was not unexpected as apple flesh does not contain substantial levels of anthocyanins. Levels of total phenolics, tartaric esters and flavonols were significantly higher ( p ≤ 0.05) in the flesh of the sunlit side of apples with mild and moderate compared to flesh taken from the sunlit side of apples without SB damage ( Fig. 6a− c). No significant differences in levels of phenolic compounds were determined for flesh samples taken from the shaded side of apples with differing levels of SB (normal, mild, and moderate) ( Fig. 6a). No significant differences were observed for levels of total phenolics in the peel samples from both the sunlit and shaded side of apples, in response to SB damage ( Fig. 7a). Levels of anthocyanins were higher ( p ≤ 0.05) in the peels samples taken from the sunlit side of the fruit with no SB damage compared to peels taken from the sunlit side of fruit with mild and moderate SB damage ( Fig. 7d), suggesting that anthocyanin level in sunlit peel decreased as sunburn intensified. This was consistent with the absence of anthocyanin-containing vacuoles in the damaged tissues under microscopic observation ( Fig. 5a). There were no significant differences determined in levels of these compounds in the peel on the sunlit side of Mild SB and Moderate SB fruits ( Fig. 7). No significant differences in levels of phenolic compounds were determined for peel samples taken from the shaded side of apples with differing levels of SB. Both levels of flavonols and tartaric esters were higher ( p ≤ 0.05) in the peel samples taken from the sunlit side of Mild and Moderate SB fruits compared to peel samples taken from the sunlit side of Normal ( Fig. 7b, c). There were no significant differences determined in levels of these compounds in the peel of fruit with mild and moderate SB damage when sample from the sunlit side of the fruit. In this case, the increase in phenolic compounds in the peel from the sunlit side of mild sunburned peel seemingly peaked or levelled off as significant differences in levels of phenolic compounds were not observed between the fruit material with mild and moderate SB damage. Browning caused by sunburn is due to enzymatic browning. In enzymatic browning, polyphenol oxidase and ortho diphenolic compounds in damaged tissue, presumably due to intense UV light and high temperatures [ 6] , are exposed to oxygen. This initiates oxidation of othro diphenols, which includes chlorogenic acid, caffeic acid, quercetin and cyanidin, to quinones which are subsequently polymerized through several reactions with proteins and/or amino acids to relatively insoluble brown pigment (melanin) [ 26] . Therefore the reduction or plateauing of measured phenolic compounds in more severely sunburned peel may in fact be a consequence of the chemical analysis as the phenolic compounds are bound polymerized state and not extracted with the 70% methanol and therefore not quantified. Similarly, esters in the polymerized state play an important role in crosslinking the inter-esterified omega hydroxy acids of cutin [ 27] , therefore there may be abundant ester bonds in the thickened cuticular layer under SB damage ( Fig. 5b, c) which were underestimated by our chemical analysis.
Again, variation in levels of phenolic compounds was not unexpected as bioactive attributes of fresh fruits can be affected by different preharvest factors such as genetic variability, mineral application, irrigation, canopy position, pruning, seasonal variation and environmental effects [ 21] . Further, it has been reported that sunburn on apples is associated with increases in phenolic compounds and antioxidant activity as a function of the cultivar and areas of the fruit [ 6, 28] . The increase in phenolic compounds is a defense mechanism developed by the fruit as a response to oxidative stress as plants have developed oxidative stress defense systems based on compounds like phenolic compounds, flavonoids, ascorbic acid, α- tocopherol, carotenoids and enzymes like peroxidase and polyphenol oxidase [ 2, 6] . It should be noted that temperatures over a certain limit will result in tissue damage and compromise bioactive levels. The main causes of sunburn damage are prolonged exposure to high temperatures and high levels of solar radiation and there are factors that predispose the appearance of sunburn damage which includes: cultivar sensitivity, water stress, the orientation of the planting rows, the training system, pruning, and nutritional imbalances [ 6, 28] . Therefore in addition to exposure to high temperatures and solar radiation, these pre-harvest factors will also impact levels of phenolic compounds in sunburned fruit.
Implications of the change in skin δ A due to SB
-
In Principal Component Analysis, the clusters of Normal (green dot), Mild SB (black dot) and Moderate SB (red dot) had clear separation in δA, phenolics and tartaric esters in peel (orange line) but not in flesh (pink line); the separation was also clear on the sunlit side (purple line) but not on the shaded side (grey line) ( Fig. 8a). Total phenolics, flavonols and tartaric esters were the strongest influences on Principal Component 1 (PC1); δA and anthocyanins exerted two strong but opposite influences on PC2. There was a weak negative correlation between anthocyanin concentration and δA ( Fig. 8b).

Figure 8.
(a) Principal Component Analysis and (b) correlation matrix of the correlations between fruit skin δA and the contents of total phenolics, flavonols, tartaric esters and anthocyanin in peel and flesh on sunlit and shaded sides of normal, mild sunburn browning (Mild SB) and Moderate SB 'Buckeye Gala' apples (n = 6, N = 72).
Linking total phenolics data with δA bsorbance data, the results indicate that fruit skin δA 670–720 nm was positively correlated with the total amount of phenolics (r = 0.76) and tartaric esters (r = 0.82) in peel and flesh ( Fig. 8b). This suggested that the accumulation of the phenolics and tartaric ester triggered by heat stress contributed to the elevated δA 670–720 nm despite the degradation/absence of chloroplasts ( Fig. 5a). This information is important for interpreting the measurements of DA meter which was initially designed to detect the loss of skin chlorophyll as an indicator for fruit maturation. Meanwhile, it demonstrated the potential use of DA meter as a rapid, non-destructive tool in assessing sunburn browning severity. This study suggested the d δA threshold of 0.17 ( Fig. 4b) for separating normal and subtle SB fruits from the majority of mild-moderate SB fruits; in future studies this preliminary threshold should be refined by multiple preharvest measurements with a larger sample pool, and should be tailored for a variety of apple cultivars which differ in skin spectrual characteristics.
The work of Puumalainen presented data on using hyperspectral imaging to detect bruising in apples [ 29] . This work indicated lower reflectance/higher absorbance values were seen in bruised fruit compared to sound fruit. It was stated there were notable absorptions at 500, 680, 840 and 960 nm, which correspond to carotenoid, chlorophyll, sugar and water absorption bands, respectively. It was also indicated there was another water absorption band at 660 nm. The work stated some of the changes in spectral profiles of bruised regions could be explained by water shifting within the fruit [ 29, 30] . Further, bruising normally happened to the tissue beneath the fruit skin and after the fruit tissue was damaged, its cells were initially filled with water and turned brownish. As time elapsed, the damaged cells started to lose moisture and eventually became desiccated [ 30] . Xing et al. stated that damage to the cellular structures may cause leakage of chlorophyll in the bruised area of the fruit [ 31] . Therefore, the values of high δA 670–720 nm detected in the sunburned fruit at harvest and after storage ( Table 1, Fig. 4) may be due to water migration into the sun damaged tissue prior to desiccation and leakage of chlorophyll making absorbance in the 660–680 nm range high causing δA results to be high in sun damaged fruit. This seems to agree with the lower (more negative) Ψ values in the sun damaged fruit ( Table 1, Fig. 3). The speculation of chlorophyll leakage was supported by the degradation of chloroplast integrity observed under confocal ( Fig. 5a). Further, the increase in phenolic compounds measured may also be contributing to all of the high readings in the sun damaged fruit (high δA, more negative water potential values and high DMC) as seen in Table 1. In future studies, a comprehensive compound analysis may help to identify more phenolics and esters which accumulate due to SB and contribute to the increase in δA, and guide our use of DA meter in detecting certain heat stress responsive compounds with speed and accuracy.
-
This study improved the understanding of the impacts of different sunburn severities on fruit quality of 'Buckeye Gala'. The impact of subtle SB was negligible, whereas Mild-Moderate SB affected fruit mass, firmness, SSC and TA, and caused inconsistency in DMC and Ψ between sunlit and shaded sides in the affected fruit. Severe SB caused significant decrease in fruit mass and Ψ, and increased the rigidity of the affected tissue which led to the increase in firmness. The concurrence of the increase in DMC and SSC and the decrease in Ψ suggested water deficit in the fruit tissues affected by SB. On the microstructural level, SB increased the cuticle thickness, and reduced the abundance of chloroplasts and anthocyanin-containing vacuoles. As SB severity intensified, skin d δA 670–720 nm increased. The d δA was positively correlated with the total amount of stress-responsive phenolics and tartaric esters in peel and flesh on the sunlit side of the affected fruit. This suggested the potential use of DA meter as a rapid, non-destructive tool in facilitating heat stress ecophysiology study and assessing sunburn browning severity in red apple varieties.
-
The authors confirm contribution to the paper as follows: conceptualization: Ross K, Xu H, Watanabe Y; methodology, formal analysis and data visualization - biochemical analysis: Ross K, Fukumoto L, Zurowski-Tiffin K; microscopy: Watanabe Y, Iritani D; ethylene emission: Yang X; fruit quality assessment: Xu H, Ediger D; data curation: Ross K, Watanabe Y, Yang X, Xu H; writing original draft preparation: Ross K, Xu H, Watanabe Y, Yang X; review and editing: Xu H, Ross K; project administration and funding acquisition: Xu H. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors thank Summerland Research and Development Centre Field Service for the help with field maintenance and Madeline MacIntosh for her technical support. The 'Buckeye Gala' trial is part of NC-140 regional rootstock project. Some research activities were funded by Agriculture and Agri-Food Canada (J-002642).
-
The authors declare no conflict of interest. Hao Xu is the Editorial Board member of Fruit Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Copyright: © His Majesty the King in Right of Canada, $!{article_year}. Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ross K, Xu H, Watanabe Y, Yang X, Fukumoto L, et al. 2023. Changes in fruit skin δ Absorbance and heat stress response compounds in relation to sunburn browning severities of 'Buckeye Gala' apple. Fruit Research 3:33 doi: 10.48130/FruRes-2023-0033
Changes in fruit skin δ Absorbance and heat stress response compounds in relation to sunburn browning severities of 'Buckeye Gala' apple
- Received: 21 July 2023
- Accepted: 04 September 2023
- Published online: 20 November 2023
Abstract: High temperature deteriorates apple tree performance and fruit growth. In the summer of 2021, heat waves affected the apple production zones in the Pacific Northwest of North America. In a 'Buckeye Gala' experimental trial in Summerland, British Columbia, Canada, heat caused fruit skin sunburn browning (SB), and affected fruit mass, dry matter content, soluble solid content, titratable acidity, firmness and flesh water potential. We studied fruit skin δAbsorbance at 670–720 nm ( δA 670–720), cuticle characteristics, and key stress response compounds in peel and flesh, to elucidate the SB mechanism. SB was associated with thickened cuticle, chloroplast degradation, decreased anthocyanin content and increased firmness on the affected sunlit side of the fruit. As sunburn damage intensified, anthocyanin content in peel decreased, while the concentration of tartaric ester and total phenolics in flesh increased. The δA 670–720, measured using Delta Absorbance (DA) meter, was positively correlated with the total amount of phenolics and tartaric esters in peel and flesh. This suggested that the accumulation of the phenolics and tartaric ester triggered by heat stress contributed to the high δA 670–720 despite the degradation/absence of chloroplasts. The study improved our understanding of the impacts of sunburn severities on fruit quality and of the heat stress responses in 'Buckeye Gala' apple, and demonstrated the potential use of DA meter as a rapid, non-destructive tool in facilitating heat stress ecophysiology study and predicting SB severity in red apple varieties.
-
Key words:
- Anthocyanin /
- Chloroplast /
- Cuticle /
- Delta Absorbance (
δA)
/ - Flavonol /
- Fruit quality /
- Heat stress /
- Phenolics /
- Sunburn browning (SB) /
- Tartaric ester












