-
Turfgrasses are among the economically, environmentally, ecologically, recreationally, and aesthetically important grass species[1]. They are generally classified into cool-season and warm-season species based on their required temperatures and precipitation for optimum plant growth and development. However, both species can be periodically subjected to abiotic stresses within their adaptation zones. The frequency and severity of stresses are expected to increase as a result of climate change, which will further limit grass management, production and environmental adaptation. Various cultural practices have been implemented in promoting turfgrass stress management. One of them is called 'chemical priming', which uses protective chemical agents in inducing defense mechanisms to alleviate stress injury and enhance stress resistance[2,3].
Gamma-aminobutyric acid (GABA) is a ubiquitous nonprotein amino acid and an endogenous signaling molecule[4]. GABA often accumulates in response to a wide range of environmental stimuli, leading to changes of physiological and biochemical responses that can modulate stress tolerance[5, 6]. Nitric oxide (NO) is a gaseous molecule that has shown diverse biological functions in plants, including delaying leaf senescence and increasing abiotic stress tolerance[7, 8]. As a signal molecule, NO levels are regulated by both endogenous and environmental cues[9]. This review emphasizes the physiological and molecular mechanisms of exogenous GABA or NO application and their interactions in the amelioration of abiotic stress tolerance on turfgrass species.
-
Water deficit can severely inhibit the growth, persistence, and physiological activities of both cool- and warm-season turfgrasses. Foliar application of GABA improves drought tolerance of turfgrass species. Creeping bentgrass (Agrostis stolonifera L.) is one of the most important cool-season turfgrass species used on golf courses. Foliar application of 500 µM GABA before or during drought stress or growing plants in a Hoagland solution containing GABA improved growth and physiological activities of this species under drought stress. Specifically, decrease in leaf electrolyte leakage and increases in leaf green color and stolon length, relative water content, chlorophyll florescence (Fv/Fm), net photosynthetic rate, contents of chlorophyll, water-soluble carbohydrate and amino acids (glycine, valine, proline, 5-oxoproline, serine, threonine, aspartic acid and glutamic acid) and organic acids (malic acid, lactic acid, gluconic acid, malonic acid and ribonic acid) were found in GABA-treated plants compared to the untreated plants under drought stress[10−13]. Antioxidants play an important role in reducing oxidative damages induced by reactive oxygen species (ROS) in plant cell under abiotic stress conditions. Application of 500 µM GABA reduced leaf superoxide (O2.−) and hydrogen peroxide (H2O2) contents and enhanced activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxide (POD), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR), contributing to drought tolerance in creeping bentgrass[14,15]. Furthermore, GABA treatment alters gene expression under drought stress. For example, creeping bentgrass plants treated with GABA before drought stress showed upregulated the expression of genes such as DREB1/2, WRKY1/24/41, DHNs, HSP70, CDPK26, MAPK1, ABF3, WRKY75, MYB13, MT1, SOD, CAT, POD, APX, MDHAR, DHAR, and GR involved in transcription regulation, osmotic adjustment, antioxidant metabolism, and protein protection under drought stress[14, 16]. The promotive effects of GABA on drought tolerance has also been found in other turfgrass species. In perennial ryegrass (Lolium perenne L.), foliar application of 50 mM GABA one day before drought stress increased leaf relative water content, and decreased wilt rate, canopy temperature depression, electrolyte leakage, and lipid peroxidation, but had no effects on SOD and CAT activity under drought stress, compared with the untreated plants[17]. It appears that some GABA-mediated physiological processes such as antioxidant metabolism vary with grass species, applied concentration of GABA, and duration and intensity of drought stress.
Sodium nitroprusside (SNP) and potassium nitrite (PN), donors of NO, are compounds that produce NO in plants. It has been found that application of NO donors improves drought tolerance of several turfgrass species. In perennial ryegrass, spray of 400 µM SNP enhanced seed germination, root and shoot length, chlorophyll and proline contents, and reduced electrolyte leakage, compared to the untreated plants exposed to polyethylene glycol-induced water stress[18]. Foliar application of 150, 200 and 250 μM SNP or PN prior to drought stress increased activities of SOD, CAT and ascorbate peroxidase (APX) in perennial ryegrass, Kentucky bluegrass (Poa pratensis L.), and bermudagrass (Cynodon spp.), with maximum activity observed with the treatment of 200 μM SNP or PN during drought stress and recovery[19]. Application of 50, 100, and 150 µM SNP generally increased drought tolerance by maintaining leaf relative water content, chlorophyll and proline contents, SOD and APX activity, and reducing electrolyte leakage in creeping bentgrass and tall fescue (Festuca arundinacea Schreb.) under drought stress[20]. Foliar spray of another NO donor, 100 mM nitrosoglutathione (GSNO), enhanced activity of sucrose : sucrose 1-fructosyltransferase for fructan biosynthesis, resulting in a 3-fold increase in fructan content in perennial ryegrass exposed to drought stress[21]. Since fructan is a major carbohydrate reserve for cool-season turfgrass species, accumulation of fructan may facilitate osmoregulation and membrane protection, thus contributing to stress tolerance. The GSNO-treated plants also had higher GR activity and reduced glutathione content, suggesting that the NO-enhanced drought tolerance was partially due to mitigating oxidative stress[21]. On the contrary, foliar spray of 200 µM NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) increased stress injury by inducing membrane permeability and accumulating ROS in tall fescue and red fescue (Festuca rubra L.) during drought stress and recovery[22]. These results demonstrate that foliar application of NO alleviates drought stress injury in some turfgrass species through maintaining chlorophyll content, membrane stability, osmoregulation, and antioxidant enzyme activity.
-
High temperatures often cause declines in the quality and growth of cool-season turfgrasses, especially when they are cultivated in transition and warm climatic regions. Low temperatures particularly limit the growth and productivity of warm-season turfgrasses, but cool-season turfgrass species may suffer from freezing injury in temperate regions as plants go through an annual cycle of overwintering. GABA-improved heat tolerance has been mainly studied in creeping bentgrass. Foliar spray of 500 µM GABA increased chlorophyll content, Fv/Fm, photosynthetic rate, osmotic adjustment, and activities of SOD, POD, and APX, and decreased electrolyte leakage and reduced accumulation of O2·−, H2O2, and malondialdehyde (MDA) in creeping bentgrass exposed to heat stresses[11, 23, 24]. Analysis of metabolites revealed that GABA-treated plants had increased levels of amino acids (glutamic acid, aspartic acid, alanine, threonine, serine, and valine), organic acids (aconitic acid, malic acid, succinic acid, oxalic acid, and threonic acid), sugars (sucrose, fructose, glucose, galactose, and maltose), and sugar alcohols such as mannitol and myo-inositol[13, 25, 26]. Foliar spray of 500 µM GABA also altered nutrient status in creeping bentgrass under heat stress, with higher leaf nitrogen, phosphorus, calcium, sodium, and copper levels, and lower contents of boron and manganese observed in GABA-treated plants than the untreated plants[27]. Heat stress largely causes leaf senescence of turfgrass plants. Exogenous GABA treatment delayed leaf senescence of creeping bentgrass exposed to heat stress by reducing chlorophyll-degrading enzyme activities, increasing the abundance of heat shock proteins of HSP70, HSP90-1, HSP101 and antioxidant proteins of Cu/ZnSOD and APX4, and other proteins such as ATP-dependent 6-phosphofructokinase 5, fructokinase 2, fructofuranosidase, galactinol-sucrose galactosyltransferase 2, asparagine synthetase, as well as transcription factor of C2H2 zinc-finger protein[23, 28]. At the gene level, a spray of 500 µM GABA upregulated expression of ABF3, POD, APX, DHN3, and MT1, and genes encoding heat shock protein such as HSP12, HSP17.8, HSP26.7, HSP70, HSP82, HSP90.1-A1, HSP90.1-B1, and HSP90-5), as well as heat shock factors such as HSFA-2c, HSFA-2d, HSFA-6a, HSFB-2b, and HSFC-2b in creeping bentgrass under heat stress[16, 24, 29]. In addition, vvi-miR845c, ama-miR156, and other novel miRNAs such as novel-24223, novel-2964, and novel-10098 could be involved in GABA-regulated heat tolerance of creeping bentgrass[30]. Collectively, exogenous application of GABA improves heat tolerance by enhancing osmoprotection, antioxidant activity, upregulating HSF pathways and post-transcriptional regulation, modulating mineral nutrient availability and amino acid metabolism, and suppressing chlorophyll degradation.
Application of NO donors can also improve heat and cold tolerance of turfgrass species. After tall fescue leaves were vacuum-infiltrated with 100 µM with SNP and subsequently exposed to 44 ºC, the SNP-treated leaves exhibited higher Fv/Fm, lower electrolyte leakage, reduced contents of O2.−, H2O2, and MDA, and induced expressions of psbA, psbB, and psbC genes encoding subunits of PSII complex protein under heat stress[31]. Bermudagrass sprayed with 100 µM SNP had lower MDA content and electrolyte leakage and higher chlorophyll content and chlorophyll fluorescence parameters, activities of SOD and POD, and expression of cold-responsive genes such as LEA and CBF under 4 ºC, but such effects were suppressed by pretreatment with the NO scavenger PTIO or PTIO plus NG-nitro-L-arginine-methyl ester (L-NAME, NO synthase inhibitor)[32]. These research findings suggest that NO-mediated heat and cold tolerance is associated with the maintenance of cell membrane stability, antioxidant enzymes activities, photosystem II efficiency, and inducing the expression of cold-responsive genes in turfgrass species.
-
Salinity is a major stress limiting growth of both cool-and warm-season turfgrass in salt-affected areas. The increasing use of non-potable water containing high salt can also lead to salinity stress[33]. Salinity imposes both osmotic and ionic stresses to the plants, and salinity tolerance is strongly associated with osmotic and ionic stress tolerance[34]. In perennial ryegrass, foliar application of 50 and 100 mM GABA increased proline and total soluble protein content and POD and SOD activity, and reduced H2O2 and MDA content and Na+/ K+ ratio under 50 and 100 mM NaCl stress[35]. Across four perennial ryegrass cultivars, foliar spray of 500 µM GABA resulted in lower leaf Na+ concentration at both 100 mM and 200 mM NaCl and maintained higher Fv/Fm at 200 mM NaCl, compared with H2O treatment[36]. Also in perennial ryegrass, the addition of 1 mM GABA to 175 mM NaCl solution increased seed germination by 32% and subsequently enhanced shoot dry weight, shoot carbon and nitrogen contents, compared to the NaCl treatment alone[37] . Creeping bentgrass irrigated with 500 µM GABA had significantly higher leaf relative water content and Fv/Fm and lower electrolyte leakage, compared to the untreated plants exposed to 250 mM NaCl[38]. Furthermore, GABA treated plants showed increased total polyamines, spermidine, amino acids such as glutamic acid, alanine, phenylalanine, aspartic acid, and glycine, and carbohydrates such as galactose, talose, trehalose and xylose under salinity stress[13]. In addition, upregulated expression of some genes involved in zinc homeostasis, starch degradation, and the biosynthesis of wax, fatty acid, chlorophyll, and abscisic acid were observed in creeping bentgrass roots exposed to 250 mM NaCl containing 500 µM GABA solution, including cytochrome P450 (CYP450), zinc transporter 29 (ZTP29), alpha-amylase 3 (AMY3), 3-ketoacyl-CoA synthase 6 (KCS6), aldehyde oxidase (AO), acetyl-CoA carboxylase 1 (ACC1), and magnesium-chelatase (Mg-CHT)[38]. It appears that GABA ameliorates salinity tolerance by improving seed germination, shoot growth, photochemical efficiency, antioxidant activities and osmotic adjustment, and reducing Na+ accumulation and oxidative injury, and regulating genes involved in chlorophyll, carbon and hormone metabolism.
Application of an NO donor enhances salinity tolerance of turfgrass species. Foliar spray of 200 µM SNP reduced chlorotic and necrotic leaf tissue, increased leaf fresh weight and dry weight and leaf photochemical efficiency, and decreased leaf Na+ concentration, compared to the plants treated with H2O in perennial ryegrass cultivars at 200 mM NaCl[36]. Bermudagrass treated with 100 µM SNP was more tolerant to 400 mM NaCl stress by maintaining higher chlorophyll content, Fv/Fm, K+/Na+, Mg2+/Na+, and Ca2+/Na+ ratio, and lower levels of electrolyte leakage, MDA, H2O2, and activities of SOD, POD, and APX activities, compared to the untreated plants, while a spray of 200 µM NO inhibitor PTIO plus 200 µM L-NAME inhibited the NO-promoted positive effects on plant performance under salinity stress[39]. The results indicate that NO plays a role in maintaining cell membrane stability and ion homeostasis as well as alleviating oxidative damage, thus contributing to plant growth and salinity tolerance.
-
There are almost no research reports illustrating exogenous GABA effects on turfgrass tolerance to other abiotic stresses. A few publications have elucidated the role of NO in enhancing tolerance to other stresses including cadmium (Cd) in perennial ryegrass[40] and tall fescue[41, 42], copper (Cu) in perennial ryegrass[43], chromium (Cr) in tall fescue[44], and shade in tall fescue[45]. In perennial ryegrass, the addition of 100 µM SNP to the growing medium reduced toxicity of Cd stress by decreasing root-to-shoot translocation of Cd and increasing the activities of antioxidant enzymes in both roots and shoots of stressed plants[40]. The treatment of 200 μM SNP decreased the Cd content by 11% in tall fescue under 50 mg·L−1 Cd stress, while 100 μM c-PTIO, 200 μM L-NAME alone, or a combination of the two, increased Cd content by 24%[41]. In addition, application of 100 µM SNP improved photosystem II efficiency of tall fescue under 50, 300, 500 mg·L−1 Cd stress, while the 100 µM of NO synthesis inhibitor L-NAME suppressed the positive effects of NO[42]. Moreover, the integrated analyses of transcriptomics and metabolomics revealed 81 differentially expressed genes and 15 differentially expressed metabolites involved in 20 NO-induced pathways including antioxidant activities, secondary metabolites, arginine and proline metabolism, ABC transporters, and nitrogen metabolism[41]. When perennial ryegrasses were exposed to 200 μM Cu stress, the addition of 50, 100, and 200 μM SNP increased chlorophyll content, photosynthesis and antioxidant enzyme activities, and reduced oxidative damages, with the more pronounced effects on plant growth noted with 100 μM SNP treatment under Cu stress[43]. Application of 100 μM SNP improved physiological and photosynthetic activities of tall fescue against Cr stress[44]. Under shade condition, 100 µM SNP treatment increased leaf widths, chlorophyll and proline concentrations, and the activities of SOD, CAT, and POD, but decreased leaf lengths of tall fescue, to a greater extent under moderate shade compared to the low shade or heavy shade conditions[45]. Collectively, NO plays a positive role in maintaining growth, cell membrane integrity, antioxidant activities and function of the photosynthetic system, and alleviating oxidative damage for plants under various stress conditions.
-
The interactive effects of GABA and NO on abiotic stress resistance have been studied in plant species, but not extensively investigated in turfgrass. The mediation of GABA on NO production or vice versa could be beneficial to improving stress resistance of the plants. Exogenous application of NO inhibitor in the presence of GABA increased arsenate toxicity but addition of NO donor alleviated the adverse effects of NO inhibitor, indicating that NO was crucial in GABA-mediated arsenate tolerance in tomato (Solanum lycopersicum L.) and brinjal (Solanum melongena L.) seedlings[46]. When soybean (Glycine max L.) sprouts were exposed to salinity stress, treatment of GABA alleviated the inhibition of NO on phenolics biosynthesis by enhancing the production of NO, while NO donor treatment also alleviated the inhibition of GABA on phenolics biosynthesis[47].
Other reports also suggested that GABA-regulated stress responses were largely associated with NO production, but interestingly, effects of NO on plant responses seemed independent from GABA, at least in some plant species. In creeping bentgrass, application of 500 mM GABA to the roots increased endogenous NO production, depending on nitrate reductase and NO-associated protein pathways, which could be associated with the enhancement of antioxidant defense and whole-plant drought tolerance[15]. Similarly, GABA treatment increased salinity tolerance of wheat (Triticum aestivum L.) by enhancing antioxidant and photosynthetic capacity, proline metabolism and N–S assimilation, ion homeostasis, and plant growth through regulation of NO production[48]. The NO-or GABA-pretreated white clover (Trifolium repens L.) had higher NO content than the untreated plants under drought stress, which were correlated with higher relative growth rate, chlorophyll content, net photosynthetic rate, total antioxidant capacity, and accumulation of some key amino acids, sugars, organic acids, and sugar alcohols involved in the TCA cycle, but lower carbonyl content and electrolyte leakage[49]. Notably, the same study showed that SNP-pretreatment did not alter GABA content compared to the untreated plants under drought stress[49]. The similar results were found in muskmelon (Cucumis melo L.) that exogenous GABA enhanced endogenous NO content, nitrate reductase activity, and NO synthase activity, but SNP treatment did not change GABA level under the salinity–alkalinity stress[50]. It was suggested that NO might act as a downstream signal of GABA to regulate metabolic activity and increase the salinity–alkalinity stress tolerance of muskmelon[50]. Moreover, SNP treatment resulted in lower GABA content in tea (Camellia sinensis L.) roots under cold stress than the control[51], indicating that NO-improved the cold resistance of tea plant was not mainly through the GABA shunt. The interactive effects of GABA and NO are positive but also complicated in mediating plant stress resistance, and the exact coordinating mechanisms between these two molecules deserve further investigation, especially in turfgrass species.
-
Plants treated with GABA or NO donor exhibit better overall growth and greater stress resistance, compared to the nontreated plants of various turfgrass species. The enhanced stress resistance by exogenous GABA or NO is strongly associated with through enhancing photosynthetic and antioxidant defense capacities, maintenance of membrane integrity, osmotic adjustment and ion hemostasis, and increasing gene and protein expressions and accumulation of certain metabolites (Fig. 1). The crosstalk between GABA and NO plays a role in regulating plant responses to stress conditions.
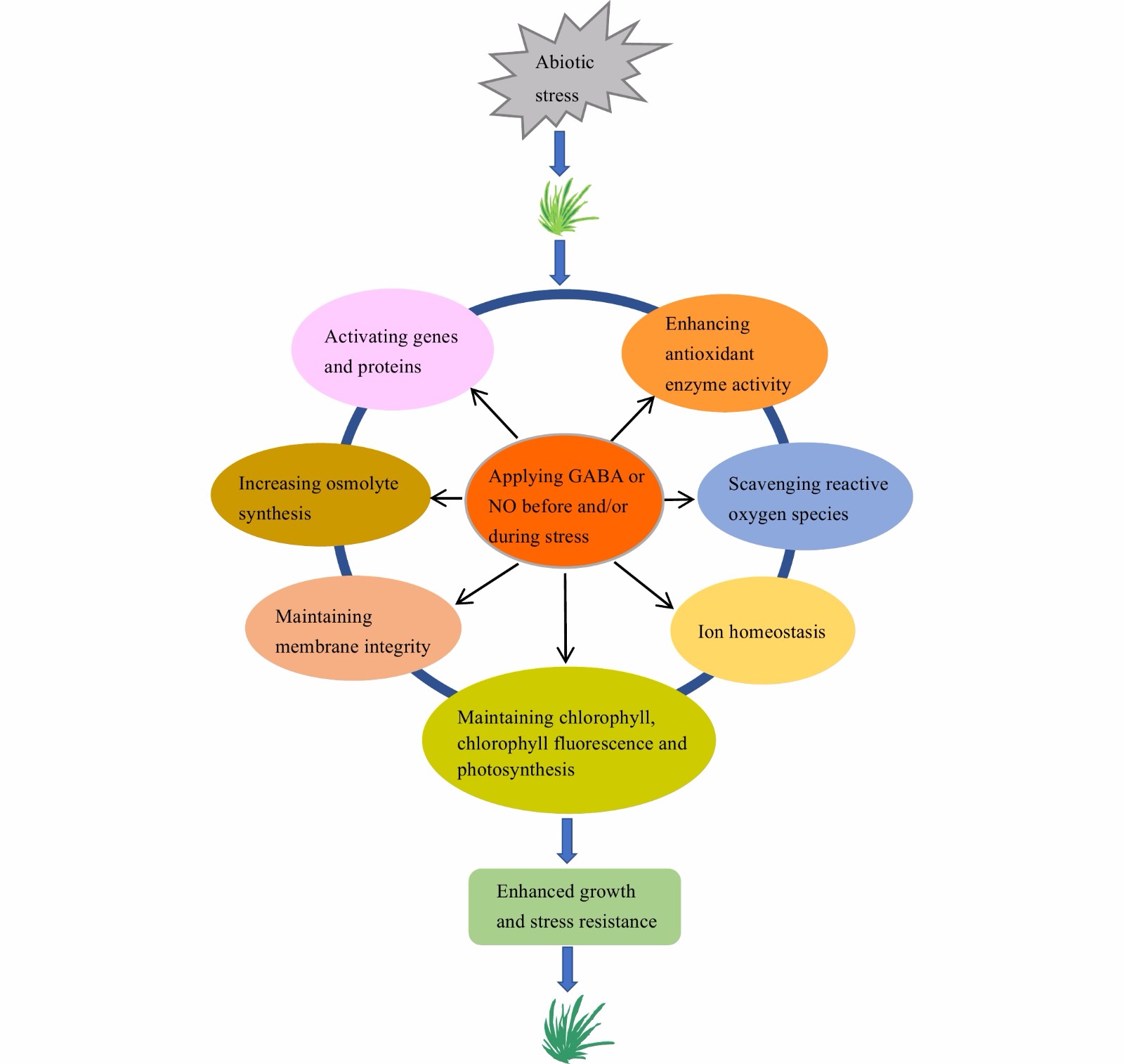
Figure 1.
A model illustrating exogenous application of γ-aminobutyric acid (GABA) and nitric oxide (NO) on improving turfgrass resistance to abiotic stresses.
To achieve maximum effects on stress resistance, multiple applications of chemicals before stress, during the stress treatment or after recovery are often conducted. However, the benefits of GABA or NO application are largely affected by chemical concentrations, stress intensity and duration, tissue type, as well as different turfgrass species and cultivars. Given the complexity of chemical action, further research work is necessary to identify GABA or NO effects on a broad spectrum of improvements in stress resistance. More specifically, it is not clear whether the GABA, NO or their interactions are effective against the combined abiotic stresses, such as drought and heat, drought and salinity, and whether there are significant genetic differences with turfgrass species and cultivars in responsiveness to one or a mixture of multiple chemical agents under abiotic stress conditions. Future research is needed to address chemical agents promoting plant resistance to multiple stresses in turfgrass species or cultivars. It is anticipated that chemical application will be more extensively used as a practical management tool to mitigate the adverse effects of abiotic stresses on turfgrass.
-
Yiwei Jiang is the Editorial Board member of Grass Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and his research group.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jiang Y. 2023. Application of gamma-aminobutyric acid and nitric oxide on turfgrass stress resistance: Current knowledge and perspectives. Grass Research 3:3 doi: 10.48130/GR-2023-0003
Application of gamma-aminobutyric acid and nitric oxide on turfgrass stress resistance: Current knowledge and perspectives
- Received: 08 December 2022
- Accepted: 15 February 2023
- Published online: 07 March 2023
Abstract: Gamma-aminobutyric acid (GABA) is a nonprotein amino acid that is well recognized as an endogenous plant signaling molecule. Nitric Oxide (NO) is a signaling molecule that has diverse biological functions in plants. There has been increasing research evidence that elucidates the protective roles of GABA and NO in improving plant growth and abiotic stress resistance of turfgrass species through enhancing photosynthetic and antioxidant defense capacities, maintenance of membrane integrity, osmotic adjustment and ion hemostasis, and increasing gene and protein expressions and accumulation of certain metabolites. Furthermore, the crosstalk between GABA and NO for modulating stress response are of interests in recent years. This review summarizes the current research advances of the physiological and molecular mechanisms involved in GABA- and NO- mitigated abiotic stress effects and discusses knowledge gaps in exploiting role of GABA an NO in turfgrass stress resistance. The review aims at promoting future research work towards a deeper understanding of GABA and NO applications in enhancing turfgrass management, production, and environmental adaptation.
-
Key words:
- Abiotic stress /
- GABA /
- Mechanism /
- Nitric oxide /
- Turfgrass












